Abstract
Purpose
Impaired cardiovascular autonomic regulation is a non-motor symptom of Parkinson's disease (PD) and may increase long-term morbidity. This study applied frequency-domain analysis of heart rate variability (HRV) to understand the progression of sympathetic and parasympathetic cardiac regulation in patients with PD.
Materials and Methods
In this cross-sectional study, 21 male and 11 female Taiwanese patients with advanced PD and 32 healthy gender- and age-matched subjects were enrolled. To minimize artifacts due to subject motion, daytime electrocardiograms for 5 minutes were recorded in awake patients during levodopa-on periods and controls. Using fast Fourier transformation, heart rate variables were quantified into a high-frequency power component [0.15-0.45 Hz, considered to reflect vagal (parasympathetic) regulation], low-frequency power component (0.04-0.15 Hz, reflecting mixed sympathetic and parasympathetic regulation), and low-frequency power in normalized units (reflecting sympathetic regulation). The significance of between-group differences was analyzed using the paired t-test. Pearson correlation analysis and stepwise regression analysis were applied to assess the correlation of patient age, PD duration, and disease severity (represented by the Unified Parkinson's Disease Rating Scale) with each heart rate variables.
Results
Impaired HRV is significantly correlated with the duration of PD, but not with disease severity and patient age. Meanwhile, parasympathetic heart rate variable is more likely than sympathetic heart rate variable to be affected by PD.
Conclusion
PD is more likely to affect cardiac parasympathetic regulation than sympathetic regulation by time and the heart rate variables have the association with Parkinsonian motor symptom duration.
Keywords: Autonomic, heart rate variability, Parkinson's disease
INTRODUCTION
Cardiovascular autonomic regulation has been reported to be impaired in Parkinson's disease (PD) and may increase the long-term morbidity of patients with this disease.1,2,3 Moreover, the deterioration of functional performance in Parkinsonian patients with impaired autonomic function may be more rapid, and these patients probably require higher dosage of levodopa supplementation.3
Heart rate change is primarily determined by cardiac autonomic regulation. Heart rate variability (HRV) is defined by irregularities in the interval between normal sinus beats.4,5 Frequency-domain analysis of HRV is a sophisticated and non-invasive tool for studying sympathetic and parasympathetic regulation of heart rate. The standard procedures and interpretation of HRV analysis were first reported in 1996.6 We have applied a modification of these procedures to investigate cardiac autonomic dysregulation in children with epilepsy.7 In this case-control study on a cohort of patients with advanced PD, we used the same technology to investigate the changes of HRV in adult Parkinsonian patients.
MATERIALS AND METHODS
Study population
We enrolled 32 Taiwanese patients with PD (21 male and 11 female; mean age: 62.2 years, range: 44-79 years), who planned to be treated by subthalamic deep brain stimulation at the Buddhist Hualien Tzu Chi General Hospital, Taiwan (Table 1). All patients met the clinical criteria for PD that at least two of the cardinal symptoms are present. The core assessment program including an acute levodopa test to measure the effects of levodopa on PD was used in all patients.8 The following was assessed: Unified Parkinson's Disease Rating Scale (UPDRS) score, behavior from videotaped clips, Hoehn and Yahr (H-Y) stage, timing of rapid alternating movements, the time required to walk a distance of 7 meters, tremorography, cognitive performance (the Mini-Mental State Examination score), and brain magnetic resonance imaging images.
Table 1.
