Abstract
Purpose
Obstructive sleep apnea (OSA) is considered an independent risk factor for hypertension. However, it is still not clear which clinical factors are related with the presence of hypertension in OSA patients. We aimed to find different physical features and compare the sleep study results which are associated with the occurrence of hypertension in OSA patients.
Materials and Methods
Medical records were retrospectively reviewed for patients diagnosed with OSA at Severance Cardiovascular Hospital between 2010 and 2013. Males with moderate to severe OSA patients were enrolled in this study. Clinical and polysomnographic features were evaluated to assess clinical variables that are significantly associated with hypertension by statistical analysis.
Results
Among men with moderate to severe OSA, age was negatively correlated with hypertension (odds ratio=0.956), while neck circumference was positively correlated with the presence of hypertension (odds ratio=1.363). Among the polysomnographic results, the lowest O2 saturation during sleep was significantly associated with the presence of hypertension (odds ratio=0.900).
Conclusion
Age and neck circumference should be considered as clinically significant features, and the lowest blood O2 saturation during sleep should be emphasized in predicting the coexistence or development of hypertension in OSA patients.
Keywords: Sleep apnea, hypertension, polysomnography, neck circumference, lowest oxygen saturation level
INTRODUCTION
Obstructive sleep apnea (OSA) is commonly associated with hypertension, and the main pathophysiologic mechanism for the blood pressure-elevating effect of OSA is the increased sympathetic activity that influences vascular resistance and cardiac output.1 Other features such as a pro-inflammatory effect, increased oxidative stress, and increased vascular stiffness are also suggested mechanisms that influence the increased risk of hypertension in OSA patients.1 Additionally, blood pressure control is critical because of its effect on cardiovascular morbidity and mortality.2,3 OSA is known as a secondary cause of hypertension but also independently associated with target-organ damage by sympathetic overactivity in patients having hypertension.4,5 Recently, the role of nocturnal rostral fluid shift in the pathogenesis of OSA has been raised and OSA can be aggravated through increase of pharyngeal resistance induced by co-existing hypertension.6,7 Therefore, early diagnosis of hypertension in OSA patients is very important.
In a previous study, patients who have hypertension or OSA were reported to have higher prevalence of diabetes mellitus, metabolic syndrome, and dyslipidemia (including low level of high density lipoprotein and high level of triglyceride).8,9,10,11 They also had distinguishing physical characteristics such as increased body mass index (BMI), neck circumference, and abdominal circumference compared to control.4 In another study, age, sex, BMI, and family history of hypertension were representatively different between OSA patients with hypertension and OSA patients having normal blood pressure.12 Although OSA is now thought to be an independent risk factor of hypertension, the characteristics associated with hypertension in patients with OSA are heterogenous and studies are still insufficient.
Many clinicians have focused on the influence of gender differences on the occurrence of hypertension in OSA patients. The Western New York Health Study reported a 66% higher prevalence of hypertension among women with subjective symptoms but subjective symptoms were not associated with the prevalence of hypertension among male patients.13 This association was independent of socioeconomic status, traditional cardiovascular risk factors, and psychiatric comorbidities.13 In another study, age was not significantly associated with the occurrence of hypertension in patients who reported a short duration of sleep.14 Obesity, especially visceral obesity, contributes to the pathogenesis of hypertension in OSA patients.15 The results from previous reports have suggested many factors that are associated with hypertension in OSA patients. However, the conclusions are inconsistent and the association between the clinical characteristics of patients with hypertension and detailed polysomnographic data has not been reported. We aimed to find different clinical characteristics and the factors of sleep study between OSA patients who are hypertensive and OSA patients who have normal blood pressure. Lipid profile and fasting glucose level were also included in the analysis because of high prevalence of metabolic syndrome in OSA patients.11 Based on these results, we tried to suggest useful predictors that might be helpful in early diagnosis of un-identified hypertension in OSA patients.
