Summary
Testing new drugs is critical to improving the treatment of tuberculosis. Quantitative cultures of Mycobacterium tuberculosis on solid media have been used in Phase 1 and 2 trials, but are time and resource intensive. Time to detection (TTD) of growth of M. tuberculosis in automated liquid culture systems is an alternative. TTD has been shown to correlate with CFU in quantitative cultures, and is faster and simpler to perform. We compared TTD in the BACTEC 460 liquid culture system with CFU in a clinical trial that included 110 subjects. Comparing all sputum cultures collected between baseline and 2 months we found a strong negative correlation between log10 CFU and TTD (rho = −0.91). In addition, when TTD at baseline was compared with 1 and 2 month sputum culture positivity, subjects whose cultures were negative after 1 and 2 months had a significantly longer median baseline TTD compared with subjects whose cultures were positive at 1 and 2 months (5 vs. 3 days and 3 vs. 2 days, respectively). TTD compares closely with CFU and represents a faster, simpler alternative to quantitative cultures.
Keywords: tuberculosis, time to detection, colony forming units, BACTEC
INTRODUCTION
Two major obstacles in the evaluation of new anti-TB drugs are the slow growth rate of Mycobacterium tuberculosis and the labor intensive nature and expense of performing quantitative sputum cultures used to assess bacteriological response in early clinical trials. Time to detection (TTD) of growth when M. tuberculosis is cultured in liquid media using the BACTEC automated systems is a promising correlate of clinical outcome. Epstein et al. demonstrated that TTD using the MGIT 960 system correlated with duration of and response to anti-TB treatment.1 Wallis et al. showed that TTD inversely correlated with duration of sputum culture positivity.2 More recently, Pheiffer et al. found a negative correlation between TTD (with the BACTEC 460 and MGIT 960 systems) and inoculum size, as well as a correlation with baseline TTD and treatment outcome assessed by two month smear conversion.3 Hesseling et al. showed that in 263 HIV-uninfected adults treated with standard short course chemotherapy, baseline TTD predicted 2 month culture conversion during treatment and recurrent TB after treatment.4 Finally, Diacon et al. showed that TTD and CFU were strongly correlated during a 7 day early bactericidal activity (EBA) study.5
To further characterize TTD in relation to accepted microbiological outcomes, we analyzed data from a prospective treatment trial6 in which quantitative cultures on solid media were performed simultaneously with liquid cultures during chemotherapy.
METHODS
Data were analyzed from a phase 2 trial of adjunctive interleukin-2 immunotherapy conducted in Uganda and reported previously.6 Ambulatory HIV-uninfected adults aged 18 to 50 years with initial episodes of smear-positive, culture-confirmed pulmonary tuberculosis were recruited at a large outpatient clinic. Eligible subjects were randomly assigned to receive standard chemotherapy (2 months of daily isoniazid (INH), rifampin, pyrazinamide and ethambutol followed by 4 months of daily INH and rifampin) plus daily intradermal injections of recombinant IL-2 or placebo during the first 30 days of treatment. Patients were hospitalized during the first month of therapy. TB treatment was subsequently supervised on an ambulatory basis. Subjects and staff were masked to treatment assignment. Subjects were followed for 1 year after the initiation of anti-TB treatment. All subjects gave written consent for participation. The protocol was approved by the institutional review boards at University Hospitals Case Medical Center and the Ugandan National AIDS Research Subcommittee.
