Abstract
BRCA1 is a phosphoprotein involved in many biological processes, including transcription, ubiquitination, checkpoint control, homologous recombination, and DNA repair. We have demonstrated that protein phosphatase 1α (PP1α) interacts with BRCA1 via a PP1-binding motif 898KVTF901, and can dephosphorylate multiple serine residues phosphorylated by checkpoint kinases. A K898E germline missense variant in the PP1-binding motif of BRCA1 has been found in an Ashkenazi patient and a non-Ashkenazi Argentinean patient with breast and ovarian cancer, but its clinical significance is still unknown. Here we report that the lysine residue in the PP1-binding motif of BRCA1 is highly conserved across many mammalian species. The K898E mutation interferes with the interaction between BRCA1 and PP1α. Moreover, while the expression of wild-type BRCA1 in Brca1-deficient cells improved cell survival after DNA damage induced by ionizing radiation (IR), expression of BRCA1 K898E proved unable to enhance cell survival. DNA damage repair mechanisms remained defective in these BRCA1 K898E-reconstituted cells, as revealed by the comet assay and IR-induced Rad51 foci formation assay. These results reflect the significance of the interaction between BRCA1 and PP1, and indicate that the K898E variant may render carriers susceptible to DNA damage and malignant transformation.
The BRCA1 gene was mapped to human chromosome 17q21 by linkage analysis of early-onset familial breast cancer in 19901, and cloned in 19942 The full-length protein encoded by the BRCA1 gene is a nuclear phosphoprotein of 1863 amino acids, which participates in many key biological processes, including gene transcription, ubiquitination, checkpoint activation, DNA damage signaling, DNA recombination and repair3,4,5. Germline mutations occur throughout the BRCA1 gene, including frame-shift, nonsense or missense mutations, or splice-site alterations, and these mutations account for most cases of familial breast and ovarian cancer6.
BRCA1 plays an essential role in the repair of double-strand breaks (DSBs), particularly via the error-free homologous recombination (HR) mechanism. BRCA1 along with BRCA2 is involved in the recruitment of the Rad51 recombinase to DNA damage sites to repair DSBs by HR3,4,5. Therefore, BRCA1 is required for accurate DNA repair and BRCA1 deficiency may lead to hypersensitivity to DNA damaging agents, and this has become the scientific basis for designing cancer therapy for BRCA1 mutation carriers.
BRCA1 is expressed and phosphorylated in a cell cycle-dependent manner, with expression and phosphorylation levels rising from the G1/S boundary and falling dramatically in G0/G17,8. BRCA1 is also phosphorylated following DNA damage, by checkpoint kinases such as ATM, ATR, and hCds1/Chk23. We have demonstrated previously that BRCA1 is mainly hypophosphorylated during mitosis, and have identified a type 1 protein phosphatase (PP1) catalytic subunit, PP1α, which can interact with BRCA1 and dephosphorylate multiple serine residues phosphorylated by hCds1/Chk2 (S988) and ATM/ATR (e.g., S1524 and S1423)9,10. PP1 holoenzymes typically consist of catalytic subunits (PP1c) and regulatory (R) subunits. R subunits may modulate PP1c activity, target PP1c to substrates, or serve as substrates themselves. Many R subunits and PP1 substrates contain a PP1-binding consensus motif (R/K)(V/I)xF, which interacts with a hydrophobic groove on the PP1 catalytic subunit; this initial contact facilitates further interactions with secondary binding sites on PP1c that determine the activity and substrate specificity of the PP1 enzyme11. In vitro studies revealed that substituting the hydrophobic valine or phenylalanine residue with alanine weakened or abolished the ability of the PP1-binding motif to interact with PP1c. Deletion of the VxF motif also disrupted the interaction of the R subunit with PP1c12.
We and others have identified a PP1-binding motif 898KVTF901 in BRCA110,13, and our studies with PP1-non-binding BRCA1 mutants F901A and DEL (deletion of the 898KVTF901 motif) have demonstrated the importance of this 898KVTF901 motif to BRCA1-mediated DNA repair14. These mutations were generated in the laboratory, but are not present in humans. However, a germline missense variant, K898E, has been found in an Ashkenazi patient and a non-Ashkenazi Argentinean patient with breast and ovarian cancer (The Breast Cancer Information Core database and ref. 15). A single nucleotide substitution (A to G) results in a change of codon 898 from lysine (AAA) to glutamic acid (GAA). The clinical significance of this mutation is currently unknown. However, since lysine and glutamic acid have very different side-chain structures and electrostatic charges, this change is expected to cause significant alterations in local protein structures and electrostatic interactions.