MATERIALS AND METHODS
Study population
This study was approved by the Institutional Review Board of Yonsei University College of Medicine (IRB No. 4-2014-0132). Medical records were retrospectively reviewed for patientsdiagnosed with OSA based on validated, ambulatory unattended polysomnography (Embletta ×100)16 performed at Severance Hospital between 2010 and 2013. Only male patients were enrolled because the characteristics of OSA in women and results in men are more heterogenous.17 As such, for subjects with asymptomatic obstructive sleep apnea, polysomnography was performed as an etiologic and risk factor assessment in hypertensives, atrial fibrillation and in patients with coronary artery disease. Patients completed the polysomnography examination, the Epworth Sleepiness Score (ESS) questionnaire to assess daytime sleepiness and the blood tests for lipid profiles and fasting glucose level. Male patients whose apnea/hypopnea index (AHI) was more than 15 (total 183 patients) were enrolled in this study. Office blood pressure was measured twice either with a mercury sphygmomanometer or an aneroid sphygmomanometer (Welch Allyn Silver Series DS45, Skaneateles Falls, NY, USA) and the average value was used. The patients were grouped as hypertension if they are diagnosed hypertension based on blood pressure following current guidelines publishedby the European Society of Hypertension18 or they are currently taking blood pressure lowering medications. The patients who do not belong to above category were grouped as non-hypertensives. Other past medical histories of enrolled patients were not considered in our study. Participants were excluded if they had sleep studies with un-interpretable measurements, inadequate sleep duration, missed physical measurements or lack of any laboratory result.
Anthropometric measurements
Anthropometric characteristics were measured by one skilled examiner according to standardized methods. Height was measured without shoes from the top of the head to the end of the first toe. Neck circumference (NC) was measured using a flexible ruler at the thyroid cartilage level. Waist circumference was measured at the midway point between the lowest portion of the rib cage and the iliac crest. Hip circumference was measured at the largest lateral extension of the hips.
Blood analysis
Blood samples were obtained after an overnight fast. Lipid profiles including total cholesterol, triglycerides (TG), high density lipoprotein (HDL), and low density lipoprotein (LDL) as well as fasting blood glucose level were measured.
Statistics
The study population was divided into OSA patients without hypertension (non-HTN group; n=67) and OSA patients with hypertension (HTN group; n=116). For the comparison of continuous variables by group, two independent sample t-tests were used. Univariate and multivariate logistic regression analyses were applied to calculate the odds ratios (OR) of variables. All variables with a p<0.05 in the univariate analysis were selected and further studied by multivariate logistic regression analysis. Adjusted OR with 95% confidence intervals were calculated for these independent variables. Pearson correlation coefficients were calculated between variables and polysomnographic findings. All analyses were done using SPSS version 20 (IBM Corporation, Armonk, NY, USA).
RESULTS
Differences in physical parameters and blood tests: non-HTN vs. HTN group
A total of 183 moderate to severe male OSA patients were analyzed in this study. The mean ages were 60.70±9.27 years for the non-HTN group and 53.26±12.73 years for the HTN group (p<0.001). HTN group patients were more obese, which was represented by increased body weight and high level of BMI. BMI was 25.18±3.04 in non-HTN group and 27.12±3.55 in HTN group (p<0.001). Neck circumference was 38.45±2.19 cm in non-HTN group and 40.27±2.71 cm in HTN group showing meaningful difference (p<0.001). Central obesity-related physical factors such as waist (93.08±8.58 cm in non-HTN group and 96.86±8.83 cm in HTN group) and hip circumferences (97.87±6.41 cm in non-HTN group and 103.02±10.33 cm in HTN group) were also significantly different between two groups (p<0.001).
The basal blood laboratory tests revealed that the TG level was the only different factor which was 127.17±73.31 mg/dL in non-HTN group and 167.26±123.81 mg/dL in HTN group (Table 1). The HDL, LDL and fasting glucose level were not significantly different.
Table 1.
