Abstract
Lung cancer is the biggest cancer killer in the UK and despite recent therapeutic advances there is a desperate need for new therapies to improve outcomes from this devastating disease. Through defining the spatial location of the airway epithelial stem or progenitor cell populations and their mechanisms of maintenance and repair of the epithelium it is becoming clear that these populations are situated at areas corresponding to those involved in lung cancer initiation. We explore the evidence for stem cells being the cancer initiator cell and for a ‘lung cancer stem cell’ within tumours that may be the cause of resistance to current therapies.
Introduction
Lung cancer is the biggest cancer killer in the UK, affecting 34 000 patients a year. Despite recent therapeutic advances, including personalized therapies targeting genetic mutations, the 5-year outcome remains poor.1
Advances in lung biology have elucidated the cells responsible for maintenance and repair of the respiratory epithelium. The anatomical locations of these respiratory stem cells correlate with the origin and type of lung tumours, with evidence emerging of an ‘origin of cancer stem cell’. This review focuses on lung stem cells in epithelial maintenance and repair, the originating cell population of lung malignancy and the concept of ‘lung cancer stem cells’. These are the cells that propagate cancers and which may be responsible for post-treatment relapses. We focus on how this new knowledge may be exploited in the development of novel treatment strategies.
Airway stem cells in lung repair and homoeostasis
The lungs are open to the outside environment and are exposed to a number of chemicals and other insults throughout an individual’s lifetime. The preservation of an intact epithelium is essential to maintain its many roles. The epithelium humidifies and warms inspired air, undertakes gaseous exchange and defends against pathogens. Proximally, the epithelium of the upper airways is lined by a pseudostratified epithelium consisting of ciliated, Clara and goblet cells. In among the columnar cells are basal cells and a small number of neuroendocrine cells. Moving into the small airways and bronchioles, the epithelium becomes more columnar, and Clara cells become more abundant. Beyond the terminal bronchi, the alveoli are lined by two epithelial cell types, the alveolar type 1 (AT1) cells forming the gas exchange surface and the AT2 cells producing surfactant.
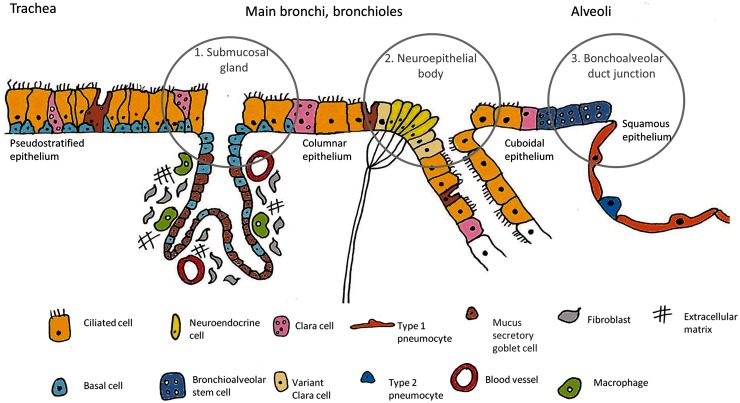
Epithelial integrity is important for its many functions, with the airway stem or progenitor cells playing a crucial role in its maintenance. Stem cells are defined by their high proliferative capacity, their ability to self-renew and their capacity to produce multiple daughter cells of varying types.2 In several organs they tend to reside among other stem cells in an area called the stem cell niche. Identification of airway stem cell niches has been made possible through use of mouse injury models. Through selective targeting and damage of a particular cell population, the cells responsible for repairing and repopulating the damaged tissue can be elucidated. In the murine airway, this strategy has identified three stem cell niches: the submucosal glands in the trachea and upper airways that harbour the basal cells, the calcitonin gene-related peptide (CGRP) marked neuroepithelial bodies (NEBs) situated at the branch points of small airways and the bronchoalveolar duct junctions (BADJs) located at the transition of the bronchi and alveoli3 (Figure 1).
Figure 1.
Schematic showing the location of the different cell types making up the epithelial surfaces of the airways. The stem cell niches are highlighted within circles. These correspond with the likely origin of lung cancers. The basal cells of the submucosal glands are suggested to give rise to SCCs and the NEBs to SCLCs. Cells of the BADJ/alveoli are the speculated origin of adenocarcinomas. The mouse airway is shown, as this is where the evidence for stem cell populations originates.
