Abstract
Purpose
To compare surgical outcomes of robotic radical hysterectomy (RRH) using 3 robotic arms with those of conventional laparoscopy in patients with early cervical cancer.
Materials and Methods
A retrospective cohort study included 102 patients with stage 1A1-IIA2 cervical carcinoma, of whom 60 underwent robotic and 42 underwent laparoscopic radical hysterectomy (LRH) with pelvic lymph node dissection performed between December 2009 and May 2013. Perioperative outcomes were compared between two surgical groups.
Results
Robotic approach consisted of 3 robotic arms including the camera arm and 1 conventional assistant port. Laparoscopic approach consisted of four trocar insertions with conventional instruments. There were no conversions to laparotomy. Mean age, body mass index, tumor size, cell type, and clinical stage were not significantly different between two cohorts. RRH showed favorable outcomes over LRH in terms of estimated blood loss (100 mL vs. 145 mL, p=0.037), early postoperative complication rates (16.7% vs. 30.9%, p=0.028), and postoperative complications necessitating intervention by Clavien-Dindo classification. Total operative time (200.5±61.1 minutes vs. 215.6±83.1 minutes, p=0.319), mean number of lymph node yield (23.3±9.3 vs. 21.7±9.8, p=0.248), and median length of postoperative hospital stay (11 days vs. 10 days, p=0.129) were comparable between robotic and laparoscopic group, respectively. The median follow-up time was 44 months with 2 recurrences in the robotic and 3 in the laparoscopic cohort.
Conclusion
Surgical outcomes of RRH and pelvic lymphadenectomy were comparable to that of laparoscopic approach, with significantly less blood loss and early postoperative complications.
Keywords: Cervical cancer, laparoscopy, robotics
INTRODUCTION
Laparoscopic surgery has been known to have advantages over laparotomy, including reduced pain, improved aesthetics, shorter length of hospital stay, and faster return to normal activities.1,2 These benefits of laparoscopy have driven efforts to achieve further development in minimally invasive surgical technology, one of which is the incorporation of robotic assistance. Since Food and Drug Administration approval for the use of the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) in gynecologic procedures in 2005, many studies have demonstrated the advantages of robotic assistance in overcoming the drawbacks of conventional laparoscopy.3,4,5 These advantages include a magnified three-dimensional view, wider range of motion with wristed instruments, and improved surgeon ergonomics with surgical stability.6,7 Therefore, when a surgeon performs an extensive gynecologic oncology procedure such as radical hysterectomy with lymph node dissection, the robotic assistance is theoretically expected to help accomplish complex tasks such as deep pelvic space dissection, adhesiolysis, ureterolysis, and multiple suturing with ease, compared to conventional straight stick laparoscopy.
For early stage cervical cancer treatment, adequate radical hysterectomy followed by tailored adjuvant therapy is of paramount importance for prognosis.8,9 Conventional laparoscopic radical hysterectomy (LRH) for these patients has come to be regarded as a reasonable alternative to open surgical method, and more recently, the application of robotic technology has shown to be feasible and safe as well.10,11,12,13,14,15 However, there is still a lack of studies addressing the surgical and oncologic outcomes of these two minimally invasive methods, since robot-assisted radical hysterectomy (RLH) is a relatively novel surgical technique that has not yet been studied in a randomized controlled trial setting.16 Also, in countries where high cost is one of the biggest concerns in adopting robotic surgery, there may be different surgical outcomes due to modification of surgical practice such as applying strict eligibility criteria for robotic approach or reducing the number of robotic arms.17
Nonetheless, minimally invasive surgical approach may provide benefit for patients with gynecological malignancies because of earlier postoperative interventions such as adjuvant chemotherapy or radiation due to less complications and a faster recovery. Therefore, the purpose of this study was to compare the surgical outcomes of robotic radical hysterectomy (RRH) using only 3 robotic arms with those of conventional four-port laparoscopic radical hysterectomy (LRH) in patients with early cervical cancer.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board at Yonsei University College of Medicine. After excluding hysterectomy for benign conditions, endometrial cancer staging, and fertility sparing trachelectomy, a total of 102 patients who received radical hysterectomy with pelvic lymph node dissection±paraaortic lymph node sampling performed between December 2009 and May 2013 were identified. Inclusion criteria were women with newly diagnosed untreated invasive cervical cancer, International Federation of Gynecology and Obstetrics (FIGO) stage IIA2 or less disease with Gynecologic Oncologic Group performance score ≤1. Another specific inclusion criteria for robotic surgery was the financial capability to pay the surgical cost ($10000). Exclusion criteria for performing laparoscopic surgery were patients with uterine size greater than 16 gestational weeks by pelvic examination and those with previous history of 3 or more open abdominal surgeries. All procedures were performed by surgeons experienced and proficient in advanced laparoscopic gynecologic procedures. The surgical team consisted of a chief resident or fellow as surgical assistants at the bedside or at the caudal part of the patient for uterine manipulation.