Clinical Features and Heart Rate Variables of Age- and Sex-Matched PD and Control Groups
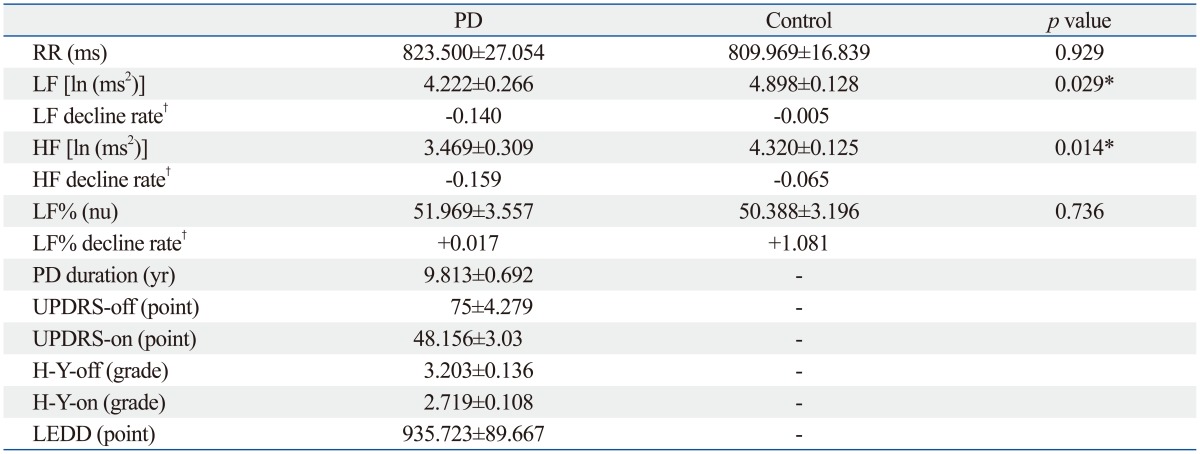
RR, interval between two neighboring R waves; LF, low frequency power; HF, high frequency power; LF%, LF/(HF+LF) in normalized units; PD, Parkinson's disease; UPDRS-off, Unified Parkinson's Disease Rating Scale in levodopa-off period; UPDRS-on, Unified Parkinson's Disease Rating Scale in levodopa-on period; H-Y-off, Hoehn and Yahr stage in levodopa-off period; H-Y-on, Hoehn and Yahr stage in levodopa-on period; LEDD, levodopa equivalent daily dose.
*p<0.05.
†The estimated change of value/year of duration.
For ruling out the autonomic deterioration from other medical issues, none of the enrolled patients had evidence of arrhythmia, ischemic heart disease, heart failure, diabetes mellitus, multiple system atrophy, pure autonomic failure, PD with dementia as well as Parkinsonism with other brain diseases, such as traumatic brain injury or stroke.9 Patients who were taking propranolol or atenolol were also excluded because of the sympatholytic effects of such medications. Thirty-two age- and gender-matched healthy subjects were enrolled as the control group. The study protocol was approved by the Institutional Review Board of the Buddhist Tzu Chi General Hospital. All of the subjects gave their written informed consent at enrollment.
Heart rate recording and frequency-domain analysis of HRV
Since many muscle tremors would be recorded in a Parkinsonian patient during a long-term heart rate recording, especially in the levodopa-off period (without levodopa or dopamine agonist, etc. for at least 12 hours), daytime electro-cardiograms (ECG) for 5 min were recorded in awake patients during levodopa-on periods (with levodopa use). Each subject lay quietly in a comfortable head-up 45-degree position during the heart rate recording. Lead I ECG signals were recorded using an analog-to-digital converter with a sampling rate of 512 Hz. Frequency-domain analysis was performed using a nonparametric method of fast Fourier transformation (FFT). The direct current component was deleted and a Hamming window was used to attenuate the leakage effect. For each time segment (288 s; 2048 data points), our algorithm estimated the power spectrum density on the basis of FFT. The resulting power spectrum was corrected for attenuation resulting from the sampling process and the use of a Hamming window.10 The power spectrum was subsequently quantified into standard frequency-domain measurements as defined by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. The frequency-domain measurements included R-R intervals (the intervals between two neighboring R waves, RR) and heart rate variables: high-frequency power (0.15-0.45 Hz, HF), low-frequency power (0.04-0.15 Hz, LF), and LF% [LF/(HF+LF) in normalized units]. The HF and LF data were logarithmically transformed to correct for any skew in the distribution. The LF reflected contributions from mixed sympathetic and parasympathetic divisions. The HF was considered to reflect vagal (parasympathetic) regulation and the LF% was considered to mirror sympathetic regulation.6,7,10
Statistical analysis
All measures are presented as the mean and standard error (SE) of the mean. Differences in clinical features and heart rate variables between the matched PD and control groups were analyzed by the paired t-test. Pearson correlation coefficient (r) was used to measure correlations of patient age, PD duration, and disease severity (represented by UPDRS score in levodopa-off period, UPDRS-off) with heart rate variables including LF, HF, and LF%. Stepwise regression analysis was conducted to identify those factors associated with heart rate variables. All analyses were performed using SPSS (now called PASW) version 17.0 (SPSS Inc., Chicago, IL, USA) and statistical assessments were evaluated at the 0.05 level of significance.