In this study, we sought to determine whether the K898E variant affected the interaction of BRCA1 with the PP1α catalytic subunit, and further assessed the impact of this variant on the DNA repair function of BRCA1.
Results
The BRCA1 K898 residue is highly conserved in mammals
A cross-species comparison of BRCA1 homologs listed in the UniProtKB database revealed that the residues surrounding the PP1-binding motif 898KVTF901 are evolutionarily conserved, and K898 in particular is retained across all primates and mammals examined (Table 1). This demonstrates that K898 is highly conserved, suggesting that a critical mutation here might have a serious impact upon protein function. Surprisingly, F901, which is considered the most critical residue in a PP1-binding motif based on in vitro binding studies11,12, is the least conserved residue in the 898KVTF901 motif among different species analyzed.
Table 1. Lysine 898 is an evolutionarily conserved residue in BRCA1.
| Species | Accession Number | Starting Residue No. | Sequence | Ending Residue No. |
|---|---|---|---|---|
| Human | P38398 | 893 | KKQSPKVTFECEQKEE | 908 |
| Gorilla | Q6J6I8 | 893 | KKQSPKVTFECEQKEE | 908 |
| Orangutan | Q6J6J0 | 893 | KKQSPKVTFECEQKEE | 908 |
| Chimpanzee | Q9GKK8 | 893 | KKQSPKVTFEREQKEQ | 908 |
| Macaque | Q6J6I9 | 892 | KKQSPKVTSECEQKEE | 907 |
| Mouse | P48754 | 879 | KELSPKVTAKGKQKER | 894 |
| Rat | O54952 | 883 | REPSPKVTSRGEQKER | 898 |
| Pig | A5A751 | 884 | REQSPKVTHEGGQKDE | 899 |
| Horse | F6SQ43 | 894 | RKRSPKVTLKCGQKEG | 909 |
| Dog | Q95153 | 891 | GKQGPKVTLECGQKEE | 906 |
UniProtKB accession numbers for each BRCA1 homolog are provided. The starting and ending residue numbers refer to the positions of the first and last amino acids shown in the sequence. The putative PP1-binding motif is highlighted in bold.
Characterization of the K898E mutation
We generated a GST fusion protein GST-BF4 K898E, comprising amino acids 759 to 1064 of BRCA1 carrying the K898E variant. GST-BF4 fusion proteins, wild-type (WT) and K898E, were incubated with recombinant PP1α catalytic subunit (recPP1α). As shown in Figure 1a, WT GST-BF4 pulled down recPP1α as expected, whereas GST-BF4 K898E failed to pull down recPP1α. When a fusion protein of GFP and full-length BRCA1 (GFPBRCA1) was expressed along with PP1αmyc in COS-7 cells, GFP immunoprecipitation (IP) showed that the interaction between GFPBRCA1 K898E and PP1αmyc was only 38% (P < 0.03) of the interaction between WT GFPBRCA1 and PP1αmyc, a significant reduction (Figure 1b) also seen with the F901A or DEL mutants10.
Figure 1. The K898E mutation disrupts binding between BRCA1 and PP1α.
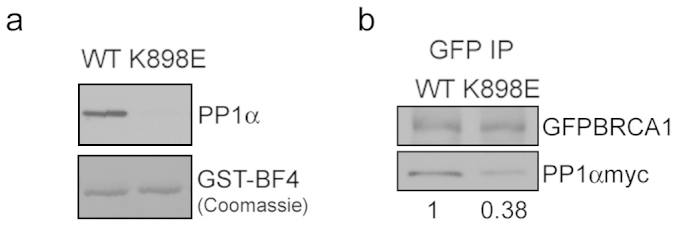
(a) GST pull-down assay. GST-BF4 proteins (WT and K898E) were employed to determine their interactions with recPP1α. Coomassie Blue staining indicates that approximately equal amounts of GST-BF4 fusion proteins were used. (b) Coimmunoprecipitation. The amount of PP1αmyc coimmunoprecipitated with GFPBRCA1 K898E was 0.38 ± 0.11 of that coimmunoprecipitated with WT GFPBRCA1 (mean ± SEM of two independent experiments, P < 0.03). Gels were run in similar conditions. The full- length immunoblots are shown in Supplementary Information section (Supplementary Fig. S2a and b online).
