Abstract
Background
The tissue-protective effects of erythropoietin (EPO) have been extensively investigated, and EPO administration can raise the hemoglobin (Hb) concentration. Recently, we reported that carbamylated erythropoietin (CEPO) protected kidneys from ischemia-reperfusion injury as well as EPO.
Methods
To investigate the clinical applications of CEPO, we next evaluated the long-term therapeutic effect of CEPO using a tubulointerstitial model rat. We randomized remnant kidney model rats to receive saline, EPO, or CEPO for 8 weeks.
Results
CEPO- and EPO-treated rats had improved serum creatinine levels compared with saline-treated remnant kidney model rats, although the Hb level was significantly increased in EPO-treated rats. Two-photon microscopy revealed that EPO/CEPO significantly ameliorated tubular epithelial cell damage assessed by endocytosis. In addition, CEPO or EPO protected endothelial cells with a sustained blood flow rate. EPO or CEPO suppressed the number of TUNEL-positive apoptotic cells with weak αSMA staining.
Furthermore, PCR analysis demonstrated that TGF-β and type I collagen expression was attenuated in EPO- or CEPO-treated rats, accompanied by a significant decrease in interstitial fibrosis.
Conclusion
We established a long-term therapeutic approach to protect tubulointerstitial injury with CEPO, and thus, the therapeutic value of this approach warrants further attention and preclinical studies.
Keywords: Carbamylated erythropoietin, Remnant kidney, Apoptosis, TGF-β
Introduction
Chronic kidney disease (CKD), characterized by protein-uria and kidney dysfunction, is now recognized as a global public health concern. Regardless of the insult, an initial critical nephron loss is frequently followed by progressive glomerular sclerosis, loss of peritubular capillaries, and hypoxia-induced tubulointerstitial fibrosis. Current therapy, aimed at slowing this progression, focuses on blood pressure control and renin–angiotensin system blockade. However, despite these important therapies, a considerable proportion of CKD patients continues to progress, and a new therapeutic tool is necessary.
The initial understanding of the biology of EPO-mediated tissue protection largely developed from the study of the nervous system, which is susceptible to ischemic injury because of its high basal metabolic rate [1]. The findings derived from nervous system studies are applicable to the kidney. The normal kidney, like the nervous system, is characterized by regions in which energy substrates are limited. Commonly, chronic renal hypoxia with subsequent tubulointerstitial injury leads to end-stage renal failure [2]. In contrast, early treatment with EPO slows the progression of renal failure [3]. However, the chronic administration of EPO also increases hemoglobin (Hb) levels, which may have protective effects on the tubulointerstitium.
In the kidney, a potential role for the non-hematopoietic activities of EPO was first suggested by the identification of the EPO receptor (EPO-R) protein that is expressed throughout the kidney, including in both the proximal and distal tubular cells [4]. However, the affinity of these receptors (~1 nM) is well below the normal plasma EPO concentration (1–10 pM). Therefore, EPO's cytoprotective effect may require higher doses than those used to treat anemia. However, recent clinical trials have suggested that higher doses of EPO are likely to be associated with adverse effects [5]. Furthermore, a recent report demonstrated that a high hemoglobin level in patients with chronic kidney disease was associated with increased risk and no incremental improvement in the quality of life [6].
Recently, a second receptor for EPO that mediates EPO's tissue protection has been identified as consisting of the EPO-R and the β-common (CD131) receptor (Cβ-R). The EPO modified by carbamylation [carbamylated EPO (CEPO)] is reported to signal only through this receptor and not through the homodimeric EPO-R [7]. It has been shown that CEPO does not stimulate erythropoiesis, but that it prevents tissue injury in spinal cord neurons [7, 8] and cardiomyocytes [9, 10]. We reported that CEPO protects the kidneys from ischemia–reperfusion injury [11] and unilateral obstruction injury [12]. Although these observations demonstrated the short-time therapeutic effect of CEPO, long-term effects of CEPO have not been studied. In this study, we examined whether treatment with CEPO could protect the kidney from critical nephron loss and thereby inhibit subsequent tubulointerstitial fibrosis.
Materials and methods
Experimental design
Thirty-five male Sprague-Dawley rats weighing 250–290 g were purchased from Japan SLC Inc. (Shizuoka, Japan) and were maintained under standard conditions until the experiments were done. All studies were performed in accordance with the principles of the Guideline on Animal Experimentation of Osaka University. Rats underwent subtotal (5/6) nephrectomy performed by infarction of approximately two-thirds of the left kidney by selective ligation of two of 3–4 extrarenal branches of the left renal artery, followed by removal of the right kidney 1 week later. One week after nephrectomy, rats were randomly allocated into three groups: (1) the saline-treatment group (saline group; n = 12); (2) the EPO-treatment group (EPO group; n = 11); (3) the CEPO-treatment group (CEPO group; n = 12). Rats received subcutaneous injections of 1 ml of saline, 1000 IU/kg recombinant human EPO (Kirin Corp., Tokyo, Japan), and 1000 IU/kg CEPO [13] once a week for 8 weeks, respectively. The rats were killed at 4 and 8 weeks.