Murine models and lineage tracing has defined basal cells as responsible for maintenance of the upper airway epithelium. A high concentration is present within the niche of the submucosal glands. When keratin 5, a basal cell surface marker is labelled in a mouse model, subsequent cell-tracking, or lineage tracing, results in labelled Clara and ciliated populations. The number of labelled cells also increases on airway damage,4 indicating a key role of the basal cells in tracheal and upper airway maintenance and repair.
Moving along the airway, the next stem-cell containing niche is the NEB consisting of two cell types: the Clara cell secretory protein (CCSP) expressing ‘variant’ Clara cells and the pulmonary neuroendocrine cells marked by CGRP. The NEBs were identified through exposure of mice to the chemical naphthalene, an agent causing selective Clara cell death. While the Clara cells were destroyed, an upregulation of activity was demonstrated in the ‘variant’ Clara cell population, thus termed due to their naphthalene resistance and their ability to subsequently repair airway damage.5
Finally, epithelial damage experiments have identified a further resistant cell population at the BADJ.6 It has been suggested through in vitro studies that a BADJ cell population marked positive for both the Clara cell marker CCSP and the AT2 cell marker surfactant protein C (SPC) are bronchioalveolar stem cells (BASCs) able to differentiate into Clara, AT2 and AT1 cells.7 However, in vivo lineage tracing of CCSP positive cells (including the CCSP and SPC positive BASCs) have shown this to be unlikely, as CCSP cells did not contribute to alveolar repair on naphthalene or oxygen exposure.8 Interestingly, exposure to either influenza or bleomycin, both causing severe alveolar damage, showed that a CCSP labelled population, regenerated the lost AT2 cells.9 Direct labelling of CCSP/SPC dual positive cells is needed to clarify the situation.
While stem cells are activated on airway damage, evidence suggests it is not the stem cells, but a group of ‘committed progenitors’ that maintain epithelial integrity.10 These are thought to be cells that have arisen from the stem cells, are partially differentiated and have a more limited ability to produce daughter lineages. Clara cell and AT2 cell populations are thought to contain this type of progenitor cell, with Clara cells suggested to maintain the epithelium through regeneration of ciliated populations,8 and AT2 cells replacing lost AT1 cells in the distal airway.11
While these murine studies give a valuable insight into the maintenance and repair of the murine airway, the translation of their findings to the human airway has recently been put in doubt by the first evidence in man that under normal conditions within the large airways, homoeostasis is performed by the stochastic division of a large number of progenitor basal cells that are not pre-programmed stem cells but rather cells that divide and differentiate or continue to proliferate by chance, but in perfect balance.12
Clinical relevance.
Chronic lung diseases, including chronic obstructive pulmonary disease and asthma, have evidence of defective epithelial repair with compromise of the stem and progenitor populations;13 an understanding of these mechanisms may create novel therapies.
Bioengineering: there is a shortage of organs for lung transplantation. Recent interest in de-cellularizing donor lung tissue and repopulating with the recipients own stem cells prior to transplantation has potential to circumvent immunosuppression challenges and the shortage of donor organs.
Lung tumours share markers and similarities to the stem cell population of the airway. Are these the cells of tumour origin?
Cell of origin in lung cancer
Stem cells are defined by their longevity, meaning that they or their daughters are present long enough to accumulate a selection of genetic mutations that can drive the development of cancers. The three common histopathological variants of lung cancer are small cell, adenocarcinoma and squamous cell carcinomas (SCCs). Therefore, an obvious question arises; do these three distinct pathological subtypes reflect the phenotype and distribution of the putative stem cell populations in the lung?