Reconstitution of BRCA1 K898E fails to enhance cell survival after IR in Brca1-deficient cells
Cells with dysfunctional BRCA1 are hypersensitive to IR and other forms of DNA damage16,17,18. However, this phenotype can be rescued upon expression of functional BRCA117,18. We proceeded to examine the impact of BRCA1 K898E on IR hypersensitivity in a Brca1-deficient mouse mammary tumor cell line 780 carrying targeted deletions of Brca1 exon 11, which is the largest exon encoding over 60% of amino acids, including the PP1-binding motif. To exclude the possibility of disruptions to BRCA1 function by a GFP tag, expression vectors of untagged WT or mutant (K898E, F901A, and DEL) full-length BRCA1 were constructed, and introduced into 780 cells by transfection. Transfection efficiency in 780 cells was consistenly in the range of 50–75% as determined using a GFP expression vector. Transient expression of WT or mutant BRCA1 did not affect cell growth or the cell cycle, as demonstrated by plating efficiencies shown in the Supplementary Information section (Supplentary Fig. S1 online). Western results shown in Figure 2a indicated that WT and mutant BRCA1 proteins were expressed at similar levels, and therefore the functional effects of the BRCA1 mutants cannot be attributed to decreased protein expression. Transfected 780 cells were then treated with 5 Gy of IR or left untreated. Cell survival was measured by colony formation 9 to 11 days after irradiation, and quantified by dividing the number of colonies in IR samples with the number of colonies in untreated control samples. As shown in Figure 2b, relative cell survival was 10.09% for cells transfected with an empty vector control (VC). This was significantly increased by more than 80%, to 18.35%, in cells expressing WT BRCA1 (VC vs. WT, P = 0.0022). However, K898E, F901A, and DEL reconstituted cells exhibited survival rates of 10.13%, 10.29%, and 10.87%, respectively, comparable to VC survival rates and significantly lower than WT (K898E vs. WT, P = 0.0029; F901A vs. WT, P = 0.011; DEL vs. WT, P = 0.014). These results were consistent with previous findings demonstrating decreased sensitivity to IR in BRCA1-deficient HCC1937 cells reconstituted with WT BRCA117, and inability of F901A and DEL mutant BRCA1 to overcome hypersensitivity to IR in Brca1-deficient 780 cells14. Importantly, BRCA1 K898E, like the F901A and DEL mutants, was unable to enhance post-IR survival in 780 cells, a phenotype also seen in a pathogenic BRCA1 C61G variant-reconstituted HCC1937 cells18.
Figure 2. Reconstitution of BRCA1 K898E is unable to reduce hypersensitivity to IR in Brca1-dysfunctional cells.
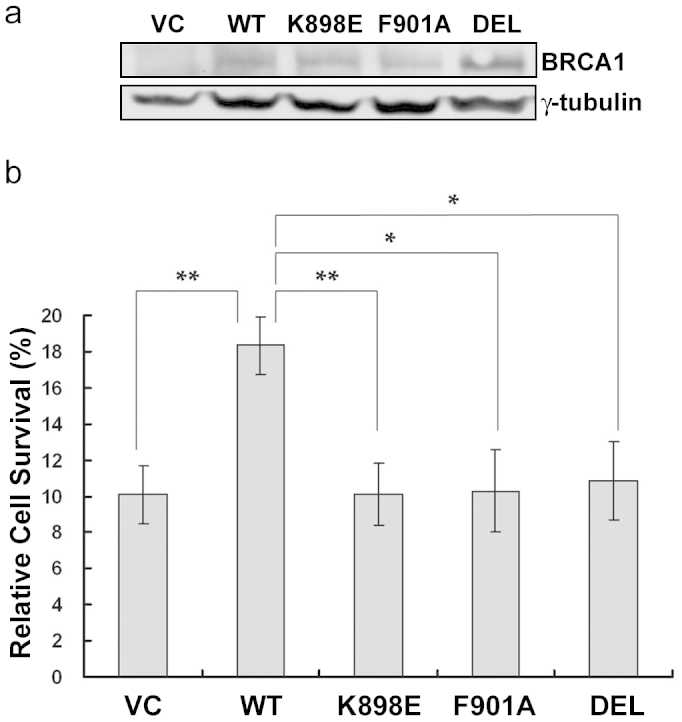
(a) WT and mutant BRCA1 proteins were expressed at similar levels in 780 cells. γ-tubulin was used as a loading control. Full length immunoblots are shown in Supplementary Information section (Supplementary Fig. S2c online). (b) Post-IR survival in WT and mutant BRCA1 expressing 780 cells measured by the colony formation assay. Results are presented as mean ± SEM of at least three independent experiments, with each experiment performed in triplicate. * P < 0.05, ** P < 0.01.