Data pertaining to patient characteristics (age, parity, body mass index, and general health status) and perioperative parameters including docking time (DT), console time (CT), total operative time (OT), estimated blood loss, number of retrieved lymph nodes, pathologic analysis, length of hospital stay, and perioperative complications were retrospectively reviewed from the prospectively entered computerized database. The total OT was the time from the first skin incision to the last port site skin closure. Number of lymph node retrieval was the number of pelvic lymph nodes identified at pathologic analysis. Complications were categorized as intraoperative and postoperative (early/late) events. Clavien-Dindo classification was used to stratify complications into five grades according to their therapeutic interventions.18
Surgical techniques
After general anesthesia, the patient was placed in a low dorsal lithotomy and steep Trendelenburg position. A nelaton catheter was inserted to drain the bladder. RUMI uterine manipulator with a Koh colpotomy ring and vaginal balloon pneumo-occluder (Cooper Surgical Inc., Trumball, CT, USA) was routinely placed for adequate pelvic exposure. Ports were placed after creating pneumoperitoneum by Veress needle insertion or by open Hasson method at the umbilicus. The surgical management of cervical cancer included radical hysterectomy with removal of bilateral pelvic lymph nodes as described in our previous report.19 The decision to perform paraaortic lymph node dissection was at the surgeon's discretion, up to the level of inframesenteric artery. Modified radical or radical hysterectomy was performed according to disease extent. Nerve sparing radical hysterectomy is not a routine practice at our institution. In brief, the procedure consisted of eight component parts: 1) development of the paravesical and pararectal spaces, 2) right and left pelvic lymphadenectomy from common iliac nodes to bilateral inguinal nodes, 3) ureteral dissection, 4) ligation and dissection of the uterine artery, 5) development of the vesicouterine and rectovaginal spaces, 6) resection of the parametria, 7) resection of the upper vagina, and 8) vaginal cuff closure. Adequacy of the component parts of this procedure was routinely determined and documented on video.
Robotic approach
All robotic surgeries were performed using the da Vinci robotic surgical system (Intuitive Surgical Inc., Sunnyvale, CA, USA) with Maryland Bipolar and Permanent Cautery Spatula or needle holder on each robotic arm. Four trocars with three robotic arms were used for port placement: a 12-mm conventional laparoscopic trocar at the umbilicus for the camera, two 8-mm lateral robotic trocars at each lower quadrant of the abdomen 2 to 3 cm below the umbilical level, and a fourth conventional trocar (either 5 or 10 mm) at mid-distance between the umbilicus and the left robotic arm for the bedside assistant (Fig. 1A). The bedside assistant assisted procedures such as suction, irrigation, retraction of tissues, and lymph node retrieval through the 5 or 10 mm trocar placed on the left side of the patient. DT was defined as the time to position the robot and install the robotic arms securely to the port sites. The CT was defined as the time spent by the surgeon at the robotic console during the main procedure. The vaginal cuff was closed intracorporeally using interrupted or continous sutures of 1-Vicryl (Ethicon, Piscataway, NJ, USA) or extracorporeally using a Clarke-Reich knot pusher. Upon completion of the procedure, the fascia of the port sites greater than 8 mm in diameter were closed with interrupted suture using 1-0 Vicryl (Ethicon, Piscataway, NJ, USA).
Fig. 1.
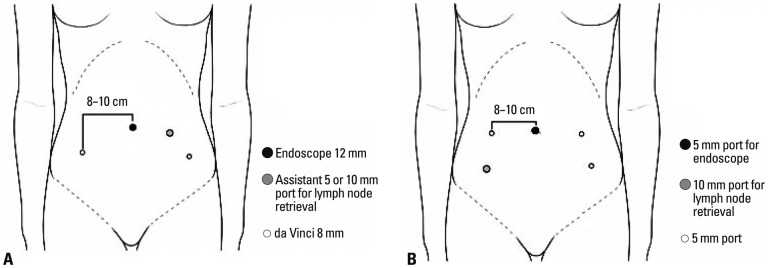
Port placement. (A) Robotic radical hysterectomy. (B) Laparoscopic radical hysterectomy.
Laparoscopic approach
For conventional LRH, a 5-mm trocar was inserted as the camera port through a vertical incision made inside the umbilicus. A 30 cm length 30-degree endoscope with 5 mm in diameter was used. The surgeon was at the left side of the patient using the two 5 mm ports on the left side. A 5 or 11 mm trocar was placed in the right lower quadrant for the first assistant (Fig. 1B). For both laparoscopic and robotic procedures, Lap bag (Sejong Medical, Paju, Korea) for intraperitoneal lymph node storage during lymphadenectomy was introduced if necessary. Since there were bulky lymph nodes that needed to be extracted safely without spillage into the trocar or in the peritoneal cavity, this method was used rather than retrieving each lymph nodes through the assistant trocar. Gathered lymph nodes were removed transvaginally altogether after the completion of hysterectomy. Conventional instruments such as monopolar hook and bipolar graspers were used. Energy devices such as Harmonic Ace (Ethicon Endo-surgery, Cincinnati, OH, USA) or LigaSure system (Valleylab Inc., Boulder, CO, USA) were used according to surgeon's preference. For vaginal cuff closure, a 1-0 Vicryl with a 40-mm round-bodied needle was introduced through the 11-mm port and closed intracorporeally in a continuous running suture method. The fascia of the ancillary port site on the right lower quadrant was approximated with 1-0 Vicryl only when a 11-mm trocar was inserted. All wounds of laparoscopic incision sites equal or less than 5 mm were closed only with Steri-Strips™ (3M, St. Paul, MN, USA).