In human airways, a proximal to distal tumour pattern exists with a predominance of SCCs found in the trachea and upper airways, small cell lung cancers (SCLCs) in the intermediate airways and distally adenocarcinomas/bronchoalveolar cell carcinomas. This suggests certain cell types, in specific environments, are capable of supporting tumour growth. Intriguingly the tumour subtypes do share characteristics with the stem cell population involved in the repair of each area.3
SCCs develop as a result of stepwise morphological changes: basal cell hyperplasia, metaplasia, dysplasia, carcinoma in situ and invasive disease.14 It is intriguing that both the preinvasive lesions and the SCC maintain a basal cell phenotype with persistent keratin 5 expression.15 SCCs are also sited at submucosal gland duct junctions or at intracartilaginous boundaries, areas where the highest basal cell concentration is found, suggestive of basal cell origins of SCC.3
Meanwhile, SCLCs localize to the mid-level bronchioles and express a range of neuroendocrine markers, including the CGRP, as expressed by neuroendocrine cells found within the NEBs. Hence again, both site and marker expression suggest that SCLCs may arise from the NEB. This observation is strongly supported by the finding that the Rb1 gene negatively regulates neuroendocrine differentiation. When both Rb1 and p53 are sequentially ‘knocked out’ in the Clara, AT2 cells and neuroendocrine cells of transgenic mice, SCLCs arise most frequently and with the shortest time lag from the neuroendocrine cells within the NEB.16
Finally, human adenocarcinomas frequently co-express the markers CCSP and SPC,7 indicating possible Clara or AT2 cell origins. About 25–50% of adenocarcinomas have a Kras mutation. When Kras is activated in all SPC or CCSP expressing airway cells of a transgenic mouse model, subsequent adenocarcinomas only develop in the distal lung. This again demonstrates only certain cells have potential to develop adenocarcinomas, despite a widespread Kras activation.17
Clinical relevance.
If cancer arises from the lung stem cells, this is the population that should be targeted early in the disease course.
An understanding of how these lung stem cells may become tumorigenic may open further treatment avenues.
Lung cancer stem cells
In recent years, evidence has accumulated that cancer not only may originate from endogenous stem cells but that they may contain within them a small population of stem cells that control tumour growth and tumorigenicity, termed a ‘cancer stem cell’ (CSC). A CSC may originate from either a mutated normal stem cell or a differentiated progenitor cell that has accumulated the necessary damage to become tumorigenic.18,19
To establish a CSC’s existence, key characteristics must be shown. These include the ability to self-renew to generate differentiated progeny and to produce malignancy in xenotransplantation models. Xenotransplantation involves isolating potential CSCs from a lesion based on cell surface markers, and then injecting the CSCs into an immunocompromised mouse. If CSC properties are present, tumours will develop. This has led to the identification of CSCs in several cancer subtypes including the brain, breast, haematopoietic system and lung cancers.20
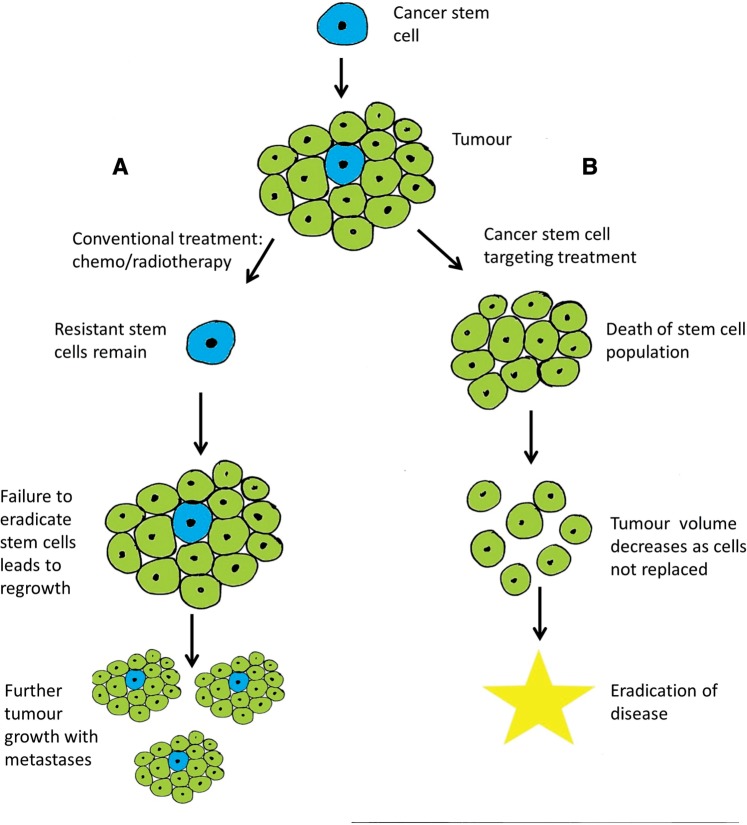
As normal stem cells have a vital role in tissue repair, they have evolved protective mechanisms that prevent lethal damage. These include high levels of anti-apoptotic proteins and the possession of ABC transporter proteins that cause toxin and, as a by-product, drug efflux from the cell. There is evidence that CSCs share these properties which affect treatment responses. To date, success of radiotherapy/chemotherapy has been assessed through a reduction in tumour size, and monitored through CT imaging. However, although there is a reduction in tumour bulk, the CSCs with their inherent resistance mechanisms may not be eradicated, and will act as a reservoir to repopulate the tumour after treatment, and thus be responsible for subsequent relapse (Figure 2). It is also intriguing that while cancer cells are often identified in the peripheral circulation, they are not always associated with the development of metastases. This is suggestive of a unique population of cells within a tumour that may have this capacity, and may be the CSC population.21
Figure 2.
