Abstract
Vaccines for early-life immunization are a crucial biomedical intervention to reduce global morbidity and mortality, yet their developmental path has been largely ad hoc, empiric, and inconsistent. Immune responses of human newborns and infants are distinct and cannot be predicted from those of human adults or animal models. Therefore, understanding and modeling age-specific human immune responses will be vital to the rational design and development of safe and effective vaccines for newborns and infants.
THE BURDEN OF INFECTION EARLY IN LIFE
More than 2 million newborns and infants under the age of 6 months die each year worldwide from infection (1–3). In this context, vaccines are second only to clean drinking water as a cost-effective measure to reduce infant morbidity and mortality. Global eradication of smallpox and the hopefully forthcoming eradication of poliomyelitis demonstrate the power and potential of immunization programs. Per World Health Organization (WHO) guidelines, children should be immunized with Bacille Calmette-Guérin (BCG) to prevent disseminated tuberculosis in endemic areas, as well as Diphtheria, Tetanus, and Pertussis (DTaP); oral or inactivated Polio vaccine (OPV or IPV, respectively); hepatitis B vaccine (HBV); measles vaccine; and Haemophilus influenzae type b (Hib) vaccine (4). However, substantial morbidity and mortality among neonates and infants continues to be caused by infections, including those that are currently vaccine-preventable. Common pathogens of infants include Streptococcus pneumoniae, H. influenza, Escherichia coli and other enteric Gram-negative bacteria, Bordetella pertussis (whooping cough), as well as Herpes Simplex Virus, Respiratory Syncitial Virus, and rotavirus (5). This burden of infection highlights early-life susceptibility, particularly among those 0 to 6 months of age, and an unmet global need for improved immunization.
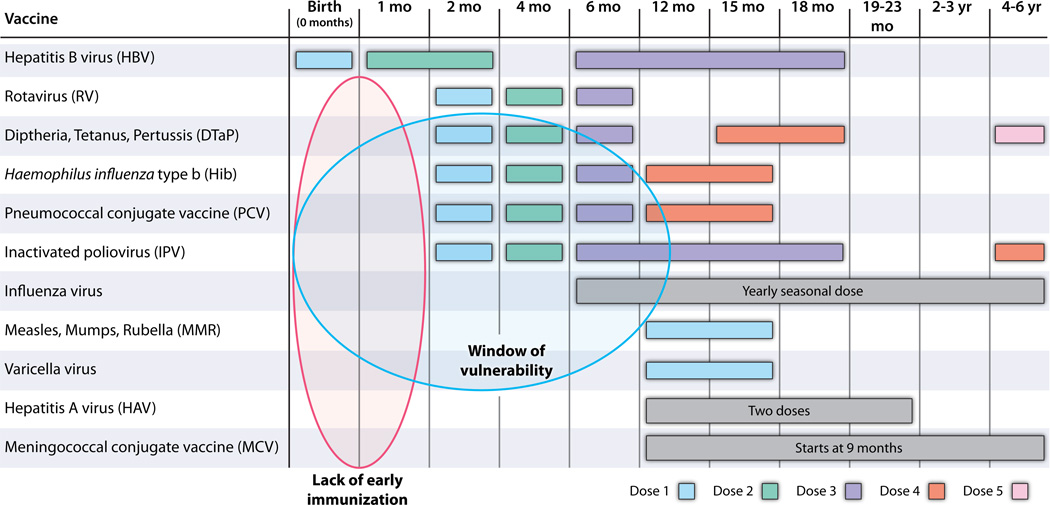
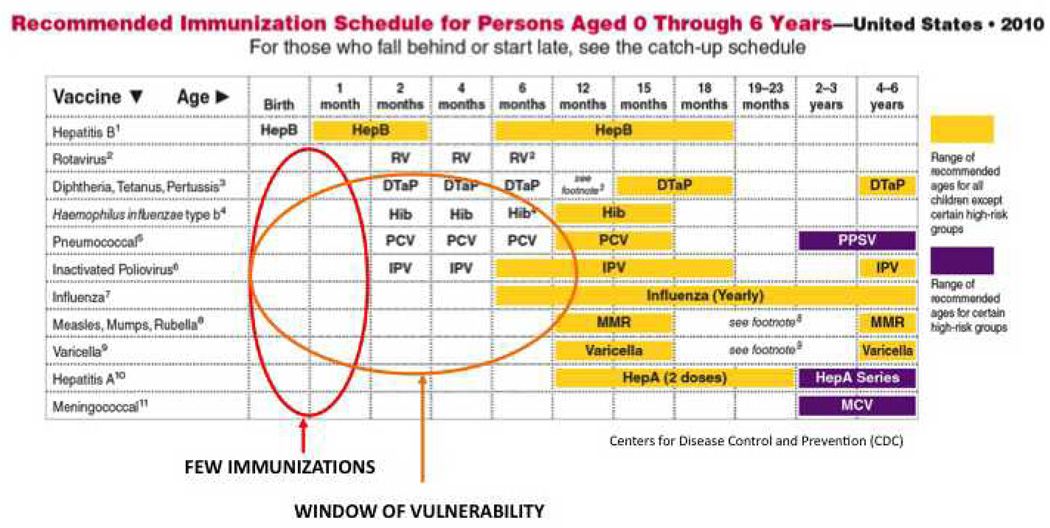
Developing new vaccines against pathogens, such as respiratory syncitial virus (RSV), malaria, HIV, and Dengue virus, as well as enhancing availability and delivery of existing, available vaccines could help mitigate the global burden of infection. However, any such approaches will need to focus on early-life immunization in order to benefit the very young, including newborns, defined as those who are ≤28 days of age. Immunization of pregnant mothers, with the consequent, passive transplacental transmission of antibodies to the fetus, could protect neonates (6). However, this promising strategy might be limited by safety and medico-legal concerns. Because birth is the most reliable point of health care contact worldwide, vaccines that are active at birth are of special and strategic importance (7). Vaccines given at birth achieve high population penetration and could substantially reduce the window of susceptibility inherent to the current vaccine schedules that largely focus on a 2/4/6 months of age schedule (Table 1) (8).
Table 1. Recommended immunization schedule for persons aged 0 through 6 years in the United States.
Only HBV is given to newborns; thus, there is a lack of early immunization (blue oval). The window of vulnerability (orange oval) reflects a phase in which both immune immaturity and dearth of vaccine protection render the young infant particularly vulnerable to infection. [Adapted from the U.S. Centers for Disease Control and Prevention (CDC) website: http://www.cdc.gov/vaccines/recs/schedules/child-schedule.htm.]
CREDIT: C. BICKEL/SCIENCE TRANSLATIONAL MEDICINE
 |
VACCINES CURRENTLY LICENSED FOR USE AT BIRTH
On a global basis, three vaccines are currently licensed for immunization at birth: HBV, BCG, and OPV. Of these, only the HBV vaccine is given in the United States, with a first dose at birth (Table 1). As with many medications, these were first developed for and tested in older individuals and then eventually evaluated in newborns. Clinical trials that investigated an accelerated vaccination schedule of these vaccines, including neonatal “birth” doses, demonstrated safety as well as efficacy, often as reflected by the production of antigen-specific antibodies, a surrogate marker of protection (Table 2).
Table 2. Vaccines that have been licensed and/or tested in human newborns and infants.