Morphological assessment
Tissue samples were fixed in 4 % (wt/vol) buffered paraformaldehyde (PFA) for 16 h and then embedded in paraffin. Four-μm tissue sections were mounted on silane (2 % 3-aminopropyltriethoxysilane)-coated slides (Muto Pure Chemicals, Tokyo, Japan), deparaffinized with xylene, and stained with PAS and Masson's trichrome. The fibrotic area stained blue by Masson's trichrome staining was calculated. Glomeruli and large vessels were not included in the microscopic fields for image analysis.
Immunohistochemical staining was done using the Envision system (Dako, Hamburg, Germany), according to the manufacturer's instructions. Endogenous peroxidase activities were blocked with 3 % H2O2 for 10 min. The first antibodies were diluted in 1 % bovine serum albumin (BSA) in phosphate-buffered saline (PBS) with 0.1 % Tween-20 (PBS-T) at specific concentrations as described below and then incubated for 16 h at 4 °C. Glomerular and peritubular capillaries were identified with JG12 antibody, raised against aminopeptidase P of microvascular endothelial cells (1:100, Bender MedSystem, San Bruno, CA). To identify myofibroblasts, we used anti-human α-smooth muscle actin (α-SMA) antibody (EPOS System: Dako). This was followed by incubation with suitable secondary antibodies. Antigen retrieval was performed for 10 min in preheated 10 mmol/l sodium citrate (pH 7) using an auto-clave. All incubations were performed in a humidified chamber. Chromogenic color was developed with DAB (Dako). The nuclei were counterstained with hematoxylin. All histological slides were examined by light microscopy using a Nikon Eclipse 80 i (Nikon, Tokyo, Japan); pictures were taken with the Nikon ACT-1 version 2.63. The area of endothelial cells stained with JG12 or α-SMA-positive myofibroblasts relative to the total area of the field was calculated as a percentage by a computer-aided manipulator. To assess the α-SMA-positive area, glomeruli and large vessels were not included in the microscope fields for image analysis. The scores of ten fields per kidney were averaged, and the mean scores for each group were then averaged.
Two-photon microscopy
For intravital multiphoton microscopy, a 10–15-mm lateral incision was made dorsally under sterile conditions. The kidney was exteriorized, and the PE50 tubing (Becton-Dickinson and Company, Sparks, MD, USA) was then inserted into the external jugular vein or femoral vein for dye infusion of 3.75 mg of FITC-conjugated dextran (500 kDa, Molecular Probes, Eugene, OR, USA), which is retained in the vasculature, and 3 mg of cascade blue dextran (10 kDa, Molecular Probes), which is freely filtered by the glomerulus and thereafter internalized into endosomes of the proximal tubule. The left kidney was imaged through a retroperitoneal window via a left-flank incision using a Bio-Rad MRC-1024MP Laser Scanning Confocal/Multiphoton scanner (Bio-Rad, Hercules, CA, USA) with an excitation wavelength of 800 nm attached to a Nikon Diaphot inverted microscope (Fryer, Huntley, IL, USA) as previously described [7]. Image processing was performed using Metamorph software (Universal Imaging, West Chester, PA, USA).
Renal blood flow was measured using a modified line scan method based on evaluation of RBC speed rate. The evaluation was performed in 20 randomly captured cortical fields and 5 randomly captured line scans of horizontal vessels within each field, and both small and large diameter vessels within the field of view were studied. The results were summarized per animal and averaged per field.
Terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL) staining
TUNEL staining was performed using the in situ Apoptosis Detection Kit (Takara Bio, Otsu, Japan), according to the manufacturer's instructions. Briefly, the sections were deparaffinized and treated with antigen retrieval in preheated 10 mmol/l sodium citrate (pH 7) using a steamer for 40 min. They were then incubated with 3 % H2O2 for 10 min, which was followed by incubation with TdT enzyme solution for 90 min at 37 °C. The reaction was terminated by incubation in a stop/wash buffer for 30 min at 37 °C. The number of TUNEL-positive cell nuclei and the total numbers of cell nuclei stained with hematoxylin were counted in ten random areas, and the percentages of the numbers of TUNEL-positive nuclei to the numbers of total cell nuclei were then calculated.
Reverse transcription-polymerase chain reaction
Total RNA was extracted from the cortex of the treated kidney with TRIzol reagent (Invitrogen) according to the manufacturer's directions. Total RNA (1 μg) was reverse-transcribed using a first strand cDNA synthesis kit (GE Healthcare, Buckinghamshire, England) following the manufacturer's instruction. An aliquot (2 μl) of reverse transcription (RT) product was used for polymerase chain reaction (PCR) amplificationin at a total volume of 25 μl. The PCR primer sets used for TGF-β, type I collagen, and β-actin cDNA amplification were as follows : TGF-β sense 5′-GCT CGC TTT GTA CAA CAG CA-3′, antisense 5′-GAG TTC TAC GTG TTG CTC CA-3′; type I collagen sense 5′-GCT GCC TTT TCT GTT CCT TT-3′, antisense 5′-GGA TTT GAA GGT GCT GGG TA-3′; β-actin 5′-AGC CAT GTA CGT AGC CAT CC-3′, antisense 5′-CTC TCA GCT GTG GTG GTG AA-3′. To inactivate contaminating enzyme activities, acc cDNA was heated to 95 °C for 5 min before PCR use. The thermal cycle profile used for TGF-β was 25 cycles of denaturation at 95 °C for 30 s, annealing at 53 °C for 1 min, and extension at 72 °C for 2.5 min. The profile for type I collagen was 25 cycles of denaturation at 95 °C for 45 s, annealing at 57 °C for 45 s, and extension at 72 °C for 2 min. For β-actin, 25 cycles of denaturation at 95 °C for 45 s, annealing at 60 °C for 45 s, and extension at 72 °C for 2 min were carried out. PCR fragments were analyzed by electrophoresis on 2 % agarose gels and stained with SYBR green (Applied Bio-system, Foster, CA).