A CSC will produce daughter cells forming a tumour. In path A, conventional treatments are administered. These target the rapidly dividing daughter cells, but have no effect on the CSC due to their resistance mechanisms and possible relative quiescence. Once the cancer treatments are withdrawn, the unaffected CSC repopulates the tumour bulk with likely improved resistance. Path B shows administration of CSC targeting therapies. These treatments eliminate the CSCs and the tumour, having lost its tumorigenic cells will reduce in size as the daughter cells are not replenished. This could lead to curative therapies.
Markers have been used to isolate putative CSCs. The best studied of these include the drug effluxing cells and the surface marker CD133, both identified in lung cancer. Due to the presence of ABC transporters, CSCs have a ‘side population’ (SP) phenotype demonstrable on a flow cytometry plot as this cell population pumps out the fluorescent nuclear dye Hoechst 33342. Ho et al. have detected a SP in SCLC and non-SCLC (NSCLC) cell lines, and after isolating these cells found the SP cells could generate both SP and non-SP cells on culture, showing their multipotential capacity. Xenotransplantation of the SP cell into immunosuppressed mice resulted in tumours at significantly lower cell concentrations than the non-SP fragment. Importantly, these SP cells also have significant chemotherapy resistance.22,23
CD133 is a stem cell marker rarely found in normal lung tissue. However, CD133+ cells increase in areas of tissue regeneration (where stem cells are activated) and in cancer, with CD133+ cells isolated from both SCLC and NSCLC. Again injection of a purified pool of CD133+ cells into immunodeficient mice results in tumour development, whereas CD133− cell injection does not. Of note, when cisplatin, a conventional lung cancer treatment agent was administered to the xenotransplanted mice the tumours reduced in size, however, the remaining chemoresistant cells were entirely CD133+,24 and thus indicative of the CSC population.
CSCs are an important potential therapeutic target. Unlike current therapies that target proliferative cells, if the CSCs can be killed (which may by their nature be more quiescent) the tumour will be unable to regenerate and the cancer cured (Figure 2). Such therapies may target key signalling pathways used by stem cells such as Hedgehog, Notch and Wnt. These pathways are important in development and stem cell self-renewal, with evidence emerging that pathway abnormalities are seen within lung tumours.25
Hedgehog signalling is important in embryonic stem cells and is overactive in SCLC. Blocking agents targeting the hedgehog pathway have had anti-tumour effects in mice and have recently entered clinical trials. The Notch signalling pathway regulates cellular proliferation and differentiation, through cell to cell communication systems, which have been found abnormal in both SCLC and NSCLCs. Inhibitors blocking Notch activation have also entered early clinical trials in a number of malignancies including NSCLC. Wnt signalling regulates normal tissue stem cells and has also been found to be perturbed in lung cancer; Wnt inhibitors remain in development.20 When manipulating the pathways that control stem cell maintenance, the effect on the normal stem cell populations with close monitoring of side effects will be needed to ensure the endogenous repair mechanisms are not detrimentally affected.
Clinical relevance.
The presence of resistant CSCs can explain treatment failure and subsequent disease relapse with the wrong cellular population targeted by conventional therapy.
Exploiting mechanisms that control the CSC population including the Hedgehog, Notch and Wnt pathway provide a new therapeutic avenue in the management of lung cancer.
Results of early clinical trials of Hedgehog and Notch inhibitors are awaited.
Summary
The role of stem cells in the cause and resistance of cancers is being gradually delineated. This should lead to the evolution of therapies that may stop cancer initiation or more likely target the tumorigenic population within cancers, making therapies more effective.
Funding
L.S. is a Wellcome Trust Clinical Research Training Fellow. S.M.J. is a Wellcome Trust Senior Fellow in Clinical Science and is supported by the Rosetrees Trust and the Roy Castle Lung Cancer Foundation. This work was supported by researchers at the National Institute for Health Research University College London Hospitals Biomedical Research Centre to S.M.J.