Statistical analysis was performed with IBM SPSS version 20 for Windows (SPSS Inc., Chicago, IL, USA), and Kolmogorov Smirnov test was used to verify standard normal distributional assumptions. Student's t-test and Mann-Whitney U test were used for parametric and non-parametric variables, respectively. Differences between proportions were compared using Fisher's test or χ2 test. A p value of less than 0.05 was regarded as statistically significant. Survival outcome was estimated using the Kaplan-Meier method and groups were compared by log-rank test.
RESULTS
One hundred two consecutive radical hysterectomy procedures performed by robotic or conventional laparoscopic approach were identified during the study period. Patient characteristics are shown in Table 1. The mean age was less than 50 years, and most of the patients (75/102, 73.5%) had clinical FIGO stage 1B1 disease with predominantly squamous cell carcinoma. Other histology included adenosquamous and small cell types. Body mass index and uterine weight were not significantly different between the two surgical cohorts. All cases were completed through robot or laparoscopic approach without conversion to laparotomy. According to Piver classification, 12 cases (28.6%) and 30 cases (71.4%) received type 2 and type 3 radical hysterectomy, respectively, in the laparoscopic cohort. There was no significant difference in radicality in the robotic group as well, with 11 cases (18.6%) and 48 cases (81.4%) of type 2 and 3 radical hysterectomy, respectively (p=0.336). Surgical types of radical hysterectomy were further divided according to the Querleu and Morrow20 classification (Table 2). Only 2 cases in each surgical cohort underwent autonomic nerve sparing radical hysterectomy (type C1) since it is not a routine practice at our institution. There was no difference in the total OT between the two groups (200.5 and 215.6 minutes for robotic and laparoscopic group, respectively; p=0.319). The mean CT of the robotic cohort was 152 minutes. There was no significant difference in the acquired number of pelvic and para-aortic lymph nodes between the two cohorts, although a significantly less amount of blood loss was seen in the robotic group (median 100 mL, range 30-600 mL, p=0.037). The case with the least amount of blood loss was measured in a patient with stage 1A1 disease with lymphovascular space invasion who underwent modified radical hysterectomy with pelvic lymph node dissection. Postoperative pathologic data including tumor size, invasion depth, and lymph node metastasis did not show significant difference between the two groups. The mean survival time in the cohorts was 40.7 months in the robotic group (2 recurrences) and 42.0 months in the laparoscopic group (3 recurrences), yielding a 3-year relapse-free survival of 96.4% and 91.9%, respectively, without statistical significance (data not known, p=0.482). One death occurred in a patient with FIGO stage 1B1 disease with small cell type who underwent RRH with bilateral pelvic lymph node dissection. Although she received postoperative chemotherapy due to high risk histologic type, pelvic recurrence occurred postoperative 5 months and subsequent death 21 months after the treatment.
Table 1.
Overall Patient Characteristics (n=102)
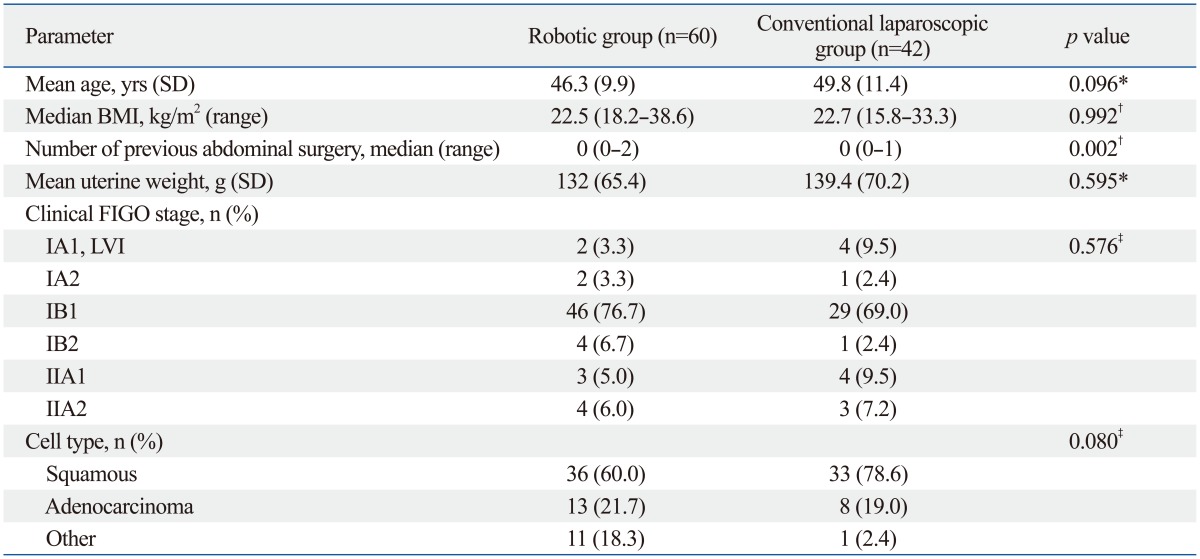
BMI, body mass index; SD, standard deviation; FIGO, International Federation of Gynecology and Obstetrics; LVI, lymphovascular space infiltration.
*Student's t-test.
†Mann-Whitney U test.
‡Fisher's exact test.
Table 2.
