Abstract
Gap junction is a cell-cell communication junction type found in virtually all mammalian epithelia and endothelia and provides the necessary “signals” to coordinate physiological events to maintain the homeostasis of an epithelium and/or endothelium under normal physiological condition and following changes in the cellular environment (e.g., stimuli from stress, growth, development, inflammation, infection). Recent studies have illustrated the significance of this junction type in the maintenance of different blood-tissue barriers, most notably the blood-brain barrier and blood-testis barrier, which are dynamic ultrastructures, undergoing restructuring in response to stimuli from the environment. In this chapter, we highlight and summarize the latest findings in the field regarding how changes at the gap junction, such as the result of a knock-out, knock-down, knock-in, or gap junction inhibition and/or its activation via the use of inhibitors and/or activators, would affect the integrity or permeability of the blood-tissue barriers. These findings illustrate that much research is needed to delineate the role of gap junction in the blood-tissue barriers, most notably its likely physiological role in mediating or regulating the transport of therapeutic drugs across the blood-tissue barriers.
INTRODUCTION
Intercellular communication is an important means to maintain tissue homeostasis in multi-cellular organisms. In animals, gap junction communication (GJIC) plays a crucial role to maintain the homeostasis of different types of epithelia as well as endothelia. Gap junctions (GJ) are sometimes compared to plasmodesmata in plants as they both allow direct transport of solutes across cells.1 However, besides intercellular transport of solutes via gap junction channels, GJ can also mediate solute transport between cells and extracellular space through GJ hemichannels.2-4 GJ proteins are the basic building blocks of GJ and include connexins in vertebrates, innexins in invertebrates and pannexins in both vertebrates and some invertebrates.5-7
In this chapter, we discuss the roles of gap junctions in regulating the junction dynamics in tissue barriers in mammals based on the latest findings in the field. It is noted that pannexins are more closely related to innexins than connexins8,9 and only form hemichannels.5,7 Pannexin-based hemichannels are found more recently in vertebrates and there are very few reports in the literature studying the relationship of pannexins and junction barriers. This chapter thus focuses on the functional relationships between connexins and blood-tissue barriers in mammals. Particular focus will be put on how GJ provides the crucial crosstalk between different junction types coexisting at the blood-testis barrier so that the immunological barrier can be maintained during blood-testis barrier restructuring at the time of preleptotene spermatocytes, many of which are connected by intercellular bridges in clones,10,11 in transit at the site.
CONNEXINS—THE BASICS: STRUCTURES, FUNCTIONS AND REGULATION
Connexin Family
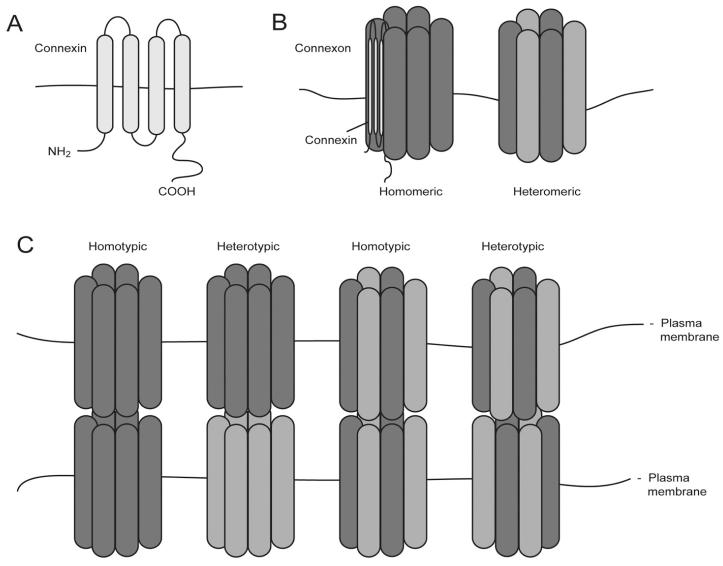
Connexon is a functional unit of gap junctions and made up of a hexamer of gap junction proteins either of the same (monomeric) or different connexins (heteromeric). A connexon on the cell surface by itself is called a hemichannel while gap junction channel refers to connexons coupled between apposing cells2,3 (Fig. 1).
Figure 1.
Schematic illustrations of the structure and organization of GJ. A) A connexin consists of four transmembrane domains, two extracellular loops and one intracellular loop. The variability of connexins lies mostly on the C-terminal tail that comes in different length and sequence and carries sites for phosphorylation and binding of interacting partners. B) Six connexins constitute a functional connexon. A connexon can be made up of the same type of connexins (homomeric) or of different types (heteromeric). An uncoupled connexon can also be called a hemichannel. C) GJ channel is formed between two compatible connexons on adjacent to that create a functional communication channel. The interaction of connexons could be homotypic or heterotypic, depending on the compatibility of individual connexins in a connexon.
There are at least 20 connexins identified in humans and rodents (Table 1). Each mammalian cell type only expresses certain members of the connexin family. These tetraspan proteins are highly conserved in their intracellular N-terminal tail, four transmembrane domains and two extracellular loops, which are for recognition and coupling.2,3,12 The variability of connexins in terms of both length and sequence lies mostly in their intracellular loop and C-terminal tail. The C-terminal tail is the region where connexins interact with different modulators and interacting partners. Most phosphorylation sites of connexins (e.g., Cx43) are found on the C-terminal tail.13
Table 1.
Connexin family members and associated defects due to mutations
| Human | Mouse | Human Hereditary Disease(s) |
Phenotype(s) of Knockout Mice | |
|---|---|---|---|---|
| GJA1 | Cx43 | Cx43 | Oculodentodigital dysplasia,113 cardiac defects,114 hearing loss115 |
Neonatal lethality (lethal at birth) with abnormal cardiac development,116,117 osteoblast dysfunction118 |
| GJA3 | Cx46 | Cx46 | Cataract119 | Cataract120 |
| GJA4 | Cx37 | Cx37 | / | Female sterility121 |
| GJA5 | Cx40 | Cx40 | Cardiac defects122 | Cardiac defects123,124 |
| GJA6 | / | Cx33 | / | / |
| GJA8 | Cx50 | Cx50 | Cataract125 | Microphthalmia, cataract126 |
| GJA9 | Cx59 | / | / | / |
| GJA10 | Cx62 | Cx57 | / | Reduction in visual field in retina127 |
| GJB1 | Cx32 | Cx32 | Charcot-Marie-Tooth disease (CMTX)128,129 |
Decreased glycogen mobilization,130 increased liver carcinogenesis,131 mild myelination defects132 |
| GJB2 | Cx26 | Cx26 | Hearing loss,133-135 epidermal disease135,136 |
Embryonic lethality137 |
| GJB3 | Cx31 | Cx31 | Non-syndromic hearing loss,138,139 epidermal disease83 |
Placental dysfunction140 |
| GJB4 | Cx30.3 | Cx30.3 | Epidermal disease,85 hearing loss115 |
/ |
| GJB5 | Cx31.1 | Cx31.1 | / | / |
| GJB6 | Cx30 | Cx30 | Hearing loss,141 ectodermal dysplasia84 |
Hearing loss,142 behavioral changes to novel environment143 |
| GJB7 | Cx25 | / | / | / |
| GJC1 | Cx45 | Cx45 | / | Embryonic lethality due to vascular and cardiac defects72,144 |
| GJC2 | Cx46.6/ | Cx47 | Pelizaeus-Merzbacher– | Mild myelination defects146 |
| Cx47 | like disease145 | |||
| GJC3 | Cx31.3/ | Cx29 | Hearing loss115,147 | / |
| Cx30.2 | ||||
| GJD2 | Cx36 | Cx36 | / | Visual transmission defects148 |
| GJD3 | Cx31.9 | Cx30.2 | / | Increase in cardiac impulse propagation149 |
| GJD4 | Cx40.1 | Cx39 | / | / |
| GJE1 | Cx23 | Cx23 | / | / |
Information about the recommended names of connexins and their corresponding molecular sizes in human and mouse is extracted from the UnitProtKB database (http://www.uniprot.org, accession date: 11 May 2010). Molecular sizes of connexins in mouse are the same as those in rats although some members are yet to be identified in rats. “/”, not identified.
Gap junction channel can be assembled between cells of the same cell type or of different cell types for heterocellular communication and the interaction of connexons can also be homotypic or heterotypic. Thus, a wide combination of gap junction channels can be formed between epithelial and endothelial cells.2,3
Despite the similarities between different connexin family members and expression of different connexins in the same cell type, different connexins seem to process unique functions as well as shared functions,14,15 as illustrated in different knockout and/or knockin mouse models and genetic diseases in humans (Tables 1 and 2). For instance, while Cx43−/− mice die at birth, homozygous Cx43 knockin Cx26 mice are viable at birth. Even though the percentage of homozygous Cx43 knockin Cx26 mice born is less than the expected Mendelian ratio and has lower survival rate, it illustrates that Cx26 can at least partially compensate for the loss of Cx43. But some vital functions of Cx43 cannot be compensated by Cx26 as gametogenesis in both homozygous males and females becomes impaired, resulting in infertility.15
Table 2.