AS01, liposomes of MPL (monophosphoryl lipid A) and QS21 (saponin from the tree Quillaja saponaria); AS02, oil-in-water emulsion with MPL and QS21; ID, intradermal; IM, intramuscular; (NANP)50, series of tetrapeptides of four or five Asn-Ala-Asn-Pro repeats of immunodominant B cell epitope of P. falciparum circumsporozoite surface protein; PC, percutaneous; PRP–OMPC, Hib capsular polysaccharide conjugates with meningococcal outer membrane protein C; PRP–CRM, Hib capsular polysaccharide conjugates with diphtheria toxoid; PRP–T, Hib capsular polysaccharide conjugates with tetanus toxoid; RTS, S/ASO1/2 (GlaxoSmithKline), a pre-erythrocytic vaccine based on P. falciparum circumsporozoite surface protein and the candidate malaria vaccine in advanced development; SC, subcutaneous; SPf66, synthetic 45-amino acid peptide vaccine containing linked blood and circumsporozoite stage sequences from four different proteins of P. falciparum.
| Youngest Age |
Route | Vaccine (series) |
Antigen | Adjuvant | Safety | Immune response |
Limitations | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Intrinsic | Extrinsic | |||||||||
| Licensed | Newborn | PC; D |
BCG (single) |
Mycobacterium bovis |
TLR2/4/ 8/9 |
Ø | Rare: disseminated |
CD4, CD8, IFNγ |
---- | 1 |
| IM | HBV (0/1/6m) |
Virus-like nanoparticles |
? | Alum | MLAE (1- 10%) |
Ab (IgG) | ----- | 2 | ||
| Oral | OPV (single) |
Live attenuated virus |
ssRNA (TLR8?) |
Ø | Rare: revertant/para lysis |
Neutralizing anti- PV Ab |
Impaired CD4/IFNγ |
3 | ||
| Infant | Oral | RV (2/4/6m) |
Live attenuated virus |
dsRNA (RIG-I?) |
Ø | Rare: intussusception |
Ab (IgA) | Surrogate protection marker |
22 | |
| IM | DTaP (2/4/6m) |
Dip, Tet & PTX, fHA |
PTX (?TLR4) |
Alum | NSAR | Ab (IgG) | ----- | 23 | ||
| IM | Hib (2/4 or /6m) |
PRP– OMPC/- CRM/-T |
OMPC (TLR2) |
Ø or with Alum |
NSAR | Ab | ----- | 24 | ||
| IM | PCV (2/4/6/12m) |
CRM197- pneumo PSs |
? | Alum | MLAE (~20%) |
T-cell dep. Ab (IgG) |
----- | 25 | ||
| IM; SC |
IPV (2/4/6m) |
Inactivated polio |
ssRNA (?TLR8) |
Ø | NSAR to MLAE |
Ab | ----- | 18 | ||
| IM | TIV (6m or 6/8m) |
Inactivated Influenza virus; |
ssRNA (TLR7/8 ?) dsRNA (TLR3;R IG-I?) |
Ø | MLAE (~10%) |
HA Ab | Surrogate protection marker |
21 | ||
| Studied | Newborn | IM | Pertussis (0/1/2/4m) |
PTX, pertactin, fHA; or Whole |
PTX (?TLR4) |
Alum | NSAR; MLAE- HSAE (whole) |
Agglutinins & IgG Abs |
Sub- optimal Ab levels |
4 |
| IM | DTaP (0/2/4/6m) |
Dip & Tet Toxoids |
? | Alum | NSAR | Ab (IgG) | Lower Ab response @7mo than those without birth dose |
9 | ||
| IM | Hib (0/4/14m) |
PRP-CRM/-T | ? | Ø | NSAR | Ab (IgG) | ----- | 16 | ||
| IM | PCV (0/1/2m) |
CRM197- pneumo PSs |
? | Alum | NSAR | CRM197 Th2>Th1 |
Polarizes subsequent TLR responses (Th2) |
12 | ||
| IM | HIV (0/1/3/5m to infants of HIV- infected mothers) |
HIV-1 gp120 | ? | MF59 or Alum |
NSAR | Ab (IgG), LP |
Alum-HIV <LP than MF59-HIV |
13 | ||
| Oral | RV (0–2–4 vs. 2–4–6m) |
4 live virus strains |
? | Ø | MLAE | Ab (IgA) | Lower, but Still acceptable, IgA levels c birth dose |
17 | ||
| IM | RTS, S/AS O1/2 (0/1/2 or 7m) |
Circumsporo zoite protein |
? | AS01/2(TL R4) |
MLAE | Ab | ---- | 26 | ||
| Infant | IM | SPf66 (1/2/7m) |
(NANP)50 and P. falciparum Lysate (IFAT). |
? | Alum | NSAR | Ab(IgG) | Narrower/less sustained Ab response than in older children |
27 | |
| ID | IPV | Inactivated Poliovirus |
ssRNA (?TLR8) |
Ø | MLAE | Ab to 3 serotypes |
Lower Ab than IM |
20 | ||
NSAR= No serious adverse reactions
MLAE= mild local adverse effects
HSAE= higher systemic adverse effects
Intramuscle= IM
Intradermal= ID
Percutaneous= PC
Subcutaneous= SC
LP = Lymphoproliferation
PRP–OMPC= Haemophilus influenzae type b capsular polysaccharide conjugates with meningococcal protein OMPC
PRP–CRM= Haemophilus influenzae type b capsular polysaccharide conjugates with diphtheria toxoid (CRM)
PRP–T= Haemophilus influenzae type b capsular polysaccharide conjugates with tetanus toxoid
PTX= Pertussis Toxin
fHA= Filamentous hemagglutinin
MF59= oil-in-water emulsion of 0.5% polysorbate 80, 0.5% sorbitan trioleate, and 0.5% squalene
MPL= monophosphoryl lipid A
QS21= a saponin from the tree Quillaja saponaria
AS02 Adjuvant System= an oil-in-water emulsion with MPL and QS21
AS01 Adjuvant System = liposomes of MPL and QS21
SPf66 = SPf66 is a synthetic peptide containing antigens from the blood stages of malaria linked together with an antigen from the sporozoite stage
1. Andersen et al. Nat Rev Microbiol. 2005;3:656–662; van Duin et al. TRENDS in Immunology 2006; 27(1):49–55
2. Van Herck et al. Pediatr Infect Dis J 2008; 27: 861–869
3. Vekemans et al. Clin Exp Immunol 2002; 127: 495–498
4. Sako. J Pediatr 1947; 30:29–40; Provenzano et al. NEJM 1965; 273(18): 959–965; Wood et al. Pediatr Infect Dis J 2010; 29: 209–215; Knuf et al. J Pediatr. 2008; 152:655– 660; Belloni et al. Pediatrics 2003; 111:1042–1045
9. Halasa et al. J Pediatr 2008; 153:327–332; Di Sant' Agnese. Pediatrics 1949; 3:20–33; Provenzano et al. NEJM 1965; 273(18): 959–965; Lieberman et al. J PEDIATR 1995; 126:198–205; http://clinicaltrials.gov/ct2/show/NCT00133445
12. van den Biggelaar et al. Vaccine. 2009; 27:1340–1347
13. McFarland et al. JID 2001; 184:1331–1335; Borkowsky et al. JID 2000; 181:890–6
16. Kurikka et al. Pediatrics 1995; 95:815–822; Lieberman et al. J PEDIATR 1995; 126:198–205
17. Vesikari et al. Pediatr Infect Dis J 2006; 25(2): 118–122
18. IPOL® vaccine insert
20. Resik et al. JID 2010; 201: 1344–1352
21. Fluzone vaccine insert
22. Rotarix® vaccine insert; RotaTeq® vaccine insert
23. Tripedia® vaccine insert
24. ActHIB® vaccine insert; PedvaxHIB® vaccine insert; van Duin et al. TRENDS in Immunology 2006; 27(1):49–55
25. Prevnar® vaccine insert; van Duin et al. TRENDS in Immunology 2006; 27(1):49–55
26. Agnandji et al. J Infect Dis 2010; 202(7): 1076–87; Malaria candidate vaccine, ClinicalTrials.gov identifier: NCT00436007. GlaxoSmithKline (GSK); Owusu-Agyei et al. PLoS ONE 2009; 4(10): e7302; van Duin et al. TRENDS in Immunology 2006; 27(1):49–55
27. Galindo et al. Parasite Immunology 2000; 22: 437–443.
Hepatitis B vaccine
The rates of tuberculosis in the United States are sufficiently low so that BCG is not indicated for neonates and polio immunization is provided as IPV beginning at 2 months of age; therefore, HBV is the only vaccine administered during the first 28 days of life that is currently recommended in the United States (Table 1) (8). HBV vaccine, available since 1982, uses recombinant DNA technology to express hepatitis B surface antigen (HBsAg)—a protein that forms viral-like nanoparticles—in yeast. Alum, a chemical compound containing aluminum salts whose mechanism of action is still under investigation (9), is added as adjuvant. A three-dose series of HBV starting at birth is safe and effective (10).
