Abstract
Purpose
Multiple genetic factors are associated with chronic obstructive pulmonary disease (COPD). The association of gene encoding vitamin D binding protein (VDBP, GC) with COPD has been controversial. We sought to investigate the types of GC variants in the Korean population and determine the association of GC variants with COPD and lung function in the Korean population.
Materials and Methods
The study cohort consisted of 203 COPD patients and 157 control subjects. GC variants were genotyped by the restriction fragment-length polymorphism method. Repeated measures of lung function data were analyzed using a linear mixed model including sex, age, height, and pack-years of smoking to investigate the association of GC genetic factors and lung function.
Results
GC1F variant was most frequently observed in COPD (46.1%) and controls (42.0%). GC1S variant (29.0% vs. 21.4%; p=0.020) and genotype 1S-1S (8.3% vs. 3.4%; p=0.047) were more commonly detected in control than COPD. According to linear mixed model analysis including controls and COPD, subjects with genotype 1S-1S had 0.427 L higher forced expiratory volume in 1 second (FEV1) than those with other genotypes (p=0.029). However, interaction between the genotype and smoking pack-year was found to be particularly significant among subjects with genotype 1S-1S; FEV1 decreased by 0.014 L per smoking pack-year (p=0.001).
Conclusion
This study suggested that GC polymorphism might be associated with lung function and risk of COPD in Korean population. GC1S variant and genotype 1S-1S were more frequently observed in control than in COPD. Moreover, GC1S variant was more common in non-decliners than in rapid decliners among COPD.
Keywords: Vitamin D binding protein, polymorphism, lung function, chronic obstructive pulmonary disease
INTRODUCTION
The most critical risk factor for the development of chronic obstructive pulmonary disease (COPD) is cigarette smoking.1 However, only 10-20% of heavy smokers develop airway obstruction and -20-30% of COPD patients never smoke.2,3,4,5,6 Moreover, previous reports of familial aggregation of COPD suggest that genetic components are likely associated with susceptibility to the development of COPD.7,8
Numerous candidate genes have been reported to be associated with COPD. These genes are generally related to protease-antiprotease interaction, antioxidant effects, xenobiotic metabolism, and inflammation and immune response pathways.9 One of the candidates involved in inflammation and immune reaction is the gene encoding vitamin D binding protein (VDBP, GC).
VDBP was first described by Hirschfeld in 1959 as a marker in gamma-globulin of human serum and characterized as a group-specific component.10 In 1975, it was identified as the plasma protein that binds vitamin D.11 The major role of VDBP is transporting 25-hydroxyvitamin D, the major circulating form of vitamin D, and 1,25-dihydroxyvitamin D, the most active vitamin D metabolite. However, when expressed by neutrophils, VDBP activates macrophages and augments monocyte and neutrophil chemotaxis.12,13,14,15 These roles of VDBP may contribute to the chronic inflammatory response in COPD.
GC, located on chromosome 4, is approximately 42 kb in size and has more than 120 reported variants.16 Most of the variants are rare except three commonly recognized variants according to single nucleotide polymorphisms at rs4588 and rs7041 in exon 11: GC1F (A/G), GC1S (A/T), and GC2 (C/G).17 The association of these VDBP polymorphisms and the risk for COPD has been debated in the literature for some time.9,18,19,20,21,22,23,24,25,26 GC2 tended to be protective variants for COPD in Caucasian populations and GC1F tended to be risk variants for COPD in Asian populations.27 However, Asian studies were conducted in the limited ethnicities, and GC variants were not evaluated in South Korea. Moreover, the studies were conducted in a small number of patients and the relationship with lung function decline was rarely investigated.20
The purpose of this study was to evaluate the frequencies of GC variants in Koreans with COPD as well as healthy subjects. We determined that certain GC variants are associated with a genetic susceptibility to COPD in Koreans and examined the correlation between GC variants and lung function in COPD patients.
MATERIALS AND METHODS
Subjects
The COPD group consisting of 203 patients who were taken from the Korean Obstructive Lung Disease (KOLD) Cohort, in which patients with COPD or asthma have been enrolled from pulmonary clinics in 17 hospitals in South Korea from June 2005 to 2011, were selected for this study. The subjects were culled from a KOLD Cohort that fulfilled the following criteria: post-bronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FEV1/FVC) <0.7, over 40 years of age, smoking history of 10 or more pack-years, and no or minimal abnormality on chest radiography. The control group was selected from a community-based prospective cohort, South Korea. Among 2534 people recruited in 2006, 157 participants had a normal pulmonary function test, 10 or more pack-years of smoking history, and were over the age of 40. The study protocol was approved by the Institutional Review Boards of the 17 hospitals included in the KOLD Cohort and the community-based prospective cohort for control group, and informed written consent was obtained from all patients.
Pulmonary function testing
Spirometry was performed according to American Thoracic Society/European Respiratory Society guidelines. Airway obstruction is defined by Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria as FEV1/FVC ratio of <0.7. The severity of the disease was based on the percent predicted FEV1 in accordance with GOLD criteria.28 For COPD patients, pulmonary function tests (PFTs) were performed at follow-up visits annually and PFT was performed at least two times in 178 (87.6%) with an average of 3.7 times during the study.