Comparison of Perioperative Outcomes by Surgical Approach
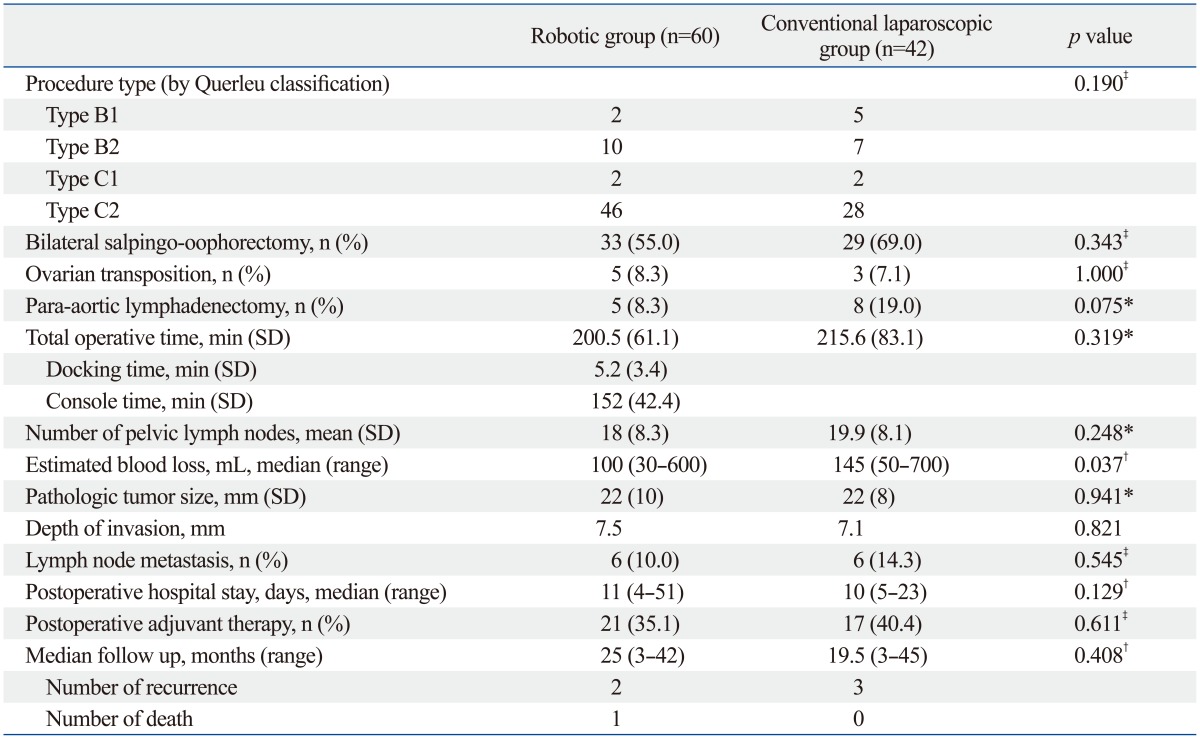
SD, standard deviation.
*Student's t-test.
†Mann-Whitney U test.
‡Fisher's exact test.
Intraoperative and postoperative complications (including all minor complications such as transient febrile event to major complications including bowel injury) occurred in 13 patients (21.7%) in the robotic cohort and in 21 patients (50.0%) in the conventional laparoscopic group (Table 3). Early postoperative complications (<6 weeks after surgery) were significantly lower in the robotic group than laparoscopic group (16.7% vs. 30.9%, respectively, p=0.028). Most of early and late postoperative complications were spontaneously resolved by conservative management (grade 1 or 2 by Clavien-Dindo classification), except one case of panperitonitis from bowel perforation, which developed postoperatively in the 59th patient of the robotic cohort that needed surgical correction (grade IIIB). An inadvertent surgical intervention needed in the conventional laparoscopic cohort was a case of remnant peritoneal drain catheter after an attempt for removal. The patient underwent emergency laparoscopic removal of the catheter tip left inside the peritoneal cavity. The overall postoperative complications after 6 weeks were found to be higher in the conventional laparoscopic group with only marginal statistical significance (p=0.054). Also, postoperative complications higher than grade 3, defined as those requiring surgical or radiological intervention, occurred in 2 patients each of robotic (3.4%) and laparoscopic (4.8%) group (p=0.021).
Table 3.
