Abstract
Toll-like receptors (TLRs) play important roles in infection. We have previously reported TLR2 is up-regulated in neonatal Gram-positive (G+) bacteremia whereas TLR4 is up-regulated in neonatal Gram-negative (G−) bacteremia. For functional signaling, TLR4 requires MD-2 and both TLR2 and TLR4 signal need MyD88. However, it is unknown whether newborns can enhance expression of MD-2 and MyD88 with bacterial infection in coordination with TLR expression. We characterized neonatal peripheral blood leukocyte expression of MD-2 and MyD88 in relation to TLR2/4 in newborns. TLR2 mRNA expression by PBMCs and TLR2 protein expression by monocytes/granulocytes were significantly increased in the G+ bacteremia group. TLR4 mRNA on PMBCs and protein expression on monocytes/granulocytes were significantly increased in the G− bacterial group. Remarkably, whereas MyD88 mRNA was increased in all patients with documented bacterial infection and correlated with both TLR2 and TLR4, MD-2 mRNA was selectively increased in G− bacterial group wherein it correlated with TLR4, but not TLR2 mRNA. Our findings demonstrate that during bacterial infection in vivo, newborns selectively and coordinately amplify the TLR2-MyD88 pathway in G+ bacterial infection and the TLR4/MD2/MyD88 pathway in G− bacterial infection, suggesting key roles for innate immune pathway in neonatal responses to bacterial infection.
Bacterial infections remain a leading cause of neonatal morbidity and mortality, especially among premature neonates. The mechanisms of immune function in newborns are not completely understood. It is assumed that high susceptibility of newborns to infections is due to functional immaturity of innate and adaptive immune responses (1). The innate immune response is important in the early stages of defense against bacterial pathogens. Defense against pathogens is in part based on leukocytes, such as granulocytes and monocytes, which express pattern recognition receptors (PRRs) that recognize specific structures present on microorganisms, termed pathogen-associated molecular patterns (PAMPs)(2,3). Among the PRRs are the Toll-like receptors (TLRs), whose importance as sentinel receptors has been increasingly appreciated (4,5). Eleven TLRs have been identified in mammals that recognize different PAMPs present in bacteria and viruses, among which TLR2 and TLR4 are the most widely studied (6). Multiple studies have shown that TLR2 mainly responds to Gram-positive (G+) bacterial peptidoglycan (7) and TLR4 mainly recognizes Gram-negative (G−) bacterial lipopolysaccharides (LPS)(8). LPS is one of the best characterized PAMPs that binds to the CD14/TLR4/MD-2 receptor complex (9). CD14 binds to LPS but lacks an intracellular component and is, thus, incapable of signaling. Myeloid differentiation-2(MD-2) is a protein necessary for LPS recognition by TLR4. MD-2 binds on TLR4 in the endoplasmic reticulum/cis-Golgi and then the TLR4-MD-2 complex moves to the cell surface(10). LPS binds MD-2 triggering changes in MD-2 conformation that are detected by TLR4 (11). Engagement of TLR4 activates intracellular signaling via the adapter protein myeloid differentiation factor 88 (MyD88)(12) ultimately leading to intracellular activation of mitogen activated protein kinase and nuclear factor-κB that activate transcription of cytokine and chemokine genes(13).
Most studies of the functional expression of the TLR system have been carried out in samples derived from murine or human adults. Studies of neonatal mice and human neonatal cord blood have demonstrated gestational age-dependent increases in expression of functional TLR4 during gestation(14,15). Studies of basal and LPS-induced TLR4 expression on neonatal blood monocytes demonstrate expression of TLR4 at birth and some ability of neonatal cells to up-regulate TLR4 in vitro but have provided contradictory data with respect to quantitative comparisons to adult cells(14,16–21). TLR4 expression in neonatal cord blood monocytes increases in a gestational age-dependent manner while that of TLR2 does not (22). Unstimulated human newborn cord blood monocytes express TLRs and MyD88 at birth (16,23). Basal expression of TLR2 is slightly lower in neonatal phagocytes compared adults (17).