Genotyping
For genotyping GC, DNA was extracted from blood and polymerase chain reaction (PCR) was performed followed by restriction fragment-length polymorphism analysis. The region that includes the two point mutation sites in exon 11 (Glu/Asp 416 and Thr/Lys 420) was amplified.27 We used upstream primer of 5'TAATGAGCAAATGAAAGAAG3' and downstream primer of 5'TGAGTAGATTGGAGTGCATAC3'.20,25 The final PCR product was a fragment of 462 base pairs (bp).
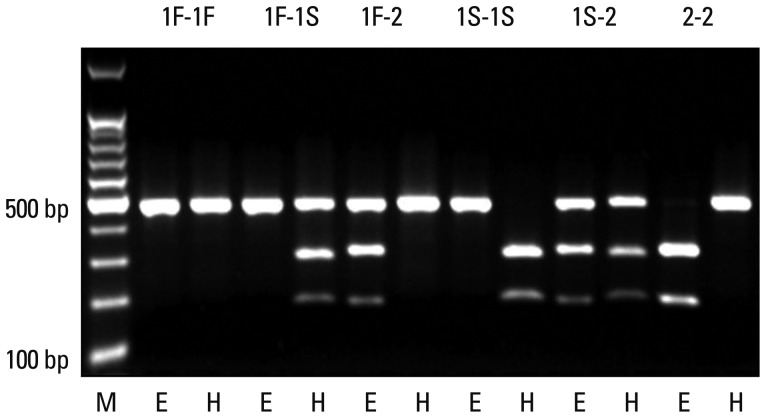
PCR was performed in a thermal cycler (DNA Thermal Cycler; Perkin Elmer Cetus; Norwalk, CT, USA). PCR reactions were carried out in a volume of 40 µL containing 100 ng DNA, 1.5 mM MgCl2, 10 mM Tris Cl (pH 8.3), 40 mM KCl, 4% dimethyl sulphoxide, 0.2 mL of each dNTP (Amersham Biosciences KK; Tokyo, Japan), 0.5 µmol/L of each primers, and 3.75 units of Taq DNA polymerase (Bioneer, Daejeon, Korea). After amplification, PCR products were digested with Hae III (Toyobo; Osaka, Japan), or Eco T14 I (Takara Bio; Otsu, Japan) at 37℃ overnight. Hae III cuts the GC1S product into two bands of 295 bp and 167 bp, whereas Eco T14 I cuts the GC2 product into two bands of 302 bp and 156 bp. GC1F PCR product remains uncut by either enzyme (Fig. 1).
Fig. 1.
Restriction fragment-length polymorphism analysis of GC. Eco T14 I cuts GC2 into two bands of 302 base pairs (bp) and 156 bp, whereas Hae III cuts GC1S into two bands of 295 bp and 167 bp. GC1F remains uncut by either of the enzymes (E: digested by Eco T14 I, H: digested by Hae III).
Definitions
Rapid decliners were defined as those with a decrease in FEV1 ≥3.0% predicted/yr and non-decliners as those with an increase in FEV1 ≥0% predicted/yr.24
Data analysis
Differences in distribution and frequency of variants and genotypes among the groups were examined by chi-squared test. Normally distributed variables are presented as means±standard deviations, and non-normally distributed variables are presented as medians (interquartile range). Genotype frequencies and Hardy-Weinberg equilibrium (HWE) for the GC between COPD and control groups were determined by chi-squared test. Statistical inference of the genotype effect on FEV1 was conducted with the linear mixed model to consider correlation between annually measured FEV1. Statistical inference of the genotype effect on FEV1 at the 0.05 significance level was conducted with the linear mixed model to consider correlation between repeated measures. COPD patients and control subjects were included for linear mixed model and COPD indicator was included as dummy variable to explain their mean differences. We also found the heteroscedasticity between COPD patients and control subjects, and a different variance-covariance matrix was applied. The optimal correlation structure between repeated observations was selected with the likelihood ratio test based on the restricted maximum likelihood method and the unstructured correlation format was chosen.29,30 Age, sex, height, pack-year, and their interactions were included as covariates and the significant interactions were selected with likelihood ratio test based on the maximum likelihood method. Marginal effects are all included in our final model. In all analyses, a p-value <0.05 was deemed to be statistically significant.