Modulation of connexins and their effects on barrier integrity
| Modulation | Barrier Integrity |
Other Observations | Tissue or Cell Line | |
|---|---|---|---|---|
| Cx26 | Overexpression | / | Prevent Na+/K+ ATPase inhibitor ouabain-induced TJ barrier disruption, even in the presence of gap junction blockers 18β-glycyrrhetinic acid or oleamide; Increase in Cldn14 expression |
Human transformed bronchial epithelial cell line Calu-3150 |
| Overexpression | Increase | Increase in Cldn4 expression; the increase in barrier integrity can be disrupted by oleic acid, taurocholic acid and 18α-glycyrrhetinic acid |
Human colonic cell line Caco-2151 |
|
| Ectopic expression with epidermis-specific promoter |
Decrease | Disruption of epidermal barrier acquisition during development and recovery of epidermal barrier after wounding; increase in ATP release |
Epidermis of genetically modified mice88 |
|
| Carriers of R134W Cx26 allele (loss of function mutant) |
/ | Increase in epidermal thickness |
Population study of human epidermis87 |
|
| Overexpression of R134W mutant (loss of function mutant) |
/ | Increase in epidermal thickness |
Coculture of human keratinocyte cell line nTERT and human cervical cancer cell line HeLa89 |
|
| Overexpression | / | Increase in invasion of enteric pathogen Shigella flexneri bacteria |
Coculture of human keratinocyte cell line nTERT and human cervical cancer cell line HeLa89 |
|
| Carriers of 35delG Cx26 allele (loss of function mutant) |
/ | Increase in epidermal thickness |
Population study of human epidermis86 |
|
| Overexpression | / | Increase the dissemination of enteropathogenic bacteria Shigella flexneri; increase in ATP release |
Human cervical cancer cell line HeLa152 |
|
| Cx30 | Knockout | Decrease | Independent of gap junction channel activity as GJ structures are lacking in normal mice |
Instrastrial fluid– blood barrier in cochlear of Cx30−/− mice153 |
| Cx32 | Overexpression | No change |
Induction of TJ strands and occludin level |
Mouse hepatocyte cell line CHST8154 |
| Ectopic expression | Slight increase |
Increase in levels and/or localization at cell borders of occludin, claudin-1, ZO-1 and ZO-1, which can be reversed by 18β-glycyrrhetinic acid; these observations are absent in Cx26 or Cx43 transfectants |
Immortalized Cx32-deficient mouse hepatocytes155 |
|
| Cx43 | Conditional knockout |
/ | Acceleration of wound closure |
Epidermis of Cx43Cre-ER(T)/flmice91 |
| Knockdown with antisense oligo DNA |
/ | Acceleration of wound closure |
Mice epidermis92 | |
| Overexpression of Cx43K258Stop |
Defective | Doubled half-life in Cx43K258Stop, which does not carry the C-terminal tail, as shown in HeLa cells |
Epidermis of Cx43K258stop knockin mice90 |
|
| Knockdown with siRNA |
No change | Disruption of barrier integrity only with a concurrent knockdown of Cx43 and desmosome protein plakophilin-2 |
Primary culture of Sertoli cells60 |
Nomenclature of Connexins
Two systems of connexin nomenclature are currently used in parallel (Table 1). The conventional one names connexins according to their molecular sizes (in kDa).16 For example, Cx43 means a connexin protein of 43 kDa. This is a commonly used system which is also used in this chapter. This system nonetheless has its drawbacks due to the differences in molecular sizes in connexin orthologs even between humans and mice. The second system involves grouping connexins according to their sequence similarities and length of their cytoplasmic tails.16 Connexins are assigned into one of the several groups, namely α, β, γ, δ and ε, and a number according to the order of discovery. Cx43 is named GJA1 in the second system, which marks it as the first member discovered in the alpha group of gap junction proteins.
Life Cycle of Connexins
Connexins typically have short half-life of about 1.5-6 hours in mammalian cells.17 Majority of connexins, with the exception of Cx26, are translated in the rough endoplasmic reticulum (ER)18 and then oligomerize to form connexons in the ER, ER–Golgi intermediate compartment or trans-Golgi network, depending on the individual connexins.18,19 Cx26, however, could be synthesized outside of the Golgi-based secretory pathway and inserted directly to the plasma membrane via microtubules.20 All connexins are capable of forming monomeric channels. When cells express more than one connexin at one time, heteromeric connexons may be formed but the compatibility of connexins to form heteromeric connexons depends on individual protein structure. Thus, it is not surprising to find heteromeric connexons consisting of connexins in the same subgroup. For instance, Cx43 and Cx46 in the α subgroup21 and Cx26 and Cx30 in the β subgroup22 have been demonstrated to form heteromeric channels.
After oligomerization, connexons are inserted into the plasma membrane.18,23 Gap junction channels are assembled upon docking of connexons with compatible connexons on the apposing cell surface. Connexons until then remain “closed” to avoid unregulated and unwanted flow of materials from the intracellular compartment or between cells. The regulation of the opening of hemichannels and gap junction channels are to be discussed below. As mentioned above, connexon interactions can be homotypic or heterotypic. Connexons would sometimes aggregate to form gap junction plaques, which can contain up to thousands of connexons, between adjacent cells.18,19 Connexons can also be targeted to specific domains on the membrane,19,23 such as to cell adhesion site by microtubules.24
In gap junction plaques, gap junction channels that need to be metabolically degraded are internalized at the central region as double membrane vesicles into one of the apposing cells while new connexons are recruited to the periphery of the plaque. The internalized structure is called annular gap junction or connexosome and is targeted to lysosomes for degradation.18
Gating of Connexons
Connexons or pannexons alike have gating mechanisms to avoid unwanted flow of solutes.7 Their openings are not “all or none” but are graded so that there are different levels of conductance, ranging between the fully “open” and “closed” state.7,25 Different connexins display different regulatory mechanisms. The factors regulating the opening of connexins include intracellular and extracellular calcium concentration, voltage, mechanical stress, intracellular pH, redox potential and phosphorylation status of connexins.7,12
Phosphorylation of connexins serves as an important means for protein kinases in different signaling pathways to regulate the connexin gating.13,26 Studies have shown different phosphorylation patterns of connexins under various physiological conditions.27,28 For Cx43, many phosphorylation sites have been identified at its intracellular C-terminal tail.13 Phosphorylation of Cx43 by kinases such as c-Src, MAPK, protein kinase C would result in a decline in GJIC whilst protein kinase A and casein kinase 1 can phosphorylate Cx43 to induce an increase in GJIC. A shift in the phosphorylation level of Cx43 at Ser-368, an inhibitory form, has been detected at different phases of cell cycle28 and during development from embryonic stage to adulthood in mice.27 Hence, changes in the phosphorylation status of connexins plays a role to induce changes in GJIC under different physiological conditions.
Selective Permeabilities of Connexins
Gap junction channels had been viewed as channels allowing passive nonspecific diffusion of solutes less than 1.0 to 1.5 kDa in molecular mass,29,30 ranging from inorganic ions, ATP, cyclic nucleotides, siRNA, glucose to polypeptides.31 However, gap junction channels made of different connexins have been shown to process selective permeabilities (also called permselectivity) even towards similar solutes.2,31-35 These selectivities include ionic charge and molecular size, while other factors are still being identified. Early studies by Goldberg et al.33,36 provide a clear demonstration of this. Cx43 channels are more permeable to metabolites like ADP and ATP than Cx32 channels while Cx43 channels are less permeable to adenosine and calcein than Cx32 channels. Another example is the in vitro passage of siRNA through Cx43 channels, but not Cx26/Cx32 channels.37 The rate of transfer is also inversely proportional to the length of siRNA.
For heteromeric channels, permeabilities are determined by their parental connexins. An early study has demonstrated the differences in permeabilities of homomeric Cx32 and heteromeric Cx26/Cx32 hemichannels.38 While homomeric Cx32 hemichannels are similarly permeable to cAMP and cGMP, heteromeric Cx26/Cx32 hemichannels are more permeable to cGMP than cAMP. Heteromeric Cx43/Cx45 channels have also been shown to have unitary conductances that vary from their respective homomeric channels.39 These functional diversities of connexins and connexons thus provide an explanation for the existence of a large number of connexins and human genetic diseases due to mutations in connexins (Table 1).