Compared to the many studies of TLR expression in vitro, much less is known about the ability of human newborns to modulate TLR system expression in vivo. A study of human neonatal sepsis, largely defined by clinical parameters with a preponderance of G+ bacteria in the culture-positive cases, found marked up-regulation of TLR2 on monocytes, with only transiently increased TLR2 expression on granulocytes(17). We have previously shown that human newborn peripheral blood mononuclear cells selectively up-regulated TLRs during bacteremia such that TLR2 was up-regulated with G+ bacteremia and TLR4 was up-regulated with G− bacteremia (24). As TLR2 and TLR4 require partnering molecules to facilitate their function, in the present study we characterized expression of TLRs, MD-2, and MyD88 on human neonatal peripheral blood leukocytes and investigated their relationships to infection. We find that human newborns demonstrate selective and coordinated expression of TLR2 and TLR4/MD-2 as well as MyD88 in a pathogen-specific manner, providing new insights into the neonatal response to bacterial infection.
MATERIALS AND METHODS
Study Design and Population
We enrolled 83 neonates with bacterial infection and 43 neonates without infection, who were admitted to the neonatal intensive care unit of the Children’s Hospital of Fudan University, Shanghai, China during 2004 to 2006. We employed the following exclusion and inclusion criteria: (a) Exclusion criteria: Neonates with evidence of major congenital malformations, inborn errors of metabolism and those who had received immunotherapy were excluded from enrollment. None of the neonates exhibited signs of hypoxia/asphyxia. All those neonates with mothers have history of infection, HIV, exposure of steroids and maternal history of autoimmune disease and smoking were also excluded. (b) Inclusion criteria: All infants were vaginally delivered. At the time of admission, neonates were suspected to have infection if they had clinical symptoms (apnea, bradycardia, instability of body temperature, feeding intolerance or desaturation and so on) and at least one abnormal test result (high C-reactive protein (CRP), high ratio of immature total neutrophils, and/or abnormal x-ray). Blood, urine and CSF cultures and blood samples for TLR pathway analysis were obtained prior to initiating antibiotic therapy. Patients with positive culture results were then enrolled in this study. Tracheal aspirate cultures were taken from neonates who were intubated and had abnormal chest radiographs. Patients with positive tracheal aspirate cultures were included in this analysis only if they had accompanying chest radiograph abnormalities. All those patients were successfully treated by supportive and standard antibiotic treatment after all these blood samples were taken, none of them received any steroids or immune modulators.
We have previously reported up-regulation of TLR2 and TLR4 in neonates with bacteremia and in those with clinical infection of any site (including pneumonia, meningitis, and urinary tract infection), but did not analyze TLR expression in the infected group with respect to microbiologic culture- i.e., we had not compared data for G− or G+ bacterial infection (24). In our current study, we focused only on those patients with positive cultures from the previous study and an additional two patients, both with Klebsiella pneumoniae bacteremia. In addition, we measured MD-2 and MyD88 mRNA expression for all patients with positive culture. In total, we analyzed neonates in three groups: control (N = 43), G+ infection (N = 41) and G− infection (N = 42).
Blood Samples for RNA Analysis
1ml venous blood was collected by peripheral venipuncture into heparinized tubes (QDKY, Qingdao, Shandong, China) and diluted 1:1 in endotoxin-free Hank’s balanced salt solution HBSS. 5 × 106 peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient centrifugation, washed three times with ice cold endotoxin-free HBSS and immediately lysed in 1 mL Trizol reagent (Invitrogen, Carlsbad, CA). The control group, was comprised of blood samples obtained from neonates whose blood was taken for the screening of inborn errors of metabolism (N = 39), or at the time of routine electrolyte testing (N=4).
Whole Blood Flow Cytometry
Flow cytometry was employed to measure cell surface TLR expression. Whole blood samples (0.5 mL, anti-coagulated with EDTA) were stained for 20 min at 4°C in the dark with isotype-matched control antibodies (BD PharMingen, SanDiego, CA), a FITC-conjugated CD14 monoclonal antibody (mAb; clone M-P9; BD Phar-Mingen), and Phycoerythrin (PE)-conjugated mouse anti-human TLR2 or -4 IgG2a mAbs (clones TL2.1 or HTA125, respectively), or corresponding isotype control mAbs (eBioscience, Frederick, MD, USA). Measurements were performed using a FACS Calibur flow cytometer and data acquired by Cell Quest software (BD Biosciences, Franklin Lakes, NJ). Granulocytes and monocytes were differentiated by scatter properties and CD14 expression (high in monocytes and low in granulocytes). MFIs(mean fluorescence intensity) were determined by subtracting the fluorescence intensity of the isotype control mAbs from that of the specific mAbs. We defined the MFI shift as the ratio of the MFI of the marker to the MFI of the isotype control. We also measured the percentage of TLR2 and TLR4-positive cells on monocyes and granulocytes.