Complications
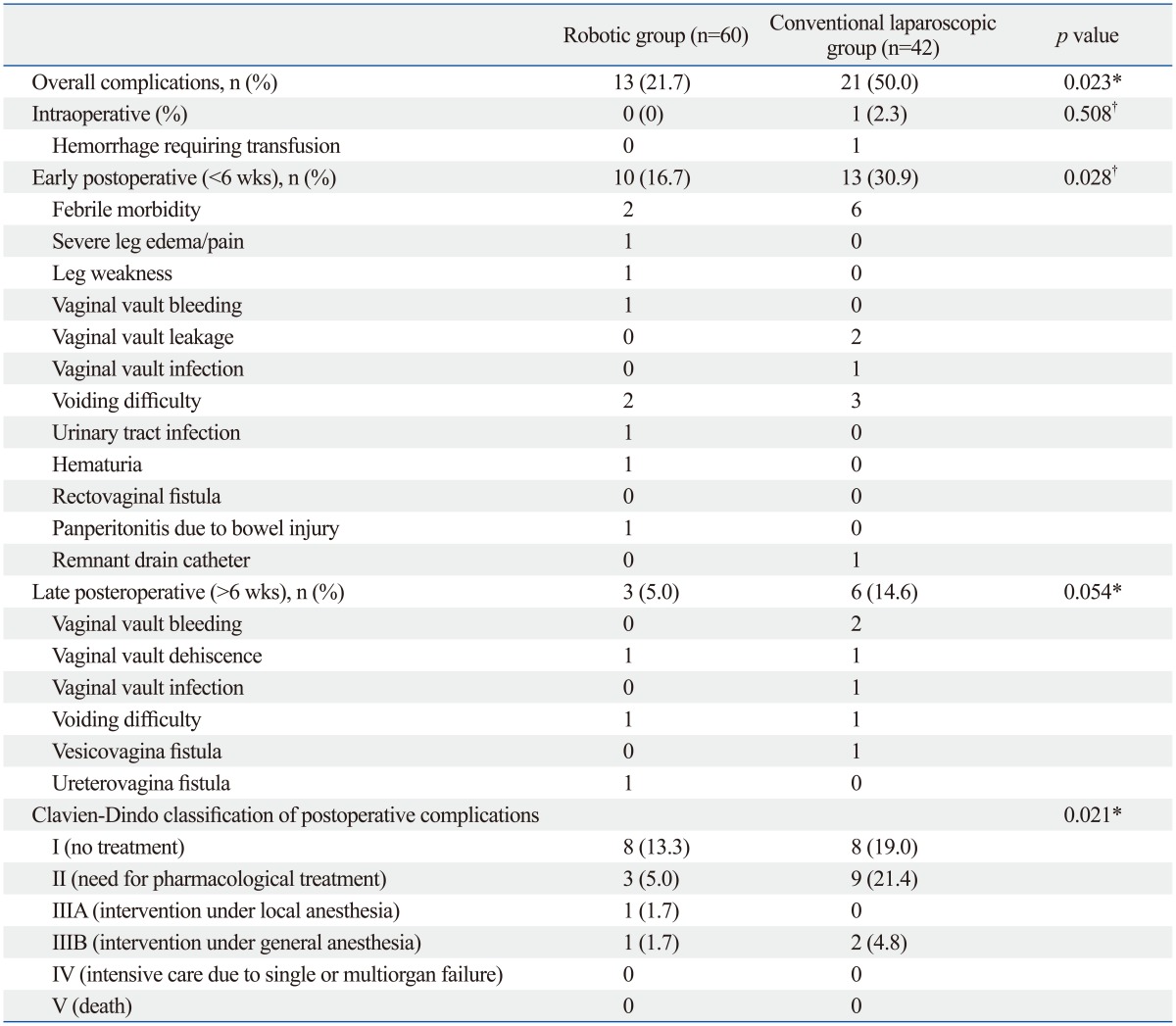
*Fisher's exact test.
†Chi-square.
DISCUSSION
The present results showed comparable surgical outcomes of RRH to that of conventional laparoscopic approach in the treatment of early cervical cancer, with lower intraoperacative blood loss and early complication rates.
The clinical impact of robotic surgery in gynecologic field is growing widely, as suggested by a recent consensus statement made by the Society of Gynecologic Oncology in 2012.21 The society has stated that the current evidence supports at least an equivalence of robotic surgery and laparoscopy in many perioperative outcomes. However, there is a lack of disease specific oncologic outcomes and the cost is still a potential barrier to the widespread acceptance of robotic surgery.19,22 The cost barrier is a problem not only in the U.S., but also in Korea especially where this relatively new technology is not covered by the national insurance. Nevertheless, the number of robotic surgeries is growing and the implementation of robotic surgery in gynecologic oncology has made a dramatic change in the proportion of minimally invasive surgery, ranging from 3.3% to 43.5%, as shown in a study by Hoekstra, et al.23 Likewise, the number of robotic surgery for gynecologic malignancies has rapidly increased from 21 to over 300 cases in 2012 at our institution since the implement of robotic platform in 2006. Despite the universal increase in robotic surgery, there have been only 27 studies evaluating the surgical outcomes of RRH until 2011 in comparison with LRH with relatively small number of subjects.16 A recent meta-analysis indicated that comparison of robotic and conventional laparoscopic surgery was not feasible due to insufficiency in studies that assessed proper 'radical' hysterectomy solely for cervical cancer.24 This suggests a need for more comparative studies with consistency in the operative procedures performed. In this study cohort, all cases were performed according to Piver classification of modified radical or radical hysterectomy by clinical stage, and further reclassified retrospectively according to the new Querleu and Morrow classification to clarify whether or not nerve preservation and paracervical nodal removal were performed. Also, this study is one of the few reports that included fairly a large number of patients in the robotic cohort consisting of only radical hysterectomy.