Modulators of Gap Junction Communication for Functional Studies
To perform functional studies of gap junction channels or hemichannels, modulation of their activities seems to be a necessity. Inhibition by RNAi or gene knockout model remains useful for the functional study of individual connexins but chemical modulators can also serve as convenient tools for functional studies (see Table 3). However, specificity of chemical modulators remains a concern.7,40 Some widely used modulators, such as 18α-glycyrrhetinic acid and oleamide, have indirect actions on connexins and likely affect other signaling pathways besides GJIC.40 In addition, these inhibitors inhibit connexin and pannexins channels at similar concentrations.7 A comprehensive list of the effective concentration of these pharmacological inhibitors on connexin channels, hemichannels, pannexins hemichannels or other membrane channels, namely P2X7 ATP channel and volume-regulated anion channel, has been provided by D’hondt et al.7
Table 3.
Gap junction blockers and their effects on barrier integrity
| Chemical Modulator(s) |
Concentration | Barrier Integrity |
Other Observations | |
|---|---|---|---|---|
| 18β- glycyrrhetinic acid |
10 μM | / | No observable changes in distribution of occludin, ZO-1 and NCAM of TJ and N-cadherin and β-catenin of AJ |
Primary culture of embryonic chicken lens epithelial cells156 |
| Oleic acid and taurocholic acid |
3 mM and 4.5 mM respectively |
Decrease | / | Human colonic cell line Caco-2151 |
| 18β- glycyrrhetinic acid or oleamide |
5-20 μM or 25-100 μM respectively |
Decrease | No significant change (in terms of protein level or distribution) in Cx40, Cx43, occludin, claudin-5, JAM-A, JAM-B, JAM-C and ZO-1 |
Primary porcine brain microvascular endothelial cells74 |
| 18β- glycyrrhetinic acid |
20 μM | Decrease | No significant change (in terms of protein level or distribution) of claudin-1 and ZO-1 |
Rat lung endothelial cell line RLE:rtTA:CL174 |
| Octanol or 18α- glycyrrhetinic acid |
500 μM or 35 μM respectively |
/ | Reduction of monocyte/macrophage transmigration across a blood brain barrier model induced by TNFα and IFNγ |
Cocultures of human fetal astrocytes and human umbilical vein endothelial cell HUVEC and freshly isolated human monocytes157 |
| Oleic acid (oleamide) or 18α-glyceric acid |
10 μM or 10 μM respectively |
/ | Decrease in enterocyte migration which is necessary for restitution of mucosal barrier |
Primary culture of mouse intestinal epithelial cells158 |
Additionally, mimetic peptides of connexins and pannexins were shown as specific inhibitors to study GJIC.40 But they were later shown to exert steric inhibition rather than sequence-specific inhibition.41 Cross-reactivity is another concern using mimetic peptides for functional studies since pannexins mimetic peptide were shown to inhibit Cx46 channels as well as pannexins hemichannels.41 Therefore, much caution is needed to interpret results derived from studies using pharmacological inhibitors or mimetic peptides.
Assessment of Gap Junction Activity
GJIC or permeability of gap junctions is assessed by measuring the unitary conductance by patch-clamp technique or the flow of fluorescent or radioactive probes across gap junction channels or hemichannels.25,31 While unitary conductance is measured with the patch-clamp technique,42 the dye transfer assay has more varieties including the choice of dyes with different properties and different ways to introduce the dye. Commonly used cell membrane impermeable dyes such as Lucifer yellow and neurobiotin, which are of different sizes and charges, can be introduced into selective cell or area of cells by microinjection, electroporation or scrape-loading.32,35,39,41,43,44 A cell membrane permeable dye named calcein AM can also be used to label epithelial cells in vitro. This dye is converted in living cells into cell membrane impermeable calcein.45 The transfer of dye between cells can be assessed by incubating labeled cells with unlabeled ones45 or using the fluorescence recovery after photobleaching technique.46 To investigate the selective permeability towards specific metabolites, metabolites labeled with radioactive probes are also used.33,36
CONNEXINS/GJ AND BARRIER FUNCTION
The physiological importance of connexins in various systems is demonstrated by the defects caused by mutations or ablation of connexins (Table 1). Our discussion in this section is limited to the roles of connexin-based gap junctions in maintaining blood-tissue barrier integrity. Readers are encouraged to consult other reviews for the roles of connexins in other systems, including the cardiac, neuronal and reproductive systems.4,47-49
Interaction of Connexins and Junction Associated Proteins
Multiple junction proteins, including integral membrane proteins, scaffolding proteins and cytoskeletal proteins have been shown to interact with connexins.23,50 This information illustrates that connexins are part of the multiprotein junction complexes, suggesting thatgap junctions may modulate different junction types in different epithelia.51
Interaction of tight junction members, such as occludin, claudin-1, claudin-5, ZO-1 and ZO-2, with connexins were mostly demonstrated by colocalization and co-immunoprecipitation.50 Direct association of Cx43 with ZO-1 and ZO-2 was also demonstrated.52,53 The interaction of ZO-1 and Cx43 is important for the stabilization of Cx43 in the plasma membrane. The association of ZO-1 and F-actin binding protein drebrin with Cx43 was suggested for anchoring gap junction plaques to the actin cytoskeleton.23 c-Src and ZO-1 also bind competitively to the C-terminal tail of Cx43.54 The binding of c-Src to Cx43 and hence dissociation of ZO-1 from Cx43 was shown to drive the internalization of gap junction plaque from the cell membrane55 and inhibit GJIC.56,57
For adherens junction, an early study showed that the assembly of adherens junction and gap junction are interdependent. Although adherens junction are assembled at the cell-cell interface prior to gap junction formation, addition of antibodies against either N-cadherin or Cx43 could abolish the assembly of both junction types.58 Cx43 can be transported to N-cadherin at existing adhesion site24 and it was shown to colocalize and co-immunoprecipitate with AJ proteins N-cadherin and β-catenin.59,60 These reports thus supported a close physical and functional association between AJ and GJ.
GJ is also working closely with desmosomes in heart and reproductive organs. For instance, arrhythmogenic right ventricular cardiomyopathy is a hereditary disease of heart muscle caused by mutations in desmosomal proteins including plakoglobin and plakophilin-2.61 Patients with this disease had a lower level of Cx43 at the cell surface.62,63 The knockdown of plakophilin-2 by RNAi was shown to cause a reduction in Cx43 level and GJIC63,64 while plakophilin-2 can physically associate with Cx43.60,64 In the ovary and testis, junction complexes bearing ultrastructural properties of both desmosomes and GJ have been identified and are named desmosome-like or desmosome-gap junction.65-68
In short, connexins interact with various junction proteins and their associated scaffolding and signaling proteins in a tissue-dependent manner. The implications of these associations in regulating the homeostasis of barrier integrity are discussed below.
Endothelial Blood-Tissue Barrier
Various blood-tissue barriers are formed by TJ barrier between endothelial vascular cells and these include the blood-brain barrier, inner blood-retinal (also known as blood-ocular) barrier.69,70 Additional reinforcement by epithelial cells, such as pericytes, also contribute to the blood-brain barrier.70 Some barriers, such as blood-aqueous barrier and blood-retinal barrier, consist of more than one layer of TJ barrier formed by both vascular and epithelial cells.71
As shown in knockout animals, some connexins are required for proper vascular development. For instance, Cx45 is present in the endothelial cells and smooth muscle cells of all blood vessels. Embryonic lethality in Cx45−/− mice was accompanied by defects in vascular development.72 Perinatal death was noted in Cx37−/−/Cx40−/− double knockout mice, but not in single Cx37−/− or Cx40−/− knockout mice. Vascular defects in Cx37−/−/Cx40−/− double knockout mice were exhibited by hemorrhages in certain tissues, in particular testis and intestine.73 These studies illustrate the importance of connexins in vascular development. In addition, studies have suggested the importance of gap junction activity in the maintenance of tight junction barrier integrity of vascular cells. In primary cultures of vascular cells from porcine brain, addition of GJ blocker, 18β-glycyrrhetinic acid (5-20 μM) or oleamide (25-100 μM), can significantly inhibit the barrier integrity.74 In another study of rat blood-brain barrier, the reversible barrier disruption induced by ultrasound was accompanied by a redistribution of gap junction plaques. An increase in size of Cx43 and Cx36 based-GJ plaques was observed during blood-brain barrier disruption.75 These reports illustrate the importance of connexins and GJ in vascular development and maintenance of endothelial vascular barrier.