Quantitative Real-time Polymerase Chain Reaction (PCR)
Total RNA was isolated from PBMCs and extracted by Trizol. All reagents and devices used for PCR were obtained from Applied Biosystems. A total of 200 ng of RNA was reverse transcribed to cDNA using TaqMan reverse transcription agents. Quantitative RT-PCR of target cDNA was conducted for TLR, MyD88 and MD-2, and values were normalized to β-actin gene expression. Primer and probes were from the TaqMan Gene Expression Assays. Experiments were performed in 96-well plates in triplicate using TaqMan Universal Master Mix. RT-PCR amplification was performed on a Gene Amp7700 Sequence Detection System. PCR conditions were 50°C for 2 min, 95°C for 10 min, and then 45 cycles at 95°C for 15s and 60°C for 1 min. mRNAs encoding s-actin, TLR, MyD88 and MD-2 were amplified using the primers shown in Table 1.
TABLE 1.
Primers for β-actin, TLR, MyD88 and MD-2 mRNA
| PCR Primers | |
|---|---|
| β-actin forward | 5′-CACCAACTGGGACGACAT-3′ |
| β-actin reverse | 5′-ATCTGGGTCATCTTCTCGC-3′ (138bp) |
| TLR2 forward | 5′-CTGCAAGCTGCGGAAGATAAT-3′ |
| TLR2 reverse | 5′-GTTACGAAGAGGCTGGAATGGT-3′ (176bp) |
| TLR4 forward | 5′-GATTGCTCAGACCTGGCAGTT-3′ |
| TLR4 reverse | 5′-TGTCCTCCCACTCCAGGTAAGT-3′ (143 bp) |
| MD-2 forward | 5′-CATTCCAAGGAGAGATTTAAAGCAA-3′ |
| MD-2 reverse | 5′-CAGATCCTCGGCAAATAACTTCTT-3′ (104bp) |
| MyD88 forward | 5′-GGATCTTGGGAGGGAATGGA-3′ |
| MyD88 reverse | 5′-GAGATGGCTTTAAAATGCCCAGTA-3′ (168bp) |
Statistical Analysis
Data are presented as mean ± SD. Groups were compared using the t test and the Mann-Whitney test. Correlations between individual parameters were determined using a Spearman rank correlation. All comparisons were made using two-sided significance levels of p < 0.05. Statistical analyses were performed using SPSS 12.0 (SPSS China; Shanghai, China).
Ethical Approval
The study was approved by the Children’s hospital of Fudan University Research Ethical Committee and informed written parental consent was obtained before neonates were entered into the study.
RESULTS
Study Population
From January 2004 to January 2006, a total of 126 neonates who had been referred to the neonatal care unit of the Children’s Hospital of Fudan University, Shanghai, P.R. China were included. Forty-three neonates were included in the non-infection group, forty-one neonates in the G+ bacterial infection group and forty-two neonates in the G− bacterial infection group. The groups were matched for gestational age, birth weight and the time of infection. A total of 67 preterm infants and 59 term infants were included. Most term infants were near 37 weeks, their mean gestational age was 38.1 ±1.1 weeks, and there were no post-dates neonates. Control samples were matched according to the age at entry of the study. The relevant clinical characteristics of the 126 infants are shown in Table 2. Bacteria isolated from positive cultures are shown in Table 3.
TABLE 2.
Characteristics of the neonatal study subjects*
| Groups | n | preterm/term | Gestional age (weeks) | Birthweight (kg) | Age at study enrollment (days) |
|---|---|---|---|---|---|
| Control | 43 | 21/22 | 35.05 ± 2.71 | 2.56 ± 1.19 | 5.47 ± 6.97 |
| G+ group | 42 | 23/19 | 33.12 ± 3.84 | 2.04 ± 0.84 | 6.33 ± 7.72 |
| G− group | 41 | 23/18 | 34.05 ± 4.56 | 2.12 ± 1.12 | 8.0 ± 7.49 |
There were no significant differences between the control group and the infection groups
TABLE 3.