The findings of this study are comparable with a recent review by Kruijdenberg, et al.,16 which compared surgical outcomes of 11 studies (342 patients) of RRH versus 18 studies (914 patients) of LRH. There was no difference in OT and lymph node yields between robotic and laparoscopic approach, however, significantly shorter hospital stay was found after RRH, similar to the results of our study. In our series, the intraoperative blood loss and complications rates were significantly lower in the robotic cohort as well. Interestingly, Kruijdenberg, et al.16 showed that although the total percentage of major intraoperative complications was comparable in both methods, the 'type' of complications differed according to the treatment method. Despite the small patient population in the RRH publications, more nerve injury was observed during robotic surgery whereas higher vascular and bladder injury was seen during conventional laparoscopic surgeries. The authors suggested that these findings are associated with improved visualization and ability to coagulate fast, and dissect more precisely with the robotic instruments due to filtered tremor and enhanced approachability to the surgical field. As for the nerve injury, they speculated possible thermal energy damage produced by predominantly monopolar and bipolar robotic cautery. Likewise, there seemed to be a trend in the complications in our series as well. Although the overall complication rate was statistically lower in the RRH cohort, the incidence of severe leg edema and leg weakness was more frequent in the RRH group. Our hypothesis is that enhanced visualization and ergonomics might have provided the ease for extensive pelvic space dissection, which led to more profound leg lymph edema due to increased lymph node dissection. However, it is difficult to support this finding with the number of lymph node yields obtained in this study, since the lymph node counting method is not standardized at our institution. This is one of the limitations of our data, that the number of lymph nodes in a bulky pelvic lymphatic tissue was not entirely counted, according to the protocol of the pathologic department. Another interesting finding is the higher number of vaginal vault-related complications such as bleeding, dehiscence, and infection after conventional laparoscopy. Since suture and knot tying are technically more difficult than that with the wristed robotic instruments, the vaginal vault would have been closed more firmly and completely through the robotic approach. However, this finding is inconclusive since vaginal vault closure per surgical method was not uniform in the entire cohort. Further analysis with a larger cohort and thorough evaluation of suture methods may clarify this matter. Nevertheless, the overall postoperative complications requiring pharmacological and surgical intervention (Clavien-Dindo classification grade of 2-3B) were significantly lower in the robotic group (5/60, 8.3%), compared with laparoscopic group (11/42, 26.2%, p=0.021).
The reason for using only 3 robotic arms for all robotic gynecologic surgery at our institution is directly linked to cost issues. High cost is the major obstacle for deciding robotic surgical approach and also for launching a randomized clinical study. The patient expense for robotic cancer surgery is approximately 4 times higher than the conventional laparoscopic surgery, since the robotic surgical cost is not reimbursed by the National health insurance. Therefore, by eliminating one robotic arm, the patient can save approximately US $500. So far, we have performed successful robotic surgeries with only 3 robotic arms without major technical difficulties. The function of a 4th arm had been performed by the bedside assistant, and we believe that this is also important for training purposes, since robotic surgery provides a unique challenge with regard to resident training because of remote location from the surgeon console.25 Three-arm usage is a unique characteristic, unlike many other studies on robotic gynecologic surgery that use mostly four robotic arms for radical hysterectomy.16,26,27 Another factor that is affected by the overall cost is the length of hospital stay. The number of days was not comparable between the two surgical methods because of routine postoperative bladder catheter training prior removal and also the patients' preference to remain hospitalized for a prolonged period of time despite a minimally invasive surgery. The biggest reason is the very low hospital admission fee due to comprehensive national insurance coverage in Korea.
Oncologic outcome and survival are important factors to analyze when implementing a novel surgical approach. Although the survival data were not analyzed in detail in this study, Kaplan-Meier survival analysis demonstrated 96.4% recurrence-free survival (RFS) at 40.7 months due to the recurrence of 2 patients in the RRH cohort and 91.9% RFS at 42 months due to 3 recurrences in the LRH cohort, without statistical difference (p=0.482). This finding is compatible with the previous few reports addressing survival. In 2010, Cantrell, et al.28 analyzed their 3-year experience of RRH for stage 1A1 to 2B cervical cancer. Median follow up time was 12.2 months, and both the progression-free and overall survival (OS) was equivalent to that of the historical open surgical cohort (94% PFS and 94% OS at 36 months in RRH cohort). Similarly, a multicenter study by Tinelli, et al.29 compared surgical outcomes and survival of robotic approach to that of laparoscopic approach on radical hysterectomy. Although the total number of patients recruited from 4 centers was small (23 cases in the robotic cohort), they found no difference in recurrence rate between the two cohorts during the 24.5 and 46.5 months of median follow up time for laparoscopic and robotic group, respectively. One important consideration that should be taken into account in the future study is the involvement of remnant or transposed ovaries in recurrence and metastasis after radical hysterectomy. Ovarian metastasis of cervical cancer has been documented in the literature, although the effect of histologic type is uncertain.30,31,32 In a large cohort of radical hysterectomy series, there was a significantly higher ovarian metastasis from adenocarcinoma (3.72%) than squamous cell types (0.22%) in stage IB cervical carcinoma.31 In our cohort, 56 patients (54.9%) were premenopausal, and 17 patients (30.3%) among them, underwent bilateral salpingo-oophorectomy, and only 8 patients received ovarian transposition. Our practice is to provide options for ovarian preservation to all premenopausal women unless there are radiologic or intraoperative evidence of ovarian metastasis. Although the possibility of ovarian micrometastasis is low, it is still an oncologic dilemma when performing fertility sparing surgeries.33 Therefore, oncologic outcome as well as patient preference should be taken into account when counseling premenopausal women.