DNA repair is defective in 780 cells reconstituted with BRCA1 K898E
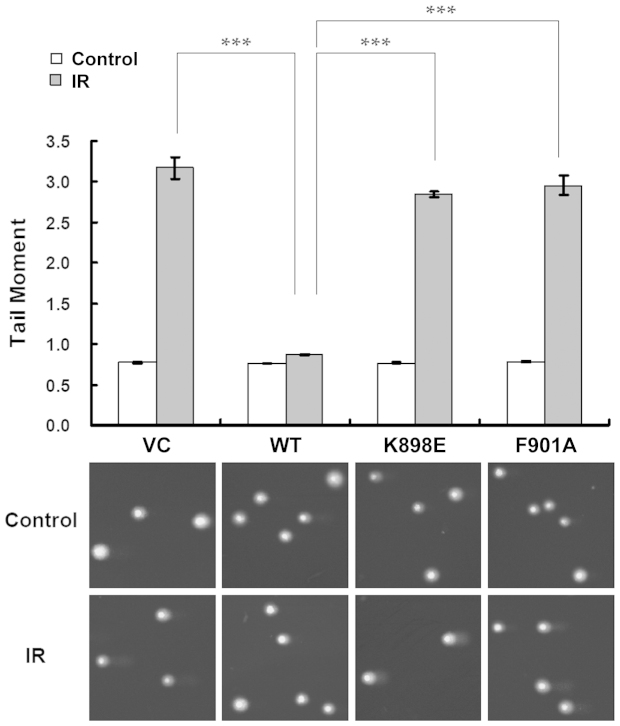
Next, we further examined the impact of K898E on the DNA repair function of BRCA1 using the comet assay. As illustrated in Figure 3, DNA damage levels, measured by tail moment, remained high for VC, K898E, and F901A-transfected 780 cells 6 h after IR, as compared to untreated controls (tail moments: VC-C = 0.770, VC-IR = 3.172; K898E-C = 0.765, K898E-IR = 2.846; F901A-C = 0.783, F901A-IR = 2.954). However, WT BRCA1-reconstituted 780 cells demonstrated robust DNA repair, with similar tail moment values for irradiated samples and untreated controls (tail moments: WT-C = 0.765, WT-IR = 0.872). Irradiated 780 cells with reconstituted WT BRCA1 displayed less visible comet tails than those transfected with VC, K898E, and F901A expression vectors, and the differences were statistically significant (two-tailed t-test for tail moments of IR samples: K898E vs. WT, P = 4 × 10−7; F901A vs. WT, P = 6 × 10−5; VC vs. WT, P = 7 × 10−5). This result suggests that, the same as VC and BRCA1 F901A-transfected 780 cells, DNA repair is defective in BRCA1 K898E-reconstituted 780 cells, further indicating that the binding interaction between BRCA1 and PP1 may be critical to DNA repair.
Figure 3. The K898E mutation affects BRCA1-mediated DNA repair determined by the comet assay.
Images were captured with a 20× objective and results are presented as mean ± SEM of at least three independent experiments. *** P < 0.001.
IR-induced Rad51 foci formation is impaired in 780 cells expressing BRCA1 K898E
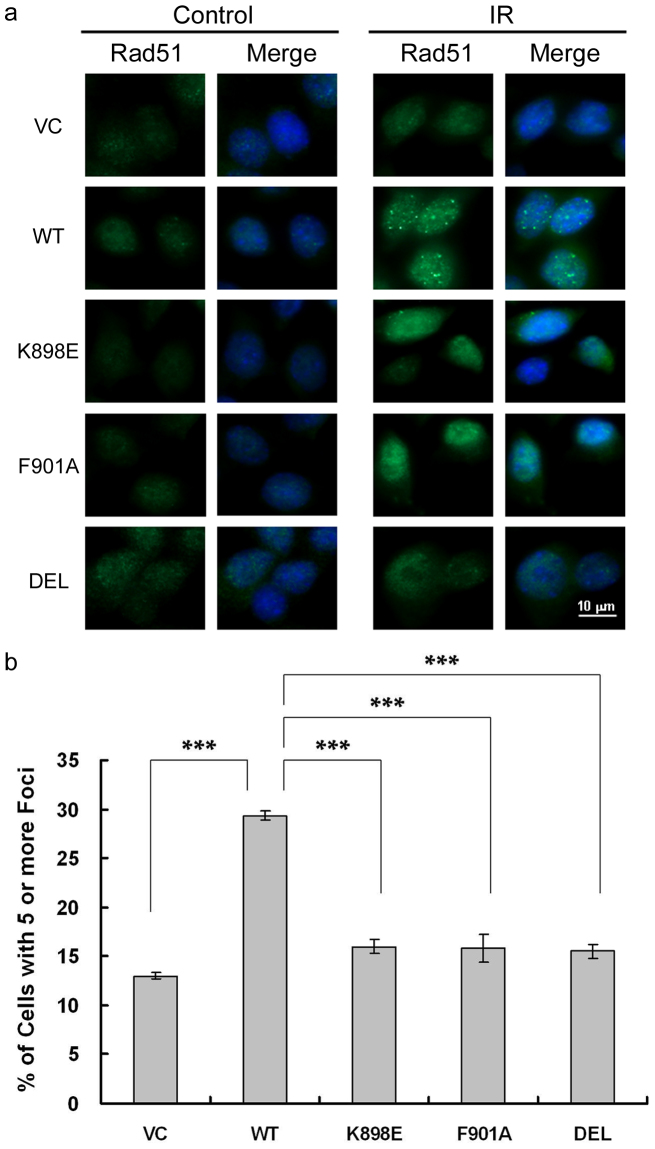
Having ascertained that the K898E mutation in BRCA1 disrupts BRCA1-mediated DNA repair, we then proceeded to take a closer look at the underlying mechanisms leading to this disruption, through the visualization of DNA repair foci after DNA damage. BRCA1 is responsible for recruiting DNA repair proteins, such as the Rad51 recombinase, to double-strand breaks (DSBs)3,4,5. Rad51 nuclear foci formation can thus serve as an indicator of DNA repair after DNA damage. WT and mutant (K898E, F901A, and DEL) BRCA1-reconstituted 780 cells were treated with 5 Gy of IR, and fixed 3 h later for immunofluorescence staining of Rad51. IR-induced Rad51 foci formation in BRCA1 K898E-reconstituted cells was diminished when compared with the WT BRCA1 expressing cells, and the same was observed for VC, as well as the F901A and DEL mutants (Figure 4a). Quantification of Rad51 foci formation revealed that the percentage of cells forming Rad51 foci after IR was 29.33% for WT, but only 13.00%, 16.00%, 15.83%, and 15.50% for VC, K898E, F901A, and DEL, respectively (Figure 4b). These significant decreases of ~50% indicate that the reduced post-IR survival and DNA repair seen in BRCA1 K898E-reconstituted 780 cells may be due to an impaired ability to recruit Rad51 in response to DNA damage.
