Abstract
The zebrafish (Danio rerio) is increasingly used as a model in neurobehavioral and neuroendocrine studies. The inhibitory avoidance paradigm has been proposed as tool to study mechanisms underlying learning and memory in zebrafish. In this paradigm subjects receive a shock after entering the black compartment of a black-white box. On the next day, latency to enter the black compartment is assessed; higher latencies are indicative of increased avoidance learning. Here, we aimed to understand the effects of different shock intensities (0, 1, 3, and 9 V) and to unravel variation in inhibitory avoidance learning in an in-house reared Tuebingen Long-Fin zebrafish (D. rerio) strain. While median latencies had increased in the 1, 3, and 9 V groups, no increase in median latency was found in the 0 V group. In addition, higher shock intensities resulted in a higher number of avoiders (latency ≥180 s) over nonavoiders (latency <60 s). Both changes are indicative of increased avoidance learning. We assessed whole-body cortisol content and the expression levels of genes relevant to stress, anxiety, fear, and learning 2 h after testing. Shock intensity was associated with whole-body cortisol content and the expression of glucocorticoid receptor alpha [nr3c1(alpha)], cocaine- and amphetamine-regulated transcript (cart4), and mineralocorticoid receptor (nr3c2), while avoidance behavior was associated with whole-body cortisol content only. The inhibitory avoidance paradigm in combination with measuring whole-body cortisol content and gene expression is suitable to unravel (genetic) mechanisms of fear avoidance learning. Our data further show differences in brain-behavior relationships underlying fear avoidance learning and memory in zebrafish. These findings serve as starting point for further unraveling differences in brain-behavior relationships underlying (fear avoidance) learning and memory in zebrafish.
Introduction
The zebrafish (Danio rerio) is increasingly used as a model in (neuro)behavioral,1,2 neurobiological,3 and genetic research.4 This teleost shows relatively high genetic homology to humans5 and has many advantages over the use of rodents, such as low (maintenance) cost, easy handling, short reproduction cycle, and high fecundity. In addition, its genome,6 transcriptome,7 and proteome8 have been described, making the species a model of choice for behavioral research linked to genetics.
An emerging field of interest in zebrafish research is learning and memory related to anxiety and fear,9,10 which has been studied, among others, using inhibitory avoidance.11–13 Inhibitory avoidance is measured by the latency of an individual to enter a conditionally aversive environment.14 Recently an inhibitory avoidance response was observed in a single-trial paradigm in which zebrafish (AB strain) learned to avoid swimming from a white to a black compartment to avoid a 3-V electric shock.11–13 It has been shown that fear-avoidance learning is mediated by activation of the N-methyl-D-aspartic acid (NMDA) receptor and that administration of NMDA antagonists, such as MK-801, prevents the formation of fear avoidance11,13 and alters behavioral performance.15 Other pharmacological studies in teleosts show that disrupting cell adhesion molecules16 or protein synthesis17 may also interfere with memory formation or consolidation. The inhibitory avoidance paradigm has therefore been proposed as tool to study mechanisms underlying learning and memory formation in zebrafish and as screen for drugs affecting these mechanisms.
