Abstract
Accumulating evidence suggests that renal tubulointerstitial fibrosis is a main cause of end-stage renal disease. Clinically, there are no beneficial treatments that can effectively reverse the progressive loss of renal functions. Caffeic acid phenethyl ester is a natural phenolic antifibrotic agent, but rapid decomposition by an esterase leads to its low bioavailability. In this study, we evaluated the effects of KS370G, a caffeic acid phenylethyl amide, on murine renal fibrosis induced by unilateral renal ischemia-reperfusion injury (IRI) and in TGF-β1 stimulated renal tubular epithelial cells (NRK52E and HK-2). In the animal model, renal fibrosis was evaluated at 14 days post-operation. Immediately following the operation, KS370G (10 mg/kg) was administered by oral gavage once a day. Our results show that KS370G markedly attenuates collagen deposition and inhibits an IRI-induced increase of fibronectin, vimentin, α-SMA and TGF-β1 expression and plasma TGF-β1 levels in the mouse kidney. Furthermore, KS370G reverses TGF-β1-induced downregulation of E-cadherin and upregulation of α-SMA and also decreases the expression of fibronectin, collagen I and PAI-1 and inhibits TGF-β1-induced phosphorylation of Smad2/3. These findings show the beneficial effects of KS370G on renal fibrosis in vivo and in vitro with the possible mechanism being the inhibition of the Smad2/3 signaling pathway.
Tubulointersitial fibrosis is a common chronic kidney disease with features characterized by tubular atrophy, myofibroblast accumulation and abnormal extracellular matrix (ECM) deposition1,2,3. Epithelial-mesenchymal transition (EMT) is a process in which renal tubular epithelial cells under pathological conditions can phenotypically convert to fibroblast-like morphology in the tubulointerstitium. This process plays a critical role in the pathogenesis of tubulointerstitial fibrosis4. During the EMT process, a repression of epithelial cell adhesion molecules, like E-cadherin and an increase of mesenchymal cell markers, such as α-smooth muscle actin (α-SMA), are essentials for the structural integrity changes occurring in the renal epithelium5. Previous studies have shown that many growth factors are involved in renal interstitial fibrosis pathogenesis6. TGF-β1 is one of the main growth factors that stimulate both EMT and ECM deposition through activating the downstream Smad signaling pathway7,8. It is well accepted that TGF-β1 mediates fibrosis by activating the phosphorylation of Smad2 and Smad39.
Excessive accumulation of ECM proteins, including collagen and fibronectin, is also a key characteristic on renal fibrosis10. TGF-β1 has been shown to stimulate the synthesis of ECM proteins and inhibit the degradation of collagen11,12. In a unilateral ureteral obstruction (UUO) model, the obstructed kidneys have higher levels of TGF-β1 thus inducing the transcription of genes that cause ECM protein accumulation13,14. In addition, TGF-β1 stimulates ECM proteins accumulation in renal cells by stimulating the expression of protease inhibitors, such as plasminogen activator inhibitor-1 (PAI-1)15,16. PAI-1, a key physiological inhibitor of tissue and urokinase plasminogen activators and is considered to be the most important inhibitor of fibrinolysis16,17. Recent studies show that PAI-1 directly promotes tissue fibrosis through increasing the migration of macrophages, transdifferentiating tubular epithelia, and myofibroblasts18.
There is much evidence indicating that polyphenolic compounds, such as resveratrol, curcumin and caffeic acid phenethyl ester (CAPE), possess anti-inflammatory, anti-oxidative, anti-carcinogenic, anti-thrombotic, and cardiovascular protective activities in various experimental models19,20,21. CAPE is one of the major components of honeybee propolis which exhibits antioxidant, anti-inflammatory and anti-diabetic effects22,23. However, rapid decomposition by esterases leads to CAPE's low bioavailability in vivo24. Caffeic acid phenylethyl amide (KS370G), a caffeamide derivative, induces hypoglycemic effects in diabetic mice and is cardiovascular protective in pressure-overload mice hearts23,24. However, it is not known whether KS370G has protective effects in renal fibrosis.
In this study, we investigated the effects of KS370G on renal fibrosis in mice using the IRI model and in human and non-human renal tubular epithelial cells (HK-2 and NRK52E) stimulated by TGF-β1. Our results reveal that KS370G inhibits renal fibrosis. We suggest that this inhibition is achieved by blocking the TGF-β/Smad signaling pathway.
Results
KS370G ameliorates fibronectin expression, renal interstitial fibrosis and collagen deposition in IRI kidneys
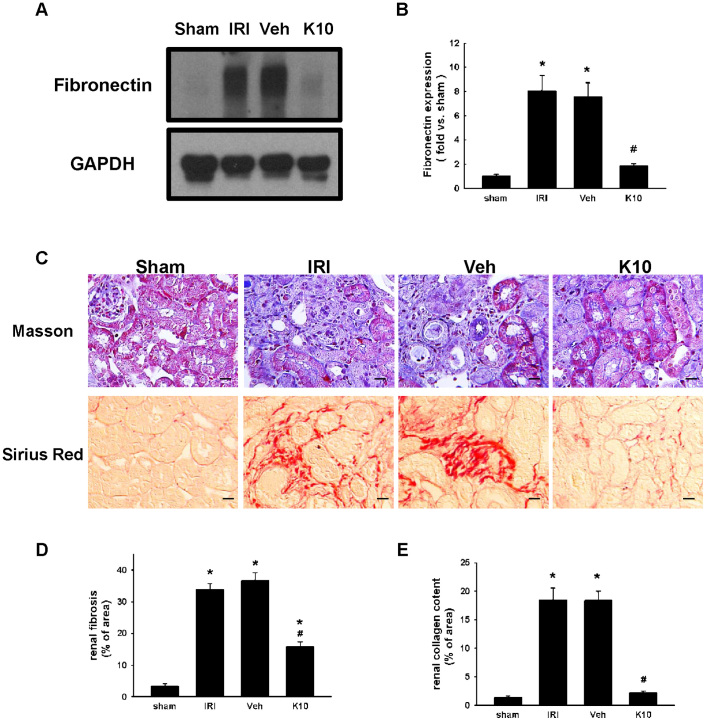
To examine the effect of KS370G on IRI-induced renal fibrosis, fibronectin, a typical marker of fibroblast, and renal interstitial fibrosis and collagen deposition were measured. Western blot analysis shows that fibronectin expression increased in the IRI and Veh groups at day14 after the operation and that KS370G (10 mg/kg once a day) decreased fibronectin expression significantly after the IRI operation (Fig. 1A and 1B). Moreover, both Masson's trichrome staining and Picrosirius Red staining also indicate that renal interstitial fibrosis and collagen deposition were elevated in the IRI and Veh groups and KS370G treatment markedly reduced renal interstitial fibrosis and collagen deposition in IRI kidneys (Fig. 1C–1E).