Figure 4. IR-induced Rad51 foci formation is impaired by the BRCA1 K898E mutation.
(a) Images of IR-induced Rad51 foci in transfected 780 cells. Scale bar: 10 μm. (b) The percentage of transfected 780 cells with 5 or more Rad51 foci. Results are presented as mean ± SEM of at least three independent experiments. *** P < 0.001.
Discussion
Our experimental results suggest that the interaction between BRCA1 and PP1 may be essential to BRCA1-mediated DNA repair. Previous studies have already shown that both an F901A mutation and an engineered deletion of the 898KVTF901 sequence in BRCA1 can abolish BRCA1-PP1 binding and disrupt BRCA1-mediated DNA damage signaling and repair10,14. While these mutations have not been identified in humans, two cases of the germline K898E variant have been identified in a Ashkenazi patient (The Breast Cancer Information Core database) and a non-Ashkenazi Argentinean patient15 with breast and ovarian cancer. Previous in silico predictions regarding the effect of mutations at the PP1-binding motif have been contradictory. Using the SIFT program and the ancestral sequence method, Pavlicek et al. predicted that F901A could be tolerated, whereas K898E could be deleterious19. In contrast, Solano et al. reported a case of the K898E variant in the Argentinean patient who also carried a deleterious BRCA2 mutation and they predicted that K898E could be tolerated using the SIFT program15. Our cross-species comparison of BRCA1 homologs has shown that the phenylalanine residue in the PP1-binding motif of BRCA1 is not evolutionarily conserved; whereas, K898E is highly conserved in mammals (Table 1). Furthermore, we also provide the first experimental evidence to demonstrate that the K898E variant causes defects in DNA repair and could possibly be a disease-causing mutation.
The K898E mutation was found to disrupt the binding interaction between BRCA1 and PP1. BRCA1 K898E was unable to mitigate hypersensitivity to IR in Brca1-deficient 780 cells. DNA repair defects and reduced Rad51 foci formation after IR were also observed in BRCA1 K898E-expressing 780 cells. We have also demonstrated that knockdown of PP1α by siRNA reduces nuclear Rad51 foci induced by DNA damage in a BRCA1-proficient human ovarian cancer cell line ES214. Taken together, these results affirm the importance of BRCA1-PP1 binding to the BRCA1-mediated DNA damage response. The dramatic impact of K898E on BRCA1-mediated DNA repair indicates that this variant has the potential to be cancer-predisposing, although additional clinical information would be necessary to confirm this. To our knowledge, this is the first demonstration that a naturally occurring mutation located in a PP1-binding motif, particularly at the lysine/arginine residue, may contribute to cancer development.