RESULTS
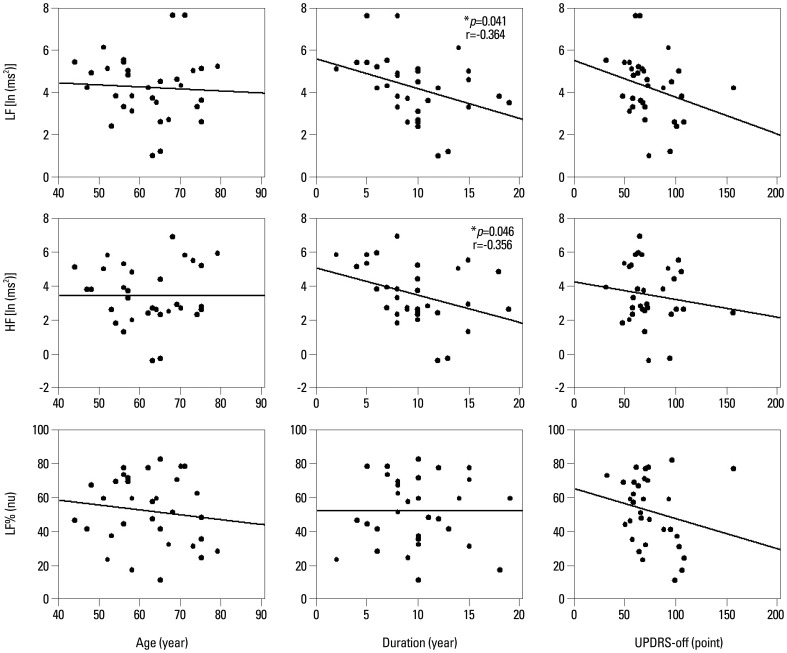
Table 1 demonstrated that the PD group had 9.813±0.692 years (mean±SE) in the duration of disease. The UPDRS scores and H-Y stages of the PD group were: 75±4.279 in UPDRS-off, 48.156±3.03 in UPDRS-on, 3.203±0.136 in H-Y-off, and 2.719±0.108 in H-Y-on. Differences in LF and HF values between PD and control groups were significant. The PD group had significantly lower LF [4.222±0.266 ln (ms2) vs. 4.898±0.128 ln (ms2), p=0.028] and lower HF [3.469±0.309 ln (ms2) vs. 4.320±0.125 ln (ms2), p=0.029] when compared to the age- and gender-matched control group. In the patient group, Pearson correlation analysis revealed that the rate of LF decline significantly correlated with PD duration (r=-0.364, p=0.041) but not with disease severity (UPDRS-off) and patient age (Fig. 1, Table 2). Similarly, the results indicated that lower HF was correlated with longer PD duration (r=-0.356, p=0.046). After adjustment for possible confounders, a stepwise regression using age, gender, PD duration, and UPDRS-off score still found significant correlation of heart rate variables such as LF and HF with long PD duration (Table 3). Furthermore, the results indicated that the duration of PD explained 13.3% and 12.7% of the variance in LF and HF measures, respectively. No variables were significantly correlated with the LF%. Meanwhile, increase in UPDRS-off was significantly correlated with PD duration with a slope of 2.578 points per year.
Fig. 1.
The correlations of patient age, PD duration, and UPDRS-off score with heart rate variables. Pearson correlation analysis showed that the rates of LF and HF decline were significantly correlated with PD duration. LF, low frequency power; HF, high frequency power; LF%, LF/(HF+LF) in normalized units; UPDRS-off, Unified Parkinson's Disease Rating Scale in levodopa-off period; PD, Parkinson's disease. *p<0.05.