Figure 1. KS370G regulates the expression of fibronectin and collagen deposition in a murine IRI model.
(A) Western blot analysis of renal fibronectin expression in sham-operated (sham), ischemia-reperfusion injury (IRI), ischemia-reperfusion injury treatment with vehicle (Veh) and ischemia-reperfusion injury treatment with KS370G 10 mg/kg (K10), 14 days after IRI. Vehicle group was treated with RO water. (B) Quantitative results presented as mean ± SEM of the signal's optical density (n = 6 samples each group). (C) Representative images of Masson's trichrome staining and Picrosirius Red staining of renal cortex sections in sham, IRI, Veh and K10 groups. Bar = 50 μm in all panels. (D and E) Quantitative results presented as mean ± SEM of the percentage of renal fibrosis area and collagen content. *P < 0.001 compared with sham group. #P < 0.001 compared with IRI and Veh groups. Original magnification × 200.
KS370G inhibits α-SMA and vimentin protein expression in IRI kidneys
Next, we determined the effect of KS370G on the expression of myofibroblast activation markers, including α-SMA and vimentin. Western blot analysis shows that the expression of α-SMA and vimentin markedly increased in the IRI and Veh groups compared with sham group, suggesting that activation of myofibroblasts is stimulated following an IRI-induced injury. However, treatment with KS370G significantly decreases α-SMA and vimentin protein expression after the IRI operation (Fig. 2).
Figure 2. KS370G regulates the expression of α-SMA and vimentin in a murine IRI model.
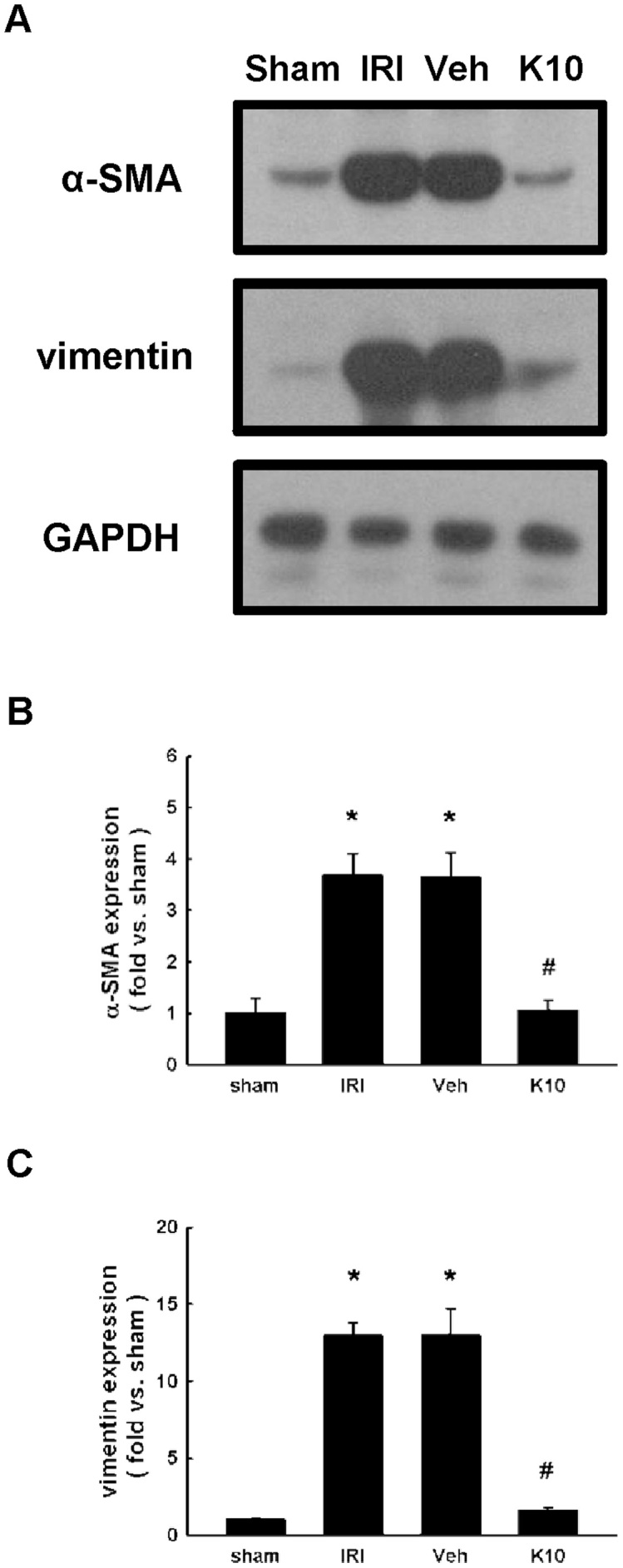
(A) Western blot analysis of renal α-SMA and vimentin expression in sham-operated (sham), ischemia-reperfusion injury (IRI), ischemia-reperfusion injury treatment with vehicle (Veh) and ischemia-reperfusion injury treatment with KS370G 10 mg/kg (K10), 14 days after IRI. Vehicle group was treated with RO water. (B and C) Quantitative results presented as mean ± SEM of the signal's optical density (n = 6 samples each group). *P < 0.005 compared with sham group. #P < 0.005 compared with IRI and Veh groups.
KS370G reduces kidney tissue TGF-β1 protein expression and plasma TGF-β1 levels in IRI kidneys
Compared with the sham group, IRI and Veh groups increased the TGF-β1 protein expression after the IRI operation. Treatment with KS370G significantly reduced TGF-β1 protein expression (Fig. 3A and 3B). Similarly, ELISA results also indicate that plasma TGF-β1 levels were increased in IRI and Veh groups compared with the sham group. Treatment with KS370G markedly decreased plasma TGF-β1 levels after the IRI operation (Fig. 3C).
Figure 3. KS370G regulates the expression of TGF-β1 and plasma TGF-β1 levels in a murine IRI model.