It has been reported recently that an autosomal dominant polycyctic kidney disease-associated mutation, occurring at the phenylalanine residue of the PP1-binding motif of polycystin-1, reduces its dephosphorylation by PP1α but does not affect binding20. However, mutations of the PP1-binding motif not only affect BRCA1 interaction with PP1α but may also reduce BRCA1 dephosphorylation as the PP1-binding motif-deleted BRCA1 protein (BRCA1GFP DEL) displayed increased S1423 phosphorylation at the basal level and after hydroxyurea treatment when expressed in the ES2 ovarian cancer cell line10. BRCA1 interacts with many proteins, and it is possible that inappropriate phosphorylation patterns may affect these interactions, thereby impacting other BRCA1 functions such as transcription or E3 ubiquitin ligase activity3,4,5,21. These additional adverse effects to BRCA1 function may underlie the DNA repair deficiencies observed for K898E. Given that the BRCT domain of BRCA1 is involved in the binding of phosphoproteins22,23, it is also possible that BRCA1 may serve as a targeting subunit for PP1 in the dephosphorylation of phosphoproteins participating in checkpoint control and the DNA damage response. Alternatively, it is also likely that the K898E variant may also cause DNA repair dysfunction via other mechanisms, such as affecting BRCA1 interactions with other proteins independent of PP1-binding.
Puzzlingly, F901A in human BRCA1 also decreases BRCA1 interaction with PP1α and causes DNA repair defects10,14 similar to the K898E variant (Figures 3–4); however, the equivalent PP1-binding motif sequence in mouse Brca1 is KVTA (Genbank accession no.: NM_009764), raising a question of why F901A is tolerated in the mouse if the PP1-binding motif is functionally significant. As mentioned above, the PP1-binding motif serves as an initial contact with PP1c to facilitate further interactions with secondary binding sites on PP1c11. It is possible that secondary binding sites in mouse Brca1 may compensate for the reduced binding affinity of the initial binding site. Further investigations are required to clarify this issue.
PP1α is a highly conserved protein with 330 amino acid residues. Human and mouse PP1α only differ in amino acid 113 (lysine in human and arginine in mouse), which is considered a conserved substitution (NP_002699.1 and NP_114074.1). In contrast, BRCA1 is among the most weakly conserved protein-coding genes in the mammalian genome, with only 55.8% amino acid sequence identity between human and mouse24. Even though the BRCA1 K898 residue is highly conserved across mammals, the amino acid sequences around the PP1-binding motif are either missing or not conserved in lower organisms24. Phosphorylation of BRCA1 S1423 is considered critical for the IR-induced G2-M checkpoint25; however, it is not conserved in the mouse either (asparagine instead)24. Thus, some regulatory mechanisms of BRCA1 functions may develop late in evolution.
Cancers with the K898E variant or other BRCA1 mutations that impair BRCA1-PP1 binding might be particularly sensitive to DNA damage, such as that induced by radiation therapy or platinum-based agents. Diminished BRCA1-PP1 binding ability might have potential as a biomarker for recommending the use of anticancer therapies targeting DNA.
Methods
Construction of vectors
GST-BF4K898E was constructed using site-specific mutagenesis by overlap extension, a two-step PCR approach, to generate the mutant BF4 DNA fragment, which was then subcloned into the BamHI and SalI sites of pGEX-5X-3 (Amersham Biosciences/GE Healthcare, Piscataway, NJ, USA) as described previously10. The untagged full-length WT human BRCA1 expression vector was constructed by subcloning the BRCA1 cDNA sequence with stop codon into pEGFP-N1-KS(−)26. The mutant full-length BRCA1 expression vectors were constructed by replacing a KpnI-ScaI fragment of ~830 bp in the WT expression vector with that from GST-BF4K898E, GST-BF4F901A, or GST-BF4DEL, which contained the mutations. The GFPBRCA1K898E expression vector was generated by replacing a 4.2 kb AflII-HindIII fragment in the GFPBRCA1 expression vector14 with that from the BRCA1K898E vector. All plasmid clones were sequence-verified.
GST pull-down assay
GST fusion proteins were expressed in Escherichia coli and purified using glutathione Sepharose beads (Amersham Biosciences/GE Healthcare, Piscataway, NJ, USA), according to the instructions provided by the manufacturer, and 1–2 μg of WT and mutant GST-BF4 proteins were respectively incubated with 0.5 μl of recPP1α (2.5 U/μl, specific activity: ~15 U/μg, New England BioLabs, Ipswich, MA, USA) and glutathione Sepharose beads in GST binding buffer containing 25 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1 mM DTT, 0.1% NP40, and 0.1 mg/ml BSA for 2 hours at 4°C, then washed with L buffer (PBS with 0.1% NP40 and 0.1% Triton X-100) as previously described9,10 and subjected to SDS-PAGE and Western analysis. A goat polyclonal PP1α antibody (C-19, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used for the detection of PP1α.