Although inhibitory avoidance is well studied in rodents, knowledge thereof is limited in zebrafish.11–13 Inhibitory avoidance in zebrafish is based on a conflict between the innate response to enter a dark (black) environment to avoid a brightly lit (white) environment (anxiety18,19) and avoiding a shock received in the dark environment (fear; shock-environment learning). We studied the effects of different shock intensities (0, 1, 3, and 9 V) on inhibitory avoidance learning in an in-house-reared Tuebingen Long-Fin (TLF) zebrafish strain to manipulate this conflict. We predicted that increasing shock intensities from 1 to 9 V would increase inhibitory avoidance as shock-induced fear would overrule innate anxiety. We assumed that no avoidance behavior would occur in a 0-V group, which served as control group for the training procedure per se. Data from our laboratory20 have suggested differences in inhibitory avoidance learning between individuals. Whether these differences are task specific or an expression of differences in coping styles21–23 is currently not clear. Therefore, we aimed at further unraveling these differences in inhibitory avoidance learning by assessing differences across shock intensities and measuring whole-body cortisol content and gene expression. We assessed whole-body cortisol content and studied gene expression levels in the telencephalon 2 h after inhibitory avoidance. This sampling moment was chosen according to Morsink et al.,24 who showed that between 1 and 3 h a sufficient number of nonimmediate early genes can be analyzed following a challenge. We further assumed that by measuring 2 h after the task, differences in whole-body cortisol content and gene expression between groups would be closely associated with differences in inhibitory avoidance behavior. In rodents, the expression of the immediate-early gene c-Fos is one such marker used to determine differences between treatments.25
The following genes relevant for behavioral differences22,23 and task-related features, such as stress, anxiety, fear, and fear conditioning,26–32 were analyzed in the telencephalon: brain-derived neurotrophic factor (bdnf), glucocorticoid receptor alpha [nr3c1(alpha)], glucocorticoid receptor beta [nr3c1(beta)], mineralocorticoid receptor (nr3c2), cocaine- and amphetamine-regulated transcript (cart4), and serotonin receptor 1ab (htr1ab). Analysis of telencephalic gene expression specifically, rather than of whole brain, was chosen as the telencephalon is involved in learning and memory and fear conditioning (lateral and medial zone of the dorsal pallium33–35). When differences in inhibitory avoidance learning are robust, this would translate to consistent differences in whole-body cortisol content and gene expression across shock intensity. In addition, these analyses could reveal differences in the stress response or expression of genes related to shock intensity per se and provide a starting point for further studies on inhibitory avoidance learning in the zebrafish.
Materials and Methods
Ethical approval
Experimental procedures were approved by the ethical committee of the Radboud University Nijmegen (RU-DEC 2013-179) and were conducted in line with Dutch law (Wet op de Dierproeven 1996) and European regulations (Directive 86/609/EEC).
Animals and housing
For our experiment we used an in-house-reared TLF zebrafish strain. Fish were a mix of offspring from two parental couples and hatched within the same week. At the age of 6 months, 120 animals (males and females) were pooled and randomly assigned to three different aquaria, each consisting of two compartments (n=18–21 fish per compartment). Fish were housed in groups and not individually to avoid confounding effects when shoaling behavior cannot be displayed.36 An aquarium (100×50×60 cm3) was separated in two halves by a blackened glass plate, resulting in two equally sized compartments (50×50×60 cm3). In each aquarium the water level was brought to 30 cm, to realize a total water volume of 75 L per compartment. Each compartment received an aeration stone and was provided with independent water inflow and outflow (26°C, pH 8.0); inflowing water had passed a biological filter (300-L volume). The photoperiod was kept at 12L:12D (lights on from 07:00 to 19:00 hour) with feeding moments at 09:00 hour (artemia) and 15:00 hour (TetraMin; Tetra, Melle, Germany).
Experimental groups
A total of six groups was used in the experiment. Three experimental groups were used to test for inhibitory avoidance and received either a 1 (n=19), 3 (n=21), or 9 V (n=19) electric shock upon entering the black compartment. Two sham treatment groups (0 V; n=20 and n=18) were included to control for procedural effects such as habituation to the experimental tank and netting.37,38 These controls were handled similarly to the experimental groups, except that they did not receive a shock when entering the black compartment. One group was tested at the beginning of the experiment while the other was tested at the end. These groups did not show statistical differences (Mann–Whitney U-test) in avoidance behavior [latencies on day 1 (U=128.5; p=0.14) and day 2 (U=156.0; p=0.49)] and data were therefore pooled for behavioral analyses (0 V; n=38). For gene expression and whole-body cortisol content, only the first group (n=20) was used. Finally, an unexposed control group (n=20) was used for whole-body cortisol content and gene expression analysis. This group was never exposed to the inhibitory avoidance paradigm. The unexposed control group provided baseline values to control for possible effects caused by introduction into the inhibitory avoidance tank (which is a novel environment).