Bacteria isolated from the study subjects
| Sample (n) | Gram-positive bacteria (N) | Gram-negative bacteria (N) |
|---|---|---|
| Blood sample(23) | coagulase-negative staphylococci (4), Enterococcus faecalis (2), Streptococcus spp. (3) | Klebsiella pneumoniae (8), Pseudomonas aeruginosa (2), Enterobacter cloacae (4) |
| Tracheal aspirate(46) | coagulase-negative staphylococci (16), Enterococcus faecalis (6), Streptococcus spp. (5) | Klebsiella pneumoniae (8), Pseudomonas aeruginosa (6), Enterobacter cloacae (5) |
| Urine sample(11) | Enterococcus faecalis (6) | Klebsiella pneumoniae (2), Enterobacter cloacae (3) |
| Cerebral fluid (3) | (0) | Enterobacter cloacae (3) |
Expression of TLR2, TLR4, MD-2 and MyD88 mRNA
In the control groupTLR2 and TLR4 mRNA expression were 5.05 ± 0.88, 4.93±1.30 (arbitrary units, ratio TLR mRNA/β-actin), respectively (See Fig. 1). There were no correlations found between mRNA levels of TLR2, TLR4, MD-2 or MyD88 mRNA with gestational age (r = 0.11, p 0.82;, r=0.04, p=0.82; r = 0.08, p = 0.75; r = 0.054, p = 0.85, respectively).
FIGURE 1. mRNA expression of TLR2, TLR4, MD-2 & MyD88 in control newborns in comparison to those with Gram-positive or Gram-negative infection.
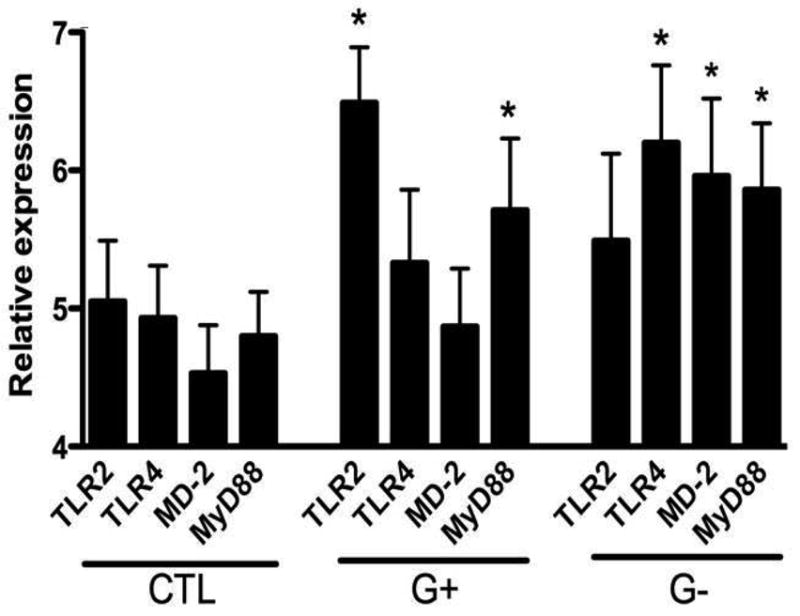
Peripheral blood was collected from control or infected newborns and analyzed for mRNA expression of TLR2, TLR4, MyD88 and MD-2 as described in Methods. Data represent mean ± SD; * p<0.05, as compared with the control group using t -test.
The expression of TLR2 mRNA in the G+ bacterial group was higher than the G− bacterial group (t = 2.28, p = 0.03). Conversely, expression of TLR4 mRNA in the G− bacterial group was higher than the G+ bacterial group (t = 2.29, p = 0.03). Expression of MyD88 mRNAin each of the infection groups was higher than the control group, but did not significantly differ between the G+ bacterial and the G− bacterial group (t = 0.79, p = 0.86). MD-2 mRNA expression in the G− group was higher than in the G+ bacterial group (p < 0.05).