Western blot analysis
Kidney tissue was homogenized in a radioimmunoprecipitation (RIPA) lysis buffer with phenylmethylsulfonylfluoride (PMSF) solution, sodium orthovanadate solution, and protease inhibitor (Santa Cruz). Homogenates were centrifuged (12000g for 10 min at 4 °C), and the super-natant total protein was measured by the Lowry protein assay (Bio-Rad, Hercules, CA). Total protein lysate (15 μg) containing 1:1 denaturing sample buffer was boiled for 3 min, resolved on 5.0–10.0 % SDS-polyacrylamide gels, and electrophoretically transferred onto an immobilon PVDF membrane (Millipore, Bedford, MA). The filter was blocked with 5 % (wt/vol) nonfat milk or 1 % BSA in 10 mM tris-buffered saline with 0.1 % Tween-20 (TBS-T), followed by overnight incubation at 4 °C with diluted primary antibodies in TBS-T or blocking buffer. After washing five times in TBS-T, the filter was incubated with secondary antibody (1:1000) (cell signaling) in TBS-T for 45 min at room temperature and developed to detect specific protein bands using ECL reagents (GE Healthcare).
Statistical analysis
Data are expressed as the mean ± SD. Statistical significance, defined as p < 0.01 or <0.05, was evaluated using one-way ANOVA with Tukey's post hoc multiple comparison tests.
Results
Effect of EPO or CEPO on erythropoiesis and renal function
The effects of EPO or CEPO on erythropoiesis and renal function in remnant kidney model rats are summarized in Fig. 1. Compared to saline-treatment (12.3 ± 1.6 and 11.9 ± 2.4 g/dl at 4 and 8 weeks, respectively), EPO-treatment significantly increased Hb concentration (15.7 ± 0.6 and 15.9 ± 0.3 g/dl at 4 and 8 weeks, respectively; p < 0.05 vs. saline group). However, CEPO-treatment neither enhanced nor reduced the Hb concentration (13.7 ± 1.0 and 12.4 ± 1.0 g/dl at 4 and 8 weeks, respectively; n.s. vs. saline group, p < 0.05 vs. EPO group), suggesting that CEPO, unlike EPO, does not stimulate erythropoiesis (Fig. 1a).
Fig. 1.
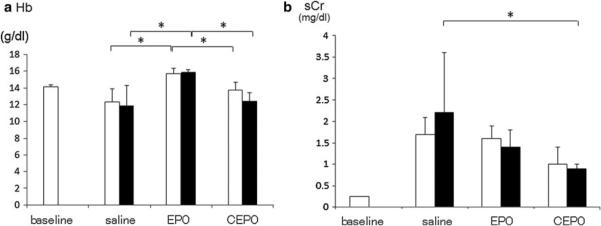
The effects of EPO or CEPO on erythropoiesis and renal function. The effects of EPO or CEPO on erythropoiesis (a) and renal function (b) in remnant kidney model rats were examined 4 weeks (white bar) and 8 weeks (black bar) after treatment with saline, EPO, or CEPO. Baseline means 0 weeks of saline group (*p < 0.05)
Saline-treated remnant kidney model rats exhibited increased serum creatinine levels (1.7 ± 0.4 and 2.2 ± 1.4 mg/dl at 4 and 8 weeks, respectively), while treatment with EPO improved serum creatinine (1.6 ± 0.3 and 1.4 ± 0.4 mg/dl at 4 and 8 weeks, respectively), and CEPO also significantly suppressed the increase of serum creatinine (1.0 ± 0.4 and 0.9 ± 0.1 mg/dl at 4 and 8 weeks, respectively; p < 0.05 vs. saline group at 8 weeks) (Fig. 1b).
Effect on tubular damage
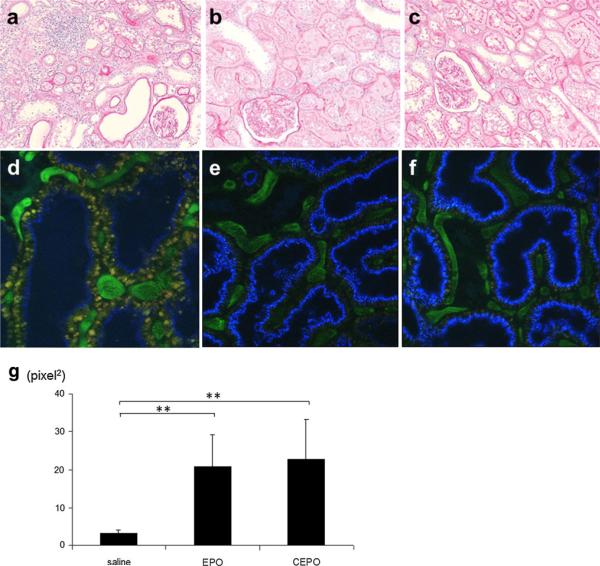
Periodic acid-Schiff (PAS) staining of the kidney sections obtained from remnant kidney treated with saline for 8 weeks revealed that subtotal nephrectomy led to signifi-cant tubular atrophy and dilation, and inflammatory cell infiltration (Fig. 2a). EPO or CEPO treatment alleviated these tubular manifestations (Fig. 2b, c).