Bacille Calmette-Guérin
Having been administered to more than 3 billion people, BCG is the most commonly used vaccine worldwide (11). BCG is a single-dose vaccine of freeze-dried, live Mycobacterium bovis. The BCG vaccine does not contain any exogenous adjuvant but is intrinsically “self-adjuvanted” because Mycobacteria activate immune responses via transmembrane Toll-like receptors (TLRs), including TLR-2, −4, and −8 (12). Although newborns typically demonstrate impaired T helper 1 (Th1) immunity to multiple stimuli, remarkably BCG can induce Th1-polarizing immune responses at birth (13). BCG has a good safety profile and has been estimated to prevent approximately 30,000 cases of tuberculous meningitis and ~11,500 cases of miliary disease during the first 5 years of life (14). Of note, BCG administration to newborns appears to have a beneficial effect on survival not solely ascribable to protection against tuberculosis, raising the possibility that this live-attenuated vaccine may have beneficial immune-enhancing effects (15).
Oral polio vaccine
In the United States, polio immunization begins with a dose of IPV at 2 months of age. In contrast, in countries where poliomyelitis has not yet been controlled the Sabin OPV—comprising live-attenuated poliovirus Sabin strains 1, 2, and 3—is administered at birth as a single dose to prevent poliomyelitis and promote herd immunity (16). Although T cell IFN-γ and proliferative recall responses to OPV are limited after immunization at birth, OPV does induce protective antibodies in neonates (17). Of note, there is no extrinsic adjuvant added with OPV, although it contains single-stranded RNA—a class of molecules that can activate human cells via TLR8 (18).
VACCINES TESTED AT BIRTH OR INFANCY
Investigators have recognized that immunization at birth represents a practical approach to reducing the global burden of infection, and accordingly, several studies have evaluated vaccines in newborns [reviewed in (19)]. A few important examples are highlighted here and in Table 2.
Pertussis
B. pertussis is the etiologic agent of whooping cough that still claims the lives of hundreds of thousands of infants worldwide and has been responsible for a recent outbreak in California, resulting in the deaths of many infants, most of whom were less than 2 months of age at disease onset (20). The particular severity of this infection in young infants has motivated studies of neonatal immunization against this pathogen (Table 2). Studies of neonatal pertussis immunization dating back to the 1940s indicate safety of immunization against pertussis at birth, but with variable efficacy (21). Using a whole-cell vaccine, immunization within 24 hours of life resulted in inadequate serum titers (22). A series starting at 1 week, continuing at 5 and 9 weeks, and followed by a booster at 6 to 12 months resulted in protective pertussis agglutinin levels in only ~60% of infants (20). Immunization starting at 3 weeks of life was apparently effective (23), possibly reflecting age-dependent maturation of antigen-presenting cell and lymphocyte function.
Whole-cell pertussis preparations have been associated with reactogenicity, including erythema and local infiltration as well as fever and irritability (24), which prompted the development of acellular pertussis (aP) vaccines containing toxoid, filamentous hemagglutinin (fHA), pertactin, and fimbriae-2 and −3. However, when given in conjunction with DTaP starting at 2 to 14 days of age aP vaccination resulted in a lower antibody response to diphtheria and to multiple pertussis antigens as compared with infants receiving the vaccine at 2/4/6/17 months only (26). These observations suggest vaccine interference, in which simultaneous administration of multiple vaccine antigens may interfere with one another’s efficacy. Such antagonistic interactions could reflect, for example, inhibition of antigen presentation and/or B lymphocyte priming—steps that are key for Ab formation. Nevertheless, aP vaccines have proven safe in newborns and have resulted in enhanced immune responses when given initially as the aP vaccine alone followed by DTaP at 3/5/11 or 2/4/6 months (25), an approach that has been associated with Th2 polarization of infant cellular immune memory (27).
Pneumococcus
A trial based in Papua New Guinea evaluated neonatal immunization with a seven-valent pneumococcal conjugate vaccine comprising pneumococcal polysaccharides coupled to the CRM197 carrier protein [a nontoxic variant of diphtheria toxin isolated from cultures of Corynebacterium diphtheriae strain C7 (β197)] adjuvanted with Alum, named PCV7 (28). At birth, PCV7 was immunogenic, but associated with somewhat lower antibody titers to multiple serogroups at 4 months of age (29). Infants that had received a dose of PCV7 at birth subsequently had greater Th2 polarization of TLR-mediated cytokine responses in vitro, suggesting a possible effect on subsequent immune system polarization (28).
Rotavirus
Rotavirus causes hundreds of thousands of infant deaths worldwide. An immunization schedule initiated in the neonatal period (2 to 7 days of age) as a 0/2/4 or 0/2/6 months schedule with live oral rhesus-human reassortant rotavirus tetravalent vaccine was associated with an immunoglobulin A (IgA) sero-response that was lower than the 2/4/6 months group but still deemed acceptable (30). Immunization schedules initiated in newborns were associated with a substantially lower frequency of febrile reactions (0% versus 18%) and a possible reduction in the small risk of intussusception, which eventually led to the withdrawal of this vaccine and subsequent replacement with different attenuated or human-bovine reassortant rotavirus vaccines (31).
Fractional intradermal IPV
A recent Cuban study evaluated a reduced dose of IPV administered at birth with a needle-free intradermal device (32). This approach carries great potential for enhanced safety and efficacy (33). The result was inadequate, as evidenced by suboptimal median polio antibody titers, especially in the fractional-dose arm. However, intradermal vaccination is in early phases of development and is a potentially important strategy to target immune responses to draining lymph nodes.
HIV
Vaccine formulations containing recombinant gp120 derived from HIV-1 adjuvanted with either Alum or with MF59—an oil-in-water emulsion comprising 0.5% polysorbate 80, 0.5% sorbitan trioleate, and 0.5% squalene—were studied in newborns of HIV-infected women (34). Infants were immunized at 0, 1, 3, and 5 months. The vaccines appeared to be safe and well-tolerated (35). Two immunizations with recombinant gp120 proved immunogenic, as measured by in vitro lymphoproliferative responses to HIV antigens in more than half of the immunized children. Although much work remains to be done in defining safe and effective HIV vaccines, including those that may be targeted to newborns, such results raise the possibility of attempting to prevent HIV transmission from mother to child by administering the vaccine shortly after perinatal exposure, which is analogous to postexposure prophylaxis by using measles, varicella, or hepatitis vaccines.
A GROWING MENU OF ADJUVANTS
The previous examples illustrate the feasibility and challenges of neonatal vaccine development. In this context, we now reflect on recent progress in adjuvant development and understanding immune ontogeny to project rational future paths for the development of neonatal and infant vaccines. Multiple adjuvant mechanisms have been described (36–38), including those that create an antigen depot; preserve antigen conformation; direct antigen to specific immune cells; activate antigen-presenting cells; induce mucosal responses; or induce cytotoxic T cell responses. It should be noted that adjuvants are not typically approved in and of themselves but as part of vaccine formulations.