Patients Characteristics
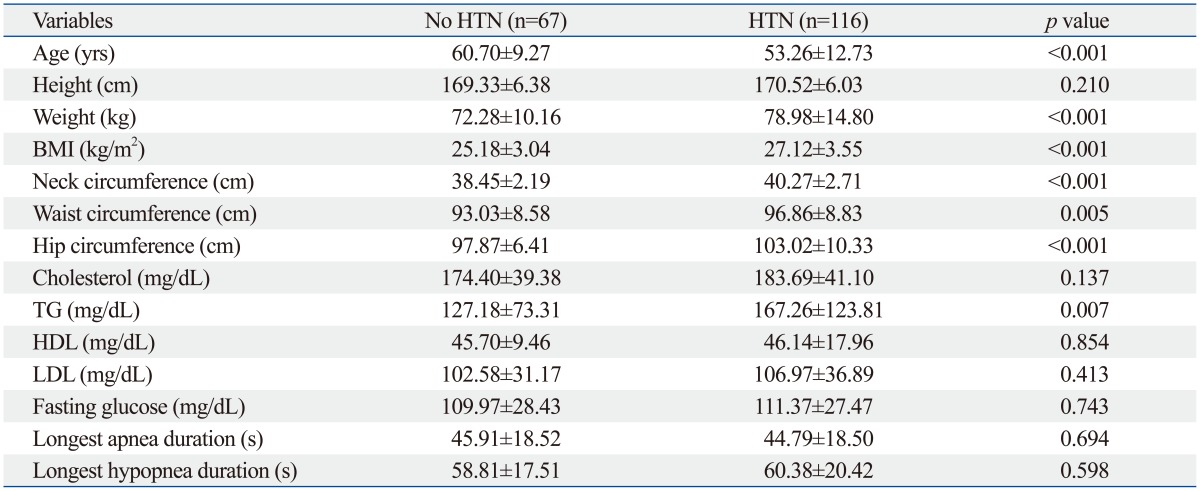
BMI, body mass index; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; HTN, hypertension.
Data are presented as mean±SD for data with normal distribution.
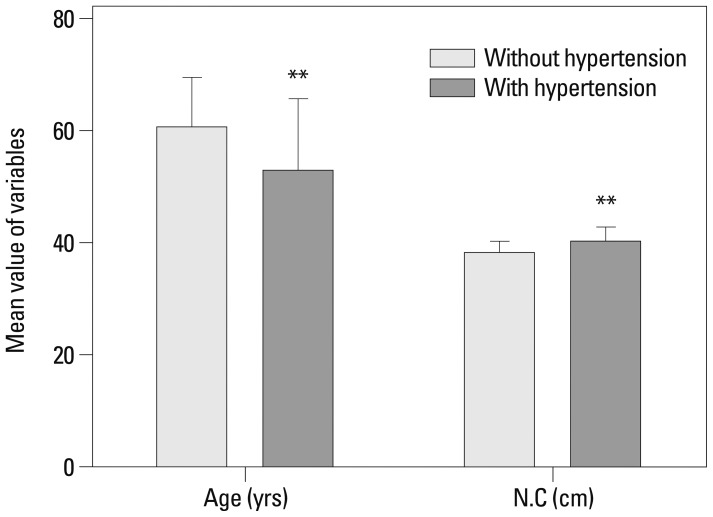
Age, weight, NC, waist and hip circumferences, and TG levels (p<0.05) which were statistically different between the non-HTN and HTN groups were selected and further evaluated by univariate and multivariate logistic regression analysis (Table 2). Age was negatively (OR=0.956) and NC was positively (OR=1.363) related to the presence of hypertension, and these clinical characteristics were also significant in multivariate analysis (p<0.05) (Fig. 1).
Table 2.
Odds Ratio of Age, Weight, Neck Circumference, Waist, Hip, and Triglyceride for Hypertension
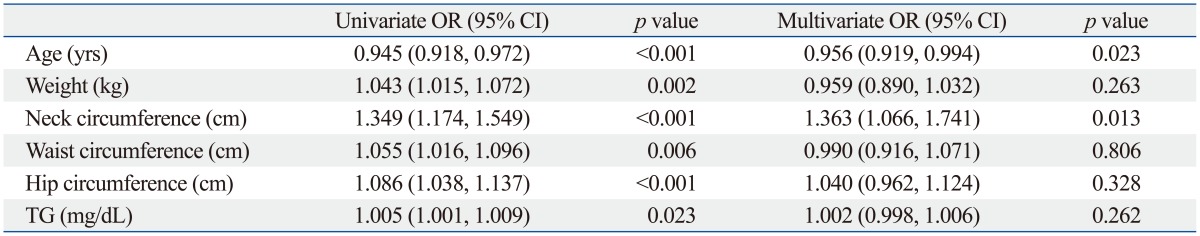
OR, odds ratio; CI, confidence interval; TG, triglyceride.
Fig. 1.
The difference of age and neck circumference in hypertensive and non-hypertensive OSA patients. Data is shown as mean±SD. **Significant (p<0.01). N.C, neck circumference; OSA, obstructive sleep apnea.