The limitation of this study is its retrospective nature. Selection bias might have been introduced since the surgical method was not randomized but determined by the surgeon. However, there was no statistical difference in the important baseline clinicopathologic characteristics that may affect surgical outcomes. One of the strengths of this study is the relatively large number of study subjects with cervical cancer who received surgical treatment with consistency in its radicality. Data available in the literature on RRH in comparison with conventional laparoscopy is limited, as evidenced by the recent review. 21
In conclusion, surgical outcomes of RRH and pelvic lymphadenectomy were comparable to that of laparoscopic approach, with favorable outcomes in regards to intraoperative blood loss and postoperative complications. Further studies on long term surgical outcome and survival are needed to confirm potential oncologic benefits of RRH compared with conventional laparoscopic treatment.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A084120).
Footnotes
Presented at the Annual Congress of the Asia-Pacific Association Gynecologic Endoscopy and Minimally Invasive Therapy (APAGE), November 2nd, 2013, Seoul, South Korea.
The authors have no financial conflicts of interest.
References
- 1.Medeiros LR, Rosa DD, Bozzetti MC, Fachel JM, Furness S, Garry R, et al. Laparoscopy versus laparotomy for benign ovarian tumour. Cochrane Database Syst Rev. 2009:CD004751. doi: 10.1002/14651858.CD004751.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Nieboer TE, Johnson N, Lethaby A, Tavender E, Curr E, Garry R, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009:CD003677. doi: 10.1002/14651858.CD003677.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Advincula AP, Wang K. Evolving role and current state of robotics in minimally invasive gynecologic surgery. J Minim Invasive Gynecol. 2009;16:291–301. doi: 10.1016/j.jmig.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Lin PS, Wakabayashi MT, Han ES. Role of robotic surgery in endometrial cancer. Curr Treat Options Oncol. 2009;10:33–43. doi: 10.1007/s11864-009-0086-4. [DOI] [PubMed] [Google Scholar]
- 5.Mendivil A, Holloway RW, Boggess JF. Emergence of robotic assisted surgery in gynecologic oncology: American perspective. Gynecol Oncol. 2009;114(2 Suppl):S24–S31. doi: 10.1016/j.ygyno.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Chen CC, Falcone T. Robotic gynecologic surgery: past, present, and future. Clin Obstet Gynecol. 2009;52:335–343. doi: 10.1097/GRF.0b013e3181b08adf. [DOI] [PubMed] [Google Scholar]
- 7.Nezhat C, Lavie O, Lemyre M, Unal E, Nezhat CH, Nezhat F. Robot-assisted laparoscopic surgery in gynecology: scientific dream or reality? Fertil Steril. 2009;91:2620–2622. doi: 10.1016/j.fertnstert.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 8.Lee TY, Jeung YJ, Lee CJ, Kim HY, Kim SH, Kim WG. Promising treatment results of adjuvant chemotherapy following radical hysterectomy for intermediate risk stage 1B cervical cancer. Obstet Gynecol Sci. 2013;56:15–21. doi: 10.5468/OGS.2013.56.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Kim YS, et al. Comparison of outcomes between radical hysterectomy followed by tailored adjuvant therapy versus primary chemoradiation therapy in IB2 and IIA2 cervical cancer. J Gynecol Oncol. 2012;23:226–234. doi: 10.3802/jgo.2012.23.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geisler JP, Orr CJ, Khurshid N, Phibbs G, Manahan KJ. Robotically assisted laparoscopic radical hysterectomy compared with open radical hysterectomy. Int J Gynecol Cancer. 2010;20:438–442. doi: 10.1111/IGC.0b013e3181cf5c2c. [DOI] [PubMed] [Google Scholar]
- 11.Nezhat CR, Nezhat FR, Burrell MO, Ramirez CE, Welander C, Carrodeguas J, et al. Laparoscopic radical hysterectomy and laparoscopically assisted vaginal radical hysterectomy with pelvic and paraaortic node dissection. J Gynecol Surg. 1993;9:105–120. doi: 10.1089/gyn.1993.9.105. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez PT, Soliman PT, Schmeler KM, dos Reis R, Frumovitz M. Laparoscopic and robotic techniques for radical hysterectomy in patients with early-stage cervical cancer. Gynecol Oncol. 2008;110(3) Suppl 2:S21–S24. doi: 10.1016/j.ygyno.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Soliman PT, Frumovitz M, Sun CC, Dos Reis R, Schmeler KM, Nick AM, et al. Radical hysterectomy: a comparison of surgical approaches after adoption of robotic surgery in gynecologic oncology. Gynecol Oncol. 2011;123:333–336. doi: 10.1016/j.ygyno.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spirtos NM, Eisenkop SM, Schlaerth JB, Ballon SC. Laparoscopic radical hysterectomy (type III) with aortic and pelvic lymphadenectomy in patients with stage I cervical cancer: surgical morbidity and intermediate follow-up. Am J Obstet Gynecol. 2002;187:340–348. doi: 10.1067/mob.2002.123035. [DOI] [PubMed] [Google Scholar]