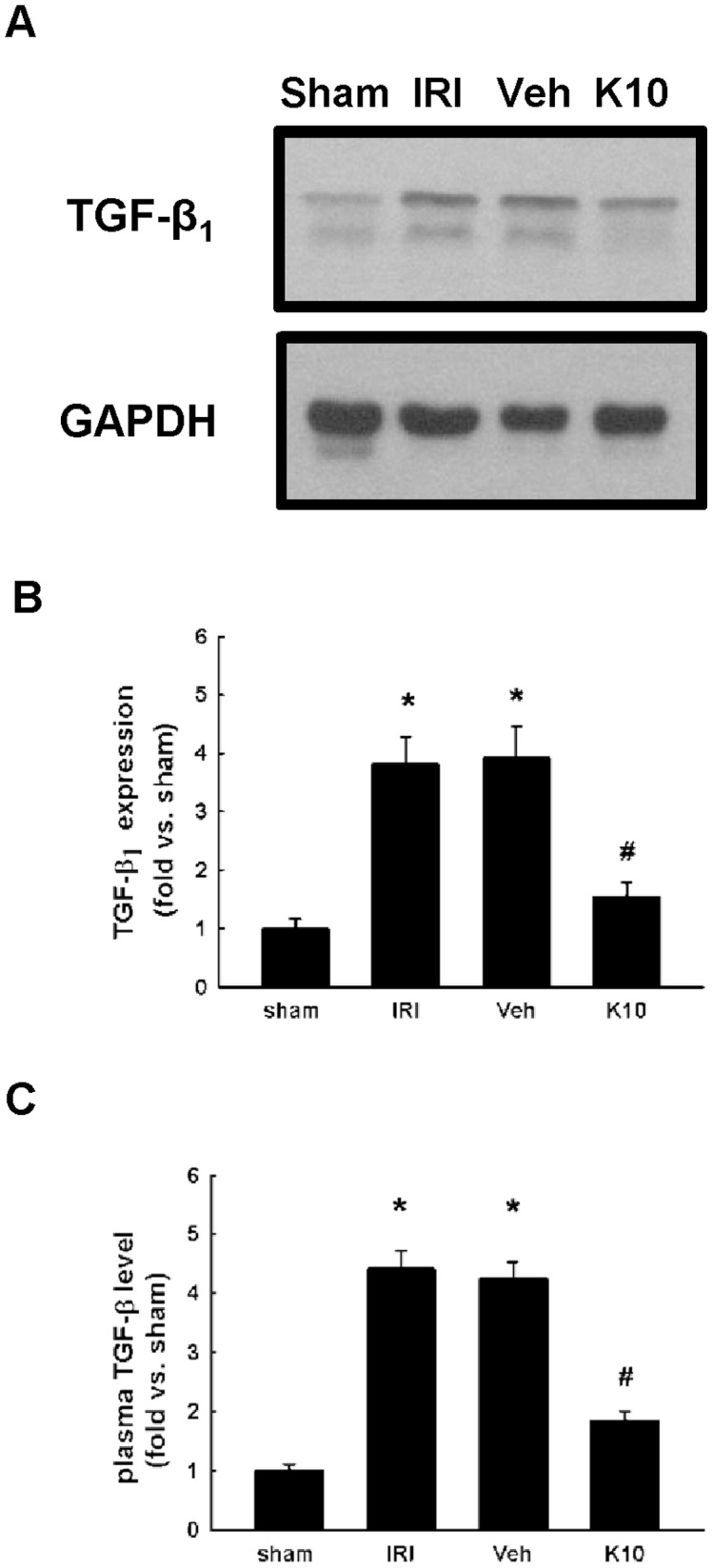
(A) Western blot analysis of renal TGF-β1 expression in sham-operated (sham), ischemia-reperfusion injury (IRI), ischemia-reperfusion injury with vehicle (Veh) or KS370G 10 mg/kg (K10) treatment groups. Vehicle group was treated with RO water. (B) Quantitative results presented as mean ± SEM of the signal's optical density (n = 6 samples each group). *P < 0.01 compared with sham group. #P < 0.01 compared with IRI and Veh groups. (C) ELISA assay analysis of plasma TGF-β1 levels in sham, IRI, Veh and K10 groups. *P < 0.05 compared with sham group. #P < 0.05 compared with IRI and Veh groups.
KS370G inhibits TGF-β1-stimulated EMT in NRK52E and HK-2 cells
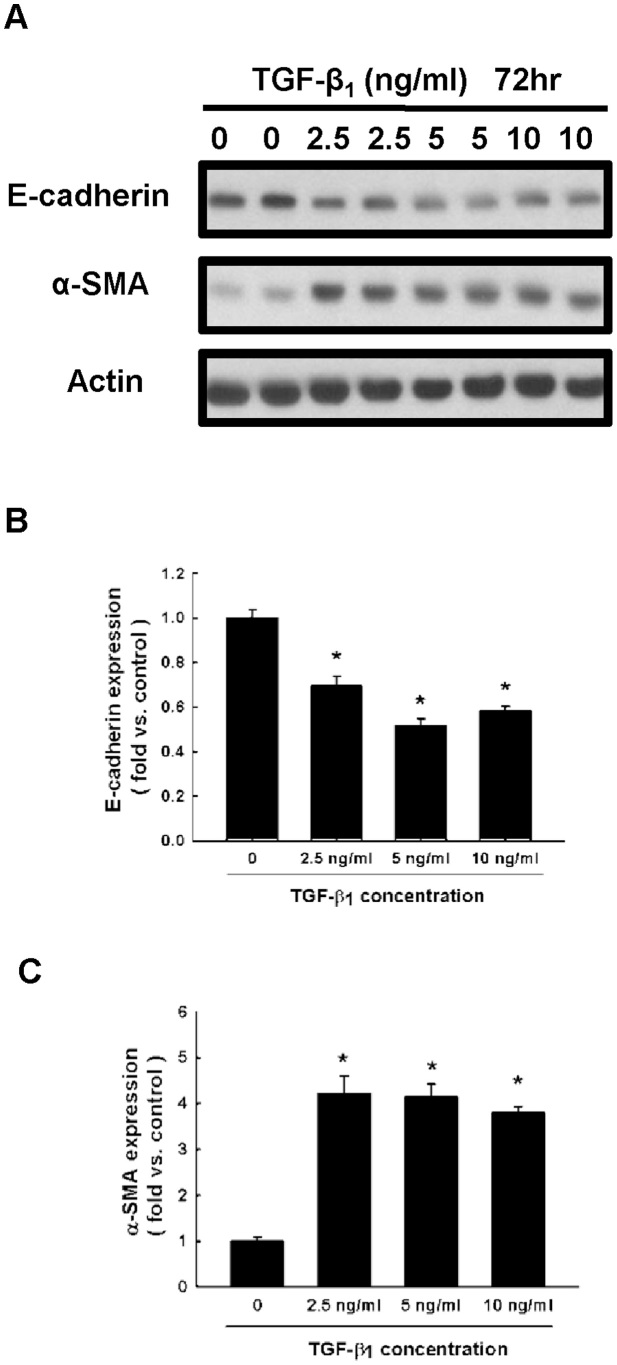
We first evaluated the suitable dose of TGF-β1 needed to induce the process of EMT in NRK52E cells. NRK52E cells were treated with different concentrations of TGF-β1 (0, 2.5, 5 and 10 ng/ml) for 72 h. The expression of two well-known markers of EMT, E-cadherin and α-SMA, were analyzed in NRK52E cells. Western blot analysis shows that the protein level of E-cadherin was downregulated and α-SMA levels were upregulated in TGF-β1 2.5 ng/ml treated cells, reaching a maximal effect in TGF-β1 5 ng/ml treated cells (Fig. 4). We therefore used 5 ng/ml of TGF-β1 in NRK52E and HK-2 cells for 72 h in subsequent experiments.