Differences in polysomnographic parameters: non-HTN vs. HTN group
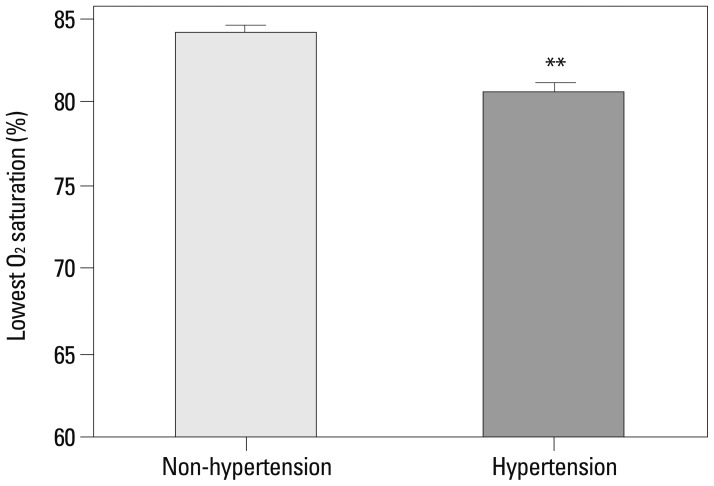
Typically, the AHI is considered diagnostic for OSA. We considered other features, including the longest apnea/hypopnea duration, average O2 saturation, and lowest O2 saturation and these were evaluated according to the presence of hypertension (Table 3). The average O2 (94.96±1.27% in non-HTN, 94.27±1.91% in HTN) and lowest O2 saturation levels (84.15±4.97% in non-HTN, 80.58±6.52% in HTN) were significant factors influencing the presence of hypertension by the t-test. Mean AHI was also significantly different between two groups (29.79±11.35 in non-HTN, 34.12±16.89 in HTN). These factors were further studied by univariate and multivariate analysis, and the lowest O2 saturation level during sleep was the only significant factor identified in multivariate analysis (p=0.006). The OR for the lowest O2 saturation was 0.900, which means that as the lowest O2 saturation level increases, the probability of co-existing hypertension decreases (Table 4, Fig. 2). Other factors of sleep study were not statistically meaningful in multivariate analysis. Excessive daytime sleepiness assessed by ESS was also not a meaningful factor. To further study the clinical significance of the lowest O2 saturation level during sleep, patients were grouped, and the correlation of clinical variables and the lowest O2 saturation level were compared (Table 5). In the HTN group, NC, BMI, and waist circumference, were negatively correlated with the lowest O2 saturation level (p<0.05); these correlations were not demonstrated in the non-HTN group. NC and waist circumference had the highest correlation coefficient in the HTN group (r=-0.276). Age was correlated with the lowest O2 saturation level among all patients (r=0.159, p=0.032). However, age did not show any significant correlation with the lowest O2 saturation level in subgroup analysis for the HTN and non-HTN groups.
Table 3.
Multiple Variables of Polysomnography in Moderate to Severe OSA Patients with or without Hypertension
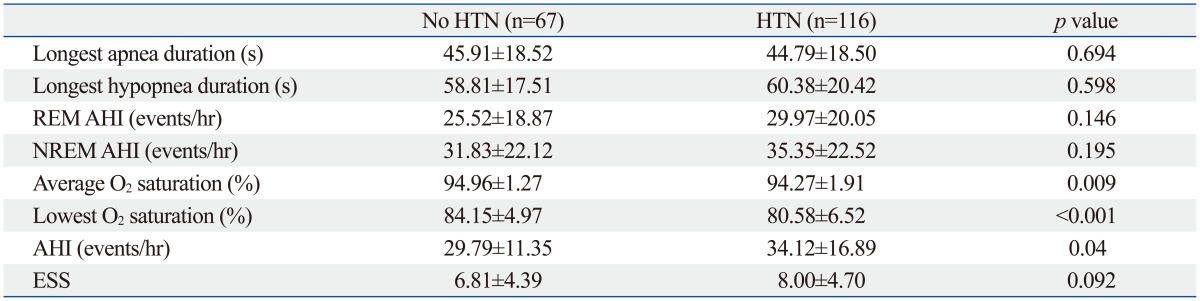
AHI, apnea/hypopnea index; ESS, epworth sleepiness score; REM AHI, apnea/hypopnea index in rapid eye movement sleep; NREM AHI, apnea/hypopnea index in non rapid eye movement sleep; OSA, obstructive sleep apnea; HTN, hypertension.