Conflict of interest: None declared.
References
- 1. CRUK Lung cancer incidence statistics. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/lung/incidence/uk-lung-cancer-incidence-statistics (6 October 2013, date last accessed)
- 2.Smith A. A glossary for epithelial stem cell biology. Nature. 2006;441:1060. [Google Scholar]
- 3.Giangreco A, Groot K, Janes S. Lung cancer and lung stem cells. Am J Respir Crit Care Med. 2007;175:547–53. doi: 10.1164/rccm.200607-984PP. [DOI] [PubMed] [Google Scholar]
- 4.Rock J, Onaitis M, Rawlins EL, Lu Y, Clark C, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–5. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds SD, Giangreco A, Power JHT, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156:269–78. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim C, Jackson E, Woolfenden A, Lawrence S, Babar I, Vogol S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Giangreco A, Reynolds S, Stripp B. Terminal bronchioles harbour a unique stem cell population that localises to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–82. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, et al. The role of Scgb1a1+ Clara cell in the long-term maintenance and repair of lung airway, but not alveolar epithelium. Cell Stem Cell. 2009;4:525–34. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng D, Limmon G, Yin L, Leung NHN, Yu H, Chow VTK, et al. Cellular pathway involved in Clara cell to alveolar type II cell differentiation after severe lung injury. PLoS One. 2013;8:e71028. doi: 10.1371/journal.pone.0071028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giangreco A, Arwet EN, Rosewell IP, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for airway homeostasis but restore airways after injury. Proc Natl Acad Sci USA. 2009;106:9286–91. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans MJ, Johnson LV, Stephens RJ, Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest. 1976;35:246–57. [PubMed] [Google Scholar]
- 12.Teixeira V, Nadarajan P, Graham T, Pipinikas C, Brown J, Falzon M, et al. Stochastic homeostasis in human airway epithelium is achieved by neutral competition of basal cell progenitors. eLife. 2013;2:e00966. doi: 10.7554/eLife.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder JC, Teisanu RM, Stripp BR. Endogenous lung stem cells and contribution to disease. J Pathol. 2009;217:254–64. doi: 10.1002/path.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George PJ, Banerjee A, Read CA, O’Sullivan C, Falzon M, Pezzella F, et al. Surveillance for the detection of early lung cancer in patients with bronchial dysplasia. Thorax. 2007;62:43–50. doi: 10.1136/thx.2005.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barth PJ, Koch S, Muller B, Unterstab F, von Wichert P, Moll R. Proliferation and number of Clara cell 10KDa protein (CC10)-reactive epithelial cells and basal cells in normal, hyperplastic and metaplastic bronchial mucosa. Virchows Arch. 2000;437:648–55. doi: 10.1007/s004280000316. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland K, Proost N, Brouns I, Adriaensen D, Song J, Berns A. Cell of origin of small cell lung cancer. Inactivation of Tp53 and Rb1 in distinct cell types of the adult mouse lung. Cancer Cell. 2011;19:754–64. doi: 10.1016/j.ccr.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Rock JR, Lu Y, Futtner C, Schwab G, Guinney J, et al. Evidence for type II cells as cells of origin of Kras-induced distal lung adenocarcinoma. Proc Natl Acad Sci USA. 2012;109:4910–5. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–22. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 19.Passegue E, Jamieson CH, Ailles LE, Weisman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci USA. 2003;100:11842–9. doi: 10.1073/pnas.2034201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Flaherty JD, Barr M, Fennell D, Richard D, Reynolds J, O’Leary J, et al. The cancer stem cell hypothesis. Its emerging role in lung cancer biology and its relevance for future therapy. J Thorac Oncol. 2012;7:1880–90. doi: 10.1097/JTO.0b013e31826bfbc6. [DOI] [PubMed] [Google Scholar]
- 21.Fleya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 22.Ho M, Ng A, Lam S, Hung J. Side population in human lung cancer cell lines and tumours is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–33. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 23.Loebinger MR, Giangreco A, Groot KR, Pritchard L, Allen K, Simpson C, et al. Squamous cell cancers contain a side population of stem-like cells that are made chemosensitive by AB transporter blockade. Br J Cancer. 2008;98:380–7. doi: 10.1038/sj.bjc.6604185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA. 2009;106:16281–6. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundin A, Driscoll B. Lung cancer stem cells: progress and prospects. Cancer Lett. 2013;338:89–93. doi: 10.1016/j.canlet.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]