Fig. 2.
Representative morphological changes. PAS staining of the kidney sections obtained from remnant kidney treated with saline (a), EPO (b), or CEPO (c) for 8 weeks. Two-photon microscopy showed that saline-treated remnant kidney showed decreased endocytosis (blue) (d), while treatment with EPO (e) or CEPO (f) preserved endocytotic function (magnification ×400). The cascade blue-positive area was calculated as tubular endocytotic function (g) (**p < 0.01)
Tubular damage was examined in vivo using dual-photon confocal microscopy. Injection of 10-kDa dextran (cascade blue) was rapidly filtered by glomeruli and accumulated in endosomes of the proximal tubule, as previously described [14]. Saline-treated remnant kidney showed tubular epithelial damage (assessed by decreased endocytosis) (Fig. 2d), while treatment with EPO (Fig. 2e) or CEPO (Fig. 2f) preserved tubular endocytotic function. Tubular endocytotic function, assessed by the cascade blue-positive area, was significantly increased in EPO-treated (20.9 ± 8.2) or CEPO-treated (22.8 ± 10.4) remnant kidney compared with saline-treated kidney (3.2 ± 0.9) (Fig. 2g).
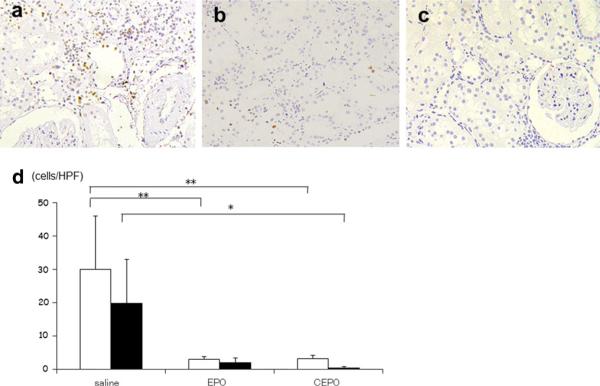
Morphological evidence of apoptosis
To investigate whether EPO or CEPO administration could protect tubular epithelial cells from apoptosis, the apoptotic bodies, labeled as an in situ end-labeled DNA fragment with the TUNEL method (Fig. 3), were examined. In parallel with the tubular damage, tubular apoptosis was persistently increased after nephrectomy (saline group, 30.0 ± 16.1 cells and 19.9 ± 13.2 cells per high power field at 4 and at 8 weeks, respectively), whereas the administration of EPO (3.0 ± 0.9 and 2.1 ± 1.3 cells at 4 and 8 weeks, respectively; p < 0.01 and n.s. vs. saline group, respectively) or CEPO (3.2 ± 0.9 and 0.5 ± 0.4 cells at 4 and 8 weeks, respectively; p < 0.01 and p < 0.05 vs. saline group, respectively) inhibited tubular apoptosis (Fig. 3d).
Fig. 3.
Effects of EPO or CEPO on apoptosis. The effect of EPO or CEPO on tubular apoptosis was examined in remnant kidney after 8-week treatment. The dark brown dots correspond to representative TUNEL-positive nuclei in saline-treated (a), EPO-treated (b), or CEPO-treated (c) remnant kidney (magnification ×400). White bar and black bar indicate the number of TUNEL-positive cells per high power field (HPF) at 4- and 8-week treatment (d) (*p < 0.05; **p < 0.01)
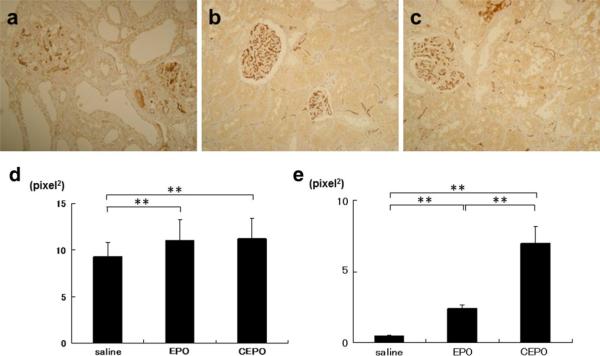
Effects on peritubular capillary damage in renal remnant kidney
The observation that EPO or CEPO treatment markedly suppressed tubular injuries suggests a possibility that protective effects may be mediated by the angiogenic property of EPO or CEPO in the interstitial microvasculature. Subtotal nephrectomy deteriorated the glomerular and peritubular capillary (PTC) endothelial cells stained with JG12 (glomerular and peritubular JG12-positive area; 8.8 ± 1.3 and 0.6 ± 0.08 pixel2, respectively, Fig. 4a, d, e). Compared to saline-treated remnant kidney, EPO treatment prevented the loss of endothelial cells in glomerular and peritubular capillaries (10.8 ± 2.4 and 2.4 ± 0.2 pixel2, p < 0.05 vs. saline group, Fig. 4b, d, e). Furthermore, CEPO treatment significantly increased the glomerular and PTC endothelial cells compared to EPO treatment (11.0 ± 2.4 and 7.2 ± 0.8 pixel2, p < 0.01 vs. saline and EPO group, Fig. 4c–e).