Inhibitory avoidance paradigm
Inhibitory avoidance learning was assessed between 11.00 and 13.00 hours under normal light conditions (∼400 lux; measured at the water surface). For inhibitory avoidance learning, a previously described protocol was used11 with minor modifications (procedure: Fig. 1A). In short, an aquarium (60×30×30 cm3; 10-cm water level) was split into two equal compartments, separated by a manually operated sliding door (schematic overview, Fig. 1B). Surfaces of one compartment were white, while surfaces of the other compartment were black. Compartments were not covered by a lid. The black compartment contained two metal plates (covered with black sound box mesh to prevent light reflection) that covered two opposite walls completely. Both plates were wired to a power source that allowed for 1-, 3-, or 9-V AC to be put between the plates in the water (measured at the middle of the tank; across a distance of 15 cm between electrodes). Inhibitory avoidance learning was assessed for each fish individually (i.e., not as a group).
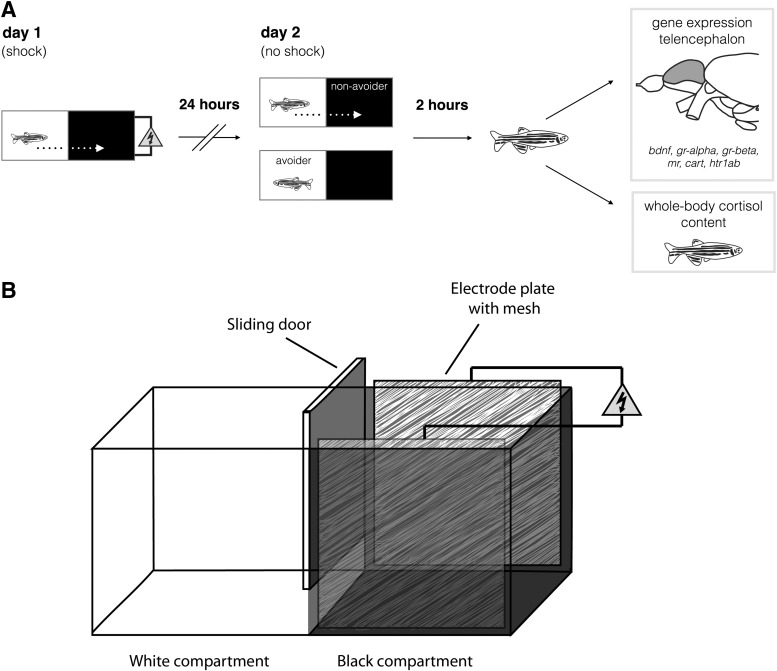
FIG. 1.
(A) Representation of the experimental setup. On day 1 fish were trained in the inhibitory avoidance paradigm by receiving a shock upon entering the dark compartment. On day 2 fish were assessed for inhibitory avoidance learning. Fish were selected on basis of their behavior: nonavoider (<60 s latency) and avoider (≥180 s latency). Two hours postinhibitory avoidance, fish were sampled for assessment of telencephalic gene expression (brain-derived neurotrophic factor [bdnf], serotonin receptor 1ab [htr1ab], cocaine- and amphetamine-regulated transcript [cart4], mineralocorticoid receptor [nr3c2], and the glucocorticoid receptor alpha [nr3c1(alpha)] and glucocorticoid receptor beta [nr3c1(beta)]) and whole-body cortisol content. (B) Schematic overview of the glass inhibitory avoidance tank (60×30×30 cm3). The two compartments were created by covering the bottom, the walls, and the corresponding side of the sliding door with black or white self-adhesive plastic film. Two electrode plates, camouflaged with black sound box mesh, covered the two opposing walls in the black compartment. An electric (1, 3, or 9 V) shock could be applied via a manually operated electric stimulator.
On day 1, fish were individually placed in the white compartment with the sliding door closed. After 60 s (acclimation period) the sliding door was lifted, giving the fish free access to the black compartment. Once the fish had completely entered the black compartment, the sliding door was closed and an electric shock (1-, 3-, or 9-V AC) was given for 5 s after which the fish was returned to its home tank. Fish that would not enter the black compartment within 180 s were excluded from further experimentation. For each fish the latency time to enter the black compartment was recorded. Higher latencies indicate that animals are less anxious in the white compartment.
