Abstract
Inflammation and oxidative stress are important mechanisms that have been implicated in the pathophysiology of major depressive disorder (MDD). Glutathione (GSH) is the most abundant antioxidant in human tissue, and a key index of antioxidant capacity and, hence, of oxidative stress. The aims of this investigation were to examine possible relationships between occipital GSH and dimensional measures of depressive symptom severity, including anhedonia – the reduced capacity to experience pleasure – and fatigue. We hypothesized that the magnitude of anhedonia and fatigue will be negatively correlated with occipital GSH levels in subjects with MDD and healthy controls (HC). Data for eleven adults with MDD and ten age- and sex-matched HC subjects were included in this secondary analysis of data from a previously published study. In vivo levels of GSH in a 3 cm × 3 cm × 2 cm voxel of occipital cortex were obtained by proton magnetic resonance spectroscopy (1H MRS) on a 3T MR system, using the standard J-edited spin-echo difference technique. Anhedonia was assessed by combining interest items from depression and fatigue rating scales, and fatigue by use of the multidimensional fatigue inventory. Across the full sample of participants, anhedonia severity and occipital GSH levels were negatively correlated (r = −0.55, p = 0.01). No associations were found between fatigue severity and GSH in this sample. These preliminary findings are potentially consistent with a pathophysiological role for GSH and oxidative stress in anhedonia and MDD. Larger studies in anhedonic depressed patients are indicated.
Keywords: Anhedonia, Glutathione, GSH, Oxidative stress, MRS
1. Introduction
Major depressive disorder (MDD) is a significant cause of disability and public health concern [4]. However, the neurobiology of the disorder remains poorly understood. A limiting factor in neurobiological research has been the use of categorical diagnostic criteria for MDD, which are based on a cluster of symptoms that are most likely derived from distinct etiologies [2]. Thus, studying specific symptoms, assessed quantitatively or dimensionally, may provide additional insight into pathophysiologies underlying MDD [11,17]. Anhedonia, the reduced capacity to experience pleasure and a core symptom of MDD, has features that make it ideal to be assessed and expressed as a quantitative or dimensional phenotype [7,8,10,19].
Convergent evidence suggests that peripheral activation of the immune system is associated with MDD [5,15,16]. We reported increased peripheral activation of the central neuroimmunological kynurenine pathway (KP) with anhedonia in adolescents, assessed both categorically and dimensionally [7]. We also reported positive associations between levels of KP neurotoxin in blood and striatal total choline (tCho, a putative index of membrane phospholipid peroxidation) in highly anhedonic adolescents [9]. These data support a role for peripheral and CNS inflammatory processes in anhedonia.
Inflammation is a major pathophysiological mechanism that mediates oxidative/nitrosative stress [3], which is increasingly implicated in severe psychiatric disorders. Glutathione (GSH) is a major intracellular antioxidant and redox regulator that protects cells against oxidative/nitrosative stress. Dysregulation of the GSH system has been hypothesized to reduce glutamatergic activity at the glutamate NMDA receptor and attenuate neurotrophin production, processes functionally linked to cognitive and affective symptoms in conditions such as MDD. Using proton magnetic resonance spectroscopy (1H MRS), we recently documented, for the first time, a 21% GSH decrease in the occipital lobe (OCC) in patients with MDD compared with healthy control (HC) subjects [24]. The OCC, primarily the precuneus, has been widely investigated in MRS studies of depression, based on technical advantages, but may be more implicated in MDD than previously thought as a result of multiple abnormalities, including GABA [22,23] and GSH [24] deficits and abnormal cortical thinning [18] that have been reported in this region.
The primary objective of the present study was to conduct an extended retrospective analysis of the above-mentioned data on occipital GSH levels in MDD [24] to assess whether there are significant associations between brain GSH levels and anhedonia and fatigue severity in MDD. We hypothesized that lower GSH will correlate with higher anhedonia severity in patients with MDD, as well as with fatigue, which often affects motivation to participate in enjoyable activities.
2. Materials and methods
2.1. Subjects
This study was approved by the Institutional Review Boards of Mount Sinai Medical Center and Weill Medical College of Cornell University. Prior to participation, study procedures were explained and informed consent was obtained.