Blood-Testis Barrier
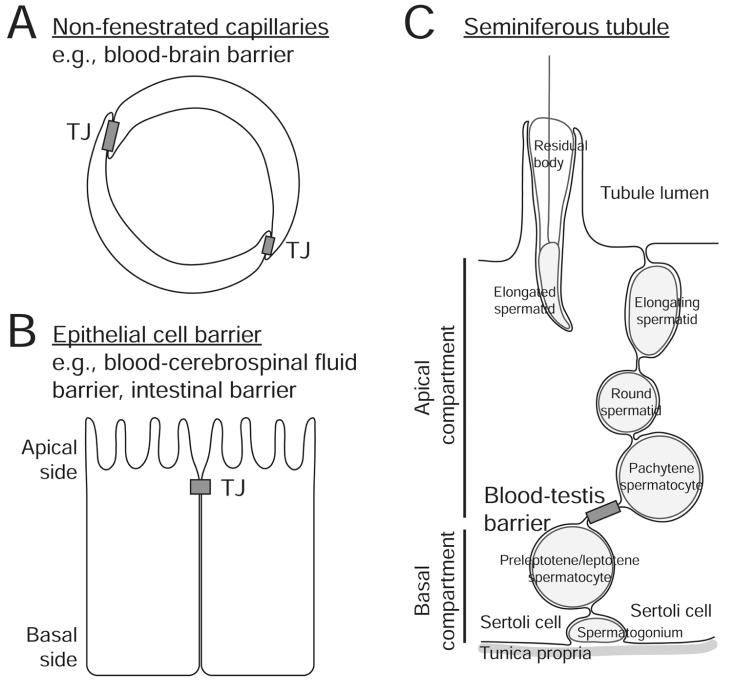
Blood-testis barrier is notably different from the endothelial blood-tissue barrier mentioned above (see Fig. 2). This barrier is constituted by adjacent Sertoli cells residing in the seminiferous epithelium, near the basement membrane in adult mammalian testes, instead of endothelial vascular cells found in the interstitium. Secondly, it is formed at the basal, instead of the apical, side of Sertoli cells. Thirdly, its structural components include not only tight junctions, but also atypical adherens junctions (basal ectoplasmic specialization), desmosome-gap junctions and GJ.66,76,77 Major functions of this barrier include providing immunological protection to developing germ cells and creating a microenvironment for the development of postmeiotic male germ cells, known as the apical compartment, during spermatogenesis77,78 (Fig. 2).
Figure 2.
This figure illustrates the major morphological features of various types of blood-tissue barriers. A) Blood-tissue barriers, including blood-brain barrier, could be constituted by TJ formed between endothelial vascular cells in nonfenestrated capillaries. B) Blood-tissue barriers could also be formed by epithelial cells. At the blood-cerebrospinal fluid barrier, the blood vessel is fenestrated (without TJ) and TJs formed at the apical region of adjacent choroid plexus epithelial cells constitute the barrier. C) The blood-testis barrier is located in the seminiferous epithelium of the seminiferous tubule, which is formed near the basal region of adjacent Sertoli cells. Different junction complexes have been identified at this site, including TJ, basal ES (an atypical AJ), desmosome-like junction and GJ. The blood-testis barrier also segregates the seminiferous epithelium into the basal and apical (or adluminal) compartment, so that meiosis and the entire events of postmeiotic germ cell development (i.e., spermiogenesis) take place behind this immunological barrier in a specialized microenvironment.
Of the various connexins expressed by Sertoli cells,48 Cx43 is a promising candidate that regulates the integrity of blood-testis barrier. While spermatogenesis defects were not reported in mice with Cx31, Cx32, Cx37, Cx40, or Cx46 knockout,48 Sertoli cell specific Cx43 knockout causes impaired spermatogenesis, leading to infertility in homozygous male mice.79 Further analysis reveals that these Sertoli cells without Cx43 stay in the proliferative phase without differentiation.80 Since the establishment of functional blood-testis barrier has been associated with differentiation of Sertoli cells,81 it is tempting to speculate that Cx43 may be a prerequisite for the establishment of blood-testis barrier.
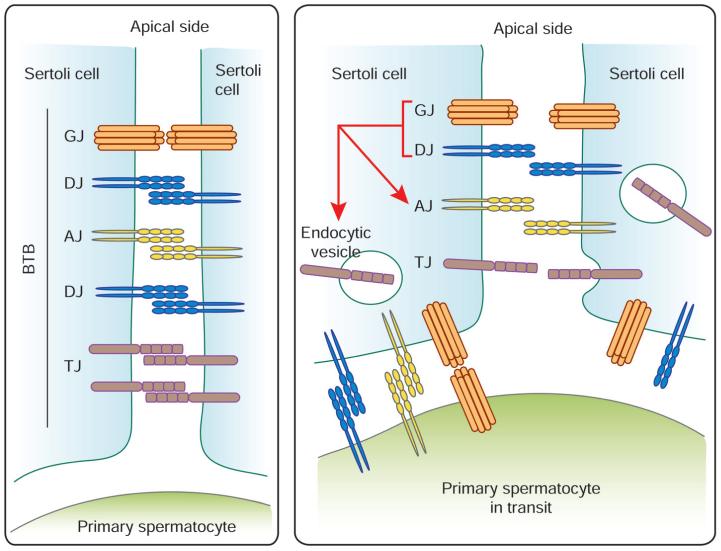
A recent report from our research group indicated that Cx43 co-operates with desmosomal protein plakophilin-2 in the maintenance of the blood-testis barrier integrity. Simultaneous knockdown of both Cx43 and plakophilin-2, but not either one alone, would disrupt the barrier integrity in primary culture of Sertoli cells.60 In addition, a dual-knockdown of desmoglein-2 and desmocollin-2, which are integral membrane proteins of desmosomes, would perturb the TJ-permeability integrity in primary Sertoli cell cultures, partly via enhancing the rate of endocytosis of TJ protein CAR.82 We postulate that when primary spermatocytes are in transit at the blood-testis barrier, such as at Stage VIII of the seminiferous epithelial cycle, the AJ, GJ and desmosome formed between Sertoli cells would be replaced by those between Sertoli cell and spermatocytes (Fig. 3). From these reports, a reduction of GJ and desmosome between adjacent Sertoli cells would induce blood-testis barrier disruption. This includes a decline in the steady-state levels of TJ and AJ proteins at the Sertoli cell surface, which is partly mediated by an increase in endocytosis of junction proteins. Thus, it resulted in an increase in the permeability at the apical region of the translocating spermatocytes to facilitate its translocation. Cx43 hence likely serves as a regulator of the blood-testis barrier homeostasis by maintaining the crucial crosstalk among different coexisting junction types at the blood-testis barrier (Fig. 3).
Figure 3.
Schematic illustration of the roles of GJ and desmosome at the blood-testis barrier. The blood-testis barrier (BTB) remains intact at most stages of the seminiferous epithelial cycle and consists of coexisting TJ, AJ, GJ and desmosome-like junction (DJ) (left panel). Primary spermatocytes migrate across the BTB at Stage VIII of the seminiferous epithelial cycle in the rat testis (right panel). The AJ, DJ and GJ formed between Sertoli cells are likely replaced by those between Sertoli cell and spermatocyte since it is now known that many of AJ, DJ and GJ proteins are also found in germ cells, such as spermatocytes.76-77 A reduction of GJ and DJ between adjacent Sertoli cells would destabilize the BTB, inducing its disruption. This involves a decline in the steady-state levels of TJ and AJ proteins at the Sertoli cell surface, which is partly mediated by an increase in endocytosis of junction proteins. The net result is an increase in the permeability at the apical region of the translocating spermatocytes to facilitate their transit at the BTB. However, “new” TJ, AJ, DJ and GJ are formed behind the spermatocytes in transit before the “old” junctions are being disrupted, so that the immunological barrier can be maintained.
Epidermal Barrier
Epidermal barrier of mammalian skin serves as the first line of defense against pathogens and other harmful substances.1 At least nine connexins are expressed at different layers of epidermis except the uppermost layer called stratum corneum.2 Influences of connexins on epidermal barrier integrity are exemplified by their effects on epidermal thickness and wound repair process. Multiple human hereditary diseases in skin with mutations in Cx26, Cx30, Cx30.3 and Cx31, have been discovered.83-85 This illustrates the necessity of connexins in maintaining the epidermis homeostasis and epidermal barrier. For instance, Cx30 mutants could result in hidrotic ectodermal dysplasia,84 with symptoms including eczematous dermatitis.
Cx26 in particular has been the focus of much research since the loss of function mutants of Cx26 that lead to nonsyndromic hearing loss would give heterozygous individuals an advantageous edge of an increase in epidermal thickness.86,87 An ectopic Cx26 overexpression in mice epidermis would however disrupt the epidermal barrier development and wound healing process.88 Another study using cocultures of keratinocytes and HeLa cells demonstrated that the invasion of enteric pathogen S. flexneri could be enhanced by overexpression of Cx26, but not its loss of function mutant.89 These reports collectively illustrate the inhibitory effect of Cx26 on the establishment, recovery and hence integrity of epidermal barrier.
Cx43, which displays a broad expression profile in epidermis, has been shown to regulate the epidermal barrier in animal studies even though Cx43 mutants are yet to be associated with skin abnormalities in humans. Knockin mice with Cx43 carrying no C-terminal tail (Cx43K258Stop) have perinatal death due to epidermal barrier defects. The truncated Cx43 mutant without C-terminal tail also form GJ channels and has a doubled half-life than Cx43.90 Mice having an epidermis-specific Cx43 knockout or knockdown display an acceleration of wound closure.91,92 These illustrate that a decline in Cx43 level is probably required for epidermal barrier establishment and wound repair.