Cell culture and transfection
COS-7 and 780 cells were cultured in DMEM supplemented with 10% FBS and 2 mM L-glutamine. Cells at 70–80% confluence were subjected to transfection using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA) and subsequent experiments were performed 24 to 48 h post-transfection.
Coimmunoprecipitation
COS-7 cells expressing exogenous GFPBRCA1 and myc-tagged PP1α were harvested and lysed in L buffer supplemented with protease inhibitors, followed by brief sonication. Cleared cell lysates were subjected to IP using a GFP antibody (Clontech, Mountain View, CA, USA), followed by SDS-PAGE and Western analysis10. GFPBRCA1 was detected using a BRCA1 antibody MS110 (Calbiochem, San Diego, CA, USA) and PP1αmyc was detected using a c-myc antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Western analysis
Cells were lysed in L buffer or RIPA buffer containing protease inhibitors and lysates were subjected to 7.5% or 4–12% SDS-PAGE. Western analysis was performed using BRCA1 (MS110, Calbiochem, San Diego, CA, USA), PP1α (C-19), c-myc (9E10), and γ-tubulin (Sigma, St Louis, MO) antibodies. Quantitative analysis was performed using Quantity One (Bio-Rad, Hercules, CA, USA) software.
Cell survival (colony formation) assay
Transfected 780 cells were trypsinized 24 h after transfection, and 500 (for untreated controls, C) or 1000 cells (for irradiated samples, IR) were plated to T25 flasks in triplicate. One day after plating, IR samples were irradiated with 5 Gy of gamma ray using a cesium irradiator (IBL 637, CIS Bio International, Saclay, France), at a dose rate of ~3 Gy/min. Colonies were fixed in cold methanol and stained with Giemsa 9 to 11 days post-IR, and those with >50 cells were counted. Relative cell survival was calculated as the ratio of IR to C.
Comet assay
Transfected 780 cells were subjected to 5 Gy of IR or left untreated and harvested 6 h after irradiation, and 3000 cells from each sample were subjected to the alkaline comet assay as described14. Following electrophoresis, DNA was stained with SYBR Green. Comet images were captured with a 20× objective on a Carl Zeiss Axioscope A1 upright fluorescent microscope (Carl Zeiss GmbH, Jena, Germany) and tail moment values for 200 randomly selected cells per sample were scored using CometScore version 1.5 software (TriTek Corp., Sumerduck, VA, USA). Tail moment is defined as the product of tail length and fraction of total DNA in the tail, and serves as a quantitative measure of DNA damage. Mean tail moment values were used to evaluate DNA repair capability.
Rad51 foci formation assay
780 cells were trypsinized 24 h after transfection and plated into 8-well chamber slides (1.5 × 105 cells per well). Two slides were prepared, and cells were allowed to attach overnight. One slide was then subjected to IR and another was left untreated as the control slide. Cells were fixed and subjected to Rad51 staining 3 h after irradiation as previously described14. A rabbit polyclonal anti-Rad51 (H-92, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as the primary antibody, and FITC-conjugated goat anti-rabbit IgG (SouthernBiotech, Birmingham, AL, USA) was used as the secondary antibody. Slides were counter-stained with DAPI and mounted with antifade (Invitrogen, Carlsbad, CA, USA). Approximately 200 cells (ranging from 195 to 220 cells) were scored for each sample, and the percentages of cells with at least five Rad51 nuclear foci were calculated. Cell images were captured with a 100× objective.
Statistical analysis
Data were presented as mean ± SEM (standard error of the mean) of at least two to three independent experiments. Two-tailed t-tests were used to assess the significance of the data, and P-values < 0.05 were considered to be statistically significant.
Author Contributions
B.Y.C., C.X.D. and L.C.H. generated research materials, conceived and designed the studies. B.Y.C. and L.C.H. performed the experiments. C.H.H., Y.H.L. and C.C.H. provided technical assistance and discussion. B.Y.C. and L.C.H. wrote the manuscript and prepared the table and figures. All authors reviewed the manuscript.
Supplementary Material
pdf file
Acknowledgments
This study was supported by NCI Grant R01CA111436, Taiwan National Science Council grants 97-2314-B-002-183- and 98-2320-B-002-055-, and funds from the National Taiwan University (to L.C.H.). We would also like to acknowledge the National Taiwan University Hospital Sequencing Core Facility for providing DNA sequencing service.