Study participants were the same as previously reported [24], excluding subjects lacking sufficient data to assess anhedonia and fatigue severity, as elaborated below.
Participants with MDD were recruited via clinician referrals and media advertisements. Prior to brain imaging, all subjects were psychotropic medication-free for at least 2 weeks (4 weeks for fluoxetine). These subjects were free of alcohol or substance abuse/dependence for 6 months or more and had no lifetime history of psychosis, mania or hypomania, pervasive developmental disorder or mental retardation, and had no current eating disorder. HC subjects were recruited from media advertisements, did not meet the criteria for any past or present psychiatric disorder, and were group-matched for sex and age with the MDD group.
2.2. Inclusion and exclusion criteria
Participant eligibility included ages 18–65 years, negative urine toxicologies at baseline and on day of scan; use of effective birth control methods; and, for females, negative day-of-scan urine pregnancy test. In addition, patients were instructed to abstain from consuming alcohol for at least 48 h before neuroimaging. Exclusion criteria for all subjects consisted of any unstable medical or neurological illness or any factors precluding MRI exposure (e.g., pacemaker, metallic prosthesis, history of work with metal or shrapnel exposure).
2.3. Clinical assessments
All participants were assessed for psychiatric disorders, including MDD, using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition and were evaluated by a board-certified psychiatrist [1]. Additional assessments, performed within 1 week of the scan, included the 16-item Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR16) [21] and the 20-item Multidimensional Fatigue Inventory (MFI) [25].
Depression severity was assessed using the QIDS-SR16. To assess relationships between anhedonia and overall depression severity, item 13 (“general interest”) was removed from depression severity, because it was included in anhedonia assessment. Anhedonia scores were computed by summing the responses on QIDS-SR16 (item 13: “general interest”) and MFI (item 4: “feel like doing nice things”). Similar approaches have been used in other investigations assessing anhedonia severity [12,19] and the scores were shown to correlate with other anhedonia assessments (e.g. Snaith-Hamilton Pleasure Scale, SHAPS) [14]. Lastly, MFI was used as a dimensional measure of fatigue severity. Relationships between fatigue and GSH were assessed with and without item 4.
2.4. 1H MRS measurements of brain GSH
In vivo levels of GSH were obtained by 1H MRS from a 3 cm × 3 cm × 2 cm voxel, positioned to contain primarily bilateral occipital cortex (OCC) gray matter posterior to the lateral ventricle, at the level of a line defined by the genu and splenium of the corpus callosum. Imaging was performed on a 3T MR system and an eight-channel phased-array head coil, using the standard J-edited spin-echo difference technique [20,26] and spectral data analysis methods that were fully described recently [24] (Fig. 1). In brief, a standard point-resolved spectroscopy sequence was turned into a volume-selective J-editing technique by inserting a pair of frequency-selective inversion RF pulses, flanked by spoiler gradient pulses of opposite signs, before and after the second 180° RF pulse of the double spin echo. The application of these “editing” RF pulses at the frequency (4.56 ppm) of the GSH α-cysteinyl resonance on alternate scans, with TE/TR = 68/1500 ms, alternately inverts the GSH β-cysteinyl resonance at 2.98 ppm by inhibiting and allowing its J modulation. Subtracting the two resulting subspectra yields the GSH resonance at 2.98 ppm with elimination of the overlapping creatine peak, which is not J modulated. The reported GSH levels are expressed semi-quantitatively as GSH peak area ratios relative to the area of the simultaneously acquired unsuppressed voxel water resonance (W).