Figure 4. TGF-β1 stimulates the expression of E-cadherin and α-SMA in NRK52E cells.
(A) E-cadherin and α-SMA expression were determined by western blot of NRK52E cells cultured for 72 h in different concentration of TGF-β1. (B and C) Quantitative results presented as mean ± SEM of the signal's optical density for E-cadherin (B; n = 5) and α-SMA (C; n = 5). *P < 0.05 compared with control group.
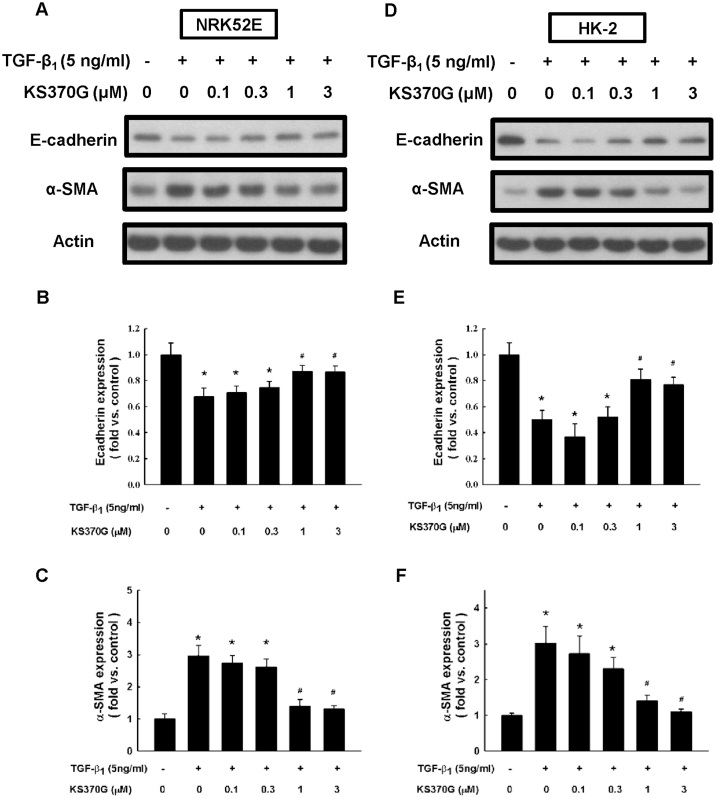
Next, the effect of KS370G in preventing TGF-β1-stimulated EMT in NRK52E and HK-2 cells were examined. Western blot analysis shows that treatment with TGF-β1 (5 ng/ml) in NRK52E cells for 72 h led to a marked decrease in E-cadherin expression and an increase in α-SMA expression. KS370G significantly prevented TGF-β1 stimulated changes of the E-cadherin and α-SMA expression in NRK52E cells at concentrations ranging from 1 to 3 μM (Fig. 5). Similar results were also obtained in HK-2 cells (Fig. 5). These results suggest that KS370G prevents the loss of the epithelial marker E-cadherin and the de novo expression of myofibroblast marker α-SMA in both human and non-human renal epithelial cells stimulated by TGF-β1.
Figure 5. KS370G regulates the expression of E-cadherin and α-SMA in NRK52E and HK-2 cells induced by TGF-β1.
(A and D) E-cadherin and α-SMA expression were determined by western blot of NRK52E and HK-2 cells cultured with different concentration of KS370G (0.1 to 3 μM) for 72 h under TGF-β1 stimulation. (B,C,E and F) Quantitative results presented as mean ± SEM of the signal's optical density for E-cadherin (B; n = 7) and α-SMA (C; n = 5) in NRK52E cells and E-cadherin (E; n = 3) and α-SMA (F; n = 3) in HK-2 cells. *P < 0.05 compared with control group. #P < 0.05 compared with TGF-β1 (5 ng/ml) groups.
KS370G ameliorates TGF-β1-stimulated fibronectin and type I collagen expression in NRK52E and HK-2 cells
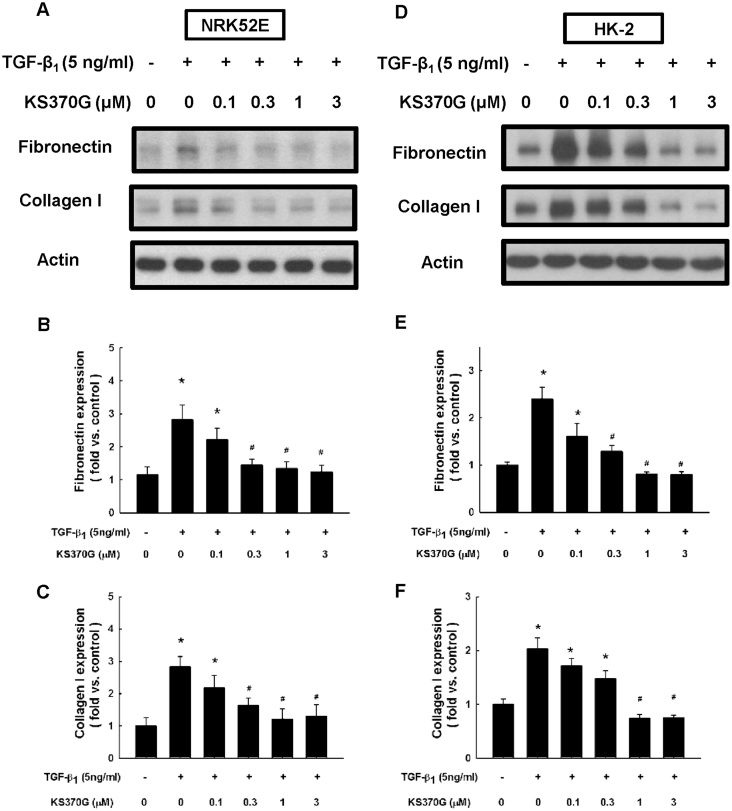
The ability of KS370G to decrease ECM proteins accumulation in NRK52E and HK-2 cells was examined. Western blot analysis shows that both fibronectin and type I collagen expression were significantly increased after TGF-β1 treatment for 72 h. By contrast, KS370G attenuated fibronectin and type I collagen expression in a dose-dependent manner, especially at concentrations ranging from 0.3 to 3 μM in NRK52E cells and 1 to 3 μM in HK-2 cells (Fig. 6).