RESULTS
Population characteristics
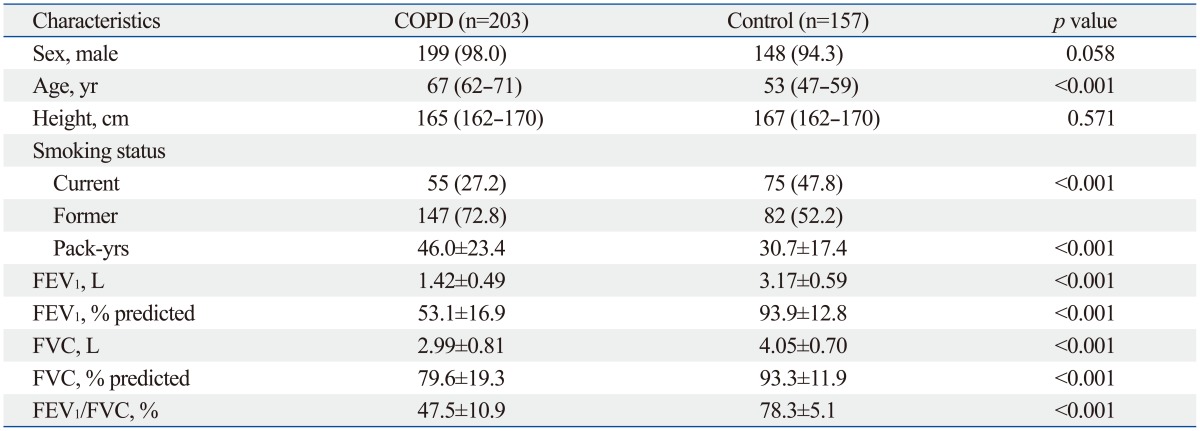
All baseline characteristics of the study population are summarized in Table 1. Both groups exhibited a similar gender distribution of significant male dominance. COPD patients were also of more advanced age than the control population. The number of current smokers was higher in controls compared to COPD group, whereas former smokers were more prevalent in the COPD group than controls. Among COPD patients, 17 (8.4%), 101 (49.8%), 73 (36.0%), and 12 (5.9%) patients were classified as GOLD class I, II, III and IV.
Table 1.
Baseline Characteristics of the Study Population*
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
*Data are presented as numbers (percentages) for categorical variables. Continuous variables are presented as means±standard deviations or median (interquartile range).
Genotyping
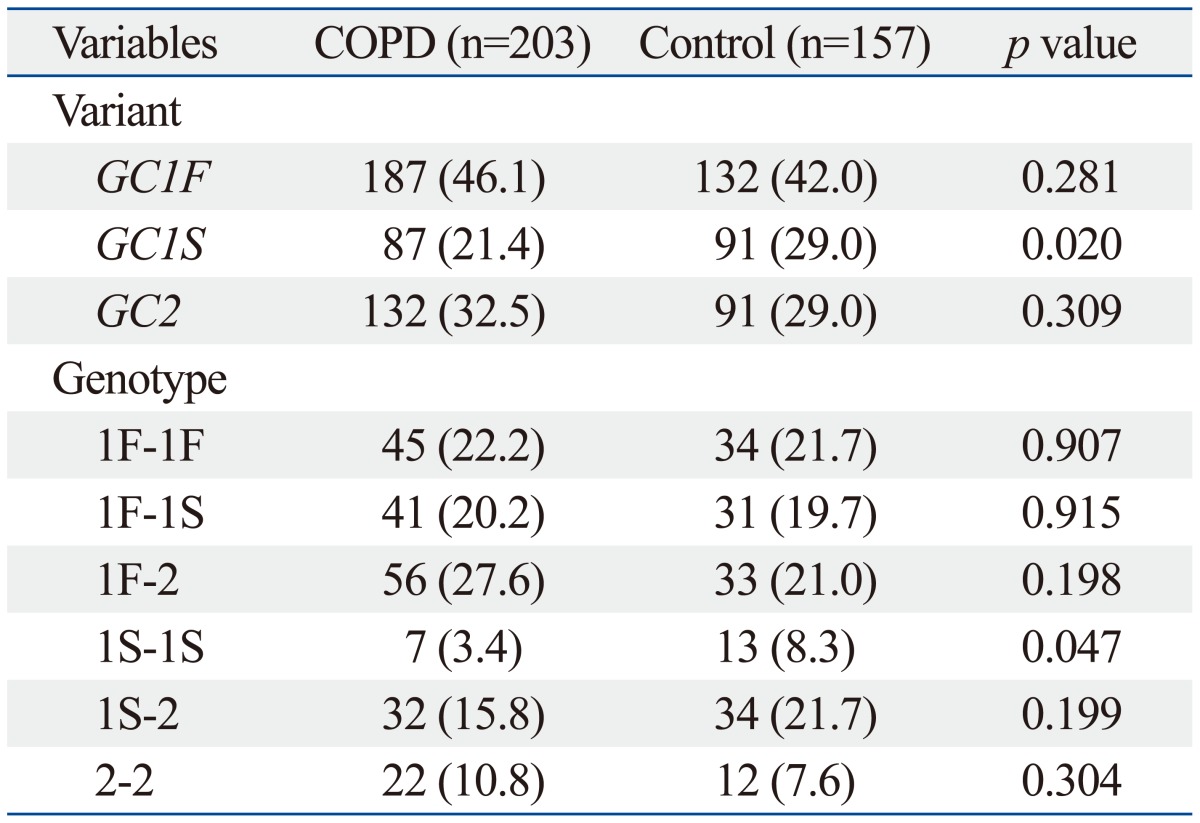
The frequency of each variant and genotype in COPD and control groups are compared in Table 2. Each of the investigated single nucleotide polymorphisms (rs4588 and rs7041) was in HWE in the controls (all p>0.05). Frequencies of GC1F, GC1S, and GC2 variants in COPD patients were 46.1%, 21.4%, and 32.5%, respectively, and 42.0%, 29.0%, and 29.0%, in controls, respectively. GC1S variant was more frequent in controls than COPD patients (29.0% vs. 21.4%; p=0.020). There were no significant differences in sex ratio, age, and smoking history between the different types of genotypes. Genotype 1F-2 was most common in both groups and genotype 1S-1S was significantly more frequent in control than COPD (8.3% vs. 3.4%; p=0.047).