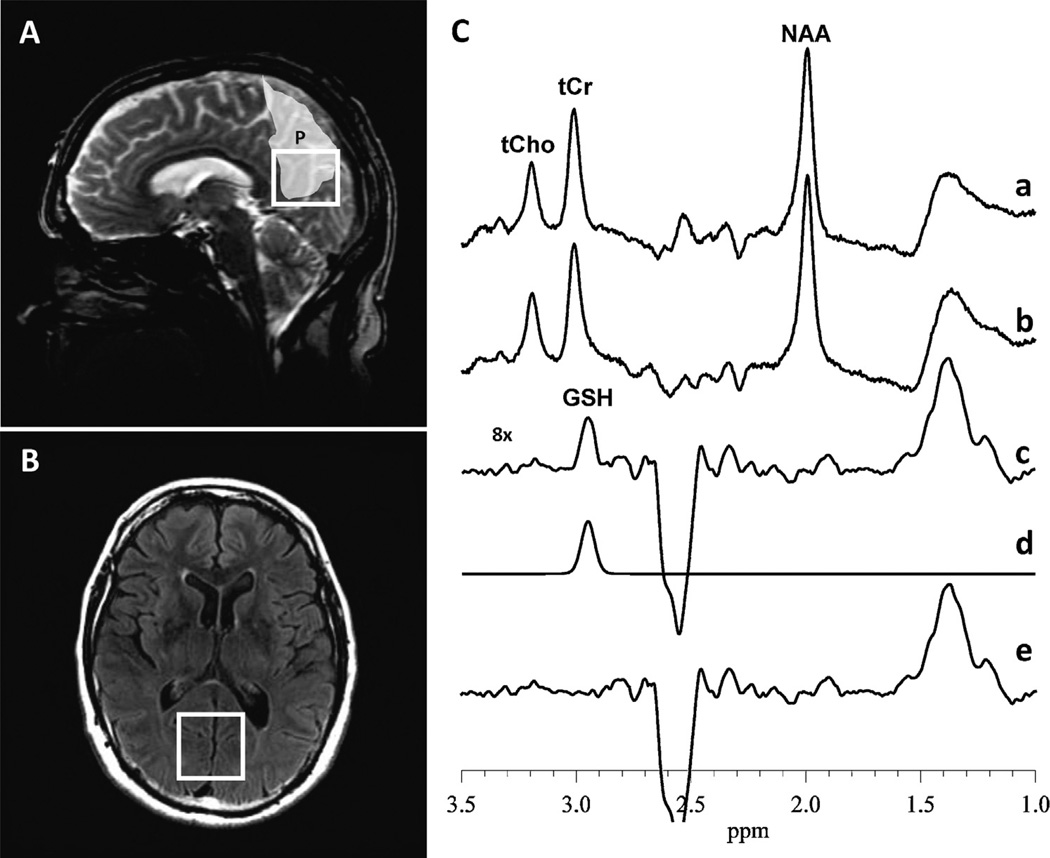
Fig. 1.
(A) Sagittal and (B) axial views of the voxel of interest (VOI) in the occipital cortex. The light shaded area depicts the precuneus [P], a large fraction of which can be seen to be contained within the VOI from which GSH was measured. (C) In vivo 1H MRS acquisition of GSH, showing: (a) and (b) subspectra that are subtracted to obtain the cleanly “edited” GSH spectrum in (c); (d) frequency-domain model-fitting of only the edited GSH peak in (c) to derive its peak, which is directly proportional to the concentration of the antioxidant in the VOI; (e) residual of the difference between (c) the measured and (d) the fitted GSH spectral peak shows the “goodness of fit”. NAA, N-acetyl-l-aspartate; tCr, total creatine; tCho, total choline.
2.5. Statistical data analysis
Demographic data was compared using t-tests and χ2 tests. All variables were normally distributed (Ryan-Joiner), and we used Pearson correlation coefficients to characterize the association of brain GSH levels with anhedonia and fatigue scores for the combined groups and MDD subjects alone. Statistical significance was defined as two-sided p ≤ 0.05, while trends toward significance were defined as p ≤ 0.1. General linear models were used to perform corrections for potential confounds. As these secondary analyses were exploratory and hypothesis-generating in nature, we did not apply statistical correction for multiple comparisons.
3. Results
3.1. Demographic and clinical characteristics
Study subjects consisted of 11 adults with MDD (ages 21–52) and 10 HC (ages 20–29), who did not differ with respect to gender, but did with respect to age. Clinical and demographic information are provided in Table 1. All subjects with MDD were in a current major depressive episode at time of scans.
Table 1.