Expression of TLR2 and TLR4 protein on granulocytes and monocytes
In the control group, 72 ± 21% of monocytes were TLR2+ with an MFI of 1.27 ± 0.75. There was no significant correlation between either % or MFI of TLR2 with gestational age; (r = 0.03, p = 0.87) and (r = 0.08, p = 0.70), respectively. 49 ± 11% of monocytes were TLR4+ with an MFI of 1.16 ± 0.36, neither of which correlated with gestational age (r = 0.19, p = 0.45, and r = 0.14, p = 0.58, respectively). 30 ± 10% of granulocytes in controls were TLR2+ with an MFI of 0.31 ± 0.25. There was no significant correlation between either % or MFI of TLR2 with gestational age (r = 0.02, p = 0.92, and r = 0.001, p = 0.95, respectively). 21.12 ± 11.13% of granulocytes were TLR4+ with an MFI of 1.21 ± 0.55, neither of which correlated with gestational age (r = 0.04, p = 0.65 and r = 0.05, p = 0.42, respectively).
Monocyte and granulocyte TLR2 expression (MFI) was higher in the G+ bacterial group than G− bacterial group and control group (p <0.05; see Fig 2). Monocyte and Granulocyte TLR4 MFI was higher in the G− group than in the control and the G+ bacterial group (p<0.05; see Fig 2). The average percentage of monocytes and granulocytes that were TLR2+ and TLR4+ in the infection group were not different from the control group (p >0.05; see Table 4).
FIGURE 2. MFI of TLR2 and TLR4 on the granulocytes and monocytes in control newborns in comparison to those with Gram-positive or Gram-negative infection.
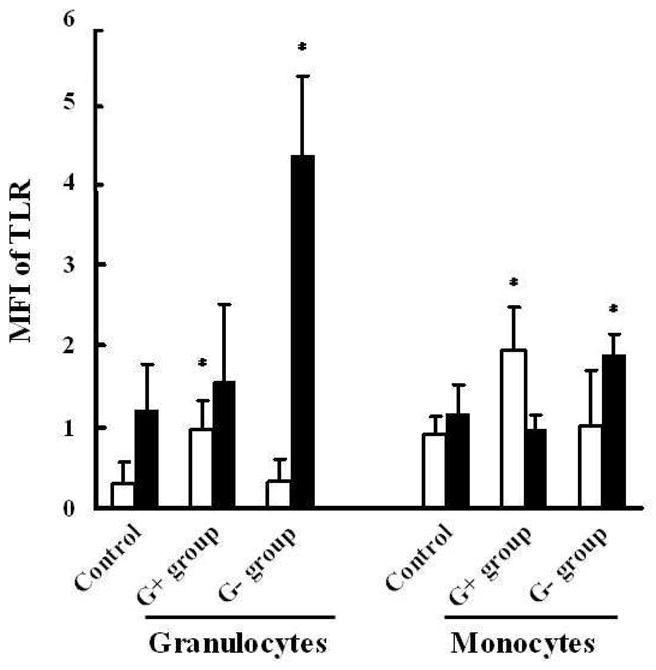
Peripheral blood was collected from control or infected newborns and analyzed for MFI of TLR2(□) and TLR4(■) as described in Methods. Data represent mean ± SD; * p<0.05, as compared with the control group using t -test.
TABLE 4.
Percentage of TLR2 and TLR4 positive cells on Monocytes(M)/Granulocytes(G)
| Groups | n | Percentage of TLR2 positive cell (%)on M | Percentage of TLR2 positive cell(%) on G | Percentage of TLR4 positive cell (%) on M | Percentage of TLR4 positive cell (%) on G |
|---|---|---|---|---|---|
| Control | 43 | 72.02 ± 21.41 | 30.1 ± 10.21 | 49.21 ± 11.23 | 21.12 ± 11.13 |
| G+ group | 42 | 75.7 ± 15.16 | 32.4 ± 12.36 | 54.13 ± 13.44 | 24.15 ± 13.64 |
| G− group | 41 | 72.39 ± 18.71 | 28.9 ± 11.74 | 62.53 ± 15.16 | 21.96 ± 12.45 |
Relationships of MyD88 and MD-2 mRNAs with TLR expression
TLR4 mRNA and MFI were significantly correlated with MD-2 mRNA in the G-bacterial infection group (r = 0.42, p = 0.02, and r = 0.54, p = 0.01, respectively). In each of the infection groups, TLR2 mRNA and TLR4 mRNA were significantly correlated with MyD88 mRNA (r = 0.95, p = 0.001, and r = 0.99, p = 0.001, respectively). Similarly, TLR2 and TLR4 protein expression on monocytes (flow cytometry, MFI) were also significantly correlated with MyD88 mRNA, (r = 0.87, p = 0.001) and (r = 0.91, p = 0.001). Among the G− and G+ bacterial infection groups, there was no significant correlation between TLR2 expression (MFI or mRNA) and MD-2 expression (mRNA): r = 0.24, p = 0.07 and r = 0.12, p = 0.12, respectively.