Use of adjuvants that activate antigen-presenting cells is a particularly effective means of enhancing vaccine efficacy. However, in order to reduce reactogenicity, vaccine design has increasingly turned to the use of protein subunit vaccines composed of single protein molecules that can aggregate to form higher-order structures, potentially at the cost of reduced immunogenicity. In this context, inclusion of adjuvants in vaccine formulations can be crucial for antigen-dose sparing, broadening epitopes, and increasing responses in populations with distinct immunity, including the very young. Indeed, expanding awareness of pattern recognition receptors (PRRs) expressed on leukocytes, including antigen-presenting cells—as well as other host cells—and their ligands (microbial and endogenous danger signals that can act as adjuvants) has opened a new era in vaccine development (36). To the extent that the ontogeny of PRR function has been evaluated, functional expression has been noted to be age-dependent, with stimulus-induced expression of Th1-polarizing cytokines increasing with age (39); yet, for several families of PRRs this correlation has yet to be characterized (40). Another unknown is whether early-life exposure to adjuvants may contribute to chronic skewing of an individual’s Th1/Th2 profile.
Importantly, TLR agonists are present in multiple vaccines that have been given to pediatric populations, including BCG (41) and the Hib vaccine that was adjuvanted with a Neisseria meningitidis group B outer membrane protein, which is a TLR2 agonist (42). The connections between this prior experience with administering TLR agonist-adjuvanted vaccines to children and current development of novel vaccine formulations, in which TLR agonists may be incorporated as adjuvants, is often underappreciated. These examples do not, of course, prove that all TLR agonists are safe and effective, but they do provide proof of concept for using PRR agonists as neonatal infant vaccine adjuvants.
THE ONTOGENY OF THE INFANT IMMUNE SYSTEM
Newborns possess a distinct innate and adaptive immune system comprising humoral components, antigen-presenting cells, and lymphocytes of distinct composition and function (Fig. 1) (39, 43, 44). Preterm newborns tend to have even more extreme differences in humoral and cellular immunity compared with those of adults and, correspondingly, an even greater susceptibility to infection (45) as well as reduced responsiveness to subunit glycoconjugate vaccines (such as pneumococcal conjugate vaccine) (46). Rational development of neonatal and infant vaccines will need to take immune ontogeny into account in preclinical development. The fetal and neonatal immune systems are biased against Th1 responses, with CD4 lymphocyte responses that are often weaker and less sustained than those of adults. Although B cell antibody responses (T cell–independent) are impaired during infancy, T cell–dependent antibody responses mature earlier; nevertheless, multiple immunizations might be needed for newborns and young infants to achieve or sustain protective titers (44). Indeed, the neonatal immune system is heavily Th2- and Th17-biased, presumably to avoid pro-inflammatory/Th1-type allo-immune responses to maternal tissues that might trigger preterm birth or spontaneous abortion (39). Birth triggers a dramatic shift in environment that challenges the neonatal immune system to mediate the transition from a sterile intrauterine compartment to a foreign antigen–rich external environment, including initial microbial colonization of the skin and gastrointestinal tract.
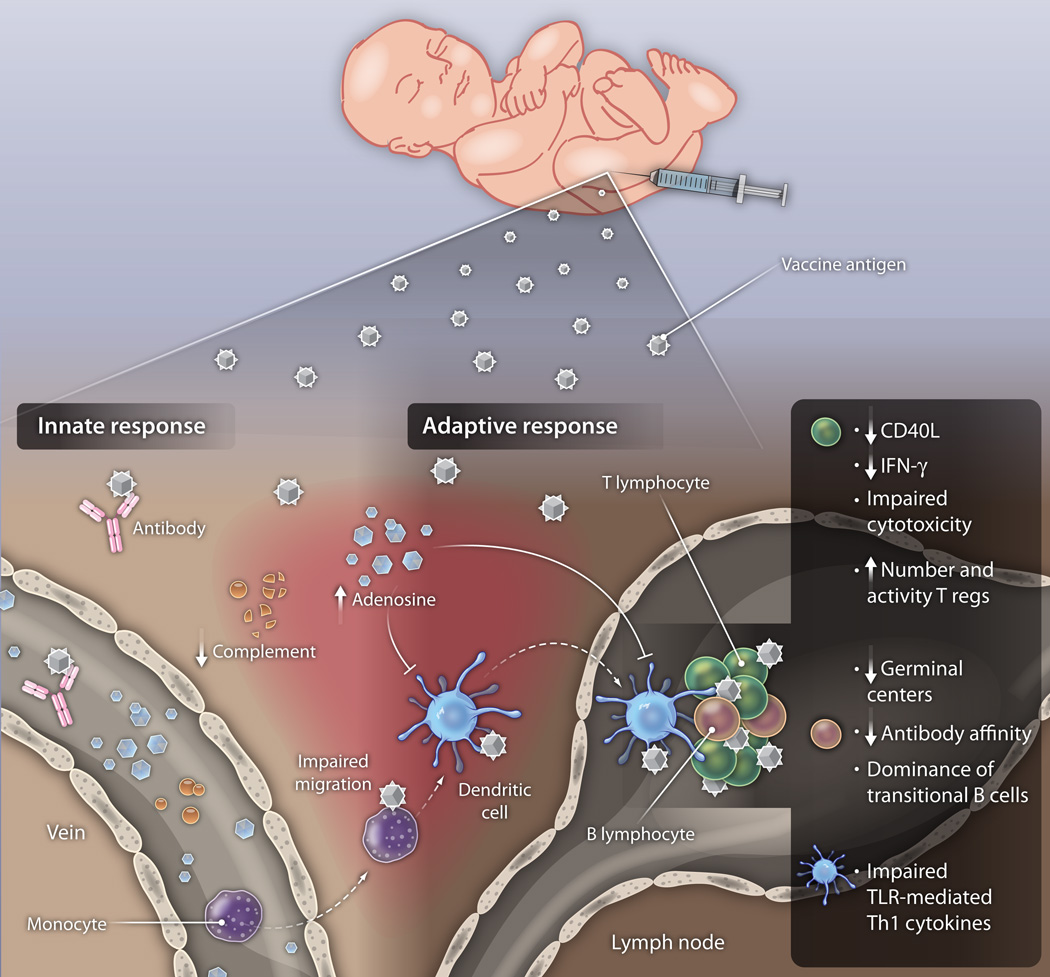
Fig. 1. Distinct humoral and cellular components of the neonatal immune system.
Neonatal blood plasma contains a different proportion of key immunomodulatory components than older individuals, including the presence of maternal antibodies, high concentrations of immunomodulatory adenosine, and reduced concentrations of complement, which are important to adaptive immune responses. Differences in neonatal leukocytes include impaired migration and reduced Th1-polarizing responses of neonatal APCs to most TLR agonists. T cell impairments include diminished CD40 ligand expression and reduced IFN-γ production. Neonatal B cells are predominantly transitional and demonstrate impairments in antibody maturation and affinity.
CREDIT: C. BICKEL/SCIENCE TRANSLATIONAL MEDICINE
Neonatal humoral components
There are marked differences in soluble immunomodulatory components of newborn and adult blood plasma (Fig. 1). Several mechanisms contribute to skewing neonatal antigen-presenting cells toward Th2-type responses—including placenta-derived mediators, such as transforming growth factor β, progesterone, and prostaglandin E2—that enhance Th2 cytokine production (47). Transplacental maternal antibodies can potentially reduce immune responses, although this effect can be overcome depending on antigen dose and epitope (or epitopes) (48, 49). Relative to adult blood plasma, neonatal plasma contains high concentrations of adenosine (Fig. 1), an endogenous purine metabolite that acts via adenosine receptors to induce intracellular cyclic adenosine monophosphate (cAMP) and selectively inhibits production of Th1-polarizing cytokines (39, 50). Newborn cord-blood also demonstrates lower plasma concentrations of antimicrobial proteins and peptides (51) and complement (45), which play important roles in innate and adaptive immune responses (52).