On day 2, fish were individually placed in the white compartment. After 60 s the sliding door was opened and the fish was given 180 s to enter or avoid the black compartment. This time, when the fish entered the black compartment no shock was given. Instead, fish were taken out of the experimental tank, either immediately after entering the black compartment or after 180 s in the white compartment, and temporarily housed in one of three tanks based on their latency time (see “Avoider/nonavoider fish” heading). Two hours after the test (see “Tissue collection and preparation” heading) fish were euthanized for sample collection. For each fish the latency to enter the black compartment was recorded. Higher latencies are indicative of increased inhibitory avoidance learning.
Operation of the sliding door was done by hand; visual observation was done in real-time and recorded on-site. Recording of latency times was done by stopwatch (by a single experimenter; R.M.). All procedures were carried out in a manner that caused the least possible disturbance of fish during the experiment.
Avoider/nonavoider fish
On day 2 of the inhibitory avoidance paradigm, after the test, fish were temporarily housed based on their latency in one of three tanks until sampling 2 h later. Based on previous experiments20 fish were classified into three categories; avoider fish did not enter the black compartment within 180 s, semiavoider fish entered the black compartment between 60 and 180 s, and nonavoider fish entered the black compartment within 60 s. In this study we included only the avoiders and nonavoiders to obtain maximal differences in whole-body cortisol content and telencephalic gene expression.39 Semiavoiders were therefore excluded from the analyses of avoider/nonavoider ratios, whole-body cortisol content, and gene expression.
Tissue collection and preparation
Anesthesia was given by placing 8–10 fish from each group in water containing 2-phenoxyethanol (0.1% v/v). Once deeply anesthetized, fish were killed by spinal transection and the telencephalon was dissected, snap-frozen in liquid nitrogen and stored at −80°C until further analysis. The remains of the fish were collected and stored at −80°C for whole-body cortisol content analysis (“Whole-body cortisol content” heading).
Whole-body cortisol content
A previously published protocol40 was modified and used for whole-body cortisol extraction. Briefly, frozen zebrafish (stored at −80°C) were thawed on ice and homogenized individually in polystyrene tubes (12 mL; Greiner-Bio-One, Frickenhausen, Germany) containing 1 mL PBS (80 mM Na2HPO4, 20 mM NaH2PO4, and 100 mM NaCl; pH=7.4) with a microblender. The homogenate was mixed with 4 mL methanol (J.T. Baker, Deventer, The Netherlands) and stored at 4°C for 1 h. Subsequently, the mixture was centrifuged (4°C, 3000 rpm, 5 min) and the supernatant was collected. The pellet was resuspended in 4 mL methanol and left at 4°C for 30 min followed by centrifugation and collection of the supernatant (this step was repeated twice.). The collected methanol (16-mL end volume) was evaporated under N2 gas, leaving a residue containing steroids, which was reconstituted in 200 μL radioimmunoassay buffer (100 mM Tris, 0.9% NaCl, 0.1% 8-anilino-1-naphthalenesulfonic acid, and 0.02% NaN3, pH=7.4).
Cortisol was measured as previously described.41 Briefly, 96-well microtiter plates were coated with monoclonal mouse antibodies to cortisol (Abcam, Cambridge, MA) in coating buffer. Plates were cleared of coating buffer and washed with a wash buffer before blocking possible nonspecific binding sites with blocking buffer. Wells were cleared of blocking buffer and 10 μL of standard or sample, together with 90 μL of 3H-cortisol tracer, was added to designated wells. Nonspecifics received assay buffer (10 μL) and tracer only. After the incubation period wells were emptied and washed before scintillation liquid was added and radioactivity was measured with a β-counter (detection limit=4 ng/mL; interassay VC=12.5% and intraassay VC=2.5%).
Gene expression analysis
Genes of interest were cart4, nr3c1(alpha), nr3c1(beta), nr3c2, bdnf, and htr1ab. Two internal standards [ribosomal protein L13 (rpl13) and elongation factor 1-alpha (elf1α)] were used for normalization. Primer sequences are shown in Table 1.
Table 1.