Table 2.
Variant and Genotype Frequency of GC in COPD and Control Groups*
COPD, chronic obstructive pulmonary disease.
*Data are presented as numbers (percentages).
Comparison between rapid decliners and non-decliners
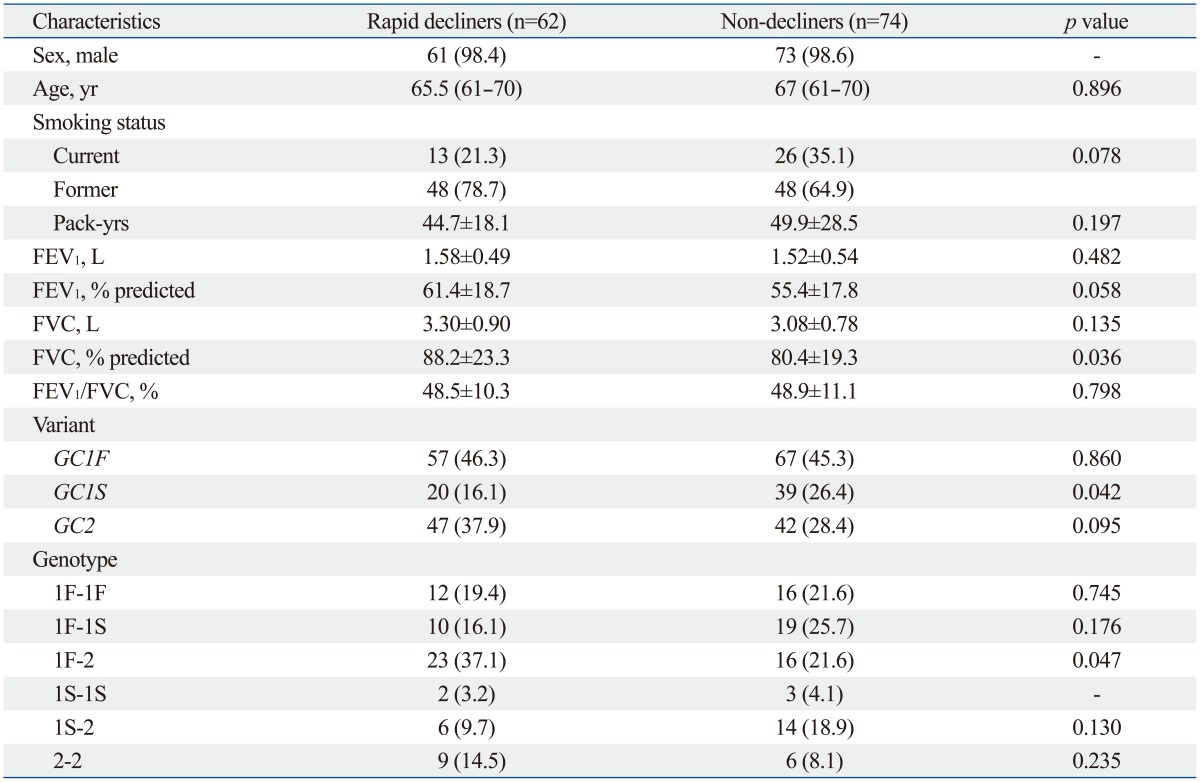
Table 3 shows the comparison of characteristics and genotypes between 62 rapid decliners and 74 non-decliners. Baseline characteristics including sex ratio, age, and spirometry data were not different between the two groups. In comparing the variants and genotypes, GC1S variant was more frequently observed in non-decliners (26.4% vs. 16.1%, p=0.042) and genotype 1F-2 was more frequently observed in rapid decliners (37.1% vs. 21.6%, p=0.047).
Table 3.
Characteristics of Subjects with COPD Classified by Annual Rates of Decline in FEV1*
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
*Data are presented as numbers (percentages) for categorical variables. Continuous variables are presented as means±standard deviations or median (interquartile range).
Parameters associated with FEV1 according to linear mixed model analysis
The difference in FEV1 between different genotypes was investigated by linear mixed model analysis including sex, age, height, and pack-years of smoking as shown in Table 4. FEV1 was 2.649 L lower in COPD compared to controls (p<0.001), 0.345 L lower in females than in males (p=0.014), and 0.033 L lower per year of age (p<0.001). Moreover, as stature increased by 1 cm, FEV1 also increased by 0.027 L (p<0.001). Among the different genotypes, subjects with genotype 1S-1S had 0.427 L higher FEV1 than those with other genotypes (p=0.029). The genotype/smoking pack-year interaction was found to be particularly significant among subjects with genotype 1S-1S; FEV1 decreased by 0.014 L per smoking pack-year (p=0.001).