Clinical and demographic characteristics of subjects with MDD and healthy volunteers (means ± SD are presented).
| Characteristic | Healthy volunteers (n = 10) | Major depressive disorder (n = 11) | p-Value |
|---|---|---|---|
| Age in years | 25.7 ± 2.6 | 34.6 ± 9.5 | 0.012 |
| Female (%) | 5/10 (50) | 6/11 (54) | 0.835 |
| Caucasian (%) | 6/10 (60) | 5/11 (45) | 0.504 |
| Hispanic (%) | 2/10 (20) | 2/11 (18) | 0.916 |
| Smoker (%) | 1/10 (10) | 3/11 (27) | 0.304 |
| Married (%) | 1/10 (10) | 1/11 (9) | n/a |
| Body mass index (kg/m2) | 23.6 ± 2.8 | 23.7 ± 4.3* | 0.95 |
| Illness duration in years | n/a | 9.2 ± 6.2 | n/a |
| Years of education | 15.3 ± 3.5 | 17.2 ± 4.1 | 0.270 |
| QIDS-SR16 | 3.3 ± 4.7 | 16.8 ± 4.7 | <0.001 |
| Anhedonia score | 1.5 ± 1.2 | 5.6 ± 1.4 | <0.001 |
QIDS-SR16 – Quick Inventory of Depressive Symptomatology Self Report 16 Item; Anhedonia Score = QIDS-SR item #13 + MFI item #4.
n = 10.
3.2. Association between depression severity and anhedonia scores
In the MDD group, anhedonia and overall depression severity (QIDS-SR minus item #13) were not significantly correlated (p > 0.1). Therefore, we did not control for depression severity in subsequent analyses.
3.3. Association between occipital GSH and anhedonia scores
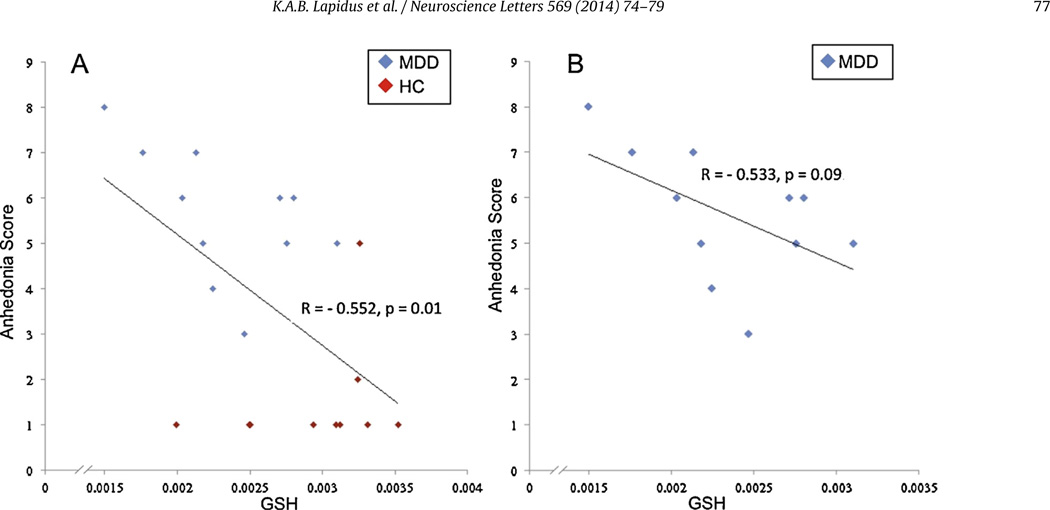
Levels of the reference unsuppressed voxel water signal did not differ between groups (p > 0.1), so we henceforth use GSH to mean GSH/W. Across the full sample, anhedonia severity was found to correlate inversely with OCC GSH levels (r = −0.55, p = 0.01, Fig. 2a), a finding that remained unchanged after controlling for age (F = 7.3, p = 0.015). Controlling for age, gender, and smoking status in a general linear model also did not alter this finding (F = 7.49, p = 0.015) (Table 2). Within only the MDD sample, there was a trend-level inverse correlation between anhedonia scores and OCC GSH levels (r = −0.53, p = 0.09, Fig. 2b).
Fig. 2.
Scatter plots indicating inverse correlations between occipital cortex GSH levels and anhedonia scores. (A) All subjects, r = −0.552, p = 0.01 and (B) MDD subjects r = −0.533, p = 0.09.
Table 2.