DISCUSSION
Neonates, and in particular preterm newborns, are susceptible to bacterial infections, which cause significant mortality and morbidity annually. Despite considerable advances in neonatal intensive care medicine, the incidence of infection is still very high. The pathogenesis of neonatal infection with its rapid progression from infection to a systemic inflammatory response still remains unclear. The characterization over the past two decades of PRRs based on insect and animal models as well as in vitro studies of human cells has informed novel concepts regarding the pathogenesis of infection. The immature innate immune system plays a key role in the first line of defense against invading microbes in neonates (1). In the present study, we characterized human neonatal peripheral blood leukocyte expression of MyD88 and MD-2 in relation to TLR expression and bacterial infections.
The frequent exposure to invasive procedures in neonatal intensive care units such as umbilical and intravascular catheterization, intubation and long-term ventilation play roles in the high neonatal infections (25). Reduced levels of TLR4 expression and impaired TLR-mediated production of Th1-polarizing cytokines might also contribute to neonatal susceptibility to bacterial infection (22). A study of blood leukocyte TLR expression after endotoxin-infusion in human adults in vivo demonstrated up-regulation of TLR2 (but not TLR4) on monocytes and down-regulation of TLR4 (but not TLR2) on neutrophils (26). TLR-signaling pathway genes are differently regulated in PBMCs and neutrophils of adult patients with clinical sepsis (27). However, TLR2, TLR4 and MyD88 mRNA all increased in adult sepsis mainly due to G+ bacteria (27). In accordance with our findings in neonates, a study of adult G− bacterial sepsis, demonstrated elevations of TLR4 and soluble MD-2 on endothelial cells (28).
Multiple studies have established that TLR4 requires MD-2 to mediate LPS signaling (29–31). Our data show significant correlations between the mRNA expression of MD-2 and TLR4, suggesting coordinate expression of these co-receptors on the cell surface during G-bacterial infection.
MyD88-deficient mice are highly susceptible to infections with a broad range of microorganisms (32) and reduced MyD88 expression levels might contribute to impaired neonatal immune responses to pathogens (18). However our study did not demonstrate any correlation between MyD88 mRNA and gestational age. We demonstrated that MyD88 mRNA expression increased greatly in both bacterial infection groups. There are at least two pathways downstream of TLR activation: the MyD88-dependent and the MyD88-independent pathways. Given the brisk up-regulation of MyD88 in the context of bacterial infection, the MyD88 pathway may play an important role in neonatal infections. Indeed, study of patients deficient in MyD88 or IRAK4 demonstrates that the TLR pathway is particularly important early in life, as susceptibility to pyogenic infection in these patients diminishes and normalizes with age (33,34)
In this study, the basal expression of TLR2, TLR4, MyD88 and MD-2 were similar in pre-term and full term neonates at both the mRNA and protein level. A previous study demonstrated similar basal TLR2 and TLR4 expression between neonatal cord blood and adult peripheral blood (24). These results indicate that infection status, more than gestation age, is a key parameter effecting TLR2 and TLR4 expression, suggesting potential utility of TLR measurement as a biomarker of sepsis. We also measured TLR2 and TLR4 expression on neonatal granulocytes and monocytes. We analyzed the MFI of TLR2 and TLR4, and found that TLR2 MFI expression was up-regulated in the G+ bacterial infection group on both monocytes and granulocytes, compared with the control group. In contrast, TLR4 protein expression (MFI) was up-regulated in the G− bacterial infection group on both monocytes and granulocytes. We also characterized expression levels of TLR2 and TLR4, demonstrating that human newborn peripheral blood monocytes expressed higher levels of TLR2 and TLR4 than granulocytes. In addition to the analysis of surface TLR expression on leukocytes, we also analyzed the TLR mRNA and its signaling pathways in culture-positive neonatal infections. We found that peripheral blood TLR2 and TLR4 mRNA significantly increased in neonatal infections. Furthermore, TLR2 was increased in the setting of G+ bacterial infection, whereas TLR4 mainly increased in the setting of to G− bacterial infection, consistent with the results of our previous study (35). With respect to the absolute increases in TLR expression by flow, we demonstrate significant increases in MFI of ~110% for TLR2 on monocytes in the setting of G+ infection and ~60% for TLR4 on monocytes in the context of G− infection. The relationships between absolute surface expression of TLRs and functionality are incompletely characterized and may be non-linear, as dimerization/multimerization of the receptors is known to be important for signaling (35).