Fig. 4.
Effects of EPO or CEPO on capillary endothelial cells. The effect of EPO or CEPO on PTC endothelial cells was examined in remnant kidney at 8 weeks. PTC endothelial cells stained with JG12 in saline-treated (a), EPO-treated (b), or CEPO-treated (c) remnant kidney (magnification ×400). The area of endothelial cells stained with JG12 was calculated quantitatively using a color image analyzer (d glomerular endothelial cells, e peritubular endothelial cells, *p < 0.05; **p < 0.01)
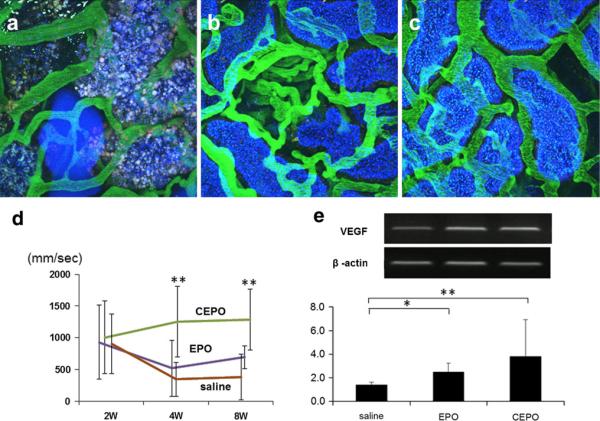
The renal microcirculation was also investigated using two-photon microscopy by injection of fluorescein isothiocyanate (FITC)-conjugated dextran (500 kDa), which was maintained in the vascular space and not filtered by the glomerulus. The microvascular blood flow of the PTC was poor or disrupted at 4 weeks (Fig. 5a). In contrast, EPO-treated (Fig. 5b) or CEPO-treated (Fig. 5c) remnant kidney showed better blood flow that was similar to that of normal kidneys. Furthermore, the blood flow rate of PTC was measured using a variant line-scan method [15]. Quantitative analysis of the blood flow rate showed that the flow rate was significantly faster in CEPO-treated remnant kidney than in saline-treated remnant kidney (Fig. 5d). These observations suggest that CEPO preserved PTC endothelial cells from subtotal nephrectomy.
Fig. 5.
Effects of EPO or CEPO on PTC blood flow. The effect of EPO or CEPO on PTC blood flow was determined by two-photon microscopy at 8 weeks. The renal microcirculation was investigated by injection of FITC-conjugated dextran in saline-treated (a), EPO-treated (b), or CEPO-treated (c) remnant kidney (magnification ×400). Quantitative analysis using the line-scan method showed the blood flow rate of PTC in saline-treated (brown line), EPO-treated (purple line), or CEPO-treated (green line) remnant kidney (d **p < 0.01). RT-PCR demonstrated that EPO or CEPO induced VEGF mRNA compared with saline-treated remnant kidney (e)
Since EPO or CEPO treatment preserved the glomerular and peritubular endothelial structure in remnant kidney, we examined the vascular endothelial growth factor (VEGF) mRNA. Reverse transcription-polymerase chain reaction (RT-PCR) demonstrated that EPO or CEPO produced substantial VEGF mRNA compared with saline-treated remnant kidney (2.49 ± 0.76 and 3.82 ± 3.09 in the EPO and CEPO group, respectively; p < 0.05 vs. 1.40 ± 0.22 in saline group, Fig. 5e).
Effects on interstitial changes
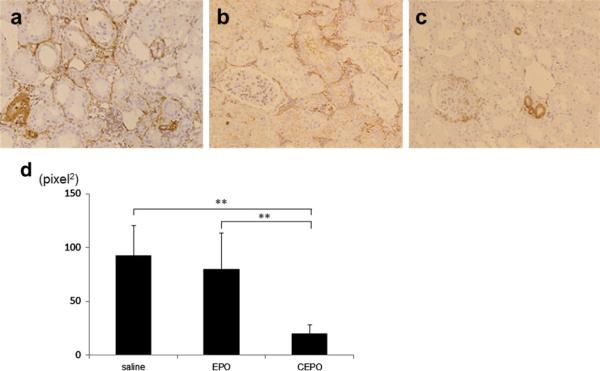
To detect interstitial myofibroblasts, which are associated with interstitial damage and fibrosis, the expression of α-SMA was examined immunohistochemically. The inter-stitial expression of α-SMA increased at 8 weeks in the saline-treated rats (92.8 ± 28.0 pixel2, Fig. 6a), whereas EPO treatment significantly suppressed interstitial expression of α-SMA (79.7 ± 33.9 pixel2, p < 0.01 vs. saline group, Fig. 6b, d). CEPO treatment suppressed interstitial expression of α-SMA even further so that the expression of α-SMA was limited to the blood vessels (20.2 ± 8.1 pixel2, p < 0.01 vs. saline group, Fig. 6c).
Fig. 6.