CONNEXIN-MEDIATED BYSTANDER EFFECTS
Connexins Mediating Harmful Signals
The above discussion illustrates the regulatory roles of connexins on the homeostasis of different blood-tissue barriers. Most barriers serve primarily as selective permeablility barrier to isolate and protect cells behind the barriers from harmful substances such as pathogens.1 While connexins could regulate barrier integrity, they could also be responsible for mediating harmful signals under pathological conditions. For instance, in intestinal barrier, Cx43 hemichannel was recently shown to mediate infection of enteric pathogen Citrobacter rodentium.93 Water loss following C. rodentium incubation, as assessed by the water content in distal colon, was significantly reduced in heterozygous Cx43+− mice. In addition, connexins have been implicated in tumorigenesis.94 Tumor cell migration and attachment during metastasis was shown to be induced by Cx43.95 This is probably due to the close structural association of connexins and adhesion molecules as discussed above so that an alteration of Cx43 would lead to changes in cell adhesion and cell migration.
Bystander Killing by Connexins
Due to their versatility, gap junction channels or hemichannels are capable of transferring harmful signals between neighboring cells. Bystander effect is a term used to describe the spread and amplification of harmful signals from cells directly exposed to insults to neighboring cells. These insults include radiation, inflammation and viral transfection.96-99 Exposure to very low influences of α-particles could induce DNA damage in non-irradiated cells in skin and lung fibroblasts cultures.99 After spinal cord injury, Cx43 was upregulated. Rats with a knockdown of Cx43 by Cx43 antisense oligodeoxynucleotides showed reduced inflammation and a faster functional recovery.98 GJIC was also shown to be responsible for mediating the transfer of apoptotic signals from HIV-infected astrocytes to non-infected ones.97
Bystander effect can be observed even across an intact barrier. A recent study has shown the damage caused by indirect exposure to cobalt-chromium nanoparticles in human fibroblast cells across an intact layer of BeWo cells.100 The DNA damage resulted in fibroblast cells was reduced by gap junction mimetic peptide GAP26 while it was potentiated by antiarrhythmic peptide AAP10,100 an upregulator of GJIC.101 Furthermore, regional X-ray irradiation of the lower body part of mice induced DNA damage and apoptosis in mouse cerebella, which are behind the blood-brain barrier. The use of GJIC inhibitor 12-O-tetradecanoylphorbol-13-acetate could reduce these bystander effects.102
Potential Uses of Connexin-Bystander Effect
Apart from the bystander deaths mediated by gap junctions, bystander effect is beneficial under certain circumstances. Preconditioning in heart and brain involves exposing bystander cells to stress but not yet damaging stimuli, which results in better resistance towards higher and damaging levels of stimuli during subsequent exposures.103,104 Studies utilizing Cx43-deficient mice reported the absence of preconditioning in heart and brain of Cx43-deficient mice, illustrating Cx43 as a prerequisite for preconditioning.105,106 The role of connexins in preconditioning in heart has been recently reviewed.107 In addition, the possibility of utilizing the bystander effect in cancer therapy has been explored. A recent review discussed the possibility of taking advantage of the bystander effect in radiation-related cancer therapy to amplify the harmful effects of radioactive isotopes or external radiation to tumor cells.108 A potential gene therapy for cancer treatment involves the targeted introduction of thymidine kinase gene by virus into tumor cells, which is necessary for the processing of an antiviral drug named ganciclovir into its toxic phosphorylated form.94,109 It has been shown that GJ would again increase the range of the toxicity due to its bystander effect.96,110 These studies draw attentions not only to the safety of medical use of nanoparticles and radiation, but also to the potential uses of gap junctions to amplify signals, such as during cancer therapy.
CONCLUSION AND FUTURE PERSPECTIVES
Herein we summarize some of the latest findings in the field regarding the role of GJ and GJIC in the normal functioning of blood-tissue barriers. Earlier morphological studies have shown that in most blood-tissue barriers with the exception of the blood-testis barrier, GJs are present in discrete cellular localization at the paracellular site, being segregated from the tight and anchoring junctions.1 Recent studies have shown that some GJ are present in the junctional complexes besides the GJ plaques to provide the necessary communications between cells to maintain the homeostasis of an epithelium including the TJ barrier function.1 We also provide an updated molecular model regarding the crucial role of GJ in the blood-testis barrier dynamics by coordinating different coexisting junction types at the blood-testis barrier to facilitate the transit of primary spermatocytes, namely preleptotene spermatocytes, while maintaining the immunological barrier integrity (see Fig. 3).
Based on the latest findings that support this model, it is very likely that GJ is working beyond its “traditional” role of serving as a channel for the transport of chemical signals between cells. Perhaps other important biomolecules, such as electrolytes, ions, small molecular drugs, and paracrine factors are being actively transported across adjacent cells in a cell epithelium to synchronize cellular events. As a result, an entire epithelium can respond to the challenge of an external cue and/or stimulus during a complex molecular event, such as growth, differentiation, development and spermatogenesis.
In light of the recent advances in the role of GJ in blood-tissue barriers, such as the blood-testis barrier, which determines and/or dictates which drug(s) and how much of a drug can traverse the barrier to enter the apical compartment, it is also possible that GJ is working in concert with drug transporters, such as influx pumps (e.g., p-glycoprotein) or efflux pumps (e.g., Oatp3), at the blood-testis barrier. This possibility is important and it should be carefully evaluated in future studies to better understand the role of GJ in drug transport at the blood-testis barrier since such studies would have significant impacts to therapeutically manage illnesses. For instance, anti-viral drugs that effectively to reduce the AIDS/HIV-1 viral loads in the blood of AIDS patients fail to reduce the viral content in the semen,111,112 making the male reproductive tract a safe haven for HIV-1 mutation. If these drugs could traverse the blood-testis barrier as effectively as in other organs, this would minimize the transmission of AIDS from infected individuals to their partners.
ACKNOwLEDGMENTS
This work was supported in part by grants from the National Institutes of Health (NICHD, R01 HD056034 to CYC; U54 HD029990 Project 5 to CYC; and R03 HD061401 to DDM). MWML was supported by the University of Hong Kong Postgraduate Studentship.
REFERENCES
- 1.Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4 th ed Garland Science; New York: 2002. [Google Scholar]
- 2.Meşe G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol. 2007;127:2516–2524. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- 3.Dbouk HA, Mroue RM, El-Sabban ME, et al. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal. 2009;7:4. doi: 10.1186/1478-811X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sáez JC, Berthoud VM, Brañes MC, et al. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 5.Shestopalova VI, Panchinc Y. Pannexins and gap junction protein diversity. Cell Mol Life Sci. 2008;65:376–394. doi: 10.1007/s00018-007-7200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stout C, Goodenough DA, Paul DL. Connexins: functions without junctions. Curr Opin Cell Biol. 2004;16:507. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 7.D’hondt C, Ponsaerts R, De Smedt H, et al. Pannexins, distant relatives of the connexin family with specific cellular functions? Bioessays. 2009;31:953–974. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- 8.Yen MR, Saier MH., Jr. Gap junctional proteins of animals: The innexin/pannexin superfamily. Prog Biophys Mol Biol. 2007;94:5–14. doi: 10.1016/j.pbiomolbio.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baranova A, Ivanov D, Petrash N, et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Fawcett DW. Intercellular bridges. Exp Cell Res. 1961;8:174–187. doi: 10.1016/0014-4827(61)90347-0. [DOI] [PubMed] [Google Scholar]
- 11.Dym M, Fawcett DW. Further observations on the numbers of spermatogonia, spermatocytes and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod. 1971;4:195–215. doi: 10.1093/biolreprod/4.2.195. [DOI] [PubMed] [Google Scholar]
- 12.Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 13.Pahujaa M, Anikin M, Goldberg GS. Phosphorylation of connexin43 induced by Src: regulation of gap junctional communication between transformed cells. Exp Cell Res. 2007;313:4083–4090. doi: 10.1016/j.yexcr.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Plum A, Hallas G, Magin T, et al. Unique and shared functions of different connexins in mice. Curr Biol. 2000;10:1083–1091. doi: 10.1016/s0960-9822(00)00690-4. [DOI] [PubMed] [Google Scholar]