References
- Hall J. M. et al. Linkage of early-onset familial breast cancer to chromosome 17q21. Science 250, 1684–1689 (1990). [DOI] [PubMed] [Google Scholar]
- Miki Y. et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266, 66–71 (1994). [DOI] [PubMed] [Google Scholar]
- Zhang J. & Powell S. N. The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol. Cancer Res. 3, 531–539 (2005). [DOI] [PubMed] [Google Scholar]
- Deng C. X. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 34, 1416–1426 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Sato K. & Wu W. The BRCA1 ubiquitin ligase and homologous recombination repair. FEBS Lett. 585, 2836–2844 (2011). [DOI] [PubMed] [Google Scholar]
- Collins F. S. BRCA1--lots of mutations, lots of dilemmas. N. Engl. J. Med. 334, 186–188 (1996). [DOI] [PubMed] [Google Scholar]
- Chen Y. et al. BRCA1 is a 220-kDa nuclear phosphoprotein that is expressed and phosphorylated in a cell cycle-dependent manner. Cancer Res. 56, 3168–3172 (1996). [PubMed] [Google Scholar]
- Ruffner H. & Verma I. M. BRCA1 is a cell cycle-regulated nuclear phosphoprotein. Proc. Natl. Acad. Sci. USA 94, 7138–7143 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Virshup D. M., White R. L. & Hsu L. C. Regulation of BRCA1 phosphorylation by interaction with protein phosphatase 1α. Cancer Res. 62, 6357–6361 (2002). [PubMed] [Google Scholar]
- Hsu L. C. Identification and functional characterization of a PP1-binding site in BRCA1. Biochem. Biophys. Res. Commun. 360, 507–512 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen M. Combinatorial control of protein phosphatase-1. Trends Biochem. Sci. 26, 426–431 (2001). [DOI] [PubMed] [Google Scholar]
- Egloff M. P. et al. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 16, 1876–1887 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter S. L., Bosnoyan-Collins L., Pinnaduwage D. & Andrulis I. L. The interaction of PP1 with BRCA1 and analysis of their expression in breast tumors. BMC Cancer 7, 85 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. M., Pace S. M., Allen S. R., Deng C. X. & Hsu L. C. A PP1-binding motif present in BRCA1 plays a role in its DNA repair function. Int. J. Biol. Sci. 4, 352–361 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano A. R. et al. BRCA1 and BRCA2 analysis of Argentinean breast/ovarian cancer patients selected for age and family history highlights a role for novel mutations of putative south-American origin. Springerplus 1, 20 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. X. et al. A targeted disruption of the murine Brca1 gene causes γ-irradiation hypersensitivity and genetic instability. Oncogene 17, 3115–3124 (1998). [DOI] [PubMed] [Google Scholar]
- Scully R. et al. Genetic analysis of BRCA1 in a defined tumor cell line. Mol. Cell 4, 1093–1099 (1999). [DOI] [PubMed] [Google Scholar]
- Ruffner H., Joazelro C. A. P., Hemmati D., Hunter T. & Verma I. M. Cancer-predisposing mutations within the RING domain of BRCA1: loss of ubiquitin protein ligase activity and protection from radiation hypersensitivity. Proc. Natl. Acad. Sci. USA 98, 5134–5139 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlicek A. et al. Evolution of the tumor suppressor BRCA1 locus in primates: implications for cancer predisposition. Hum. Mol. Genet. 13, 2737–2751 (2004). [DOI] [PubMed] [Google Scholar]
- Parnell S. C., Puri S., Wallace D. P. & Calvet J. P. Protein phosphatase-1α interacts with and dephosphorylates polycystin-1. PLoS One 7, e36798 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran S., Crone D. E., Palazzo R. E. & Parvin J. D. Aurora-A kinase regulates breast cancer associated gene 1 inhibition of centrosome-dependent microtubule nucleation. Cancer Res. 67, 11186–11194 (2007). [DOI] [PubMed] [Google Scholar]
- Manke I. A., Lowery D. M., Nguyen A. & Yaffe M. B. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science 302, 636–639 (2003). [DOI] [PubMed] [Google Scholar]
- Yu X., Chini C. C., He M., Mer G. & Chen J. The BRCT domain is a phospho-protein binding domain. Science 302, 639–642 (2003). [DOI] [PubMed] [Google Scholar]
- Abkevich V. et al. Analysis of missense variation in human BRCA1 in the context of interspecific sequence variation. J. Med. Genet. 41, 492–507 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Kim S. T. & Kastan M. B. Involvement of Brca1 in S-phase and G2-phase checkpoints after ionizing irradiation. Mol. Cell. Biol. 21, 3445–3450 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L. C., Doan T. P. & White R. L. Identification of a γ-tubulin-binding domain in BRCA1. Cancer Res. 61, 7713–7718 (2001). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
pdf file