Nucleotide Sequences of the Forward and Reverse Primers Used for qPCR
| Gene | Accession number | Forward primer | Reverse primer |
|---|---|---|---|
| elf1α | AY422992 | CTGGAGGCCAGCTCAAACAT | TCAAGAAGAGTAGTACCGCTAGCATTAC |
| rpl13 | NM_212784 | TCTGGAGGACTGTAAGAGGTATGC | AGACGCACAATCTTGAGAGCAG |
| bdnf | NM_131595 | AGAGCGGACGAATATCGCAG | GTTGGAACTTTACTGTCCAGTCG |
| nr3c1(alpha) | EF436284.1 | ACTCCATGCACGACTTGGTG | GCATTTCGGGAAACTCCACG |
| nr3c1(beta) | EF436285.1 | GATGAACTACGAATGTCTTA | GCAACAGACAGCCAGACAGCTCACT |
| nr3c2 | NM_001100403 | CTTCCAGGTTTCCGCAGTCTAC | GGAGGAGAGACACATCCAGGAAT |
| cart4 | NM_001082932 | GCTGAGGCACTCGATGAACT | GAAGAAAGTGTTGCAGGCGG |
| htr1ab | NM_001145766 | GGACATTAAAACGCGCTGCT | ATGCAAGTCTTGGGTTGAGACT |
Elongation factor 1-alpha (elf1α) and ribosomal protein L13 (rpl13) were used as housekeeping genes. Genes of interest were brain-derived neurotrophic factor (bdnf), serotonin receptor 1ab (htr1ab), cocaine- and amphetamine-regulated transcript (cart4), mineralocorticoid receptor (nr3c2), and the glucocorticoid receptor alpha [nr3c1(alpha)] and glucocorticoid receptor beta [nr3c1(beta)].
Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions with the addition of an additional ethanol and sodium acetate precipitation step. RNA concentration and purity was measured by nanodrop spectrophotometry. Any genomic DNA was removed by treatment with DNase I (Invitrogen) as follows: 1 μL of DNase I (1 U/μL) and 1 μL of DNase I reaction buffer (10×) were added to 8 μL total RNA (500 ng) and incubated in a total volume of 10 μL at room temperature for 15 min. Subsequently, DNase was inactivated by addition of 1 μL EDTA (25 mM) and incubation for 10 min at 65°C. To synthesize cDNA, each sample received 1 μL random primers (250 ng/μL), 4 μL first-strand buffer (5×), 1 μL dNTP mix (10 mM), 1 μL DTT (0.1 M), 1 μL RNaseOUT (10 U), and 0.5 μL Superscript II Reverse Transcriptase (100 U/μL) (all from Invitrogen) and incubated at 25°C for 10 min, followed by 50 min at 42°C and finally 15 min at 70°C. Afterward, cDNA was diluted five times with ultrapure water and stored at −20°C until analysis.
Relative gene expression was assessed by real-time qPCR. Briefly, 4 μL of diluted cDNA was used as template in a reaction with 10 μL iQ SYBR Green Supermix (BioRad, Hercules, CA), 0.8 μL forward and reverse primers (10 μM; Table 1), and 4.4 μL ultrapure water. qPCR (3 min at 95°C, 40 cycles of 15 s at 95°C and 1 min at 60°C) was carried out on a CFX 96 (BioRad) qPCR machine. Data were analyzed with the “ΔΔCt method” and normalized for two reference genes according to Vandesompele et al.42
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics 21 for Mac (IBM, Armonk, NY). Data were plotted with GraphPad Prism 5.0 for Mac (GraphPad Software, Inc., La Jolla, CA).
As we introduced a cutoff point of 180-s differences in latency times between groups were analyzed with nonparametric tests: a Kruskal–Wallis test (followed by pair-wise comparisons) and a Mann–Whitney U-test for within-group analysis between days 1 and 2 as we could not individually label the fish, which prevented paired analyses. In addition, latency times on days 1 and 2 were analyzed with trend analysis. Differences in the number of avoiders and nonavoiders related to the shock intensity were analyzed with a chi-square test [followed by pair-wise comparisons; alpha adjusted (alpha 0.05/6 comparisons): p≤0.0083] and a chi-square test for trend.