- 15.Winterhager E, Pielensticker N, Freyer J, et al. Replacement of connexin43 by connexin26 in transgenic mice leads to dysfunctional reproductive organs and slowed ventricular conduction in the heart. BMC Dev Biol. 2007;7:26. doi: 10.1186/1471-213X-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Söhl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Adhes Commun. 2003;10:173–180. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- 17.Hervé JC, Derangeon M, Bahbouhi B, et al. The connexin turnover, an important modulating factor of the level of cell-to-cell junctional communication: comparison with other integral membrane proteins. J Membr Biol. 2007;217:21–33. doi: 10.1007/s00232-007-9054-8. [DOI] [PubMed] [Google Scholar]
- 18.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koval M. Pathways and control of connexin oligomerization. Trends Cell Biol. 2006;16:159–166. doi: 10.1016/j.tcb.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin PEM, Blundell G, Ahmad S, et al. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J Cell Sci. 2001;114:3845–3855. doi: 10.1242/jcs.114.21.3845. [DOI] [PubMed] [Google Scholar]
- 21.Das Sarma J, Meyer RA, Wang F, et al. Multimeric connexin interactions prior to the trans-Golgi network. J Cell Sci. 2001;114:4013–4024. doi: 10.1242/jcs.114.22.4013. [DOI] [PubMed] [Google Scholar]
- 22.Yum SW, Zhang J, Valiunas V, et al. Human connexin26 and connexin30 form functional heteromeric and heterotypic channels. Am J Physiol Cell Physiol. 2007;293:C1032–C1048. doi: 10.1152/ajpcell.00011.2007. [DOI] [PubMed] [Google Scholar]
- 23.Olk S, Zoidl G, Dermietzel R. Connexins, cell motility and the cytoskeleton. Cell Motil Cytoskeleton. 2009;66:1000–1016. doi: 10.1002/cm.20404. [DOI] [PubMed] [Google Scholar]
- 24.Shaw RM, Fay AJ, Puthenveedu MA, et al. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González D, Gómez-Hernández JM, Barrio LC. Molecular basis of voltage dependence of connexin channels: an integrative appraisal. Prog Biophys Mol Biol. 2007;94:66–106. doi: 10.1016/j.pbiomolbio.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Moreno AP, Lau AF. Gap junction channel gating modulated through protein phosphorylation. Prog Biophys Mol Biol. 2007;94:107–119. doi: 10.1016/j.pbiomolbio.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King TJ, Lampe PD. Temporal regulation of connexin phosphorylation in embryonic and adult tissues. Biochim Biophys Acta. 2005;1719:24–35. doi: 10.1016/j.bbamem.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solan JL, Fry MD, TenBroek EM, et al. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci. 2003;116:2203–2211. doi: 10.1242/jcs.00428. [DOI] [PubMed] [Google Scholar]
- 29.Simpson I, Rose B, Loewenstein WR. Size limit of molecules permeating the junctional membrane channels. Science. 1977;195:294–296. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- 30.Loewenstein WR. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981;61:829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- 31.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber PA, Chang HC, Spaeth KE, et al. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J. 2004;87:958–973. doi: 10.1529/biophysj.103.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg GS, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol. 1999;1:457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- 34.Kanaporis G, Mese G, Valiuniene L, et al. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol. 2008;131:293–305. doi: 10.1085/jgp.200709934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elfgang C, Eckert R, Lichtenberg-Fraté H, et al. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldberg GS, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem. 2002;277:36725–36730. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- 37.Valiunas V, Polosina YY, Miller H, et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol (Lond) 2005;568:459–468. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bevans CG, Kordel M, Rhee SK, et al. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem. 1998;273:2808–2816. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- 39.Martínez AD, Hayrapetyan V, Moreno AP, et al. Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ Res. 2002;90:1100–1107. doi: 10.1161/01.res.0000019580.64013.31. [DOI] [PubMed] [Google Scholar]
- 40.Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans. 2001;29:606–612. doi: 10.1042/bst0290606. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Ma M, Locovei S, et al. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007;293:C1112–C1119. doi: 10.1152/ajpcell.00097.2007. [DOI] [PubMed] [Google Scholar]
- 42.Neyton J, Trautmann A. Single-channel currents of an intercellular junction. Nature. 1985;317:331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- 43.El-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer: a rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168:422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 44.Risley MS, Tan IP, Farrell J. Gap junctions with varied permeability properties establish cell-type specific communication pathways in the rat seminiferous epithelium. Biol Reprod. 2002;67:945–952. doi: 10.1095/biolreprod67.3.945. [DOI] [PubMed] [Google Scholar]
- 45.Goldberg GS, Bechberger JF, Naus CC. A preloading method of evaluating gap junctional communication by fluorescent dye transfer. Biotechniques. 1995;18:490–497. [PubMed] [Google Scholar]
- 46.Wade MH, Trosko JE, Schindler M. A fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science. 1986;232:525–528. doi: 10.1126/science.3961495. [DOI] [PubMed] [Google Scholar]
- 47.Bloomfield SA, Völgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci. 2009;10:495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pointis G, Gilleron J, Carette D, et al. Physiological and physiopathological aspects of connexins and communicating gap junctions in spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1607–1620. doi: 10.1098/rstb.2009.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nattel S, Maguy A, Le Bouter S, et al. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 50.Hervé JC, Bourmeyster N, Sarrouilhe D, et al. Gap junctional complexes: from partners to functions. Prog Biophys Mol Biol. 2007;94:29–65. doi: 10.1016/j.pbiomolbio.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Derangeona M, Sprayb DC, Bourmeystera N, et al. Reciprocal influence of connexins and apical junction proteins on their expressions and functions. Biochim Biophys Acta. 2009;1788:768–778. doi: 10.1016/j.bbamem.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toyofuku T, Yabuki M, Otsu K, et al. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- 53.Singh D, Solan JL, Taffet SM, et al. Connexin 43 interacts with zona occludens-1 and -2 proteins in a cell cycle stage-specific manner. J Biol Chem. 2005;280:30416–30421. doi: 10.1074/jbc.M506799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toyofuku T, Akamatsu Y, Zhang H, et al. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J Biol Chem. 2001;276:1780–1788. doi: 10.1074/jbc.M005826200. [DOI] [PubMed] [Google Scholar]
- 55.Gilleron J, Fiorini C, Carette D, et al. Molecular reorganization of Cx43, ZO-1 and Src complexes during the endocytosis of gap junction plaques in response to a nongenomic carcinogen. J Cell Sci. 2008;121:4069–4078. doi: 10.1242/jcs.033373. [DOI] [PubMed] [Google Scholar]
- 56.Postma FR, Hengeveld T, Alblas a, et al. Acute loss of cell-cell communication caused by G protein-coupled receptors: a critical role for c-Src. J Cell Biol. 1998;140:1199–1209. doi: 10.1083/jcb.140.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giepmans BNG, Hengeveld T, Postma FR, et al. Interaction of c-Src with gap junction protein connexin-43. J Biol Chem. 2001;276:8544–8549. doi: 10.1074/jbc.M005847200. [DOI] [PubMed] [Google Scholar]
- 58.Meyer RA, Laird DW, Revel JP, et al. Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. J Cell Biol. 1992;119:179–189. doi: 10.1083/jcb.119.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei CJ, Francis R, Xu X, et al. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J Biol Chem. 2005;280:19925–19936. doi: 10.1074/jbc.M412921200. [DOI] [PubMed] [Google Scholar]
- 60.Li MWM, Mruk DD, Lee WM, et al. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basso C, Corrado D, Marcus FI, et al. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 62.Kaplan SR, Gard JJ, Protonotarios N, et al. Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos disease) Heart Rhythm. 2004;1:3–11. doi: 10.1016/j.hrthm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Fidler LM, Wilson GJ, Liu F, et al. Abnormal connexin43 in arrhythmogenic right ventricular cardiomyopathy caused by plakophilin-2 mutations. J Cell Mol Med. 2008;13:4219–4228. doi: 10.1111/j.1582-4934.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oxford EM, Musa H, Maass K, et al. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res. 2007;101:703–711. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- 65.Russell L. Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat. 1977;148:301–312. doi: 10.1002/aja.1001480302. [DOI] [PubMed] [Google Scholar]