- 15.Yim GW, Kim SW, Nam EJ, Kim YT. Role of robot-assisted surgery in cervical cancer. Int J Gynecol Cancer. 2011;21:173–181. doi: 10.1097/IGC.0b013e318200f7a7. [DOI] [PubMed] [Google Scholar]
- 16.Kruijdenberg CB, van den Einden LC, Hendriks JC, Zusterzeel PL, Bekkers RL. Robot-assisted versus total laparoscopic radical hysterectomy in early cervical cancer, a review. Gynecol Oncol. 2011;120:334–339. doi: 10.1016/j.ygyno.2010.12.342. [DOI] [PubMed] [Google Scholar]
- 17.Jung YW, Lee DW, Kim SW, Nam EJ, Kim JH, Kim JW, et al. Robot-assisted staging using three robotic arms for endometrial cancer: comparison to laparoscopy and laparotomy at a single institution. J Surg Oncol. 2010;101:116–121. doi: 10.1002/jso.21436. [DOI] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YT, Kim SW, Hyung WJ, Lee SJ, Nam EJ, Lee WJ. Robotic radical hysterectomy with pelvic lymphadenectomy for cervical carcinoma: a pilot study. Gynecol Oncol. 2008;108:312–316. doi: 10.1016/j.ygyno.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Querleu D, Morrow CP. Classification of radical hysterectomy. Lancet Oncol. 2008;9:297–303. doi: 10.1016/S1470-2045(08)70074-3. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez PT, Adams S, Boggess JF, Burke WM, Frumovitz MM, Gardner GJ, et al. Robotic-assisted surgery in gynecologic oncology: a Society of Gynecologic Oncology consensus statement. Developed by the Society of Gynecologic Oncology's Clinical Practice Robotics Task Force. Gynecol Oncol. 2012;124:180–184. doi: 10.1016/j.ygyno.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Sarlos D, Kots L, Stevanovic N, Schaer G. Robotic hysterectomy versus conventional laparoscopic hysterectomy: outcome and cost analyses of a matched case-control study. Eur J Obstet Gynecol Reprod Biol. 2010;150:92–96. doi: 10.1016/j.ejogrb.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Hoekstra AV, Morgan JM, Lurain JR, Buttin BM, Singh DK, Schink JC, et al. Robotic surgery in gynecologic oncology: impact on fellowship training. Gynecol Oncol. 2009;114:168–172. doi: 10.1016/j.ygyno.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill M, Moran PS, Teljeur C, O'Sullivan OE, O'Reilly BA, Hewitt M, et al. Robot-assisted hysterectomy compared to open and laparoscopic approaches: systematic review and meta-analysis. Arch Gynecol Obstet. 2013;287:907–918. doi: 10.1007/s00404-012-2681-z. [DOI] [PubMed] [Google Scholar]
- 25.Thiel DD, Lannen A, Richie E, Dove J, Gajarawala NM, Igel TC. Simulation-based training for bedside assistants can benefit experienced robotic prostatectomy teams. J Endourol. 2013;27:230–237. doi: 10.1089/end.2012.0382. [DOI] [PubMed] [Google Scholar]
- 26.Reynisson P, Persson J. Hospital costs for robot-assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy. Gynecol Oncol. 2013;130:95–99. doi: 10.1016/j.ygyno.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Vizza E, Patrizi L, Saltari M, Sindico S, Cimino M, Corrado G. Robotic radical hysterectomy after neoadjuvant chemotherapy in locally advanced cervical cancer. Minim Invasive Ther Allied Technol. 2012;21:206–209. doi: 10.3109/13645706.2012.672426. [DOI] [PubMed] [Google Scholar]
- 28.Cantrell LA, Mendivil A, Gehrig PA, Boggess JF. Survival outcomes for women undergoing type III robotic radical hysterectomy for cervical cancer: a 3-year experience. Gynecol Oncol. 2010;117:260–265. doi: 10.1016/j.ygyno.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Tinelli R, Malzoni M, Cosentino F, Perone C, Fusco A, Cicinelli E, et al. Robotics versus laparoscopic radical hysterectomy with lymphadenectomy in patients with early cervical cancer: a multicenter study. Ann Surg Oncol. 2011;18:2622–2628. doi: 10.1245/s10434-011-1611-9. [DOI] [PubMed] [Google Scholar]
- 30.Brockbank EC, Evans J, Singh N, Shepherd JH, Jeyarajah AR. Ovarian recurrence from a Stage 1b1 cervical adenocarcinoma previously treated with radical vaginal trachelectomy: a case report. Gynecol Oncol Case Rep. 2012;2:51–53. doi: 10.1016/j.gynor.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimada M, Kigawa J, Nishimura R, Yamaguchi S, Kuzuya K, Nakanishi T, et al. Ovarian metastasis in carcinoma of the uterine cervix. Gynecol Oncol. 2006;101:234–237. doi: 10.1016/j.ygyno.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Gizzo S, Ancona E, Patrelli TS, Saccardi C, Anis O, Donato D, et al. Fertility preservation in young women with cervical cancer: an oncologic dilemma or a new conception of fertility sparing surgery? Cancer Invest. 2013;31:189. doi: 10.3109/07357907.2013.767343. [DOI] [PubMed] [Google Scholar]
- 33.Sicam RV, Huang KG, Lee CL, Chen CY, Ueng SH. Treatment of fallopian tube metastasis in cervical cancer after laparoscopic ovarian transposition. J Minim Invasive Gynecol. 2012;19:262–265. doi: 10.1016/j.jmig.2011.12.006. [DOI] [PubMed] [Google Scholar]