In conclusion, our study reveals that during bacterial infections, human neonatal blood leukocytes demonstrate pathogen-specific and coordinated up-regulation of TLR2, TLR4/MD-2, and the MyD88 adaptor molecule. Characterizing neonatal TLR pathway expression, provides insights into the development of neonatal innate immune function and host defense against infection. Indeed, adjunctive therapy with TLR-based immunomodulators has demonstrated reduction of mortality after polymicrobial peritonitis in a neonatal animal model (36). A better understanding of neonatal host defense mechanisms may thus lead not only to improved diagnostics but also preventative and/or therapeutic options (37).
Acknowledgments
This study was funded by Chinese Education Ministry 211 Project, and the New Teacher Foundation of the Ministry of Education of China (20090071120078); Children’s Hospital of Fudan University. Dr. Levy’s laboratory is funded by NIH RO1 AI067353-01A1 and by the Bill & Melinda Gates Foundation.
We thank the patients and their families for their participation in this study. We thank Liat Stoler-BarakZhiheng Huang and Jianguo Zhou for assistance with data analysis.
Abbreviations
- G+
Gram-positive
- G−
Gram-negative
- MD-2
myeloid differentiation protein-2
- MFI
mean fluorescence intensity
- MyD88
Myeloid differentiation 88
- PAMPs
pathogen-associated molecular patterns
- PBMCs
peripheral blood mononuclear cells
- PRRs
pattern recognition receptors
- TLRs
Toll-like receptors
References
- 1.Levy O. Innate Immunity of the Human Newborn: Basic Mechanisms and Clinical Correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins KA, Mansell A. TIR-containing adaptors in Toll-like receptor signalling. Cytokine. 2010;49:237–244. doi: 10.1016/j.cyto.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Strunk T, Temming P, Gembruch U, Reiss I, Bucsky P, Schultz C. Differential maturation of the innate immune response in human fetuses. Pediatr Res. 2004;56:219–226. doi: 10.1203/01.PDR.0000132664.66975.79. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchiet O. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 7.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poltorak A, He X, Smirnova I, Liu M-Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 9.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903–914. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 10.Nishitani C, Mitsuzawa H, Hyakushima N, Sano H, Matsushima N, Kuroki Y. The Toll-like receptor 4 region Glu24-Pro34 is critical for interaction with MD-2. Biochem Biophys Res Commun. 2005;328:586–590. doi: 10.1016/j.bbrc.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci USA. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Lakhani SA, Bogue CW. Toll-like receptor signaling in sepsis. Curr Opin Pediatr. 2003;15:278–282. doi: 10.1097/00008480-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Förster-Waldl E, Sadeghi K, Tamandl D, Gerhold B, Hallwirth U, Rohrmeister K, Hayde M, Prusa AR, Herkner K, Boltz-Nitulescu G, Pollak A, Spittler A. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res. 2005;58:121–124. doi: 10.1203/01.PDR.0000163397.53466.0F. [DOI] [PubMed] [Google Scholar]
- 15.Harju K, Glumoff V, Hallman M. Ontogeny of Toll-like receptors Tlr2 and Tlr4 in mice. Pediatr Res. 2001;49:81–83. doi: 10.1203/00006450-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Yan SR, Qing G, Byers DM, Stadnyk AW, Al-Hertani W, Bortolussi R. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect Immun. 2004;72:1223–1229. doi: 10.1128/IAI.72.3.1223-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viemann D, Dubbel G, Schleifenbaum S, Harms E, Sorg C, Roth J. Expression of toll-like receptors in neonatal sepsis. Pediatr Res. 2005;58:654–659. doi: 10.1203/01.PDR.0000180544.02537.FD. [DOI] [PubMed] [Google Scholar]
- 18.Henneke P, Osmers I, Bauer K, Lamping N, Versmold HT, Schumannet RR. Impaired CD14-dependent and independent response of polymorphonuclear leukocytes in preterm infants. J Perinat Med. 2003;31:176–183. doi: 10.1515/JPM.2003.024. [DOI] [PubMed] [Google Scholar]