- 66.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 67.Szöllösi D. Desmosomes: their occurrence between adjacent primary oocytes in polyovular follicles in the rabbit. Cell Tissue Res. 1978;191:115–119. doi: 10.1007/BF00223220. [DOI] [PubMed] [Google Scholar]
- 68.Anderson E, Beams HW. Cytological observations on the fine structure of the guinea pig ovary with special reference to the oogonium, primary oocyte and associated follicle cells. J Ultrastruct Res. 1960;3:432–446. doi: 10.1016/s0022-5320(60)90021-6. [DOI] [PubMed] [Google Scholar]
- 69.Vinores SA. Assessment of blood-retinal barrier integrity. Histol Histopathol. 1995;10:141–154. [PubMed] [Google Scholar]
- 70.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Hornof M, Toropainen E, Urtti A. Cell culture models of the ocular barriers. Eur J Pharm Biopharm. 2005;60:207–225. doi: 10.1016/j.ejpb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 72.Krüger O, Plum A, Kim JS, et al. Defective vascular development in connexin 45-deficient mice. Development. 2000;127:4179–4193. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- 73.Simon AM, McWhorter AR. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol. 2002;251:206–220. doi: 10.1006/dbio.2002.0826. [DOI] [PubMed] [Google Scholar]
- 74.Nagasawa K, Chiba H, Fujita H, et al. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J Cell Physiol. 2006;208:123–132. doi: 10.1002/jcp.20647. [DOI] [PubMed] [Google Scholar]
- 75.Alonso A, Reinz E, Jenne JW, et al. Reorganization of gap junctions after focused ultrasound blood-brain barrier opening in the rat brain. J Cereb Blood Flow Metab. 2010;30:1394–1402. doi: 10.1038/jcbfm.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulate spermatogenesis. Nat Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pelletier RM, Byers SW. The blood-testis barrier and Sertoli cell junctions: structural considerations. Microsc Res Tech. 1992;20:3–33. doi: 10.1002/jemt.1070200104. [DOI] [PubMed] [Google Scholar]
- 79.Brehm R, Zeiler M, Ruttinger C, et al. A Sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am J Pathol. 2007;171:19–31. doi: 10.2353/ajpath.2007.061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sridharan S, Simon L, Meling DD, et al. Proliferation of adult sertoli cells following conditional knockout of the gap junctional protein GJA1 (connexin 43) in mice. Biol Reprod. 2007;76:804–812. doi: 10.1095/biolreprod.106.059212. [DOI] [PubMed] [Google Scholar]
- 81.Walker WH. Molecular mechanisms controlling Sertoli cell proliferation and differentiation. Endocrinology. 2003;144:3719–3721. doi: 10.1210/en.2003-0765. [DOI] [PubMed] [Google Scholar]
- 82.Lie PPY, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 2010;42:975–986. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Richard G, Smith LE, Bailey RA, et al. Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis. Nat Genet. 1998;20:366–369. doi: 10.1038/3840. [DOI] [PubMed] [Google Scholar]
- 84.Lamartine J, Munhoz Essenfelder G, Kibar Z, et al. Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat Med. 2000;26:142–144. doi: 10.1038/79851. [DOI] [PubMed] [Google Scholar]
- 85.Macari F, Landau M, Cousin P, et al. Mutation in the gene for connexin 30.3 in a gamily with erythrokeratodermia variabilis. Am J Hum Genet. 2000;67:1296–1301. doi: 10.1016/s0002-9297(07)62957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D’Adamo P, Guerci VI, Fabretto A, et al. Does epidermal thickening explain GJB2 high carrier frequency and heterozygote advantage? Eur J Hum Genet. 2009;17:284–286. doi: 10.1038/ejhg.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meyer CG, Amedofu GK, Brandner JM, et al. Selection for deafness? Nat Med. 2002;8:1332–1333. doi: 10.1038/nm1202-1332. [DOI] [PubMed] [Google Scholar]
- 88.Djalilian AR, McGaughey D, Patel S, et al. Connexin 26 regulates epidermal barrier and wound remodeling and promotes psoriasiform response. J Clin Invest. 2006;116:1243–1253. doi: 10.1172/JCI27186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Man YKS, Trolove C, Tattersall D, et al. A deafness-associated mutant human connexin 26 improves the epithelial barrier in vitro. J Membr Biol. 2007;218:29–37. doi: 10.1007/s00232-007-9025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maass K, Ghanem A, Kim JS, et al. Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol Biol Cell. 2004;15:4597–4608. doi: 10.1091/mbc.E04-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kretz M, Euwens C, Hombach S, et al. Altered connexin expression and wound healing in the epidermis of connexin-deficient mice. J Cell Sci. 2003;116:3443–3452. doi: 10.1242/jcs.00638. [DOI] [PubMed] [Google Scholar]
- 92.Qiu C, Coutinho P, Frank S, et al. Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol. 2003;13:1697–1703. doi: 10.1016/j.cub.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 93.Guttman JA, Lin AEJ, Li Y, et al. Gap junction hemichannels contribute to the generation of diarrhoea during infectious enteric disease. Gut. 2010;59:218–226. doi: 10.1136/gut.2008.170464. [DOI] [PubMed] [Google Scholar]
- 94.Naus CC, Laird DW. Implications and challenges of connexin connections to cancer. Nat Rev Cancer. 2010;10:435–441. doi: 10.1038/nrc2841. [DOI] [PubMed] [Google Scholar]
- 95.Elzarrad MK, Haroon A, Willecke K, et al. Connexin-43 upregulation in micrometastases and tumor vasculature and its role in tumor cell attachment to pulmonary endothelium. BMC Med. 2008;6:20. doi: 10.1186/1741-7015-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mesnil M, Piccoli C, Tiraby G, et al. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci USA. 1996;93:1831–1835. doi: 10.1073/pnas.93.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci. 2007;27:12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cronin M, Anderson PN, Cook JE, et al. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol Cell Neurosci. 2008;39:152–160. doi: 10.1016/j.mcn.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 99.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junctionmediated intercellular communication in the transmission of damage signals from α-particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bhabra G, Sood A, Fisher B, et al. Nanoparticles can cause DNA damage across a cellular barrier. Nat Nanotechnol. 2009;4:876–883. doi: 10.1038/nnano.2009.313. [DOI] [PubMed] [Google Scholar]
- 101.Müller A, Schaefer T, Linke W, et al. Actions of the antiarrhythmic peptide AAP10 on intercellular coupling. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:76–82. doi: 10.1007/pl00005031. [DOI] [PubMed] [Google Scholar]
- 102.Mancuso M, Pasquali E, Leonardi S, et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc Natl Acad Sci USA. 2008;105:12445–12450. doi: 10.1073/pnas.0804186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 104.Dahl NA, Balfour WM. Prolonged anoxia survival due to anoxia pre-exposure: brain ATP, lactate and pyruvate. Am J Physiol. 1964;207:452–456. doi: 10.1152/ajplegacy.1964.207.2.452. [DOI] [PubMed] [Google Scholar]
- 105.Lin JHC, Lou N, Kang N, et al. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schwanke U, Konietzka I, Duschin A, et al. No ischemic preconditioning in heterozygous connexin43-deficient mice. Am J Physiol Heart Circ Physiol. 2002;283:H1740–H1742. doi: 10.1152/ajpheart.00442.2002. [DOI] [PubMed] [Google Scholar]
- 107.Miura T, Miki T, Yano T. Role of the gap junction in ischemic preconditioning in the heart. Am J Physiol Heart Circ Physiol. 2010;298:1115–1125. doi: 10.1152/ajpheart.00879.2009. [DOI] [PubMed] [Google Scholar]
- 108.Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moolten FL, Wells JM. Curability of Tumors Bearing Herpes Thymidine Kinase Genes Transfered by Retroviral Vectors. J Natl Cancer Inst. 1990;82:297–300. doi: 10.1093/jnci/82.4.297. [DOI] [PubMed] [Google Scholar]
- 110.Trepel M, Stoneham CA, Eleftherohorinou H, et al. A heterotypic bystander effect for tumor cell killing after adeno-associated virus/phage-mediated, vascular-targeted suicide gene transfer. Mol Cancer Ther. 2009;8:2383–2391. doi: 10.1158/1535-7163.MCT-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang H, Dornadula G, Beumont M, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 112.Marcelin AG, Tubiana R, Lambert-Niclot S, et al. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma. AIDS. 2008;22:1677–1679. doi: 10.1097/QAD.0b013e32830abdc8. [DOI] [PubMed] [Google Scholar]
- 113.Paznekas WA, Boyadjiev SA, Shapiro RE, et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dasguptaa C, Martinezb AM, Zuppanb CW, et al. Identification of connexin43 (α1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE) Mutat Res. 2001;479:173–186. doi: 10.1016/s0027-5107(01)00160-9. [DOI] [PubMed] [Google Scholar]
- 115.Yang JJ, Huang SH, Chou KH, et al. Identification of mutations in members of the connexin gene family as a cause of nonsyndromic deafness in Taiwan. Audiol Neurootol. 2007;12:198–208. doi: 10.1159/000099024. [DOI] [PubMed] [Google Scholar]
- 116.Vaidya D, Tamaddon HS, Lo CW, et al. Null mutation of connexin43 causes slow propagation of ventricular activation in the late stages of mouse embryonic development. Circ Res. 2001;88:1196–1202. doi: 10.1161/hh1101.091107. [DOI] [PubMed] [Google Scholar]