Table 4.
Odds Ratio of Average and Lowest O2 Saturation and Various AHIs for Hypertension
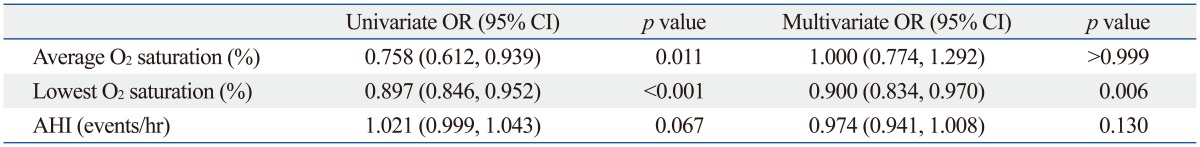
AHI, apnea/hypopnea index; OR, odds ratio; CI, confidence interval.
Fig. 2.
The difference of the lowest O2 saturation during sleep in hypertensive and non-hypertensive OSA patients. Data is shown as mean±SD. **Significant (p<0.01). OSA, obstructive sleep apnea.
Table 5.
Correlation between Lowest O2 Saturation and Clinical Variables in Total Hypertensives and Non-Hypertensives
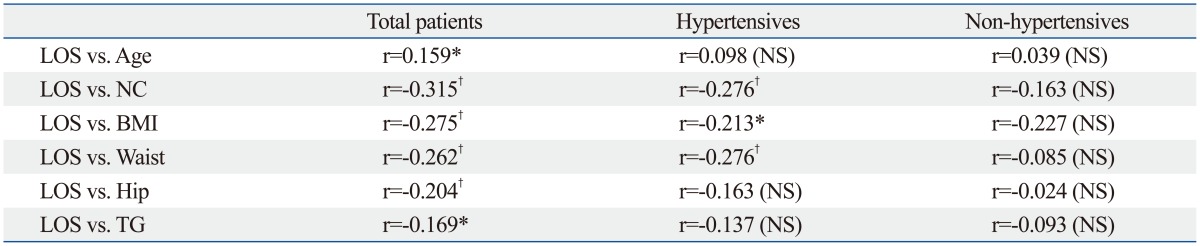
LOS, lowest O2 saturation level during sleep; NC, neck circumference; BMI, body mass index; TG, triglyceride; NA, not significant.
r: Pearson correlation.
*Significant (p<0.05).
†Significant (p<0.01).
DISCUSSION
Growing evidence suggests that OSA is one of the major causes of hypertension.19,20 In our study of 183 men with moderate to severe OSA, 116 men had hypertension (63% prevalence). Recently, it was reported that portable devices showed good diagnostic performance compared with level 1 sleep tests in adult patients21 and our result also shows that Embletta ×100 which is a level 2 portable device is useful in diagnosis of OSA. The prevalence of hypertension in OSA patients was higher compared to previous reports.22 This may be because most of the hypertensive patients referred to Severance Hospital have severe hypertension and are often difficult to treat. Furthermore, the exclusion of mild OSA may have influenced this prevalence.
In this study, age was the most meaningful factor that predicted the co-existence of hypertension in OSA patients. Interestingly, the mean age was 7 years younger in the HTN group compared to the non-HTN group. Similar results were previously reported and the OR of hypertension decreased as age increased in severe OSA patients.23 However, increased age has been positively associated with sleep-disordered breathing,24 and a contradictory study reported that age had no significant association with hypertension in male OSA patients.13 Based on our study, middle-aged patients with an AHI >15 should be carefully evaluated for concurrent hypertension. However, care should be taken when interpreting these results as there is the potential for selection bias in this study. When the average BMI of younger (age <60 yr) and older patients (age ≥60 yr) were compared, younger patients had higher BMI levels compared to the older patients, which might explain why age was negatively correlated with hypertension.