Figure 6. KS370G regulates the expression of fibronectin and collagen I in NRK52E and HK-2 cells induced by TGF-β1.
(A) Fibronectin and type I collagen expression were determined by western blotting of NRK52E and HK-2 cells cultured with different concentration of KS370G (0.1 to 3 μM) for 72 h under TGF-β1 stimulation. (B,C,E and F) Quantitative results presented as mean ± SEM of the signal's optical density for fibronectin (B; n = 5) and type I collagen (C; n = 5) in NRK52E cells and fibronectin (E; n = 3) and type I collagen (F; n = 3) in HK-2 cells. *P < 0.05 compared with control group. #P < 0.05 compared with TGF-β1 (5 ng/ml) groups.
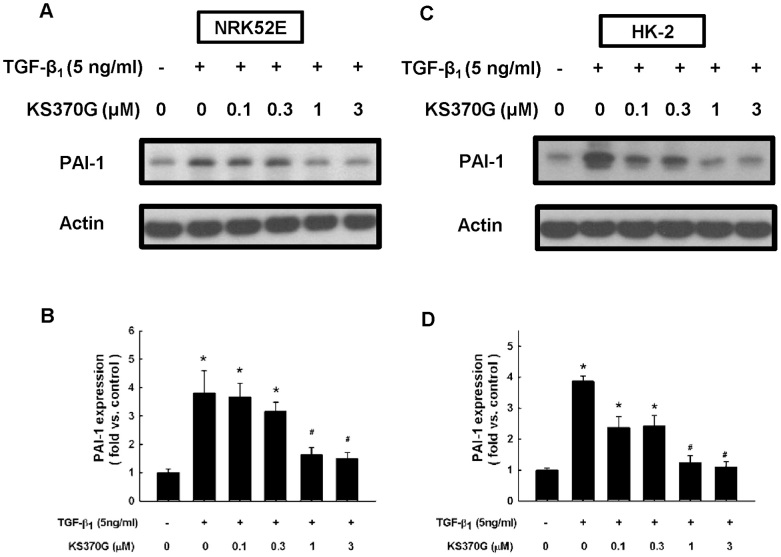
KS370G attenuates TGF-β1-stimulated PAI-1 expression in NRK52E and HK-2 cells
Western blot analysis indicates that PAI-1 expression was markedly elevated after TGF-β1 stimulation for 72 h. KS370G significantly reduced TGF-β1-induced PAI-1 expression in both NRK52E and HK-2 cells at concentrations ranging from 1 to 3 μM (Fig. 7).
Figure 7. KS370G reduces the expression of PAI-1 in NRK52E and HK-2 cells induced by TGF-β1.
(A and C) PAI-1 expression were determined by western blotting of NRK52E and HK-2 cells cultured with different concentration of KS370G (0.1 to 3 μM) for 72 h under TGF-β1 stimulation. (B and D) Quantitative results presented as mean ± SEM of the signal's optical density in NRK52E cells (B; n = 5) and in HK-2 cells (D; n = 3). *P < 0.05 compared with control group. #P < 0.05 compared with TGF-β1 (5 ng/ml) groups.
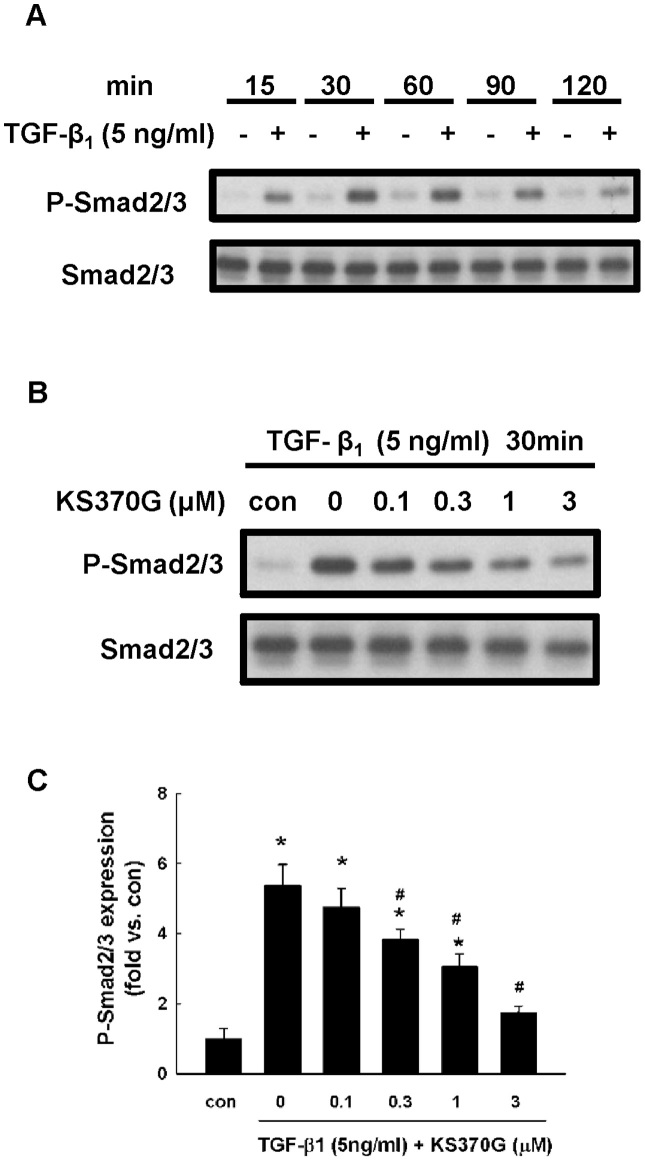
KS370G blocks TGF-β1-stimulated phosphorylation of Smad2/3 in NRK52E cells
Western blot analysis shows that TGF-β1 triggered the phosphorylation of Smad2/3 in NRK52E cells at the first 15 minutes of incubation and reached peak expression at 30 minutes. It then gradually decreased after prolonged TGF-β1 stimulation (Fig. 8A). We chose 30 minutes to be the time point to investigate the regulatory role of KS370G on TGF-β1-induced Smad2/3 phosphorylation. KS370G inhibited the phosphorylation of Smad2/3 in a dose-dependent manner. Concentrations higher than 0.3 μM significantly blocked Smad2/3 phosphorylation protein expression (Fig. 8B and 8C).