Neonatal antigen-presenting cells
There are both quantitative and qualitative differences between neonatal and adult antigen-presenting cells. Newborn cord-blood monocytes, dendritic cells (DCs), and monocyte-derived dendritic cells (MoDCs) demonstrate robust TLR-mediated response to support Th17- and Th2-type immunity [such as interleukin 6 (IL-6) and IL-23], which promotes defense against extracellular pathogens. However, neonatal monocytes, DCs, and MoDCs exhibit reduced Th1-type responses [such as tumor necrosis factor (TNF), interferon α (IFN-α), and IFN-γ], including reduced single-cell polyfunctional responses (Fig. 1), which are important for defense against intracellular pathogens (53). This neonatal polarization might partly reflect high cytosolic concentrations of inhibitory cAMP in newborn cord-blood mononuclear cells (39). The patterns of neonatal cytokine production appear to be relevant in vivo: During the first week of life, human neonatal peripheral serum levels of TNF remain low (relative to human adult serum), whereas levels of IL-6 increase (54). For many TLR agonists, cytokine responses increase to adult levels during the first months of life (55). One apparent exception to this pattern is the family of TLR8 agonists, which when tested in vitro induces adult levels of TNF and up-regulation of co-stimulatory molecules in newborn and infant whole blood, as well as cord-blood monocytes and MoDCs (56, 57).
Neonatal lymphocytes
Fetal T cells are distinct from adult T cells and arise from different populations of hematopoietic stem cells present at distinct developmental stages and biased toward immune tolerance (58). Neonatal lymphocytes demonstrate a high proportion of recent thymic emigrants and have distinct cellular function, including diminished proliferative and impaired IFN-γ responses (Fig. 1), the latter ascribed to promoter hypermethylation. Neonatal CD4+ cells show reduced stimulus-induced CD40 ligand up-regulation and an impaired capacity to provide help for B cell function. Moreover, newborns have an increased number and activity of inhibitory regulatory T cells (Treg cells) that limit adaptive immune responses at birth and promote tolerance (Fig. 1) (59).
The B cell compartment is also distinct in early life. Naïve B cells predominate in early life, whereas CD27+ memory B cells increase during the first 6 months of life (60). Newborns and young infants use a biased antibody gene repertoire with a low frequency of somatic mutations, which might contribute to poor affinity maturation and impaired functional antibody responses (61). Despite these many limitations, neonatal lymphocytes can be activated under specific conditions with certain stimuli (43, 62, 63). For example, human newborns can mount CD8+ memory responses during congenital cytomegalovirus infection (64) and upon BCG immunization, as well as antibody responses to OPV and HBV (19). Much remains to be learned regarding the immunologic and molecular rules governing effective activation of neonatal and infant immune responses. Overall, rational approaches to the development of new vaccines for the very young must take into account immune ontogeny by ensuring that vaccine formulations targeting newborns and infants effectively engage their distinct immune systems.
INTEGRATING IMMUNE ONTOGENY WITH MODERN VACCINOLOGY
The present process of vaccine development for newborns and infants essentially focuses on ad hoc evaluation of vaccines originally developed for use in older individuals. However, the importance of early-life immunization is increasingly evident from practical (1), biomedical (63), and economic (65) perspectives. In this context, the characterization of immune ontogeny in relation to age-specific adjuvant effects will inform a new era of targeted vaccine development.
Development of novel vaccine formulations must take into account not only age-specific differences but also the distinct immune systems of nonhuman animals, including mice (66). Accordingly, for preclinical evaluation of pediatric vaccine components, including adjuvants, in vitro work using human neonatal and infant primary cells in media containing the relevant composition of human humoral components (such as autologous plasma) will be important for modeling the distinct immune responses of neonatal and infant monocytes, APCs, and lymphocytes (67). Related approaches have been recently described in adult settings (68). Results from such in vitro studies should inform the selection of appropriate preclinical animal models, to see whether the adjuvants identified in vitro are bioactive in neonates of the test species. Clinical trials should ensure that rigorous biomarker evaluation, including genome-wide transcriptional and proteomic approaches, are used to further refine age-specific markers of safety and efficacy (69). The accelerated schedule approach, in which trials are designed to extend to earlier ages of initial immunization, remains important. However, if responses to a given vaccine formulation administered early in life are inadequate then addition of different adjuvant systems and formulations should be considered. Studies will need to take into account not only safety and efficacy but also potential vaccine-vaccine interactions that can lead to interference (70). Optimizing neonatal and infant vaccine formulations will also entail evaluation of distinct routes of administration (71), combination vaccines (72), live vector vaccines (73), and the possibility of genetic immunization (74).
Safety considerations, which are important for all biopharmaceutical development, are especially critical for vaccines because they are given to healthy individuals. The use of these agents in infants places all the more emphasis on rigorous safety evaluation. Proof-of-concept for safe and effective neonatal immunization exists in the form of vaccines such as BCG and HBV, which are given to millions of newborns and which have good safety profiles. Nevertheless, safety concerns are paramount in the development of any new biologic agent, particularly ones to be given to healthy newborns and infants. The potential benefits of neonatal vaccination are thus tempered by appropriate social and medical concerns about safety. Biopharmaceutical development of neonatal vaccines will have to proceed with caution within a viable development pathway, given the urgent unmet needs and great potential benefits of early-life immunization (7, 19). Although animal models will continue to be important in preclinical development, they do not necessarily reflect human immunology accurately. Moreover, there are few if any gold-standard safety biomarkers with respect to preclinical in vitro studies; some biomarkers that have been studied in this context include cytokines, acute-phase reactants, and prostaglandins (75). It will be important to benchmark the ability of novel vaccine formulations to induce responses from human neonatal and infant cells against existing vaccines in order to develop an understanding of potential correlates of protection and reactogenicity.
Correlates of protection are crucial vaccine study end-points, including antibody titers for protection against encapsulated bacteria and cytotoxic T cell responses for protection against intracellular pathogens. In some instances, vaccine formulations may be approved on the basis of clinical safety and surrogate markers of efficacy. Post-approval phase IV clinical evaluation can ultimately verify that immune responses known to be protective in adults or older children are also protective against disease in neonates and young infants.
ENSURING PROGRESS AND TRANSLATION
Although there are several challenges in developing vaccines for newborns and infants, proof-of-concept exists that this approach can be safe and effective and represents a promising strategy to reduce infant mortality (19). Most vaccine formulations that have been studied at birth have used Alum as an adjuvant (Tables 1 and 2); as such, novel adjuvants that are active at birth might be key in developing new and more effective neonatal vaccines (40). Progress will require support for basic and translational research in neonatal and infant immunology and vaccinology. Ongoing optimization of practical regulatory guidelines for vaccine formulation development will also be crucial to ensuring that safe and effective neonatal vaccines are developed to meet the urgent global challenge posed by infection.
Given the importance of early-life immunization and the vast amounts of new information regarding adjuvant formulations, routes of delivery, and immune ontogeny, a conceptual and practical framework for age-specific vaccine development is very much needed. The high disease burden early in life because of respiratory viral infections, including respiratory syncitial virus and influenza, suggests that early-life immunization, preferably at birth, might be the key to reducing the burden of these diseases as well. At particular risk are preterm newborns whose markedly distinct immune responses render them at especially high risk of infection (46, 76). National and international regulatory agencies will need to work with academia and industry to help define and validate specific biomarkers for vaccine safety and efficacy in newborns and young infants. Funding support from both government sources and private foundations will be key components for such progress. In this regard, the recent coordination by the United States National Institutes of Health and The Bill and Melinda Gates Foundation to develop and host a workshop on “Challenges in Infant Immunity” (June 2010; Bethesda, Maryland) was a welcomed and important development in the field (63). Moreover, international collaboration will be crucial to ensure that formulations, adjuvants, and biomarkers identified apply to diverse populations throughout the world. Although progress has been made in reducing infant infection, more than 200 newborns and young infants die each hour from infection worldwide; therefore, the challenging but feasible task ahead must therefore be approached in a thoughtful and prudent yet urgent manner.
Fig. 2.
Acknowledgments
The authors acknowledge the mentorship and support of M. Wessels, R. Malley, R. Geha, E. Guinan, and G. Fleisher. The authors also acknowledge the advice and support of G. vanden Bossche and C. Wilson of the Bill & Melinda Gates Foundation.