- 117.Reaume AG, de Sousa P, Kulkarni S, et al. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 118.Lecandaa F, Warlowa PM, Sheikha S, et al. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities and osteoblast dysfunction. J Cell Biol. 2000;151:931–944. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mackay D, Ionides A, Kibar Z, et al. Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet. 1999;64:1357–1364. doi: 10.1086/302383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gong X, Li E, Klier G, et al. Disruption of α3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 121.Simon AM, Goodenough DA, Li E, et al. Female infertility in mice lacking connexin 37. Nature. 1997;385:525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- 122.Gollob MH, Jones DL, Krahn AD, et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med. 2006;354:2677–2688. doi: 10.1056/NEJMoa052800. [DOI] [PubMed] [Google Scholar]
- 123.Tamaddon HS, Vaidya D, Simon AM, et al. High-resolution optical mapping of the right bundle branch in connexin40 knockout mice reveals slow conduction in the specialized conduction system. Circ Res. 2000;87:929–936. doi: 10.1161/01.res.87.10.929. [DOI] [PubMed] [Google Scholar]
- 124.van Rijen HVM, van Veen TAB, van Kempen MJA, et al. Impaired conduction in the bundle branches of mouse hearts lacking the gap junction protein connexin40. Circ Res. 2001;103:1591–1598. doi: 10.1161/01.cir.103.11.1591. [DOI] [PubMed] [Google Scholar]
- 125.Shiels A, Mackay D, Ionides A, et al. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet. 1998;62:526–532. doi: 10.1086/301762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol. 1998;143:815–825. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shelley J, Dedek K, Schubert T, et al. Horizontal cell receptive fields are reduced in connexin57-deficient mice. Eur J Neurosci. 2006;23:3176–3186. doi: 10.1111/j.1460-9568.2006.04848.x. [DOI] [PubMed] [Google Scholar]
- 128.Fairweather N, Bell C, Cochrane S, et al. Mutations in the connexin 32 gene in X-linked dominant Charcot-Marie-Tooth disease (CMTX1) Hum Mol Genet. 1994;3:29–34. doi: 10.1093/hmg/3.1.29. [DOI] [PubMed] [Google Scholar]
- 129.Nelis E, Van Broeckhoven C, De Jonghe P, et al. Estimation of the mutation frequencies in Charcot-Marie-Tooth disease type 1 and hereditary neuropathy with liability to pressure palsies: a European collaborative study. Eur J Hum Genet. 1996;4:25–33. doi: 10.1159/000472166. [DOI] [PubMed] [Google Scholar]
- 130.Nelles E, Bützler C, Jung D, et al. Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc Natl Acad Sci USA. 1996;93:9565–9570. doi: 10.1073/pnas.93.18.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Temme A, Buchmann A, Gabriel HD, et al. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr Biol. 1997;7:713–716. doi: 10.1016/s0960-9822(06)00302-2. [DOI] [PubMed] [Google Scholar]
- 132.Sutor B, Schmolke C, Teubner B, et al. Connexin 47 (cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of cx47 and display vacuolized myelin in the CNS. Cereb Cortex. 2000;10:684–697. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gabriel H, Kupsch P, Sudendey J, et al. Mutations in the connexin26/GJB2 gene are the most common event in nonsyndromic hearing loss among the German population. Hum Mutat. 2001;17:521–522. doi: 10.1002/humu.1138. [DOI] [PubMed] [Google Scholar]
- 134.Kelsell DP, Dunlop J, Stevens HP, et al. Connexin 26 mutations in hereditary nonsyndromic sensorineural deafness. Nature. 1997;387:80–83. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- 135.Richard G, Rouan F, Willoughby CE, et al. Missense mutations in GJB2 encoding connexin-26 cause the ectodermal dysplasia keratitis-ichthyosis-deafness syndrome. Am J Hum Genet. 2002;70:1341–1348. doi: 10.1086/339986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maestrini E, Korge BP, Ocaña-Sierra J, et al. A missense mutation in connexin26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel’s syndrome) in three unrelated families. Hum Mol Genet. 1999;8:1237–1243. doi: 10.1093/hmg/8.7.1237. [DOI] [PubMed] [Google Scholar]
- 137.Gabriel HD, Jung D, Bützler C, et al. Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J Cell Biol. 1998;140:1453–1461. doi: 10.1083/jcb.140.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu XZ, Xia XJ, Xu LR, et al. Mutations in connexin31 underlie recessive as well as dominant nonsyndromic hearing loss. Hum Mol Genet. 2000;9:63–67. doi: 10.1093/hmg/9.1.63. [DOI] [PubMed] [Google Scholar]
- 139.Xia J, Liu C, Tang B, et al. Mutations in the gene encoding gap junction protein β-3 associated with autosomal dominant hearing impairment. Nat Genet. 1998;20:370–373. doi: 10.1038/3845. [DOI] [PubMed] [Google Scholar]
- 140.Plum A, Winterhager E, Pesch J, et al. Connexin31-deficiency in mice causes transient placental dysmorphogenesis but does not impair hearing and skin differentiation. Dev Biol. 2001;231:334–347. doi: 10.1006/dbio.2000.0148. [DOI] [PubMed] [Google Scholar]
- 141.del Castillo I, Villamar M, Moreno-Pelayo MA, et al. A deletion involving the connexin 30 gene in nonsyndromic hearing impairment. N Engl J Med. 2002;346:243–249. doi: 10.1056/NEJMoa012052. [DOI] [PubMed] [Google Scholar]
- 142.Teubner B, Michel V, Pesch J, et al. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet. 2003;12:13–21. doi: 10.1093/hmg/ddg001. [DOI] [PubMed] [Google Scholar]
- 143.Dere E, De Souza-Silva MA, Frisch C, et al. Connexin30-deficient mice show increased emotionality and decreased rearing activity in the open-field along with neurochemical changes. Eur J Neurosci. 2003;18:629–638. doi: 10.1046/j.1460-9568.2003.02784.x. [DOI] [PubMed] [Google Scholar]
- 144.Kumai M, Nishii K, Nakamura K, et al. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development. 2000;127:3501–3512. doi: 10.1242/dev.127.16.3501. [DOI] [PubMed] [Google Scholar]
- 145.Uhlenberg B, Schuelke M, Rüschendorf F, et al. Mutations in the gene encoding gap junction protein α12 (connexin 46.6) cause Pelizaeus-Merzbacher-like disease. Am J Hum Genet. 2004;75:251–260. doi: 10.1086/422763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Odermatt B, Wellershaus K, Wallraff A, et al. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci. 2003;23:4549–4559. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang WH, Yang JJ, Lin YC, et al. Identification of novel variants in the Cx29 gene of nonsyndromic hearing loss patients using buccal cells and RFLP method. Audiol Neurootol. 2010;15:81–87. doi: 10.1159/000231633. [DOI] [PubMed] [Google Scholar]
- 148.Güldenagel M, Ammermüller J, Feigenspan A, et al. Visual transmission deficits in mice with targeted disruption of the gap junction gene connexin36. J Neurosci. 2001;21:6036–6044. doi: 10.1523/JNEUROSCI.21-16-06036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kreuzberg MM, Schrickel JW, Ghanem A, et al. Connexin30.2 containing gap junction channels decelerate impulse propagation through the atrioventricular node. Proc Natl Acad Sci USA. 2006;103:5959–5964. doi: 10.1073/pnas.0508512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Go M, Kojima T, Takano K, et al. Connexin 26 expression prevents down-regulation of barrier and fence functions of tight junctions by Na+/K+-ATPase inhibitor ouabain in human airway epithelial cell line Calu-3. Exp Cell Res. 2006;312:3847–3856. doi: 10.1016/j.yexcr.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 151.Morita H, Katsuno T, Hoshimoto A, et al. Connexin 26-mediated gap junctional intercellular communication suppresses paracellular permeability of human intestinal epithelial cell monolayers. Exp Cell Res. 2004;298:1–8. doi: 10.1016/j.yexcr.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 152.Nhieu GTV, Clair C, Bruzzone R, et al. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol. 2003;5:720–726. doi: 10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- 153.Cohen-Salmon M, Regnault B, Cayet N, et al. Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci USA. 2007;104:6229–6234. doi: 10.1073/pnas.0605108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kojima T, Sawada N, Chiba H, et al. Induction of tight junctions in human connexin 32 (hCx32)-transfected mouse hepatocytes: connexin 32 interacts with occludin. Biochem Biophys Res Commun. 1999;266:222–229. doi: 10.1006/bbrc.1999.1778. [DOI] [PubMed] [Google Scholar]
- 155.Kojima T, Spray DC, Kokai Y, et al. Cx32 formation and/or Cx32-mediated intercellular communication induces expression and function of tight junctions in hepatocytic cell line. Exp Cell Res. 2002;276:40–51. doi: 10.1006/excr.2002.5511. [DOI] [PubMed] [Google Scholar]
- 156.Le ACN, Musil LS. Normal differentiation of cultured lens cells after inhibition of gap junction-mediated intercellular communication. Dev Biol. 1998;204:80–96. doi: 10.1006/dbio.1998.9030. [DOI] [PubMed] [Google Scholar]
- 157.Eugenin EA, Branes MC, Berman JW, et al. TNF-α plus IFN-γ induce connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses. J Immunol. 2003;170:1320–1328. doi: 10.4049/jimmunol.170.3.1320. [DOI] [PubMed] [Google Scholar]
- 158.Leaphart CL, Qureshilow F, Cetinlow S, et al. Interferon-γ inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology. 2007;132:2395–2411. doi: 10.1053/j.gastro.2007.03.029. [DOI] [PubMed] [Google Scholar]