Figure 8. KS370G inhibits the phosphorylation of Smad2/3 in NRK52E cells induced by TGF-β1.
(A) Smad2/3 phosphorylation was determined by western blotting of NRK52E cells stimulated by TGF-β1 (5 ng/ml) in a time course experiment. (B) Smad2/3 phosphorylation was determined by western blotting of NRK52E cells cultured with different concentration of KS370G (0.1 to 3 μM) for 30 minutes under TGF-β1 stimulation. (C) Quantitative results presented as mean ± SEM of the signal's optical density (n = 5). *P < 0.05 compared with control group. #P < 0.05 compared with TGF-β1 (5 ng/ml) groups.
Discussion
This study was undertaken to address whether KS370G attenuates renal interstitial fibrosis in vivo and in vitro and to investigate the underlying mechanisms. Here, we show that IRI injury significantly induces the expression of fibronectin and collagen deposition, promotes myofibroblast activation and elevates plasma levels of TGF-β1 and renal TGF-β1 protein expression. Exposure to TGF-β1 for 72 h in NRK52E and HK-2 cells induce a downregulation of E-cadherin and an upregulation of α-SMA. TGF-β1 also increases ECM protein levels and PAI-1 expression in NRK52E and HK-2 cells. However, KS370G significantly reverses all of above changes in vivo and in vitro with the possible mechanism being through inhibiting the TGF-β1/Smad2/3 signaling pathway.
TGF-β1 and its downstream signaling pathway were shown to play a critical role in activating cellular pathological mechanisms in renal tubulointerstitial fibrosis through the induction of interstitial cell activation and the expression of several pro-fibrotic genes25. After ligand binding, the TGF-β1 receptor, a transmembrane Ser/Thr kinase receptor, interacts with receptor-regulated Smads, such as Smad2/3. Phosphorylated Smads enter the nucleus, where they propagate TGF-β1 signaling and regulate the promoter activities of TGF-β1 target genes26. Previous studies have examined the blockade of TGF-β1 signaling as a means to attenuate renal fibrosis27. Our results demonstrate that KS370G reduces TGF-β1 induction and plasma TGF-β1 levels in the IRI kidney. In addition, KS370G inhibits downstream Smad2/3 phosphorylation in NRK52E cells. The exact mechanism for the suppression effects of KS370G on renal TGF-β1 production in the IRI mice model needs to be further elucidated.
Renal tubulointerstitial fibrosis is the final consequence of chronic kidney disease which leads to the destruction of the kidney's parenchyma and end-stage renal failure28,29. Renal fibrosis is associated with tubular epithelial cells transition to mesenchymal cells via a process known as EMT30. EMT is an important process in the pathogenesis of tubulointerstitial fibrosis and involves a loss of epithelial cell characteristics and an increase of mesenchymal cell markers stimulated by various profibrotic cytokines31. Therefore, blocking renal EMT may prevent renal fibrosis. TGF-β1 is a well-known profibrotic cytokine in several renal diseases and plays a critical role in the renal EMT process2. In this study, we used an IRI mice model and both human (HK-2) and non-human (NRK52E) renal epithelial cells stimulated by TGF-β1 to examine the effects of KS370G on myofibroblast activation in vivo and renal EMT in vitro. We found that KS370G reduces upregulation of α-SMA and vimentin in the IRI kidney. KS370G also decreases α-SMA expression and increases E-cadherin expression in HK-2 and NRK52E cells stimulated by TGF-β1. According to these results, we suggest that KS370G prevents renal fibrosis by inhibiting myofibroblast activation in vivo and TGF-β1-mediated renal EMT in vitro.
The abnormal ECM production in renal fibrosis is not only related to the overexpression of normal ECM, such as fibronectin, but also due to an accumulation of pathological ECM components, such as type I collagen32. These proteins are involved in the renal scarring process and are irreversibly deposited in renal fibrotic tissues25. Increasing evidence indicates that TGF-β1 expression is induced in human and animal renal fibrosis models and TGF-β1 expression has been seen as the main mediator in ECM protein accumulation in renal interstitial fibrosis and diabetic nephropathy33,34. Our results show that renal fibronectin expression and collagen deposition are elevated in kidneys from IRI mice in vivo and that type I collagen and fibronectin levels increase in TGF-β1-stimulated cells in vitro. KS370G treatment beneficially attenuates ECM deposition both in vivo and in vitro. Normally, the ECM is continuously degraded. The pathogenic accumulation of ECM may also result from a loss in ECM degradation32. PAI-1, a main inhibitor of plasmin generation, inhibits ECM degradation and stimulates its accumulation, thereby contributing to renal fibrotic disease35,36. PAI-1 is also a prominent downstream target of the TGF-β1/Smad signaling pathway and is considered to be a contributor to fibrogenesis in several organs37. It has been demonstrated that activation of TGF-β1 signaling triggers a dramatic induction of Smad2/3 phosphorylation and PAI-1 protein expression in the obstructive kidney38. PAI-1 deficiency ameliorates the fibrotic injury in a UUO model36. A previous study also indicates that PAI-1 mRNA is also upregulated in NRK52E cells treated with TGF-β116. In this study, we have shown in HK-2 and NRK52E cells that KS370G treatment successfully inhibits TGF-β1-stimulated target gene expression, including matrix proteins and PAI-1. Our combined results suggest that KS370G attenuates renal interstitial fibrosis through both reducing ECM synthesis and elevating ECM degradation.
In conclusion, our study demonstrates that KS370G attenuates renal injury in an IRI animal model, preventing myofibroblast activation, ECM deposition and renal interstitial fibrosis. KS370G also inhibits renal EMT and ECM protein expression in NRK52E and HK-2 cells induced by TGF-β1. The possible mechanism involves the suppression of the TGF-β1/Smad2/3 pathway and the subsequent inhibition of PAI-1 expression.