Funding: O.L. received a Bill & Melinda Gates Foundation Grand Challenges Explorations Award and is currently funded by NIH NIAIAD RO1 AI067353-01A1, American Recovery and Reinvestment Act NIH Administrative Supplement 3R01AI067353-05S1, and by Global Health Grant OPPGH5284 from The Bill & Melinda Gates Foundation.
Competing interests: O.L. has also received reagent and/or sponsored research support from 3M Drug Delivery Systems and VentiRx, companies that develop TLR agonists as immunomodulatory agents. O.L. is named as an inventor on a patent application for use of certain TLR agonists as neonatal vaccine adjuvants.
Footnotes
Insights into immune ontogeny will inform translation of new vaccines that are safe and effective for newborns and infants.
References and Notes
- 1.Lawn JE, Cousens S, Zupan J. Lancet Neonatal Survival Steering Team, 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. Medline. [DOI] [PubMed] [Google Scholar]
- 2.Bryce J, Daelmans B, Dwivedi A, Fauveau V, Lawn JE, Mason E, Newby H, Shankar A, Starrs A, Wardlaw T. Countdown Coverage Writing Group Countdown to 2015 Core Group, Countdown to 2015 for maternal, newborn, and child survival: The 2008 report on tracking coverage of interventions. Lancet. 2008;371:1247–1258. doi: 10.1016/S0140-6736(08)60559-0. Medline. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Neonatal and perinatal mortality: Country, regional and global estimates. Geneva: 2006. [Google Scholar]
- 4.Burton A, Monasch R, Lautenbach B, Gacic-Dobo M, Neill M, Karimov R, Wolfson L, Jones G, Birmingham M. WHO and UNICEF estimates of national infant immunization coverage: Methods and processes. Bull. World Health Organ. 2009;87:535–541. doi: 10.2471/BLT.08.053819. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thaver D, Zaidi AK. Burden of neonatal infections in developing countries: A review of evidence from community-based studies. Pediatr. Infect. Dis. J. 2009;28(Suppl):S3–S9. doi: 10.1097/INF.0b013e3181958755. Medline. [DOI] [PubMed] [Google Scholar]
- 6.Gall SA. Maternal immunization to protect the mother and neonate. Expert Rev. Vaccines. 2005;4:813–818. doi: 10.1586/14760584.4.6.813. Medline. [DOI] [PubMed] [Google Scholar]
- 7.Siegrist CA. In: The Vaccine Book. Bloom B, Lambert PH, editors. Boston: Academic Press; 2003. pp. 73–83. [Google Scholar]
- 8.CDC. Recommended Immunization Schedule for Persons Aged 0 Through 6 Years—United States. Department of Health and Human Services, Centers for Disease Control and Prevention. 2010 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5851a6.htm.
- 9.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009;9:287–293. doi: 10.1038/nri2510. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, Bell BP, Alter MJ. Advisory Committee on Immunization Practices (ACIP), A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: Immunization of infants, children, and adolescents. MMWR Recomm. Rep. 2005;54(RR-16):1–31. Medline. [PubMed] [Google Scholar]
- 11.Andersen P, Doherty TM. The success and failure of BCG—Implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005;3:656–662. doi: 10.1038/nrmicro1211. Medline. [DOI] [PubMed] [Google Scholar]
- 12.Heldwein KA, Liang MD, Andresen TK, Thomas KE, Marty AM, Cuesta N, Vogel SN, Fenton MJ. TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. J. Leukoc. Biol. 2003;74:277–286. doi: 10.1189/jlb.0103026. Medline. [DOI] [PubMed] [Google Scholar]
- 13.Vekemans J, Amedei A, Ota MO, D’Elios MM, Goetghebuer T, Ismaili J, Newport MJ, Del Prete G, Goldman M, McAdam KPWJ, Mayant A. Neonatal bacillus Calmette-Guérin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur. J. Immunol. 2001;31:1531–1535. doi: 10.1002/1521-4141(200105)31:5<1531::AID-IMMU1531>3.0.CO;2-1. Medline. [DOI] [PubMed] [Google Scholar]
- 14.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. Medline. [DOI] [PubMed] [Google Scholar]
- 15.Roth A, Jensen H, Garly ML, Djana Q, Martins CL, Sodemann M, Rodrigues A, Aaby P. Low birth weight infants and Calmette-Guérin bacillus vaccination at birth: Community study from Guinea-Bissau. Pediatr. Infect. Dis. J. 2004;23:544–550. doi: 10.1097/01.inf.0000129693.81082.a0. Medline. [DOI] [PubMed] [Google Scholar]
- 16.Halsey N, Galazka A. The efficacy of DPT and oral poliomyelitis immunization schedules initiated from birth to 12 weeks of age. Bull. World Health Organ. 1985;63:1151–1169. Medline. [PMC free article] [PubMed] [Google Scholar]
- 17.Vekemans J, Ota MO, Wang EC, Kidd M, Borysiewicz LK, Whittle H, McAdam KP, Morgan G, Marchant A. T cell responses to vaccines in infants: Defective IFNgamma production after oral polio vaccination. Clin. Exp. Immunol. 2002;127:495–498. doi: 10.1046/j.1365-2249.2002.01788.x. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philbin VJ, Levy O. Immunostimulatory activity of Toll-like receptor 8 agonists towards human leucocytes: Basic mechanisms and translational opportunities. Biochem. Soc. Trans. 2007;35:1485–1491. doi: 10.1042/BST0351485. Medline. [DOI] [PubMed] [Google Scholar]
- 19.Demirjian A, Levy O. Safety and efficacy of neonatal vaccination. Eur. J. Immunol. 2009;39:36–46. doi: 10.1002/eji.200838620. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roehr B. Whooping cough outbreak hits several US states. BMJ. 2010 Aug 24;341:c4627. doi: 10.1136/bmj.c4627. Medline. [DOI] [PubMed] [Google Scholar]
- 21.Di Sant’Agnese PA. Combined immunization against diphtheria tetanus and pertussis in newborn infants; production of antibodies in early infancy. Pediatrics. 1949;3:20–33. Medline. [PubMed] [Google Scholar]
- 22.Provenzano RW, Wetterlow LH, Sullivan CL. Immunization and antibody response in the newborn infant. I. Pertussis inoculation within twenty-four hours of birth. N. Engl. J. Med. 1965;273:959–965. doi: 10.1056/NEJM196510282731804. Medline. [DOI] [PubMed] [Google Scholar]
- 23.Provenzano RW, Wetterlow LH, Ipsen J. Pertussis immunization in pediatric practice and in public health. N. Engl. J. Med. 1959;261:473–478. doi: 10.1056/NEJM195909032611001. Medline. [DOI] [PubMed] [Google Scholar]
- 24.David S, Vermeer-de Bondt PE, van der Maas NA. Reactogenicity of infant whole cell pertussis combination vaccine compared with acellular pertussis vaccines with or without simultaneous pneumococcal vaccine in the Netherlands. Vaccine. 2008;26:5883–5887. doi: 10.1016/j.vaccine.2008.07.105. Medline. [DOI] [PubMed] [Google Scholar]
- 25.Knuf M, Schmitt HJ, Wolter J, Schuerman L, Jacquet JM, Kieninger D, Siegrist CA, Zepp F. Neonatal vaccination with an acellular pertussis vaccine accelerates the acquisition of pertussis antibodies in infants. J. Pediatr. 2008;152:655–660. doi: 10.1016/j.jpeds.2007.09.034. 660, e1. Medline. [DOI] [PubMed] [Google Scholar]
- 26.Halasa NB, O’Shea A, Shi JR, LaFleur BJ, Edwards KM. Poor immune responses to a birth dose of diphtheria tetanus and acellular pertussis vaccine. J. Pediatr. 2008;153:327.el–332.e1. doi: 10.1016/j.jpeds.2008.03.011. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White OJ, Rowe J, Richmond P, Marshall H, McIntyre P, Wood N, Holt PG. Th2-polarisation of cellular immune memory to neonatal pertussis vaccination. Vaccine. 2010;28:2648–2652. doi: 10.1016/j.vaccine.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 28.van den Biggelaar AH, Richmond PC, Pomat WS, Phuanukoonnon S, Nadal-Sims MA, Devitt CJ, Siba PM, Lehmann D, Holt PG. Neonatal pneumococcal conjugate vaccine immunization primes T cells for preferential Th2 cytokine expression: A randomized controlled trial in Papua New Guinea. Vaccine. 2009;27:1340–1347. doi: 10.1016/j.vaccine.2008.12.046. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pomat W, et al. Alternative pneumococcal vaccination scheduled for high risk populations: Immunogenicity of neoantal pneumococcal conjugate vaccine and pneumococcal polysaccharide vaccine in Papua New Guinean children, in Pneumococci and Pneumococcal Diseases Symposium. Tel Aviv; Israel. 2010. [Google Scholar]