Effect on phenotypic changes. Interstitial phenotypic changes are assessed by immunohistochemical staining of α-SMA in saline-treated (a), EPO-treated (b), or CEPO-treated (c) remnant kidney at 8 weeks (magnification ×400) (**p < 0.01)
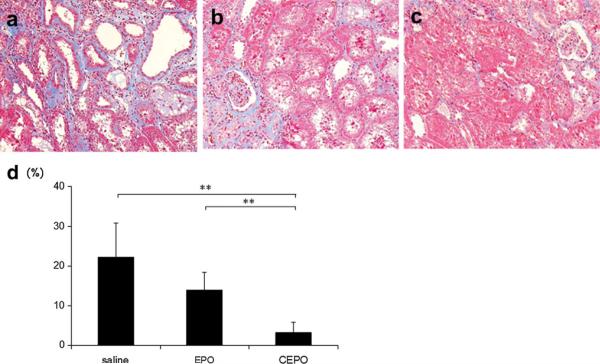
The interstitial fibrotic area at 8 weeks was stained blue on Masson's trichrome staining (Fig. 7), and a color image analyzer estimated the area quantitatively. The saline-treated rats showed progression of interstitial fibrosis (22.3 ± 8.6 %). In parallel with the findings on PAS staining, EPO or CEPO administration significantly suppressed the development of interstitial fibrosis (13.9 ± 4.5 and 3.2 ± 2.6 % in EPO and CEPO group, respectively; p < 0.01 vs. saline group).
Fig. 7.
Effect on interstitial fibrosis. The interstitial fibrotic area was stained blue on Masson's trichrome in saline-treated (a), EPO-treated (b), or CEPO-treated (c) remnant kidney at 8 weeks (magnification ×400). The interstitial fibrotic area was quantitatively assessed using a color image analyzer (d) (**p < 0.01)
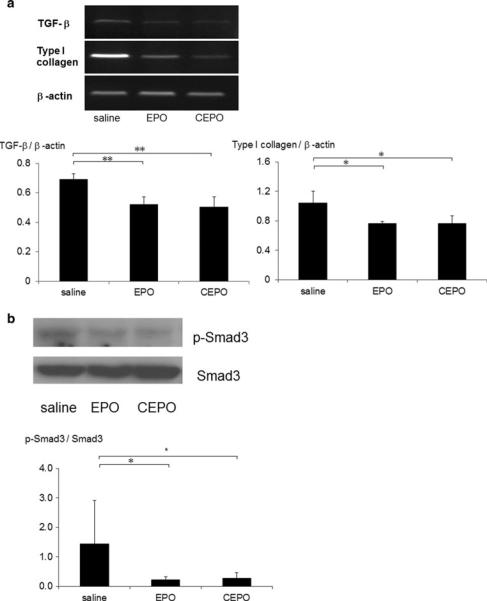
Reverse transcription-polymerase chain reaction demonstrated that renal transforming growth factor-b1 (TGF-β1) and collagen I mRNA were markedly upregulated in saline-treated remnant kidney, whereas EPO or CEPO treatment resulted in substantial inhibition of TGF-β1 and collagen I mRNA (Fig. 8a). Western blot analysis of Smad3 and phosphorylated Smad3 protein also demonstrated that treatment with EPO or CEPO inhibited the phosphorylation of Smad3 compared to saline-treated remnant kidney (Fig. 8b).
Fig. 8.
Anti-fibrotic effect of EPO or CEPO in remnant kidney. To elucidate the intracellular signaling implicated in interstitial fibrosis, we examined the expression of TGF-β1 and collagen I mRNA (a). We also examined Smad3 and phosphorylated Smad3 protein (b). (*p < 0.05; **p < 0.01)
Discussion
In this study, the long-term therapeutic effect of CEPO on a tubulointerstitial model was demonstrated. CEPO suppressed tubular and capillary damage, phenotypic interstitial alteration, and interstitial fibrosis in the remnant kidney model as well as EPO. Earlier study [16] reported that EPO administration to remnant kidney model rats was shown to accelerate the progression of chronic renal disease, especially in relation to the increased blood pressure. Recently, low-dose darbepoetin treatment was reported to confer vascular and tissue protection in remnant kidney without changing the Hb concentration [17]. In the present study, subtotal nephrectomy caused renal dysfunction, whereas high-dose EPO or CEPO administration (1000 IU/kg, weekly) significantly improved renal function. Furthermore, high-dose EPO treatment significantly increased the Hb concentration as previously reported, whereas high-dose CEPO treatment neither enhanced nor reduced the Hb concentration. Considering the adverse effects of excessive erythropoiesis, CEPO seems a useful tool for long-term renoprotection.
Several in vivo studies have shown that EPO can protect kidneys from acute renal injury caused by oxidative stress or nephrotoxic injury by reducing apoptosis or accelerating cell proliferation. The EPO doses used in many of these studies were extremely high [18]. For instance, Parsa et al. [19] used 5000 U/kg body wt of EPO in their preconditioning experiment. The dose corresponds to 350000 U in a 70-kg man. Such a dose is not conceivable clinically. Continuous treatment with high doses of EPO may lead to hypertension and vascular thrombosis, perhaps related to abruptly increased Hb levels. We previously showed that EPO treatment inhibited tubular apoptosis and aSMA expression in obstructed kidney; however, EPO increased Hb concentrations and induced a wedge-shaped infarction [12]. In this study, continuous treatment with high-dose EPO significantly increased the Hb concentration, but CEPO treatment did not. Considering long-term treatment with EPO, a nonerythropoietic derivative of EPO could be a promising therapeutic strategy for tissue protection in renal diseases.