Correlation analyses between GSH and anhedonia or fatigue, with and without corrections for age, gender, and smoking status.
| Anhedonia vs. GSH MDD and HC (n = 21) |
Anhedonia vs. GSH MDD only (n = 11) |
Fatigue vs. GSH MDD and HC (n = 21) |
Fatigue vs. GSH MDD only (n = 11) |
|
|---|---|---|---|---|
| Uncorrected | (r = −0.552, p = 0.01) | (r = −0.533, p = 0.09) | (r = −0.026, p = 0.91) | (r = −0.396, p = 0.228) |
| Controlled for age | (F = 7.3, p = 0.015) | (F = 2.47, p = 0.154) | (F = 0.06, p = 0.813) | (F = 1.0, p = 0.348) |
| Controlled for age, gender, and smoking | (F = 7.49, p = 0.015) | (F = 0.63, p = 0.456) | (F = 0.14, p = 0.711) | (F = 0.77, p = 0.414) |
GSH, glutatione; MDD, major depressive disorder; HC, healthy control; vs., versus.
3.4. Association between occipital GSH and fatigue scores
No associations were found between fatigue severity and GSH either for the full sample (r = −0.026, p = 0.910) or within the MDD group alone (r = −0.396, p = 0.228).
4. Discussion
The present study is, to our knowledge, the first to investigate the relationship between in vivo brain GSH levels, anhedonia, and fatigue severity, in MDD. Consistent with our hypothesis, this secondary analysis found that occipital GSH levels were inversely correlated with anhedonia severity in the combined group of MDD and HC, and there was a trend-level inverse correlation between the two measures within only the MDD group. The results of this study did not support an association between brain GSH levels and fatigue severity.
Our finding that decreased GSH is associated with increased anhedonia severity is consistent with the postulated involvement of neuroinflammation and oxidative stress in MDD. There exist clinical data linking inflammation to both oxidative stress and to anhedonia [6]. This suggests that inflammation is the link between anhedonia and the observed occipital GSH deficits. Mechanistically, we postulate that on one hand inflammation acts via a powerful mediator, oxidative and nitrosative stress, depleting GSH levels by increasing consumption of antioxidant reserves (Fig. 3) [3]. On the other hand, inflammation is known to be involved in the activation of the immune system, particularly via the central neuroimmunological kynurenine pathway that also increases oxidative stress, a process that we previously showed to correlate with anhedonia in adolescents with MDD [7–9].
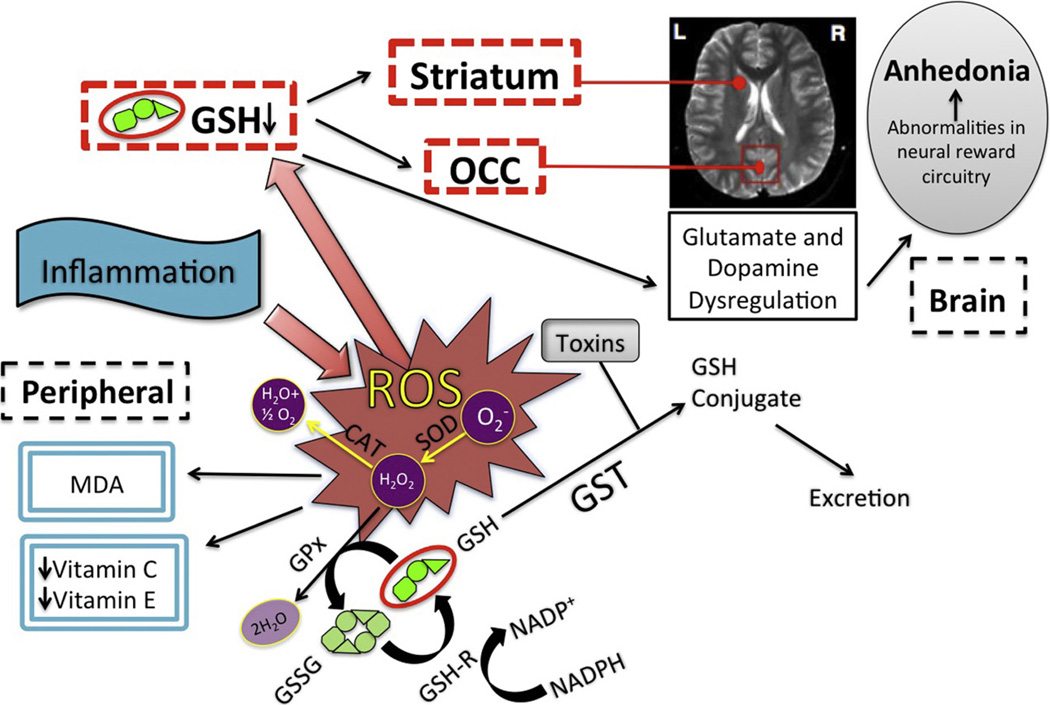
Fig. 3.