Methods
Animals and experimental design
The investigation was performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85–23, revised 1996), and was approved by the Institutional Animal Care and Use Committee of the National Taiwan University. 7-week-old male ICR mice (BioLasco Taiwan Co., Ltd) were housed at National Taiwan University College of Medicine Experimental Animal Center, maintained in a temperature- and humidity-controlled (22 ± 1°C and 60 ± 5%) environment with a 12 h light-dark cycle and given free access to food and water. After 1 week of acclimatization, mice were randomly allocated into 4 groups: (1) sham-operation group (sham); (2) IRI-operation group (IRI); (3) IRI group with oral gavage of vehicle once a day (Veh) and (4) IRI group with oral gavage of KS370G 10 mg/kg once a day (K10).
To establish the unilateral IRI model, the mice were anesthetized with sodium pentobarbital (80 mg/kg intraperitoneal). The left renal artery and vein were identified through dorsal incisions and clamped for 30 minutes to stop renal blood flow. Reperfusion was visually confirmed upon releasing the clamps before wound closing. Sham animals were subjected to the same surgical procedure except the left renal artery and vein were not clamped.
KS370G (10 mg/kg) and vehicle (RO water) were administered from the day after the operation for 13 days. All animals were sacrificed on day 14 after IRI or sham operation. The kidneys were then rapidly removed, rinsed in ice-cold 0.9% NaCl solution. Blood samples were centrifuged at 10000 rpm for 5 minutes and the plasma was stored at −80°C until assaying.
Cell culture
NRK52E and HK-2 cells were purchased from American Type Culture Collection (ATCC). NRK52E cells were grown in DMEM supplemented with 5% FBS and HK-2 cells were grown in DMEM/F12 medium, supplemented with 10% FBS in a humidified incubator at 37°C in an atmosphere of 5% CO2 and 95% air. NRK52E and HK-2 cells were incubated into 6-well plates at a density of 5 × 104 cells for 24 h and then divided into the following six treatment groups: control, TGF-β1 5 ng/ml, TGF-β1 5 ng/ml + KS370G 0.1 μM, TGF-β1 5 ng/ml + KS370G 0.3 μM, TGF-β1 5 ng/ml + KS370G 1 μM and TGF-β1 5 ng/ml + KS370G 3 μM. After another 72 h, cells were harvested and processed for western blot analysis.
Chemicals
KS370G was obtained from Professor Kuo's lab and was synthesized using an amide binding coupling method as previously described23. Briefly, benzotriazol-1-yloxy-tris (dimethylamino) phosphonium hexafluorophosphate (BOP) (1.2 eq) in dichloromethane (CH2Cl2) (5 mL) was added to a mixture of caffeic acid (100 mg). To this solution, R-NH2 (1.2 eq) and triethylamine (Et3N) (0.08 mL) in dimethylformamide (DMF) (1.0 mL) were were added. The mixture was stirred at 0°C for 30 min and then stirred at room temperature for 12 h. This reaction mixture was evaporated in vacuo, and the residue was partitioned between ethyl acetate (AcOEt) and H2O. Successive washings of the AcOEt layer with 3N aqueous HCl and 10% NaHCO3 (aq) were performed. The residue was dried over MgSO4 and concentrated in vacuo. The residue was further purified by column chromatography with an eluting solution (CH2Cl2–AcOEt 1:1, v/v) on silica gel (70–230 and 230–400 mesh, Merck 7734). The final product (82–88% yield) was recrystallized from AcOEt to obtain pure crystals. 1H and 13C NMR spectra were recorded on a Bruker Avance 500 spectrometer. Electron impact mass spectrometries (EIMS) were determined on a Finnigan TSQ-46C mass spectrometer. IR spectra were recorded on a Nicolet Magna-IR 550 spectrophotometer.
Histological analysis
Kidney sections were immersion-fixed in 10% buffered formalin. Sections were embedded in paraffin, sliced into 4 μm thick sections and mounted on glass slides. Deparaffinized and rehydrated sections were stained with Masson's trichrome or Picrosirius Red to investigate the level of renal fibrosis and the content of collagen in vivo. Tissue sections were examined using a microscope and photographed with a digital camera.
Plasma TGF-β enzyme-linked immunosorbent assay (ELISA)
Plasma level of TGF-β1 was measured using ELISA commercial kits (R&D systems, Inc., Minneapolis, MN, USA) according to the manufacturer's instruction.
Western blot analysis
The protein expression in kidney tissue and two renal tubular epithelial cell lines were analyzed by western blotting. Equal amounts of protein samples were loaded on sodium dodecyl sulfate-polyacrylamide (SDS) gels for electrophoresis and then transferred to polyvinylidene difluoride (PVDF) membranes and blotted with fibronectin (Cell Signaling, USA), α-SMA (abcam, UK), vimentin (Genscript, USA), E-cadherin (BD Biosciences, Canada), p-Smad2/3 (Cell Signaling, USA), Smad2/3 (Cell Signaling, USA), PAI-1 (Cell Signaling, USA), Collagen I (Santa Cruz, USA), β-actin (Santa Cruz, USA) and GAPDH (Santa Cruz, USA) primary antibodies, followed by the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies. The proteins were detected using western lightning series HRP chemiluminescent substrates and captured on light-sensitive X-ray film.
Statistical analysis
All data are shown as the mean ± S.E.M. Statistical analysis was performed by one-way analysis of variance (ANOVA) using Dunnett's post-hoc test. P < 0.05 is regarded as statistically significant.
Author Contributions
S.T.C. and M.J.S. conceived and designed the experiments; Y.H.K. synthesized the compound; S.T.C. performed the experiments; S.T.C., Y.H.K. and M.J.S. analyzed data; S.T.C. and M.J.S. wrote the manuscript; M.J.S. contributed reagents and materials, and all authors reviewed the manuscript.
Acknowledgments
This study was supported by National Science Council grant No. 100-2323-B-002-066 and grant No. 101-2325-B-002-070.