In this study, we examined whether CEPO may have therapeutic effects on tubulointerstitial injury in a remnant kidney model. CEPO treatment protected tubular epithelial cells by preventing apoptosis with concomitant conserved tubular function assessed by endocytosis and cast formation. One possible signal transduction pathway to suppress tubular apoptosis involves the activation of phosphoinositide 3-kinase (PI3K). In fact, we previously reported that CEPO treatment induced marked expression of PI3K at 24 h, which continued until 1 week after ischemia-reperfusion injury [11]. Consequently, phosphorylated Akt was also upregulated in CEPO-treated kidneys. Activation of the Akt pathway is a powerful mechanism to protect cells from ischemic injury. VEGF is also an important Akt pathway stimulator [20]. Our results suggest that CEPO protected the kidney by activating Akt both directly and indirectly by inducing VEGF.
Another mechanism is that CEPO improved the hypoxic status by preserving PTC blood flow. The most well-known consequence of EPO's action is stimulation of erythropoiesis, which potentially enhances tissue protection against ischemic injury by augmenting the O2-carrying capacity of blood. However, it has been reported that the renoprotective benefits of EPO occur prior to, or in the absence of, significant changes in Hb [21, 22]. In addition, CEPO treatment did not increase the Hb concentration, suggesting that the renoprotective effects of CEPO do not depend on erythropoiesis. Some of this benefit may be related to mobilization of endothelial progenitor cells (EPCs) from the bone marrow and subsequent promotion of neovascularization [23]. Increased neovascularization by EPCs has been shown to improve cardiac function after experimental myocardial ischemia and clinical outcomes after myocardial infarction [23]. Recently, EPO was reported to protect the heart after myocardial infarction by inducing angiogenic cytokine expression [24]. We also previously demonstrated that CEPO treatment induced more capillary-like formation than EPO treatment in an in vitro angiogenesis assay including both human umbilical vein endothelial cells (HUVEC) and neonatal normal human dermal fibroblasts (NHDF) [13], suggesting that CEPO could directly induce angiogenesis.
In this study, CEPO showed more renoprotective effects on capillary endothelial cells than EPO; CEPO treatment significantly increased the glomerular and PTC endothelial cells compared to EPO treatment. In addition, the blood flow rate was significantly faster in CEPO-treated remnant kidney. Probably due to the improvement of hypoxic status in tubulointerstitium in CEPO group, CEPO treatment suppressed interstitial expression of α-SMA and the fibrotic area more than EPO treatment. In this study, we demonstrated that CEPO showed a tendency to produce more VEGF mRNA than EPO. We previously demonstrated that CEPO treatment induced more capillary-like formation than EPO treatment in an in vitro angiogenesis assay including both HUVEC and NHDF [13], suggesting that CEPO could directly induce angiogenesis.
In the present study, CEPO administration decreased TGF-β1 and type I collagen mRNA levels in remnant kidneys. Increased expression of TGF-β is a characteristic feature of many human kidney diseases and of the remnant kidney model [25]. CEPO administration prevents epithelial cell death and remodeling of renal tissue with injury or fibrosis, which is completely opposite to the role of TGF-β. Recently, Park et al. [26] showed that EPO treatment inhibits the progression of renal fibrosis in obstructed kidney and attenuates the TGF-β1-induced epithelial-tomesenchymal transition (EMT). It is unclear whether CEPO suppresses TGF-β expression directly or indirectly, but the addition of CEPO results in suppression of TGF-β expression and inhibition of interstitial fibrosis.
In conclusion, CEPO administration reduced tubulointerstitial injury in two ways: protection of tubular epithelial cells and of endothelial cells. Furthermore, high-dose EPO treatment significantly increased the Hb concentration, whereas high-dose CEPO-treatment neither enhanced nor reduced the Hb concentration. These observations suggest the benefit of the therapeutic application of CEPO for patients with chronic progression of tubulointerstitial injury.
Footnotes
Conflict of interest None.