The prevalence of obesity in the general population is increasing and becoming a significant health burden. In this study, several physical measures such as body weight, NC, waist, and hip circumferences were significantly higher among patients with hypertension. Although obesity and OSA are independently associated with hypertension, studies show they are interrelated. Obesity is an important risk factor for both hypertension and OSA.25,26,27,28 In our study, BMI in the HTN group was significantly higher than in the non-HTN group. When we defined obesity as BMI above 25, obese patients had much higher value of NC, suggesting the relation of obesity with neck circumference (data not shown). NC, one clinical characteristics related to higher mortality of acute ischemic stroke, has been shown to be an independent predictor of coronary artery disease.29,30 NC was identified in our study as a significant clinical characteristic by multivariate regression analysis and was associated with the presence of hypertension in moderate to severe OSA patients. In recent studies, rostral fluid displacement in hypertensive patients has been linked to OSA by contributing to the narrowing of the upper airway,31,32 and NC could be increased by this fluid shift from the lower body.22,29,30,33,34 This result suggests that NC should not be overlooked in evaluating OSA patients. Furthermore, when we plotted Receiver Operating Characteristic (ROC) Curve of NC to the presence of HTN, the value of the Area Under the ROC Curve was 0.693 and the cut-off value was 39.750 (data not shown). We suggest that the patients whose NC is more than 39.75 have increased risk for the presence of concurrent HTN.
Lipid profile and fasting glucose level were not associated with the presence of hypertension. Subjective daytime sleepiness estimated by the ESS was not associated with the incidence of hypertension, while one study reported that prescreening of OSA by ESS was useful in predicting post-cardiac operative atrial fibrillation.35
AHI defines only the number of apneic or hypopneic events per hour. Diagnosis of OSA is based on AHI and does not consider the total apnea-hypopnea duration or the level of blood O2 desaturation during sleep. Studies about HTN in OSA patients have been focused on AHI or respiratory disturbance index in polysomnography result although one study reported that mean minimum saturation during sleep was lower in hypertension male patients compared to control.36 Therefore, we evaluated other factors of sleep study that possibly influenced the occurrence of hypertension. The average AHI of patients with moderate to severe OSA was higher in the HTN group demonstrating that the degree of OSA in HTN group was more severe than non-HTN group; however, this finding was not statistically significant in the multivariate regression analysis. The average AHI in severe OSA patients with HTN was 48.62 and 42.08 without HTN group (data not shown). The difference between severe OSA patients with HTN and without HTN was larger than moderate to severe patients. Therefore, mean AHI in severe OSA patients might be meaningful in multivariate analysis. The duration of hypopnea or apnea was not different between the two groups; however, the average and lowest blood O2 saturation were meaningful features in the t-test. The lowest blood O2 saturation level during sleep was the only significant factor in multivariate regression analysis. These results coincide with our opinion that not only AHI but also other factors of sleep study should be considered in evaluation of OSA patients. OSA-associated intermittent hypoxia activates NADPH oxidase and other reactive oxygen species-producing enzymes and leads to cardiovascular complications.37,38 AHI and oxygen desaturation events per hour in OSA were studied and found to be positively correlated with the level of superoxide radical in blood or 8-hydroxy-2'-deoxyguanosine (8-OHdG) in urine suggesting increased oxidative stress.38,39 Although it is still unclear that OSA patients with severe lowest O2 saturation is more affected by oxidative stress, it could be other features reflecting the oxidative condition during sleep and should be considered as critical values in predicting coexisting hypertension. AHI is traditionally an important factor in the diagnosis of OSA, but it does not reflect the total apnea/hypopnea duration or O2 desaturation. Thus, comprehensive evaluation of polysomnographic data including AHIs, apnea/hypopnea duration, and O2 desaturation level during sleep should be performed.
In this study, many factors of clinical and sleep study were different between non-HTN and HTN group in OSA patients. Among them, NC and lowest O2 saturation were meaningful factors by multivariate test and we suggest that these should be considered in OSA patients. This result should allow the clinician to better predict which patients with OSA are at higher risk for complications.
ACKNOWLEDGEMENTS
We are very thankful for Jae Hee Kim and the other researchers at the Cardiology laboratory for helping with data collection.