Table 2.
Correlations of LF, HF, and LF% with Patient Age, PD Duration and UPDRS-Off Score
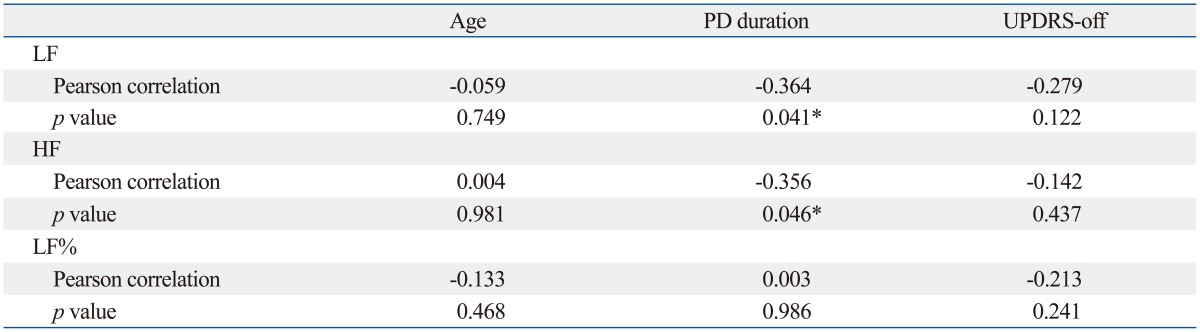
LF, low frequency power; HF, high frequency power; LF%, LF/(HF+LF) in normalized units; PD, Parkinson's disease; UPDRS-off, Unified Parkinson's Disease Rating Scale in levodopa-off period.
*p<0.05.
Table 3.
Relationships between LF, HF, and PD Duration
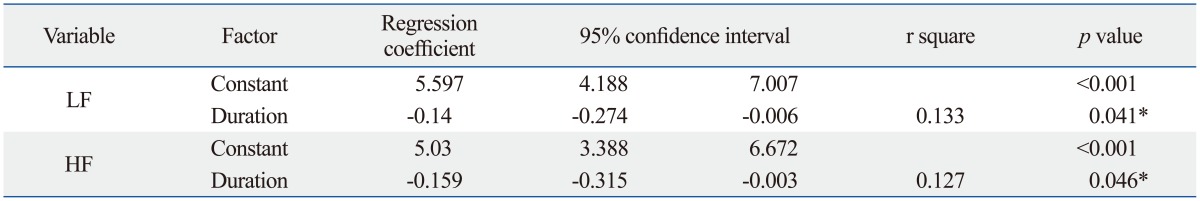
LF, low frequency power; HF, high frequency power; PD, Parkinson's disease.
*p<0.05.
DISCUSSION
Cardiovascular autonomic dysregulation in PD has been attributed to either central or peripheral autonomic regulatory impairment,1,2,3,11,12,13,14 involving the hypothalamus, insular cortex, locus coeruleus, dorsal motor nucleus of the vagus, intermediolateral nucleus of the thoracic cord, sympathetic ganglia, and sacral parasympathetic nuclei. Thus, PD affects both sympathetic and parasympathetic divisions, each of which can be evaluated by frequency-domain analysis of HRV.4,5,6,7 In this study, although the sympathetic indicator of autonomic cardiac regulation, LF%, was not significantly changed, the total and parasympathetic indicators (LF and HF) decreased significantly and correlated with disease duration. Some previous studies reported that levodopa replacement in Parkinsonian patients could decrease central sympathetic outflow, an unusual effect caused by the central D2 agonist action of levodopa.15,16 Based on it, some authors recommended monoamine oxidase B inhibitors to avoid the effect of levodopa on sympathetic regulation.17 We thought that the discordant changes in autonomic sympathetic and parasympathetic divisions in our results might stem from the effects of levodopa treatment and leave the cardiac parasympathetic dysregulation being more sensitive in the HRV study.