Contributor Information
Ryoichi Imamura, Department of Urology, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
Yoshitaka Isaka, Department of Geriatric Medicine and Nephrology, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
Ruben M. Sandoval, Department of Medicine, Division of Nephrology, Indiana University, 950 West Walnut Street, R2 202, Indianapolis, IN 46202, USA
Naotsugu Ichimaru, Department of Urology, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
Toyofumi Abe, Department of Urology, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
Masayoshi Okumi, Department of Urology, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
Koji Yazawa, Department of Urology, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
Harumi Kitamura, Department of Geriatric Medicine and Nephrology, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
Jyunya Kaimori, Department of Advanced Technology for Transplantation, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
Norio Nonomura, Department of Urology, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
Hiromi Rakugi, Department of Geriatric Medicine and Nephrology, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
Bruce A. Molitoris, Department of Medicine, Division of Nephrology, Indiana University, 950 West Walnut Street, R2 202, Indianapolis, IN 46202, USA
Shiro Takahara, Department of Advanced Technology for Transplantation, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
References
- 1.Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–94. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- 2.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 3.Rossert J, Froissart M, Jacquot C. Anemia management and chronic renal failure progression. Kidney Int Suppl. 2005;99:S76–81. doi: 10.1111/j.1523-1755.2005.09914.x. [DOI] [PubMed] [Google Scholar]
- 4.Brines M, Cerami A. Discovering erythropoietin's extra-hematopoietic functions: biology and clinical promise. Kidney Int. 2006;70:246–50. doi: 10.1038/sj.ki.5001546. [DOI] [PubMed] [Google Scholar]
- 5.Henke M, Laszig R, Rube C, Schafer U, Haase KD, Schilcher B, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–60. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 6.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–98. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 7.Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–42. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 8.Savino C, Pedotti R, Baggi F, Ubiali F, Gallo B, Nava S, et al. Delayed administration of erythropoietin and its non-erythropoietic derivatives ameliorates chronic murine autoimmune encephalomyelitis. J Neuroimmunol. 2006;172:27–37. doi: 10.1016/j.jneuroim.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA. 2004;101:14907–12. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiordaliso F, Chimenti S, Staszewsky L, Bai A, Carlo E, Cuccovillo I, et al. A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2005;102:2046–51. doi: 10.1073/pnas.0409329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imamura R, Isaka Y, Ichimaru N, Takahara S, Okuyama A. Carbamylated erythropoietin protects the kidneys from ischemiareperfusion injury without stimulating erythropoiesis. Biochem Biophys Res Commun. 2007;353:786–92. doi: 10.1016/j.bbrc.2006.12.099. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura H, Isaka Y, Takabatake Y, Imamura R, Suzuki C, Takahara S, et al. Nonerythropoietic derivative of erythropoietin protects against tubulointerstitial injury in a unilateral ureteral obstruction model. Nephrol Dial Transplant. 2008;23:1521–8. doi: 10.1093/ndt/gfm842. [DOI] [PubMed] [Google Scholar]
- 13.Imamura R, Okumi M, Isaka Y, Ichimaru N, Moriyama T, Imai E, et al. Carbamylated erythropoietin improves angiogenesis and protects the kidneys from ischemia-reperfusion injury. Cell Transplant. 2008;17:135–41. doi: 10.3727/000000008783907044. [DOI] [PubMed] [Google Scholar]
- 14.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, et al. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol. 2002;283:C905–16. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- 15.Molitoris BA, Sandoval RM. Intravital multiphoton microscopy of dynamic renal processes. Am J Physiol Renal Physiol. 2005;288:F1084–9. doi: 10.1152/ajprenal.00473.2004. [DOI] [PubMed] [Google Scholar]
- 16.Garcia DL, Anderson S, Rennke HG, Brenner BM. Anemia lessens and its prevention with recombinant human erythropoietin worsens glomerular injury and hypertension in rats with reduced renal mass. Proc Natl Acad Sci USA. 1988;85:6142–6. doi: 10.1073/pnas.85.16.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahlmann FH, Song R, Boehm SM, Mengel M, von Wasielewski R, Lindschau C, et al. Low-dose therapy with the long-acting erythropoietin analogue darbepoetin alpha persistently activates endothelial Akt and attenuates progressive organ failure. Circulation. 2004;110:1006–12. doi: 10.1161/01.CIR.0000139335.04152.F3. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee PK. Pleiotropic renal actions of erythropoietin. Lancet. 2005;365:1890–2. doi: 10.1016/S0140-6736(05)66622-6. [DOI] [PubMed] [Google Scholar]
- 19.Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, et al. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–50. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 21.Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2115–24. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]
- 22.Vesey DA, Cheung C, Pat B, Endre Z, Gobe G, Johnson DW. Erythropoietin protects against ischaemic acute renal injury. Nephrol Dial Transplant. 2004;19:348–55. doi: 10.1093/ndt/gfg547. [DOI] [PubMed] [Google Scholar]
- 23.Bahlmann FH, de Groot K, Haller H, Fliser D. Erythropoietin: is it more than correcting anaemia? Nephrol Dial Transplant. 2004;19:20–2. doi: 10.1093/ndt/gfg455. [DOI] [PubMed] [Google Scholar]
- 24.Ueda K, Takano H, Niitsuma Y, Hasegawa H, Uchiyama R, Oka T, et al. Sonic hedgehog is a critical mediator of erythropoietin-induced cardiac protection in mice. J Clin Invest. 2010;120:2016–29. doi: 10.1172/JCI39896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu LL, Cox A, Roe CJ, Dziadek M, Cooper ME, Gilbert RE. Transforming growth factor beta 1 and renal injury following subtotal nephrectomy in the rat: role of the renin-angiotensin system. Kidney Int. 1997;51:1553–67. doi: 10.1038/ki.1997.214. [DOI] [PubMed] [Google Scholar]
- 26.Park SH, Choi MJ, Song IK, Choi SY, Nam JO, Kim CD, et al. Erythropoietin decreases renal fibrosis in mice with ureteral obstruction: role of inhibiting TGF-beta-induced epithelial-tomesenchymal transition. J Am Soc Nephrol. 2007;18:1497–507. doi: 10.1681/ASN.2005080866. [DOI] [PubMed] [Google Scholar]