Model of inflammation-induced oxidative stress and role of GSH in anhedonia. Inflammation acts to increase OS, leading to the production of reactive oxygen species (ROS), which can cause cellular damage and death. One principal mechanism for management of these and other toxins is via the principal antioxidant in human tissue, GSH. Glutathione eliminates peroxides by cycling from reduced (GSH) to oxidized (GSSG) state via glutathione peroxidase (GPx), and reduced GSH is regenerated by glutathione reductase (GSH-R) in a reaction that concurrently oxidizes nicotinamide adenine dinucleotide phosphate from its reduced (NADPH) to oxidized (NADp+) form. Inflammation exerts both central and peripheral effects, and GSH reduction in brain regions including striatum and occipital cortex (OCC) leads to glutamate and dopamine dysregulation. These abnormalities affect the reward circuitry, leading to anhedonia.
This study did not find an association between fatigue and GSH levels, although we found such an association in patients with chronic fatigue syndrome (CFS). Since individuals affected with CFS experience primarily physical fatigue, the failure of the present study to find an association between GSH and “fatigue” in MDD, might reflect etiological differences in physical and mental manifestations of fatigue [13]. Thus, fatigue in affective illnesses such as MDD may be pathophysiologically different from fatigue in disorders such as CFS [24], although further study is needed.
This study has a number of limitations. First, the modest sample size limited the statistical power to find a stronger correlation between anhedonia and GSH within only the MDD group. Second, the lack of discrete values for anhedonia beyond the cutoff point representing the normal value led to a “floor effect” by truncating the range of anhedonia scores for HC group. This indicates a clear benefit for developing and using an anhedonia scale with a broader range. Moreover, our composite scale for anhedonia has not been compared with validated scales for assessing anhedonia. Lastly, the retrospective nature of this analysis was limiting in that the data were fixed for only the OCC, precluding examination or extrapolation to other regions that may be more relevant to MDD pathophysiology.
5. Conclusion
In summary, the results of the present study support a role for GSH and, potentially, oxidative stress and inflammation specifically in anhedonia in MDD, and further suggest the need to incorporate both dimensional and categorical measures in biological research of neuropsychiatric disorders.
HIGHLIGHTS.
We performed in vivo 1H MRS in subjects with major depression and healthy controls.
This data was used to examine oxidative stress and glutathione (GSH).
We analyzed anhedonia and fatigue severity in these subjects.
Anhedonia is negatively correlated with brain GSH levels.
Acknowledgements
Contract/grant sponsors included the Chronic Fatigue and Immune Dysfunction Syndrome (CFIDS) Association of America, Inc. and the National Institutes of Health (contract/grant number: R01 MH-075895, K23 MH-069656). We thank the patients who participated in this study and Mr Josefino Borja for his valuable contribution in operating the MRI scanner. Dr. Lapidus receives support from a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation. Dr. Gabbay and Ms. Johnson are supported by R01 MH-095807. Dr. Mathew is supported by the Marjorie Bintliff Johnson and Raleigh White Johnson, Jr. Chair for Research in Psychiatry at Baylor College of Medicine, and with resources and the use of facilities at the Michael E. DeBakey VA Medical Center, Houston, TX. Dr. Murrough is supported by NIH grant K23 MH-094707.
Dr. Lapidus has received research support from the Brain and Behavior Research Foundation, APIRE/Janssen, and the Le Foundation; he has received consulting fees from LCN consulting and serves on the advisory board for Halo Neuro, Inc. Dr. Murrough has received research support from the National Institutes of Health, the National Institute of Mental Health, the Department of Veterans Affairs, the Doris Duke Charitable Foundation, the American Foundation for Suicide Prevention, the Brain and Behavior Research Foundation, Janssen Research and Development, and Avanir Pharmaceuticals; he has served on advisory boards for Janssen Research and Development and Genentech and has provided consultation services for ProPhase, LLC and Impel Neuropharma. Dr. Mathew has received consulting fees or research support in the past 12 months from AstraZeneca, Bristol-Myers Squibb, Genentech, and Naurex.