- 30.Vesikari T, Karvonen A, Forrest BD, Hoshino Y, Chanock RM, Kapikian AZ. Neonatal administration of rhesus rotavirus tetravalent vaccine. Pediatr. Infect. Dis. J. 2006;25:118–122. doi: 10.1097/01.inf.0000199288.98370.71. Medline. [DOI] [PubMed] [Google Scholar]
- 31.Dennehy PH. Rotavirus vaccines: An overview. Clin. Microbiol. Rev. 2008;21:198–208. doi: 10.1128/CMR.00029-07. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resik S, Tejeda A, Lago PM, Diaz M, Carmenates A, Sarmiento L, Alemañi N, Galindo B, Burton A, Friede M, Landaverde M, Sutter RW. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J. Infect. Dis. 2010;201:1344–1352. doi: 10.1086/651611. Medline. [DOI] [PubMed] [Google Scholar]
- 33.Lambert PH, Laurent PE. Intradermal vaccine delivery: Will new delivery systems transform vaccine administration? Vaccine. 2008;26:3197–3208. doi: 10.1016/j.vaccine.2008.03.095. Medline. [DOI] [PubMed] [Google Scholar]
- 34.Borkowsky W, Wara D, Fenton T, McNamara J, Kang M, Mofenson L, McFarland E, Cunningham C, Duliege AM, Francis D, Bryson Y, Burchett S, Spector SA, Frenkel LM, Starr S, Van Dyke R, Jimenez E. Lymphoproliferative responses to recombinant HIV-1 envelope antigens in neonates and infants receiving gp120 vaccines. AIDS Clinical Trial Group 230 Collaborators. J. Infect. Dis. 2000;181:890–896. doi: 10.1086/315298. Medline. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham CK, Wara DW, Kang M, Fenton T, Hawkins E, McNamara J, Mofenson L, Duliege AM, Francis D, McFarland EJ, Borkowsky W. Pediatric AIDSClinical Trials Group 230 Collaborators Safety of 2 recombinant human immunodeficiency virus type 1 (HIV-1) envelope vaccines in neonates born to HIV-1-infected women. Clin. Infect. Dis. 2001;32:801–807. doi: 10.1086/319215. Medline. [DOI] [PubMed] [Google Scholar]
- 36.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: Putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr. Opin. Immunol. 2009;21:23–29. doi: 10.1016/j.coi.2009.01.004. Medline. [DOI] [PubMed] [Google Scholar]
- 38.Leroux-Roels G. Unmet needs in modern vaccinology: Adjuvants to improve the immune response. Vaccine. 2010;28(Suppl 3):C25–C36. doi: 10.1016/j.vaccine.2010.07.021. Medline. [DOI] [PubMed] [Google Scholar]
- 39.Levy O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007;7:379–390. doi: 10.1038/nri2075. Medline. [DOI] [PubMed] [Google Scholar]
- 40.Philbin VJ, Levy O. Developmental biology of the innate immune response: Implications for neonatal and infant vaccine development. Pediatr. Res. 2009;65(Supplement):98R–105R. doi: 10.1203/PDR.0b013e31819f195d. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Méndez-Samperio P, Belmont L, Miranda E. Mycobacterium bovis BCG Toll-like receptors 2 and 4 cooperation increases the innate epithelial immune response. Arch. Med. Res. 2008;39:33–39. doi: 10.1016/j.arcmed.2007.06.019. Medline. [DOI] [PubMed] [Google Scholar]
- 42.Latz E, Franko J, Golenbock DT, Schreiber JR. Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J. Immunol. 2004;172:2431–2438. doi: 10.4049/jimmunol.172.4.2431. Medline. [DOI] [PubMed] [Google Scholar]
- 43.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 2004;4:553–564. doi: 10.1038/nri1394. Medline. [DOI] [PubMed] [Google Scholar]
- 44.Wilson CB, Kollmann TR. Induction of antigen-specific immunity in human neonates and infants. Nestle Nutr. Workshop Ser. Pediatr. Program. 2008;61:183–195. doi: 10.1159/000113493. Medline. [DOI] [PubMed] [Google Scholar]
- 45.Wynn JL, Levy O. Role of innate host defenses in susceptibility to early-onset neonatal sepsis. Clin. Perinatol. 2010;37:307–337. doi: 10.1016/j.clp.2010.04.001. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baxter D. Vaccine responsiveness in premature infants. Hum. Vaccin. 2010;6:506–511. doi: 10.4161/hv.6.6.12083. Medline. [DOI] [PubMed] [Google Scholar]
- 47.Saito S. Cytokine network at the feto-maternal interface. J. Reprod. Immunol. 2000;47:87–103. doi: 10.1016/s0165-0378(00)00060-7. Medline. [DOI] [PubMed] [Google Scholar]
- 48.Morein B, Blomqvist G, Hu K. Immune responsiveness in the neonatal period. J. Comp. Pathol. 2007;137(Suppl 1):S27–S31. doi: 10.1016/j.jcpa.2007.04.008. Medline. [DOI] [PubMed] [Google Scholar]
- 49.Jónsdóttir I. Maturation of mucosal immune responses and influence of maternal antibodies. J. Comp. Pathol. 2007;137(Suppl 1):S20–S26. doi: 10.1016/j.jcpa.2007.04.007. Medline. [DOI] [PubMed] [Google Scholar]
- 50.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J. Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strunk T, Doherty D, Richmond P, Simmer K, Charles A, Levy O, Liyanage K, Smith T, Currie A, Burgner D. Reduced levels of antimicrobial proteins and peptides in human cord blood plasma. Arch. Dis. Child. Fetal Neonatal Ed. 2009;94:F230–F231. doi: 10.1136/adc.2008.143438. Medline. [DOI] [PubMed] [Google Scholar]
- 52.Morgan BP, Marchbank KJ, Longhi MP, Harris CL, Gallimore AM. Complement: Central to innate immunity and bridging to adaptive responses. Immunol. Lett. 2005;97:171–179. doi: 10.1016/j.imlet.2004.11.010. Medline. [DOI] [PubMed] [Google Scholar]
- 53.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J. Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Angelone DF, Wessels MR, Coughlin M, Suter EE, Valentini P, Kalish LA, Levy O. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr. Res. 2006;60:205–209. doi: 10.1203/01.pdr.0000228319.10481.ea. Medline. [DOI] [PubMed] [Google Scholar]
- 55.Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, Kimpen JL, Bont L. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: Low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin. Immunol. 2009;133:228–237. doi: 10.1016/j.clim.2009.07.003. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–1290. doi: 10.1182/blood-2005-12-4821. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burl S, Townend J, Njie-Jobe J, Cox M, Adetifa UJ, Touray E, Philbin VJ, Mancuso C, Kampmann B, Whittle H, Jaye A, Flanagan KL, Levy O. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS ONE. 2011;6:e18185. doi: 10.1371/journal.pone.0018185. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaëlsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandez MA, Puttur FK, Wang YM, Howden W, Alexander SI, Jones CA. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. J. Immunol. 2008;180:1556–1564. doi: 10.4049/jimmunol.180.3.1556. Medline. [DOI] [PubMed] [Google Scholar]
- 60.Avanzini MA, Maccario R, Belloni C, Carrera G, Bertaina A, Cagliuso M, La Rocca M, Valsecchi C, Mantelli M, Castellazzi AM, Quinti I, De Silvestri A, Marconi M. B lymphocyte subsets and their functional activity in the early months of life. Int. J. Immunopathol. Pharmacol. 2010;23:247–254. doi: 10.1177/039463201002300122. Medline. [DOI] [PubMed] [Google Scholar]
- 61.Williams JV, Weitkamp JH, Blum DL, LaFleur BJ, Crowe JE., Jr The human neonatal B cell response to respiratory syncytial virus uses a biased antibody variable gene repertoire that lacks somatic mutations. Mol. Immunol. 2009;47:407–414. doi: 10.1016/j.molimm.2009.08.024. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Randolph DA. The neonatal adaptive immune system. Neoreviews. 2005;6:e454–e462. [Google Scholar]
- 63.PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist CA. Challenges in infant immunity: Implications for responses to infection and vaccines. Nat. Immunol. 2011;12:189–194. doi: 10.1038/ni0311-189. Medline. [DOI] [PubMed] [Google Scholar]
- 64.Holt PG. Functionally mature virus-specific CD8(+) T memory cells in congenitally infected newborns: Proof of principle for neonatal vaccination? J. Clin. Invest. 2003;111:1645–1647. doi: 10.1172/JCI18805. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.A smarter jab: Big drugs companies see a bright future for vaccines. The Economist. 2010 Oct 14; http://www.economist.com/node/17258858.