PD is not a rare degenerative disorder in aged population. However, aged people usually suffer from multiple degenerative diseases and many of those might affect the cardiovascular autonomic regulation. In this study with focus on pure PD itself, we excluded the patients with cardiac arrhythmia, ischemic heart disease, heart failure, diabetes mellitus, multiple system atrophy, pure autonomic failure, PD with dementia as well as Parkinsonism with other brain diseases, such as traumatic brain injury or stroke, at the enrollment. All of them were ever reported to affect the autonomic regulation and might interfere the study results. Although we had only 32 patients left in the study group, the statistically significant results made us believe that the study number was enough to make the unquestionable conclusions in the association between the heart rate variables and PD duration. These results are important because cardiovascular autonomic dysfunction is a relatively under-recognized problem of PD and is related to the management in disease progression. It may be associated with the patients' life quality, and life span, and then may increase care-givers' burdens.
The progressive loss of cardiac autonomic regulation, both in sympathetic and parasympathetic divisions from middle age to old age is correlated with the aging itself.10,18 In the present study, the estimated annual rate of LF (-0.140 vs. -0.005) and HF (-0.159 vs. -0.065) decline was faster in the PD patients aged 44 to 79 years (Table 1). Meanwhile, the UPDRS-off increased at an estimated rate of 2.578 points per year (in agreement with a report from western countries),19 and UPDRS-off significantly correlates with disease duration. Since there is no standard way to grade the function of the autonomic nervous system using the UPDRS, applying heart rate variables as progressing biomarkers in PD may be suggested.
There were 32 patients with mean age of 62.2 years and mean disease duration of 9.8 years enrolled in this study. Their mean H-Y stage was 3.2 and mean UPDRS was 75 during the levodopa-off period because all enrolled patients at our hospital were receiving subthalamic deep brain stimulation for advanced PD. The major limitations of this study were; first, no subjects with early stage PD were enrolled because the cost of deep brain stimulation is still relatively high and cannot routinely be given in early stage of the disease; second, the use of a combination of anti-Parkinsonian medications for advanced PD could be a confounding factor affecting the results of cardiac autonomic regulation in the patients of this study; third, to minimize the artifacts from muscle tremor, we decided that the better experimental design was to combine short-term heart rate recordings with HRV spectral analysis during the levodopa-on period.20,21 However, all heart rate variables were recorded and analyzed during daytime to avoid major circadian effects.22 Obtaining recordings and heart rate variables in gender- and age-matched subjects in the same position can minimize inter-individual anthropometric and posture confounding effects. Therefore, we believe that the results of this case-control study show that PD duration is the main factor affecting changes in HRV. In the future, we are planning to study patients either in the early or late stages of their disease to elucidate more about the development of autonomic dysregulation in patients with PD.
In conclusion, PD is more likely to affect cardiac parasympathetic regulation than sympathetic regulation by time, and the heart rate variables have the association with Parkinsonian motor symptom duration.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Jost WH. Autonomic dysfunctions in idiopathic Parkinson's disease. J Neurol. 2003;250(Suppl 1):I28–I30. doi: 10.1007/s00415-003-1105-z. [DOI] [PubMed] [Google Scholar]
- 2.Oka H, Mochio S, Onouchi K, Morita M, Yoshioka M, Inoue K. Cardiovascular dysautonomia in de novo Parkinson's disease. J Neurol Sci. 2006;241:59–65. doi: 10.1016/j.jns.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Lucetti C, Gambaccini G, Del Dotto P, Ceravolo R, Logi C, Rossi G, et al. Long-term clinical evaluation in patients with Parkinson's disease and early autonomic involvement. Parkinsonism Relat Disord. 2006;12:279–283. doi: 10.1016/j.parkreldis.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Vanderlei LC, Pastre CM, Hoshi RA, Carvalho TD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc. 2009;24:205–217. doi: 10.1590/s0102-76382009000200018. [DOI] [PubMed] [Google Scholar]