This research was supported by grants NRF-2013R1A1A 1010151 to H.J. Cho from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology. This study was also supported by the Cardiovascular Research Center, Seoul, Korea.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Calhoun DA. Obstructive sleep apnea and hypertension. Curr Hypertens Rep. 2010;12:189–195. doi: 10.1007/s11906-010-0112-8. [DOI] [PubMed] [Google Scholar]
- 2.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–709. [PubMed] [Google Scholar]
- 3.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drager LF, Genta PR, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105:1135–1139. doi: 10.1016/j.amjcard.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Manolis AJ, Poulimenos LE, Kallistratos MS, Gavras I, Gavras H. Sympathetic overactivity in hypertension and cardiovascular disease. Curr Vasc Pharmacol. 2014;12:4–15. doi: 10.2174/15701611113119990140. [DOI] [PubMed] [Google Scholar]
- 6.White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591(Pt 5):1179–1193. doi: 10.1113/jphysiol.2012.245159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White LH, Motwani S, Kasai T, Yumino D, Amirthalingam V, Bradley TD. Effect of rostral fluid shift on pharyngeal resistance in men with and without obstructive sleep apnea. Respir Physiol Neurobiol. 2014;192:17–22. doi: 10.1016/j.resp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Sharma SK, Agrawal S, Damodaran D, Sreenivas V, Kadhiravan T, Lakshmy R, et al. CPAP for the metabolic syndrome in patients with obstructive sleep apnea. N Engl J Med. 2011;365:2277–2286. doi: 10.1056/NEJMoa1103944. [DOI] [PubMed] [Google Scholar]
- 9.Cuhadaroğlu C, Utkusavaş A, Oztürk L, Salman S, Ece T. Effects of nasal CPAP treatment on insulin resistance, lipid profile, and plasma leptin in sleep apnea. Lung. 2009;187:75–81. doi: 10.1007/s00408-008-9131-5. [DOI] [PubMed] [Google Scholar]
- 10.Lin QC, Zhang XB, Chen GP, Huang DY, Din HB, Tang AZ. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome in nonobese adults. Sleep Breath. 2012;16:571–578. doi: 10.1007/s11325-011-0544-7. [DOI] [PubMed] [Google Scholar]
- 11.Adedayo AM, Olafiranye O, Smith D, Hill A, Zizi F, Brown C, et al. Obstructive sleep apnea and dyslipidemia: evidence and underlying mechanism. Sleep Breath. 2014;18:13–18. doi: 10.1007/s11325-012-0760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drager LF, Pereira AC, Barreto-Filho JA, Figueiredo AC, Krieger JE, Krieger EM, et al. Phenotypic characteristics associated with hypertension in patients with obstructive sleep apnea. J Hum Hypertens. 2006;20:523–528. doi: 10.1038/sj.jhh.1002012. [DOI] [PubMed] [Google Scholar]
- 13.Stranges S, Dorn JM, Cappuccio FP, Donahue RP, Rafalson LB, Hovey KM, et al. A population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal women. J Hypertens. 2010;28:896–902. doi: 10.1097/HJH.0b013e328335d076. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Garcia E, Faubel R, Guallar-Castillon P, Leon-Muñoz L, Banegas JR, Rodriguez-Artalejo F. Self-reported sleep duration and hypertension in older Spanish adults. J Am Geriatr Soc. 2009;57:663–668. doi: 10.1111/j.1532-5415.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- 15.Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42:1067–1074. doi: 10.1161/01.HYP.0000101686.98973.A3. [DOI] [PubMed] [Google Scholar]
- 16.Chung F, Liao P, Sun Y, Amirshahi B, Fazel H, Shapiro CM, et al. Perioperative practical experiences in using a level 2 portable polysomnography. Sleep Breath. 2011;15:367–375. doi: 10.1007/s11325-010-0340-9. [DOI] [PubMed] [Google Scholar]
- 17.Mohsenin V. Gender differences in the expression of sleep-disordered breathing: role of upper airway dimensions. Chest. 2001;120:1442–1447. doi: 10.1378/chest.120.5.1442. [DOI] [PubMed] [Google Scholar]
- 18.Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158. doi: 10.1097/HJH.0b013e328333146d. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 21.Gjevre JA, Taylor-Gjevre RM, Skomro R, Reid J, Fenton M, Cotton D. Comparison of polysomnographic and portable home monitoring assessments of obstructive sleep apnea in Saskatchewan women. Can Respir J. 2011;18:271–274. doi: 10.1155/2011/408091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fusetti M, Fioretti AB, Valenti M, Masedu F, Lauriello M, Pagliarella M. Cardiovascular and metabolic comorbidities in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2012;32:320–325. [PMC free article] [PubMed] [Google Scholar]