Table 4.
Frequency of GC Polymorphism and the Risk of Chronic Obstructive Pulmonary Disease in Other Countries
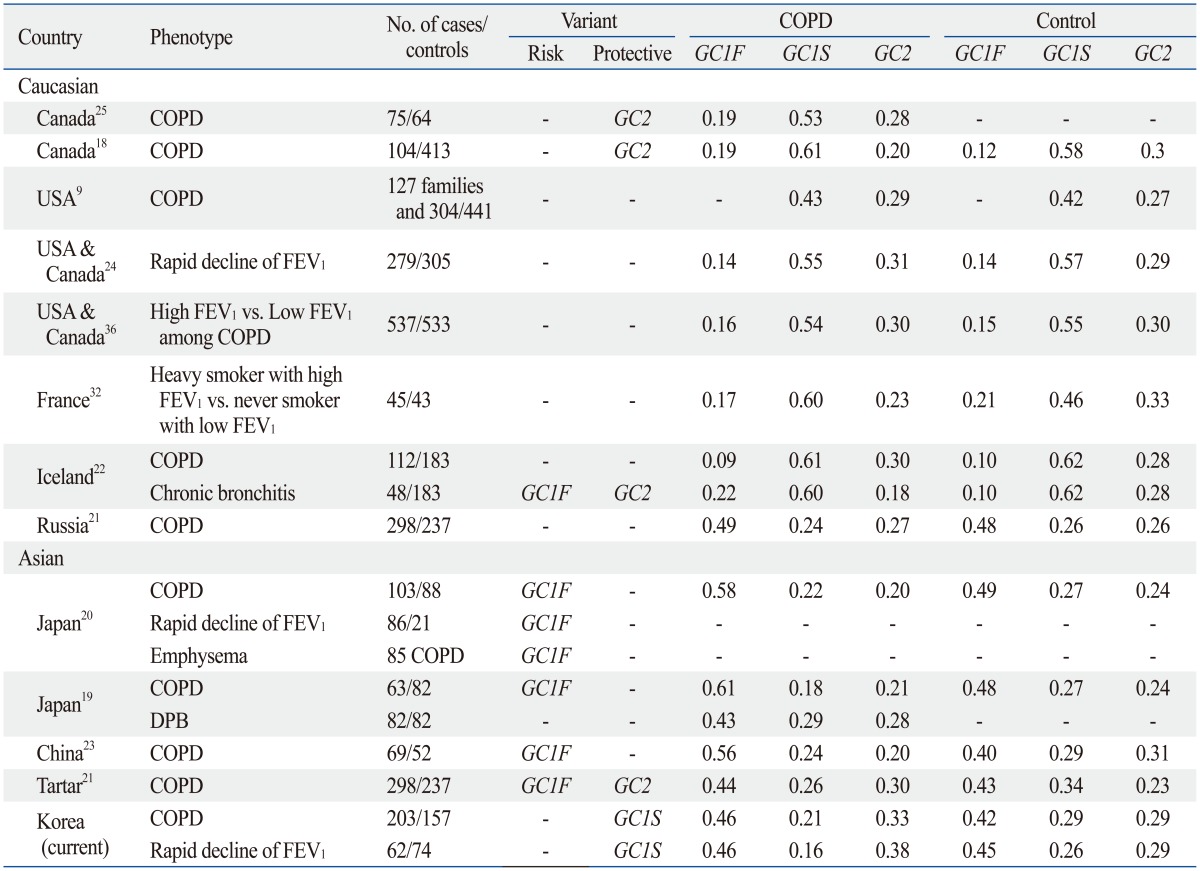
COPD, chronic obstructive pulmonary disease; USA, United States of America; FEV1, forced expiratory volume in 1 s; DPB, diffuse panbronchiolitis.
According to the linear mixed model analysis, the final equations were obtained as follows: FEV1=0.488-0.033×age (year)+0.027×height (cm)-0.012×smoking pack-years+0.427 (for control group with genotype 1S-1S); FEV1=0.488-0.033×age (year)+0.027×height (cm)+0.002×smoking pack-years (for control group without genotype 1S-1S); FEV1=0.488-0.013×age (year)+0.027×height (cm)-0.012×smoking pack-years-1.711 (for COPD group with genotype 1S-1S); FEV1=0.488-0.013×age (year)+0.027×height (cm) +0.002×smoking pack-years-2.649 (for COPD group without genotype 1S-1S).
DISCUSSION
The potential association of polymorphisms in GC with the risk of COPD and lung function was investigated for the first time in a Korean population. Subjects with GC1S variant and genotype 1S-1S were more commonly observed in control than in COPD. Moreover, GC1S variant was more frequent in non-decliners than in rapid decliners among COPD. According to the linear mixed model analysis, genotype 1S-1S showed higher initial FEV1 than other genotypes. However, FEV1 decreased more in 1S-1S patients than in other genotypes as smoking pack-years increased.