- 66.Mestas J, Hughes CC. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. Medline. [DOI] [PubMed] [Google Scholar]
- 67.Siegrist CA. The challenges of vaccine responses in early life: Selected examples. J. Comp. Pathol. 2007;137(Suppl 1):S4–S9. doi: 10.1016/j.jcpa.2007.04.004. Medline. [DOI] [PubMed] [Google Scholar]
- 68.Higbee RG, Byers AM, Dhir V, Drake D, Fahlenkamp HG, Gangur J, Kachurin A, Kachurina O, Leistritz D, Ma Y, Mehta R, Mishkin E, Moser J, Mosquera L, Nguyen M, Parkhill R, Pawar S, Poisson L, Sanchez-Schmitz G, Schanen B, Singh I, Song H, Tapia T, Warren W, Wittman V. An immunologic model for rapid vaccine assessment—A clinical trial in a test tube. Altern. Lab. Anim. 2009;37(Suppl 1):19–27. doi: 10.1177/026119290903701S05. Medline. [DOI] [PubMed] [Google Scholar]
- 69.Pulendran B. Learning immunology from the yellow fever vaccine: Innate immunity to systems vaccinology. Nat. Rev. Immunol. 2009;9:741–747. doi: 10.1038/nri2629. Medline. [DOI] [PubMed] [Google Scholar]
- 70.Reikie BA, Smolen KK, Fortuno ES, 3rd, Loeffler DI, Cai B, Blimkie D, Kollmann TR. A single immunization near birth elicits immediate and lifelong protective immunity. Vaccine. 2010;29:83–90. doi: 10.1016/j.vaccine.2010.10.013. Medline. [DOI] [PubMed] [Google Scholar]
- 71.Mahon BP. The rational design of vaccine adjuvants for mucosal and neonatal immunization. Curr. Med. Chem. 2001;8:1057–1075. doi: 10.2174/0929867013372571. Medline. [DOI] [PubMed] [Google Scholar]
- 72.Santos JI, Martin A, De Leon T, Rivera L, Gaitán ME, Del Rio C, Oselka G, Cervantes Y, Rubio P, Clemens SA, de Mendonça JS. DTPw-HB and Hib primary and booster vaccination: Combined versus separate administration to Latin American children. Vaccine. 2002;20:1887–1893. doi: 10.1016/s0264-410x(01)00512-6. Medline. [DOI] [PubMed] [Google Scholar]
- 73.Smolen KK, Loeffler DI, Reikie BA, Aplin L, Cai B, Fortuno ES, 3rd, Kollmann TR. Neonatal immunization with Listeria monocytogenes induces T cells with an adult-like avidity sensitivity, TCR-Vbeta repertoire and does not adversely impact the response to boosting. Vaccine. 2009;28:235–242. doi: 10.1016/j.vaccine.2009.09.091. Medline. [DOI] [PubMed] [Google Scholar]
- 74.Bot A, Bona C. Genetic immunization of neonates. Microbes Infect. 2002;4:511–520. doi: 10.1016/s1286-4579(02)01566-6. Medline. [DOI] [PubMed] [Google Scholar]
- 75.Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010;33:516–529. doi: 10.1016/j.immuni.2010.10.006. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: Prematurity means more than immaturity. J. Matern. Fetal Neonatal Med. 2011;24:25–31. doi: 10.3109/14767058.2010.482605. Medline. [DOI] [PubMed] [Google Scholar]
- 77.Van Herck K, Van Damme P. Benefits of early hepatitis B immunization programs for newborns and infants. Pediatr. Infect. Dis. J. 2008;27:861–869. doi: 10.1097/INF.0b013e318173966f. Medline. [DOI] [PubMed] [Google Scholar]
- 78.Sutter RW, John TJ, Jain H, Agarkhedkar S, Ramanan PV, Verma H, Deshpande J, Singh AP, Sreevatsava M, Malankar P, Burton A, Chatterjee A, Jafari H, Aylward RB. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: A randomised double-blind controlled trial. Lancet. 2010;376:1682–1688. doi: 10.1016/S0140-6736(10)61230-5. Medline. [DOI] [PubMed] [Google Scholar]
- 79.Wood N, McIntyre P, Marshall H, Roberton D. Acellular pertussis vaccine at birth and one month induces antibody responses by two months of age. Pediatr. Infect. Dis. J. 2010;29:209–215. doi: 10.1097/INF.0b013e3181bc98d5. Medline. [DOI] [PubMed] [Google Scholar]
- 80.Kurikka S, Käyhty H, Peltola H, Saarinen L, Eskola J, Mäkelä PH. Neonatal immunization: Response to Haemophilus influenzae type b-tetanus toxoid conjugate vaccine. Pediatrics. 1995;95:815–822. Medline. [PubMed] [Google Scholar]
- 81.Agnandji ST, Asante KP, Lyimo J, Vekemans J, Soulanoudjingar SS, Owusu R, Shomari M, Leach A, Fernandes J, Dosoo D, Chikawe M, Issifou S, Osei-Kwakye K, Lievens M, Paricek M, Apanga S, Mwangoka G, Okissi B, Kwara E, Minja R, Lange J, Boahen O, Kayan K, Adjei G, Chandramohan D, Jongert E, Demoitié MA, Dubois MC, Carter T, Vansadia P, Villafana T, Sillman M, Savarese B, Lapierre D, Ballou WR, Greenwood B, Tanner M, Cohen J, Kremsner PG, Lell B, Owusu-Agyei S, Abdulla S. Evaluation of the safety and immunogenicity of the RTS, S/AS01E malaria candidate vaccine when integrated in the expanded program of immunization. J. Infect. Dis. 2010;202:1076–1087. doi: 10.1086/656190. Medline. [DOI] [PubMed] [Google Scholar]
- 82.Galindo CM, Acosta CJ, Schellenberg D, Aponte JJ, Roca A, Oettli A, Urassa H, Armstrong Schellenberg J, Kahigwa E, Ascaso C, Mshinda H, Lwilla F, Vidal J, Menendez C, Tanner M, Alonso PL. Humoral immune responses during a malaria vaccine trial in Tanzanian infants. Parasite Immunol. 2000;22:437–443. doi: 10.1046/j.1365-3024.2000.00322.x. Medline. [DOI] [PubMed] [Google Scholar]