Early morning spontaneously produced spot sputum specimens of at least 5 ml in volume were collected for qualitative acid fast bacillus (AFB) smear, quantitative culture on Middlebrook 7H-10 agar plates, and culture in the BACTEC 460TB system (Becton, Dickinson, and Company, Franklin Lakes, NJ). All cultures were performed in duplicate at an on-site laboratory. Sputum was collected at baseline, 2 days, 4 days, then weekly from weeks 1-4, at 6 weeks, and then monthly during the remainder of treatment. Quantitative cultures were performed after homogenization with N-acetyl cysteine/sodium citrate and decontamination with 2% sodium hydroxide (NaOH) using previously published methods.7 Recent studies using the BACTEC and MGIT systems to examine TTD have used NaOH concentrations of 1-2%.5, 8 Serial 10-fold dilutions of processed sputum were plated on Middlebrook 7H10 plates supplemented with oleic acid albumin-dextrose-catalase. The medium was made selective by the addition of final concentrations of 200 U/ml of polymyxin B, 50 mg/ml of carbenicillin, 20 mg/ml of trimethoprim, and 10 mg/ml of amphotericin B. The plates were sealed with gas permeable tape, incubated at 37°C in 5 to 10% CO2, and examined after 1, 2, 4 and 6 weeks. Colonies were counted on plates with dilutions yielding distinct visible colonies and expressed as log10 CFU/ml of undiluted sputum. BACTEC 12B vials used in the BACTEC 460 TB system were supplemented with 0.1 ml of reconstituted PANTA Plus antibiotic (containing polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin) according to the manufacturer’s instructions. BACTEC 460 vials were incubated at 37°C and the growth index (GI) was recorded until cultures became positive (GI > 10) or for a maximum of 42 days. GI was monitored daily for the first 10 days, and then weekly. Specimens demonstrating growth, but with a GI < 10 were selected for a reading prior to the next weekly interval. Positive cultures were stained with Ziehl-Neelsen stain and sub-cultured on blood agar plates to assess for contamination. Mycobacteria were identified as M. tuberculosis complex using the BACTEC ρ-nitro-α-acetylamino-β-hydroxy-propiophenone (NAP) test.9 If either the liquid or solid media culture was contaminated, the pair of cultures was excluded from analysis. Susceptibility testing against INH, rifampin, ethambutol, and pyrazinamide was performed using standard BACTEC methods.10
Primary study outcomes were 1 and 2 month sputum culture conversion to negative, defined as having negative cultures at that time point and no subsequent positive cultures during treatment. Comparison was made between TTD in BACTEC culture and log10 CFU on solid media for all cultures from 0 to 60 days using a Spearman correlation. TTD of all lengths was used for this analysis. Since TTD was measured daily for the first 10 days after BACTEC culture inoculation, only TTD of 10 days or less was used for calculating medians since inclusion of TTD beyond 10 days could artificially lengthen median TTD. Median TTD and log10 CFU at baseline were compared with one and two month sputum culture outcomes using the Wilcoxon signed-rank test. Statistical analysis was performed using SAS software (SAS, version 9.2, SAS Institute, Cary, NC) and R statistical software version 2.10.0 (http://www.r-project.org).
RESULTS
This study included 107 of the 110 subjects enrolled in the original trial. One patient with HIV infection and two patients found to have multidrug resistant TB were excluded. The mean age was 27 + 7 yrs and 67% (72) were males. Ninety-two per cent of subjects had a baseline grade 3+ or 4+ sputum smear.11 Ninety-six percent had cavitary disease, and 75% had far advanced TB on initial chest radiograph.12 The median baseline sputum CFU was 6.2 log10 cfu/ml (interquartile range [IQR] 5.6 to 6.7) and the median baseline TTD was 3.0 days (IQR 2.0 to 4.0). For AFB smear grades 0-2, 3, and 4 the median baseline log10 cfu/ml sputum values were 4.5, 5.5, and 6.4 respectively and the median baseline TTD in days was 7.0, 4.0, and 2.0 respectively. Baseline CFU and TTD did not differ between patients with INH mono-resistance and those fully susceptible to INH, rifampin, ethambutol and pyrazinamide. Results of TTD did not differ between treatment arms in the parent trial, so study results were reported as a combined analysis. Two subjects relapsed after treatment.
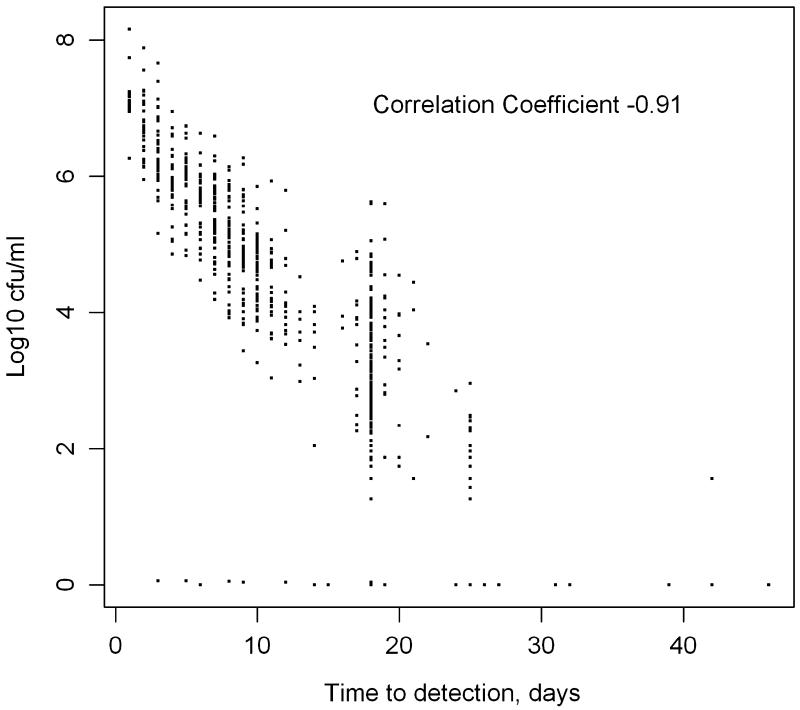
TTD and CFU were compared by subject at all culture time points up to and including day 60 demonstrating greater log10 CFU was correlated with shorter TTD (Figure; rho = −0.91, Spearman, p<0.0001). Correlations between TTD and CFU were also calculated for individual culture time points as shown in Table 1. While TTD and CFU were significantly negatively correlated at all culture time points, there was a decrease in the strength of the association the further into therapy that the culture was obtained.