Frequencies of the different genotypes previously discussed vary based on ethnicity (Table 4). In the Japanese population, GC1F variant is most commonly observed, comprising approximately half of the population (0.58-0.61 in COPD and 0.48-0.49 in control). In Caucasians, GC1S variant is most common with a frequency of 0.53-0.61 in COPD and 0.58 in controls.19,20 The Korean population in this study showed a similar distribution to the Japanese population.18,25 GC1F variant was most frequent in COPD (0.46) and control group (0.42), although its frequency was smaller than that in a Japanese population.
Prior investigations have demonstrated different results regarding the association between polymorphisms in GC and risk of COPD; however, studies focusing on Asian populations have only been in Chinese and Japanese cohorts. Ishii, et al.19 reported GC1F variant and genotype 1F-1F were significantly higher in COPD than in control (85.7% vs. 75.6%, p=0.036; 36.5% vs. 20.7%, p=0.035) in a Japanese population. In another Japanese study, Ito, et al.20 reported a higher frequency of genotype 1F-1F in COPD patients than in controls (32% vs. 17%, p=0.014). In a Chinese population, Lu, et al.23 suggested that GC1F variant and genotype 1F-1F were risk factors for COPD (55.8% vs. 40.4%, p=0.018; 33.3% vs. 11.5%, p=0.005). In a Canadian Caucasian population, Horne, et al.18 reported GC2 variant and genotype 2-2 were less frequently observed in a COPD group. Relative risks of genotype 2-2, 2-1S, 2-1F for COPD were 0.8, 0.7, and 0.5, respectively. Moreover, they stated that GC1F variant was a significant risk factor for COPD (relative risk=4.8). Schellenberg, et al.25 were able to confirm that genotype 2-2 was protective against COPD (odds ratio=0.17, 95% CI 0.03-0.83). In an American population, Kueppers, et al.31 also showed genotype 2-2 as a protective factor for COPD (relative risk=0.2). Despite all these reports, several studies have failed to show association between GC polymorphisms and COPD.24,32
Most of these studies were conducted with a cross-sectional design, such that only the frequencies of certain GC polymorphisms were compared between COPD and control groups. A few studies demonstrated the relationship of GC polymorphisms with lung function decline through longitudinal analysis.20,24 Although Sandford, et al.24 reported no difference between fast decliners and non-decliners, Ito, et al.20 showed that GC1F variant still is a risk factor for rapid lung function decline even in a longitudinal analysis. However, in the latter, the number of subjects with available follow-up lung function data was small and the follow-up duration of 1 year may not be long enough to accurately analyze lung function decline. The size of the subjects in our study is too small to demonstrate the effect of GC polymorphisms on COPD and lung function. However, we have analyzed all the follow-up lung function data of subjects with longer duration of follow-up using a linear mixed model.
There are several possible explanations for the association between GC polymorphisms and lung function decline. The major role of VDBP is to bind and transport vitamin D. Vitamin D is essential for maintaining normal bone growth and calcium homeostasis, but it also contributes to immune modulation. Epidemiologic research has demonstrated vitamin D deficiency is associated with many types of chronic disease and these chronic diseases are all co-morbidities of COPD. Moreover, vitamin D deficiency is a risk factor of lung function decline in COPD through dysregulation of adaptive and innate immunity. Janssens, et al.33 reported that vitamin D deficiency correlates with the severity of COPD and certain types of GC polymorphisms are associated with reduced vitamin D levels.34 Moreover, VDBP enhances the chemotactic activity of C5-derived peptides on human neutrophils and monocytes. Enzymatic processing of the carbohydrate side-chain of VDBP transforms the molecule into a potent macrophage-activating factor (MAF). In turn, VDBP-MAF stimulates macrophage activity. Recently, Wood, et al.35 reported that the GC2 genotype is less efficient at converting VDBP to VDBP-MAF.
However, there are limitations to the present study. First, the sex and age between the two groups were not exactly matched. The COPD group was comprised of slightly more males and older subjects compared to the control group. Though the effect of age was adjusted for by statistical methods, some of the younger control subjects may yet develop COPD in the future. Moreover, cautious interpretation of the results is needed because of the relatively small sample size, especially GC1S variant and genotype 1S-1S. Analysis of lung function in control subjects was limited because follow-up lung function data were only available in five subjects of control group. Finally, vitamin D status is also an important factor in investigating the association of GC genotypes and lung function, but it was not included in this study.
In conclusion, this study suggested that GC polymorphisms might be associated with COPD and lung function in Korean population. Subjects with GC1S variant and genotype 1S-1S were more common in control than in COPD. GC1S variant was more frequent in non-decliners than in rapid decliners among COPD. The subjects with genotype 1S-1S showed higher initial FEV1 than other genotypes. However, genotype 1S-1S was associated with lower lung function in the context of cigarette smoking. As the amount of smoking in subjects with genotype 1S-1S increases, decrease in lung function became more pronounced. To elucidate the relationship of GC polymorphisms and lung function in COPD, additional studies with larger number of subjects are required.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health and Welfare (A102065), a grant of the Korea Centers for Disease Control and Prevention (2006-E71011-00), a faculty research grant of Yonsei University College of Medicine for 2011 (6-2011-0192), and National Research Foundation of Korea Grant funded by the Korean Government (2013R1A1A 2010437).