Abbreviations
- 1H MRS
proton magnetic resonance spectroscopy
- MDD
major depressive disorder
- GSH
glutathione
- HC
healthy control
- MR
magnetic resonance
- T
tesla
- KP
kynurenine pathway
- tCho
total choline
- CNS
central nervous system
- NMDA
N-methyl-d-aspartate
- MRI
magnetic resonance imaging
- QIDS-SR16
Quick Inventory of Depressive Symptomatology-Self Report
- MFI
multidimensional fatigue inventory
- OCC
occipital cortex
- W
voxel water resonance
- CFS
chronic fatigue syndrome
- SHAPS
Snaith-Hamilton Pleasure Scale
Footnotes
Conflict of interest
The other authors have no conflicts to report.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. Task Force on DSM-IV; p. xxxvii.p. 943. [Google Scholar]
- 2.Andrews G, Brugha T, Thase ME, Duffy FF, Rucci P, Slade T. Dimensionality and the category of major depressive episode. Int. J. Methods Psychiatr. Res. 2007;16(Suppl. 1):S41–S51. doi: 10.1002/mpr.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch. Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 4.Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, Anderson W, Dhansay MA, Phillips A, Shurin S, Walport M, Ewart W, Savill SJ, Bordin IA, Costello EJ, Durkin M, Fairburn C, Glass RI, Hall W, Huang Y, Hyman SE, Jamison K, Kaaya S, Kapur S, Kleinman A, Ogunniyi A, Otero-Ojeda A, Poo MM, Ravindranath V, Sahakian BJ, Saxena S, Singer PA, Stein DJ. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front. Neuroendocrinol. 2012;33:315–327. doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabbay V, Ely BA, Babb J, Liebes L. The possible role of the kynurenine pathway in anhedonia in adolescents. J. Neural Transm. 2012;119:253–260. doi: 10.1007/s00702-011-0685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabbay V, Klein RG, Katz Y, Mendoza S, Guttman LE, Alonso CM, Babb JS, Hirsch GS, Liebes L. The possible role of the kynurenine pathway in adolescent depression with melancholic features. J. Child Psychol. Psychiatry. 2010;51:935–943. doi: 10.1111/j.1469-7610.2010.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabbay V, Liebes L, Katz Y, Liu S, Mendoza S, Babb JS, Klein RG, Gonen O. The kynurenine pathway in adolescent depression: preliminary findings from a proton MR spectroscopy study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:37–44. doi: 10.1016/j.pnpbp.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, Alonso CM, Shungu DC. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch. Gen. Psychiatry. 2012;69:139–149. doi: 10.1001/archgenpsychiatry.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 12.Joiner TE, Brown JS, Metalsky GI. A test of the tripartite model’s prediction of anhedonia’s specificity to depression: patients with major depression versus patients with schizophrenia. Psychiatry Res. 2003;119:243–250. doi: 10.1016/s0165-1781(03)00131-8. [DOI] [PubMed] [Google Scholar]
- 13.Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80:409–416. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J. Clin. Psychol. 2006;62:1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J. Affect. Disord. 2012;139:230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris SE, Cuthbert BN. Research domain criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin. Neurosci. 2012;14:29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, Durkin K, Adams PB, Wickramaratne P, Weissman MM. Cortical thinning in persons at increased familial risk for major depression. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6273–6278. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol. Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc. Natl. Acad. Sci. U. S. A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 22.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 23.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 24.Shungu DC, Weiduschat N, Murrough JW, Mao X, Pillemer S, Dyke JP, Medow MS, Natelson BH, Stewart JM, Mathew SJ. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. 2012;25:1073–1087. doi: 10.1002/nbm.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 26.Terpstra M, Henry PG, Gruetter R. Measurement of reduced glutathione (GSH) in human brain using LC model analysis of difference-edited spectra. Magn. Reson. Med. 2003;50:19–23. doi: 10.1002/mrm.10499. [DOI] [PubMed] [Google Scholar]