Figure.
Paired sputum bacillary load (log10 cfu/ml) versus time to detection in 768 sputum samples from 107 patients collected on days 0, 2, 4, 7, 14, 28, 42, and 60 of anti-TB treatment.
Table 1.
Spearman Correlation of log10 CFU vs. TTD at combined and individual culture time points.
| Culture Day | Total subjects | Correlation (rho) | P-value |
|---|---|---|---|
| 0-60 | 107 | −0.91 | <0.0001 |
| 0 | 103 | −0.83 | <0.0001 |
| 2 | 103 | −0.77 | <0.0001 |
| 4 | 101 | −0.83 | <0.0001 |
| 7 | 100 | −0.67 | <0.0001 |
| 14 | 95 | −0.59 | <0.0001 |
| 21 | 94 | −0.54 | <0.0001 |
| 28 | 85 | −0.64 | <0.0001 |
| 60 | 87 | −0.42 | <0.0001 |
Baseline TTD of 10 days or less was compared with solid media culture conversion. As seen in Table 2, subjects with negative sputum cultures at one month had a median baseline TTD of 5.0 days vs. 2.0 days (p=<0.001, Wilcoxon) in subjects with positive sputum cultures at one month. Likewise, median baseline TTD was also longer in subjects with negative 2 month sputum cultures (3.0 days) compared with those with positive cultures (2.0 days, p=0.042, Wilcoxon). Subjects with positive sputum cultures at one month were more likely to have higher baseline median sputum CFU (6.3 vs 5.6 log10 CFU, p=0.001, Wilcoxon). Baseline CFU was not significantly different when stratified by 2 month culture outcomes. All subjects had negative sputum cultures at 3 months, so no comparison could be made.
Table 2.
Median TTD at baseline stratified by culture outcome at month of follow-up.
| Month | Total Subjects | Median (25-75% interquartile range) TTD in days at baseline stratified by culture outcome at month of follow-up |
P Value | |
|---|---|---|---|---|
| Positive | Negative | |||
| 1 | 83 | 2.0 (2.0-3.0) | 5.0 (3.0-6.0) | P = <0.0001 |
| 2 | 84 | 2.0 (1.5-2.5) | 3.0 (2.0-4.5) | P = 0.042 |
| 3 | 86 | 0 | 3.0 (2.0-4.0) | |
DISCUSSION
In this retrospective analysis of a large tuberculosis treatment trial, we found a strong negative correlation between TTD in liquid media and log10 CFU on solid media. This association was strongest early during treatment when sputum bacillary load was highest but was present and significant at all culture time points assessed during the first 2 months of treatment. This suggests that TTD as a measure of bacillary load is least variable early in treatment and might be considered as a measure to replace quantitative cultures during phase 1 EBA studies of new drugs and combinations. TTD in liquid culture media is beginning to be reported as a secondary endpoint in EBA studies13, but requires further validation. Baseline TTD was also associated with 1 and 2 month culture status, measures used in phase 2 trials of TB drugs and regimens, with significantly shorter baseline TTD in subjects whose sputum cultures were still positive at 1 and 2 months. Our findings confirm previous studies in other countries and settings3-5, 14, and support the use of TTD in liquid media as an alternative to colony counting in TB drug evaluation.
Our study has several limitations. Since TTD was only read on a daily basis for the first 10 days we excluded two subjects with baseline TTD beyond 10 days in the calculation of median TTD. The results were similar when these two subjects were included in the analysis. The radiometric BACTEC 460 system was used in this study and has been replaced by the more sensitive, non-radiometric, fluorescent quenching BACTEC MGIT 960 system. Strengths of our study include a large sample size (over 100 subjects), detailed assessment of the baseline severity of TB disease, and comprehensive serial measurement of bacteriological responses during treatment using quantitative and enriched liquid culture methods.6
Our results show that TTD using automated liquid culture systems compares closely with the results of CFU counting on solid media, and represent a viable alternative to traditional quantitative cultures in assessing response to TB treatment. The substantial reduction in labor, time, and opportunities for laboratory error are additional advantages of liquid culture. Further studies comparing quantitative cultures with TTD using the BACTEC MGIT 960 system are underway, which may allow for the acceptance of automated liquid media as the primary microbiological method for culture of M. tuberculosis in clinical trials of new anti-TB drugs and regimens.