All members of the Korean Obstructive Lung Disease (KOLD) Study Group contributed to the recruitment of COPD patients and also to the collection of data and samples: Ji-Hyun Lee, Eun Kyung Kim, Tae-Hyung Kim, Tae Rim Shin, Kwang Ha Yoo, Seung Soo Sheen, Jin Hwa Lee, Seong Yong Lim, Sang Yeub Lee, Ho Il Yoon, Yong Bum Park, Yong Il Hwang, Young Sam Kim, Ji Ye Jung, Yoonki Hong, Seung Won Ra, Joon Beom Seo, Sang Min Lee, Sei Won Lee, Jae Seung Lee, Jin Won Huh, Ji Yong Moon, Hye Kyeong Park, Hye Yun Park, Jin Woo Kim, Chin Kook Rhee, Hyoung Kyu Yoon, Woo Jin Kim, Yeon-Mok Oh, and Sang-Do Lee.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Snider GL. Chronic obstructive pulmonary disease: risk factors, pathophysiology and pathogenesis. Annu Rev Med. 1989;40:411–429. doi: 10.1146/annurev.me.40.020189.002211. [DOI] [PubMed] [Google Scholar]
- 2.Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005;128:1239–1244. doi: 10.1378/chest.128.3.1239. [DOI] [PubMed] [Google Scholar]
- 3.Bridevaux PO, Probst-Hensch NM, Schindler C, Curjuric I, Felber Dietrich D, Braendli O, et al. Prevalence of airflow obstruction in smokers and never-smokers in Switzerland. Eur Respir J. 2010;36:1259–1269. doi: 10.1183/09031936.00004110. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DS, Kim YS, Jung KS, Chang JH, Lim CM, Lee JH, et al. Prevalence of chronic obstructive pulmonary disease in Korea: a population-based spirometry survey. Am J Respir Crit Care Med. 2005;172:842–847. doi: 10.1164/rccm.200502-259OC. [DOI] [PubMed] [Google Scholar]
- 6.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–763. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury MJ, Beaty TH, Newill CA, Bryant S, Cohen BH. Genetic-environmental interactions in chronic airways obstruction. Int J Epidemiol. 1986;15:65–72. doi: 10.1093/ije/15.1.65. [DOI] [PubMed] [Google Scholar]
- 8.Tager I, Tishler PV, Rosner B, Speizer FE, Litt M. Studies of the familial aggregation of chronic bronchitis and obstructive airways disease. Int J Epidemiol. 1978;7:55–62. doi: 10.1093/ije/7.1.55. [DOI] [PubMed] [Google Scholar]
- 9.Hersh CP, Demeo DL, Lange C, Litonjua AA, Reilly JJ, Kwiatkowski D, et al. Attempted replication of reported chronic obstructive pulmonary disease candidate gene associations. Am J Respir Cell Mol Biol. 2005;33:71–78. doi: 10.1165/rcmb.2005-0073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirschfeld J. Immune-electrophoretic demonstration of qualitative differences in human sera and their relation to the haptoglobins. Acta Pathol Microbiol Scand. 1959;47:160–168. doi: 10.1111/j.1699-0463.1959.tb04844.x. [DOI] [PubMed] [Google Scholar]
- 11.Daiger SP, Schanfield MS, Cavalli-Sforza LL. Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D. Proc Natl Acad Sci U S A. 1975;72:2076–2080. doi: 10.1073/pnas.72.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binder R, Kress A, Kan G, Herrmann K, Kirschfink M. Neutrophil priming by cytokines and vitamin D binding protein (Gc-globulin): impact on C5a-mediated chemotaxis, degranulation and respiratory burst. Mol Immunol. 1999;36:885–892. doi: 10.1016/s0161-5890(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 13.Kew RR, Sibug MA, Liuzzo JP, Webster RO. Localization and quantitation of the vitamin D binding protein (Gc-globulin) in human neutrophils. Blood. 1993;82:274–283. [PubMed] [Google Scholar]
- 14.Piquette CA, Robinson-Hill R, Webster RO. Human monocyte chemotaxis to complement-derived chemotaxins is enhanced by Gc-globulin. J Leukoc Biol. 1994;55:349–354. doi: 10.1002/jlb.55.3.349. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto N, Homma S. Vitamin D3 binding protein (group-specific component) is a precursor for the macrophage-activating signal factor from lysophosphatidylcholine-treated lymphocytes. Proc Natl Acad Sci U S A. 1991;88:8539–8543. doi: 10.1073/pnas.88.19.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooke NE, Willard HF, David EV, George DL. Direct regional assignment of the gene for vitamin D binding protein (Gc-globulin) to human chromosome 4q11-q13 and identification of an associated DNA polymorphism. Hum Genet. 1986;73:225–229. doi: 10.1007/BF00401232. [DOI] [PubMed] [Google Scholar]