References
- Zhou D., Tan R., Zhou L., Li Y. & Liu Y. Kidney tubular β-catenin signaling controls interstitial fibroblast fate via epithelial-mesenchymal communication. Sci Rep 3, 1878 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. et al. Norcantharidin inhibits renal interstitial fibrosis by blocking the tubular epithelial-mesenchymal transition. PloS one 8, e66356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. S. et al. Polyphenols of Hibiscus sabdariffa improved diabetic nephropathy via attenuating renal epithelial mesenchymal transition. J Agric Food Chem 61, 7545–7551 (2013). [DOI] [PubMed] [Google Scholar]
- Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21, 212–222 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan H., Fadillioglu E., Yagmurca M., Ucar M. & Irmak M. K. Protein oxidation and lipid peroxidation after renal ischemia-reperfusion injury: protective effects of erdosteine and N-acetylcysteine. Urol Res 34, 41–46 (2006). [DOI] [PubMed] [Google Scholar]
- Hung T. J. et al. 20-Hydroxyecdysone attenuates TGF-beta1-induced renal cellular fibrosis in proximal tubule cells. J Diabetes Complications 26, 463–469 (2012). [DOI] [PubMed] [Google Scholar]
- Chen H. Y. et al. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes 60, 590–601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manotham K. et al. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int 65, 871–880 (2004). [DOI] [PubMed] [Google Scholar]
- Lopez-Hernandez F. J. & Lopez-Novoa J. M. Role of TGF-beta in chronic kidney disease: an integration of tubular, glomerular and vascular effects. Cell Tissue Res 347, 141–154 (2012). [DOI] [PubMed] [Google Scholar]
- Zhou T. B., Qin Y. H., Lei F. Y., Huang W. F. & Drummen G. P. Prohibitin attenuates oxidative stress and extracellular matrix accumulation in renal interstitial fibrosis disease. PloS one 8, e77187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Jim B. & Ziyadeh F. N. Diabetic nephropathy and transforming growth factor-beta: transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol 23, 532–543 (2003). [DOI] [PubMed] [Google Scholar]
- Verrecchia F. & Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol 118, 211–215 (2002). [DOI] [PubMed] [Google Scholar]
- Klahr S. & Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283, F861–F875 (2002). [DOI] [PubMed] [Google Scholar]
- Chevalier R. L., Forbes M. S. & Thornhill B. A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75, 1145–1152 (2009). [DOI] [PubMed] [Google Scholar]
- Krag S., Danielsen C. C., Carmeliet P., Nyengaard J. & Wogensen L. Plasminogen activator inhibitor-1 gene deficiency attenuates TGF-beta1-induced kidney disease. Kidney Int 68, 2651–2666 (2005). [DOI] [PubMed] [Google Scholar]
- Jung G. S. et al. Clusterin attenuates the development of renal fibrosis. J Am Soc Nephrol 23, 73–85 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder B. R. et al. Plasminogen activator inhibitor 1: physiological and pathophysiological roles. News Physiol Sci 17, 56–61 (2002). [DOI] [PubMed] [Google Scholar]
- Matsuo S. et al. Multifunctionality of PAI-1 in fibrogenesis: evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int 67, 2221–2238 (2005). [DOI] [PubMed] [Google Scholar]
- Chen K. H. et al. Resveratrol ameliorates early diabetic nephropathy associated with suppression of augmented TGF-beta/smad and ERK1/2 signaling in streptozotocin-induced diabetic rats. Chem Biol Interact 190, 45–53 (2011). [DOI] [PubMed] [Google Scholar]
- Rah D. K. et al. Protection of rabbit kidney from ischemia/reperfusion injury by green tea polyphenol pretreatment. Arch Pharm Res 30, 1447–1454 (2007). [DOI] [PubMed] [Google Scholar]
- Giovannini L. et al. Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. J Cardiovasc Pharmacol 37, 262–270 (2001). [DOI] [PubMed] [Google Scholar]
- Lu D. Y. et al. Anti-neuroinflammatory effect of a novel caffeamide derivative, KS370G, in microglial cells. Mol Neurobiol 48, 863–874 (2013). [DOI] [PubMed] [Google Scholar]
- Weng Y. C. et al. KS370G, a synthetic caffeamide derivative, improves left ventricular hypertrophy and function in pressure-overload mice heart. Eur J Pharmacol 684, 108–115 (2012). [DOI] [PubMed] [Google Scholar]
- Weng Y. C. et al. Caffeic Acid Phenylethyl Amide Protects against the Metabolic Consequences in Diabetes Mellitus Induced by Diet and Streptozocin. Evid Based Complement Alternat Med 2012, 984780 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C. K. et al. Honokiol ameliorates renal fibrosis by inhibiting extracellular matrix and pro-inflammatory factors in vivo and in vitro. Br J Pharmacol 163, 586–597 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Zhang Y. & Feng X. H. Smads: transcriptional activators of TGF-beta responses. Cell 98, 737–740 (1998). [DOI] [PubMed] [Google Scholar]
- Hwang M. et al. TGF-beta1 siRNA suppresses the tubulointerstitial fibrosis in the kidney of ureteral obstruction. Exp Mol Pathol 81, 48–54 (2006). [DOI] [PubMed] [Google Scholar]
- Jung K. J., Kim J., Park Y. K., Yoon Y. R. & Park K. M. Wen-pi-tang-Hab-Wu-ling-san reduces ureteral obstructive renal fibrosis by the reduction of oxidative stress, inflammation, and TGF-beta/Smad2/3 signaling. Food Chem Toxicol 48, 522–529 (2010). [DOI] [PubMed] [Google Scholar]
- Zeng J., Dou Y., Guo J., Wu X. & Dai Y. Paeoniflorin of Paeonia lactiflora prevents renal interstitial fibrosis induced by unilateral ureteral obstruction in mice. Phytomedicine 20, 753–759 (2013). [DOI] [PubMed] [Google Scholar]
- Iwano M. et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110, 341–350 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson E. G. Mechanisms of disease: Fibroblasts--a new look at an old problem. Nat Clin Pract Nephrol 2, 101–108 (2006). [DOI] [PubMed] [Google Scholar]
- Fogo A. B. Mesangial matrix modulation and glomerulosclerosis. Exp Nephrol 7, 147–159 (1999). [DOI] [PubMed] [Google Scholar]
- Hills C. E. & Squires P. E. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am J Nephrol 31, 68–74 (2010). [DOI] [PubMed] [Google Scholar]
- Cheng J. & Grande J. P. Transforming growth factor-beta signal transduction and progressive renal disease. Exp Biol Med (Maywood) 227, 943–956 (2002). [DOI] [PubMed] [Google Scholar]
- Eddy A. A. Serine proteases, inhibitors and receptors in renal fibrosis. Thromb Haemost 101, 656–664 (2009). [PMC free article] [PubMed] [Google Scholar]
- Oda T. et al. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 60, 587–596 (2001). [DOI] [PubMed] [Google Scholar]
- Ha H., Oh E. Y. & Lee H. B. The role of plasminogen activator inhibitor 1 in renal and cardiovascular diseases. Nat Rev Nephrol 5, 203–211 (2009). [DOI] [PubMed] [Google Scholar]
- Samarakoon R., Overstreet J. M., Higgins S. P. & Higgins P. J. TGF-beta1 --> SMAD/p53/USF2 --> PAI-1 transcriptional axis in ureteral obstruction-induced renal fibrosis. Cell Tissue Res 347, 117–128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]