- 19.Qing G, Rajaraman K, Bortolussi R. Diminished priming of neonatal polymorphonuclear leukocytes by lipopolysaccharide is associated with reduced CD14 expression. Infect Immun. 1995;63:248–252. doi: 10.1128/iai.63.1.248-252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy E, Xanthou G, Petrakou E, Zacharioudaki V, Tsatsanis C, Fotopoulos S, Xanthou M. Distinct roles of TLR4 and CD14 in LPS-induced inflammatory responses of neonates. Pediatr Res. 2009;66:179–184. doi: 10.1203/PDR.0b013e3181a9f41b. [DOI] [PubMed] [Google Scholar]
- 21.Yerkovich ST, Wikström ME, Suriyaarachchi D, Prescott SL, Upham JW, Holt PG. Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatr Res. 2007;62:547–552. doi: 10.1203/PDR.0b013e3181568105. [DOI] [PubMed] [Google Scholar]
- 22.Sadeghi K, Berger A, Langgartner M, Prusa AR, Hayde M, Herkner K, Pollak A, Forster-Waldl E. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 23.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol. 2004;173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 24.Zhang JP, Chen C, Yang Y. Changes and clinical significance of Toll-like receptor 2 and 4 expression in neonatal infections. Zhonghua Er Ke Za Zhi. 2007;45:130–133. [PubMed] [Google Scholar]
- 25.Orsi GB, d’Ettorre G, Panero A, Chiarini F, Vullo V, Venditti M. Hospital-acquired infection surveillance in a neonatal intensive care unit. Am J Infect Control. 2009;37:201–203. doi: 10.1016/j.ajic.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Marsik C, Mayr F, Cardona F, Derhaschnig U, Wagner OF, Jilma B. Endotoxaemia modulates Toll-like receptors on leucocytes in humans. Br J Haematol. 2003;121:653–656. doi: 10.1046/j.1365-2141.2003.04350.x. [DOI] [PubMed] [Google Scholar]
- 27.Salomao R, Brunialti MK, Gomes NE, Mendes ME, Diaz RS, Komninakis S. Toll-like receptor pathway signaling is differently regulated in neutrophils and peripheral mononuclear cells of patients with sepsis, severe sepsis, and septic shock. Crit Care Med. 2009;37:132–139. doi: 10.1097/CCM.0b013e318192fbaf. [DOI] [PubMed] [Google Scholar]
- 28.Wolfs TG, Dunn-Siegrist I, van’t Veer C, Hodin CM, Germeraad WT, van Zoelen MA. Increased release of sMD-2 during human endotoxemia and sepsis: a role for endothelial cells. Mol Immunol. 2008;45:3268–3277. doi: 10.1016/j.molimm.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Akashi S, Saitoh S, Wakabayashi Y, Kikuchi T, Takamura N, Nagai Y, Kusumoto Y, Fukase K, Kusumoto S, Adachi Y. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J Exp Med. 2003;198:1035–1042. doi: 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 31.Ishihara S, Rumi MA, Kadowaki Y, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 32.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 33.Ku CL, von Bernuth H, Picard C, Zhang SY, Chang HH, Yang K, Chrabieh M, Issekutz AC, Cunningham CK, Gallin J, Holland SM, Roifman C, Ehl S, Smart J, Tang M, Barrat FJ, Levy O, McDonald D, Day-Good NK, Miller R, Takada H, Hara T, Al-Hajjar S, Al-Ghonaium A, Speert D, Sanlaville D, Li X, Geissmann F, Vivier E, Maródi L, Garty BZ, Chapel H, Rodriguez-Gallego C, Bossuyt X, Abel L, Puel A, Casanova JL. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor AB, Herrero-Mata MJ, Aróstegui JI, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M, Fortuny C, Yagüe J, Antón J, Pascal M, Chang HH, Janniere L, Rose Y, Garty BZ, Chapel H, Issekutz A, Maródi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova JL. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 36.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, Ungaro R, Levy O, Moldawer LL. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wynn JL, Neu J, Moldawer LL, Levy O. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J Perinatol. 2009;29:79–88. doi: 10.1038/jp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]