- 17.Cleve H, Constans J. The mutants of the vitamin-D-binding protein: more than 120 variants of the GC/DBP system. Vox Sang. 1988;54:215–225. doi: 10.1111/j.1423-0410.1988.tb03908.x. [DOI] [PubMed] [Google Scholar]
- 18.Horne SL, Cockcroft DW, Dosman JA. Possible protective effect against chronic obstructive airways disease by the GC2 allele. Hum Hered. 1990;40:173–176. doi: 10.1159/000153926. [DOI] [PubMed] [Google Scholar]
- 19.Ishii T, Keicho N, Teramoto S, Azuma A, Kudoh S, Fukuchi Y, et al. Association of Gc-globulin variation with susceptibility to COPD and diffuse panbronchiolitis. Eur Respir J. 2001;18:753–757. doi: 10.1183/09031936.01.00094401. [DOI] [PubMed] [Google Scholar]
- 20.Ito I, Nagai S, Hoshino Y, Muro S, Hirai T, Tsukino M, et al. Risk and severity of COPD is associated with the group-specific component of serum globulin 1F allele. Chest. 2004;125:63–70. doi: 10.1378/chest.125.1.63. [DOI] [PubMed] [Google Scholar]
- 21.Korytina GF, Akhmadishina LZ, Ianbaeva DG, Viktorova TV. [Genotypes of vitamin-D-binding protein (DBP) in patients with chronic obstructive pulmonary disease and healthy population of Republic Bashkortostan] Mol Biol (Mosk) 2006;40:231–238. [PubMed] [Google Scholar]
- 22.Laufs J, Andrason H, Sigvaldason A, Halapi E, Thorsteinsson L, Jónasson K, et al. Association of vitamin D binding protein variants with chronic mucus hypersecretion in Iceland. Am J Pharmacogenomics. 2004;4:63–68. doi: 10.2165/00129785-200404010-00007. [DOI] [PubMed] [Google Scholar]
- 23.Lu M, Yang B, Cai YY. [The relationship between vitamin D binding protein gene polymorphism and chronic obstructive pulmonary disease] Zhonghua Nei Ke Za Zhi. 2004;43:117–120. [PubMed] [Google Scholar]
- 24.Sandford AJ, Chagani T, Weir TD, Connett JE, Anthonisen NR, Paré PD. Susceptibility genes for rapid decline of lung function in the lung health study. Am J Respir Crit Care Med. 2001;163:469–473. doi: 10.1164/ajrccm.163.2.2006158. [DOI] [PubMed] [Google Scholar]
- 25.Schellenberg D, Paré PD, Weir TD, Spinelli JJ, Walker BA, Sandford AJ. Vitamin D binding protein variants and the risk of COPD. Am J Respir Crit Care Med. 1998;157(3 Pt 1):957–961. doi: 10.1164/ajrccm.157.3.9706106. [DOI] [PubMed] [Google Scholar]
- 26.Bakke PS, Zhu G, Gulsvik A, Kong X, Agusti AG, Calverley PM, et al. Candidate genes for COPD in two large data sets. Eur Respir J. 2011;37:255–263. doi: 10.1183/09031936.00091709. [DOI] [PubMed] [Google Scholar]
- 27.Chishimba L, Thickett DR, Stockley RA, Wood AM. The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax. 2010;65:456–462. doi: 10.1136/thx.2009.128793. [DOI] [PubMed] [Google Scholar]
- 28.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease (revised 2011) [accessed on 2012 March 9]. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdf.
- 29.Neyman J, Pearson ES. On the problem of the most efficient tests of statistical hypotheses. Philos Trans R Soc Lond A. 1933;231:289–337. [Google Scholar]
- 30.Harville DA. Maximum likelihood approaches to variance component estimation and to related problems. J Am Stat Assoc. 1977;72:320–338. [Google Scholar]
- 31.Kueppers F, Miller RD, Gordon H, Hepper NG, Offord K. Familial prevalence of chronic obstructive pulmonary disease in a matched pair study. Am J Med. 1977;63:336–342. doi: 10.1016/0002-9343(77)90270-4. [DOI] [PubMed] [Google Scholar]
- 32.Kauffmann F, Kleisbauer JP, Cambon-De-Mouzon A, Mercier P, Constans J, Blanc M, et al. Genetic markers in chronic air-flow limitation. A genetic epidemiologic study. Am Rev Respir Dis. 1983;127:263–269. doi: 10.1164/arrd.1983.127.3.263. [DOI] [PubMed] [Google Scholar]
- 33.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 34.Gomme PT, Bertolini J. Therapeutic potential of vitamin D-binding protein. Trends Biotechnol. 2004;22:340–345. doi: 10.1016/j.tibtech.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Wood AM, Bassford C, Webster D, Newby P, Rajesh P, Stockley RA, et al. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax. 2011;66:205–210. doi: 10.1136/thx.2010.140921. [DOI] [PubMed] [Google Scholar]
- 36.Kasuga I, Paré PD, Ruan J, Connett JE, Anthonisen NR, Sandford AJ. Lack of association of group specific component haplotypes with lung function in smokers. Thorax. 2003;58:790–793. doi: 10.1136/thorax.58.9.790. [DOI] [PMC free article] [PubMed] [Google Scholar]