- 23.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Leiby BE, Vela-Bueno A, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160:2289–2295. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 24.Morrell MJ, Finn L, McMillan A, Peppard PE. The impact of ageing and sex on the association between sleepiness and sleep disordered breathing. Eur Respir J. 2012;40:386–393. doi: 10.1183/09031936.00177411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu JC, Berger P., 3rd Sleep apnea and obesity. S D Med. 2011;Spec No:28-34. [PubMed] [Google Scholar]
- 26.Slater G, Pengo MF, Kosky C, Steier J. Obesity as an independent predictor of subjective excessive daytime sleepiness. Respir Med. 2013;107:305–309. doi: 10.1016/j.rmed.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Narang I, Mathew JL. Childhood obesity and obstructive sleep apnea. J Nutr Metab. 2012;2012:134202. doi: 10.1155/2012/134202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broström A, Sunnergren O, Nilsen P, Fridlund B, Ulander M, Svanborg E. Gender differences in respiratory disturbance, sleep and daytime sleepiness in hypertensive patients with different degrees of obesity. Eur J Cardiovasc Nurs. 2013;12:140–149. doi: 10.1177/1474515112438163. [DOI] [PubMed] [Google Scholar]
- 29.Zen V, Fuchs FD, Wainstein MV, Gonçalves SC, Biavatti K, Riedner CE, et al. Neck circumference and central obesity are independent predictors of coronary artery disease in patients undergoing coronary angiography. Am J Cardiovasc Dis. 2012;2:323–330. [PMC free article] [PubMed] [Google Scholar]
- 30.Medeiros CA, Bruin VM, Castro-Silva Cd, Araújo SM, Chaves Junior CM, Bruin PF. Neck circumference, a bedside clinical feature related to mortality of acute ischemic stroke. Rev Assoc Med Bras. 2011;57:559–564. doi: 10.1590/s0104-42302011000500015. [DOI] [PubMed] [Google Scholar]
- 31.Friedman O, Bradley TD, Chan CT, Parkes R, Logan AG. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug-resistant hypertension. Hypertension. 2010;56:1077–1082. doi: 10.1161/HYPERTENSIONAHA.110.154427. [DOI] [PubMed] [Google Scholar]
- 32.Friedman O, Bradley TD, Logan AG. Influence of lower body positive pressure on upper airway cross-sectional area in drug-resistant hypertension. Hypertension. 2013;61:240–245. doi: 10.1161/HYPERTENSIONAHA.112.203547. [DOI] [PubMed] [Google Scholar]
- 33.Ursavas A, Karadag M, Nalci N, Ercan I, Gozu RO. Self-reported snoring, maternal obesity and neck circumference as risk factors for pregnancy-induced hypertension and preeclampsia. Respiration. 2008;76:33–39. doi: 10.1159/000107735. [DOI] [PubMed] [Google Scholar]
- 34.Fink B, Manning JT, Neave N. The 2nd-4th digit ratio (2D:4D) and neck circumference: implications for risk factors in coronary heart disease. Int J Obes (Lond) 2006;30:711–714. doi: 10.1038/sj.ijo.0803154. [DOI] [PubMed] [Google Scholar]
- 35.Mungan U, Ozeke O, Mavioglu L, Ertan C, Karaca IO, Keskin G, et al. The role of the preoperative screening of sleep apnoea by Berlin Questionnaire and Epworth Sleepiness Scale for postoperative atrial fibrillation. Heart Lung Circ. 2013;22:38–42. doi: 10.1016/j.hlc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Sjöström C, Lindberg E, Elmasry A, Hägg A, Svärdsudd K, Janson C. Prevalence of sleep apnoea and snoring in hypertensive men: a population based study. Thorax. 2002;57:602–607. doi: 10.1136/thorax.57.7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumitrascu R, Heitmann J, Seeger W, Weissmann N, Schulz R. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxid Med Cell Longev. 2013;2013:234631. doi: 10.1155/2013/234631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franco CM, Lima AM, Ataíde L, Jr, Lins OG, Castro CM, Bezerra AA, et al. Obstructive sleep apnea severity correlates with cellular and plasma oxidative stress parameters and affective symptoms. J Mol Neurosci. 2012;47:300–310. doi: 10.1007/s12031-012-9738-0. [DOI] [PubMed] [Google Scholar]
- 39.Yamauchi M, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Suzuki T, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127:1674–1679. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]