Acknowledgements
The authors thank the patients and staff of the Ugandan National Tuberculosis Treatment Centre, Mulago Hospital, the Ugandan National TB and Leprosy Programme; and the TB laboratory at the Joint Clinical Research Centre, Kampala, Uganda for their help with the original clinical trial on which the current study is based.
Funding This project was funded by the Tuberculosis Research Unit (TBRU) at Case Western Reserve University (CWRU), established with funds from the United States National Institutes of Allergy and Infectious Diseases, National Institutes of Health and Human Services, under Contract No. NO1-AI95383 and HHSN266200700022C/NO1-AI-70022.
Footnotes
Conflict of interest statement The authors have no conflict of interest.
References
- 1.Epstein MD, Schluger NW, Davidow AL, Bonk S, Rom WN, Hanna B. Time to detection of Mycobacterium tuberculosis in sputum culture correlates with outcome in patients receiving treatment for pulmonary tuberculosis. Chest. 1998;113:379–86. doi: 10.1378/chest.113.2.379. [DOI] [PubMed] [Google Scholar]
- 2.Wallis RS, Patil S, Cheon SH, et al. Drug tolerance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1999;43:2600–6. doi: 10.1128/aac.43.11.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pheiffer C, Carroll NM, Beyers N, et al. Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int J Tuberc Lung Dis. 2008;12:792–8. [PubMed] [Google Scholar]
- 4.Hesseling AC, Walzl G, Enarson DA, et al. Baseline sputum time to detection predicts month two culture conversion and relapse in non-HIV-infected patients. Int J Tuberc Lung Dis. 2010;14:560–70. [PubMed] [Google Scholar]
- 5.Diacon AH, Maritz JS, Venter A, et al. Time to detection of the growth of Mycobacterium tuberculosis in MGIT 960 for determining the early bactericidal activity of antituberculosis agents. Eur J Clin Microbiol Infect Dis. 2010;29:1561–5. doi: 10.1007/s10096-010-1043-7. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JL, Ssekasanvu E, Okwera A, et al. Randomized trial of adjunctive interleukin-2 in adults with pulmonary tuberculosis. Am J Respir Crit Care Med. 2003;168:185–91. doi: 10.1164/rccm.200211-1359OC. [DOI] [PubMed] [Google Scholar]
- 7.Joloba ML, Johnson JL, Namale A, et al. Quantitative sputum bacillary load during rifampin-containing short course chemotherapy in human immunodeficiency virus-infected and non-infected adults with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:528–36. [PubMed] [Google Scholar]
- 8.Weiner M, Prihoda TJ, Burman W, et al. Evaluation of time to detection of Mycobacterium tuberculosis in broth culture as a determinant for end points in treatment trials. J Clin Microbiol. 48:4370–6. doi: 10.1128/JCM.00757-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqi SH, Hwangbo CC, Silcox V, Good RC, Snider DE, Jr., Middlebrook G. Rapid radiometric methods to detect and differentiate Mycobacterium tuberculosis/M. bovis from other mycobacterial species. Am Rev Respir Dis. 1984;130:634–40. doi: 10.1164/arrd.1984.130.4.634. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqi S. BACTEC 460 TB (Radiometric) System. Indirect susceptibility testing for Mycobacterium tuberculosis. In: Isenberg H, editor. Clinical Microbiology Procedures Handbook. American Society for Microbiology; Washington, DC: 2004. [Google Scholar]
- 11.Strong BS, Kubica GP. Isolation and identification of Mycobacterium tuberculosis: A guide for the level II laboratory. U.S. Department of Health and Human Services - Centers for Disease Control and Prevention; Atlanta, Georgia: 1981. [Google Scholar]
- 12.Falk AOJ, Pratt PC, Webb WR, Wier JA. Diagnostic standards and classification of tuberculosis. 12 ed National Tuberculosis and Respiratory Disease Association; New York: 1969. Wolinsky E Classification of pulmonary tuberculosis; pp. 68–76. [Google Scholar]
- 13.Diacon AH, Dawson R, Hanekom M, et al. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob Agents Chemother. 2010;54:3402–7. doi: 10.1128/AAC.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallis RS, Perkins MD, Phillips M, et al. Predicting the outcome of therapy for pulmonary tuberculosis. Am J Respir Crit Care Med. 2000;161:1076–80. doi: 10.1164/ajrccm.161.4.9903087. [DOI] [PMC free article] [PubMed] [Google Scholar]