- 5.Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J. 1994;127:1376–1381. doi: 10.1016/0002-8703(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 6.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 7.Harnod T, Yang CC, Hsin YL, Shieh KR, Wang PJ, Kuo TB. Heart rate variability in children with refractory generalized epilepsy. Seizure. 2008;17:297–301. doi: 10.1016/j.seizure.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 9.Takase B, Kurita A, Noritake M, Uehata A, Maruyama T, Nagayoshi H, et al. Heart rate variability in patients with diabetes mellitus, ischemic heart disease, and congestive heart failure. J Electrocardiol. 1992;25:79–88. doi: 10.1016/0022-0736(92)90112-d. [DOI] [PubMed] [Google Scholar]
- 10.Kuo TB, Lin T, Yang CC, Li CL, Chen CF, Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Physiol. 1999;277(6 Pt 2):H2233–H2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- 11.Benarroch EE, Schmeichel AM, Parisi JE. Involvement of the ventrolateral medulla in parkinsonism with autonomic failure. Neurology. 2000;54:963–968. doi: 10.1212/wnl.54.4.963. [DOI] [PubMed] [Google Scholar]
- 12.Papapetropoulos S, Mash DC. Insular pathology in Parkinson's disease patients with orthostatic hypotension. Parkinsonism Relat Disord. 2007;13:308–311. doi: 10.1016/j.parkreldis.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi K, Takahashi H. Neuropathology of autonomic nervous system in Parkinson's disease. Eur Neurol. 1997;38(Suppl 2):2–7. doi: 10.1159/000113469. [DOI] [PubMed] [Google Scholar]
- 14.Isaias IU, Marotta G, Pezzoli G, Sabri O, Schwarz J, Crenna P, et al. Enhanced catecholamine transporter binding in the locus coeruleus of patients with early Parkinson disease. BMC Neurol. 2011;11:88. doi: 10.1186/1471-2377-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludwig J, Remien P, Guballa C, Binder A, Binder S, Schattschneider J, et al. Effects of subthalamic nucleus stimulation and levodopa on the autonomic nervous system in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:742–745. doi: 10.1136/jnnp.2006.103739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouhaddi M, Vuillier F, Fortrat JO, Cappelle S, Henriet MT, Rumbach L, et al. Impaired cardiovascular autonomic control in newly and long-term-treated patients with Parkinson's disease: involvement of L-dopa therapy. Auton Neurosci. 2004;116:30–38. doi: 10.1016/j.autneu.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Löhle M, Reichmann H. Controversies in neurology: why monoamine oxidase B inhibitors could be a good choice for the initial treatment of Parkinson's disease. BMC Neurol. 2011;11:112. doi: 10.1186/1471-2377-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrne EA, Fleg JL, Vaitkevicius PV, Wright J, Porges SW. Role of aerobic capacity and body mass index in the age-associated decline in heart rate variability. J Appl Physiol (1985) 1996;81:743–750. doi: 10.1152/jappl.1996.81.2.743. [DOI] [PubMed] [Google Scholar]
- 19.Jankovic J, Kapadia AS. Functional decline in Parkinson disease. Arch Neurol. 2001;58:1611–1615. doi: 10.1001/archneur.58.10.1611. [DOI] [PubMed] [Google Scholar]
- 20.Kallio M, Suominen K, Bianchi AM, Mäkikallio T, Haapaniemi T, Astafiev S, et al. Comparison of heart rate variability analysis methods in patients with Parkinson's disease. Med Biol Eng Comput. 2002;40:408–414. doi: 10.1007/BF02345073. [DOI] [PubMed] [Google Scholar]
- 21.Lucreziotti S, Gavazzi A, Scelsi L, Inserra C, Klersy C, Campana C, et al. Five-minute recording of heart rate variability in severe chronic heart failure: correlates with right ventricular function and prognostic implications. Am Heart J. 2000;139:1088–1095. doi: 10.1067/mhj.2000.106168. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa M, Iwao T, Ishida S, Yonemochi H, Fujino T, Saikawa T, et al. Circadian rhythm of the signal averaged electrocardiogram and its relation to heart rate variability in healthy subjects. Heart. 1998;79:493–496. doi: 10.1136/hrt.79.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]