Data for whole-body cortisol content and gene expression were subjected to Grubbs' test for outliers (extreme studentized deviate) using a stringent criterion (p=0.01). Changes in whole-body cortisol content and telencephalic gene expression were analyzed using a one-sample Student's t-test against the unexposed control group [alpha adjusted (alpha 0.05/8 comparisons): p≤0.00625] and a two-way ANOVA with avoidance category (avoider/nonavoider) and shock intensity (0, 1, 3, and 9 V) as factors. To increase insight in the effects of avoidance learning in relation to shock per se, we also ran a two-way ANOVA excluding the 0-V group. Post hoc testing, following significance for shock intensity, was done by Student–Newman–Keuls (SNK).43 In case of a significant main effect in avoidance category or a significant interaction, avoidance category differences were assessed by Student's t-test per shock intensity.
To assess interrelationships between gene expression levels and whole-body cortisol content, we ran a principal component analysis (PCA) with orthogonal rotation (varimax with Kaiser normalization). In case of missing samples, data were excluded list-wise. The number of factors to retain was based on their eigenvalue (>1) and a visual inspection of the scree plot. Additionally, the Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy and Bartlett's test of sphericity were run to ensure that the data obeyed analysis criteria. Factor scores were saved and used for further analysis. The factor loading cutoff point was −0.400 or 0.400.43
In all cases, significance was accepted when p≤0.05 (two-tailed) unless otherwise stated (i.e., adjusted alpha in case of multiple comparisons).
Results
Inhibitory avoidance protocol
General observations
No weight differences were observed between groups (mean±standard deviation, weight=0.527±0.026 g; n=120). No animals were removed from the experiment for not entering the black compartment on day 1. Although not quantified, visual observation during the experiment suggested that individuals within a group responded similarly to a given electric shock. However, between-shock intensity differences in behavioral responses were observed. At 1 V almost no change in behavior was observed when the shock was given. At 3 V fish responded strongly to the shock, displaying erratic movements, seeking escape routes, and in some cases even jumping (partially) out of the water. At 9 V behavioral responses were similar to those observed at 3 V, but some individuals showed loss of muscle control, that is, stopped swimming transiently and lost equilibrium. Once the electric pulse stopped, fish immediately continued to swim normally again.
Although not quantified, visual observation during the experiment suggested that avoiders and nonavoiders from all groups showed normal swimming behavior on day 1 (before shock) and day 2 (after a single shock) in the white compartment before the sliding door was opened after 60 s. We did not observe any abnormal behavior or periods of freezing behavior44 on any of the test days.
Group latencies
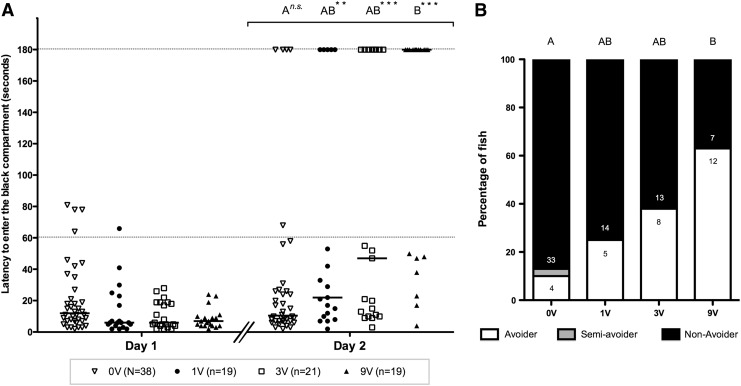
To assess inhibitory avoidance learning, latency times of fish were recorded on day 1 (before shock) and on day 2 (after shock; Fig. 2A). All fish entered the black compartment within 60 s on day 1, except for four fish in the 0-V group and one fish in the 1-V group. Median values of groups ranged from 6 to 12 s. No significant differences were found between groups.
FIG. 2.
(A) Comparison of latencies (in seconds) to enter the black compartment of fish trained with a 0 V (open triangles), 1 V (closed circles), 3 V (open squares), or 9 V (closed triangles) electric shock. Data are presented as individual latencies (single dots) as well as group medians (black bar). Day 1 shows the initial latencies recorded without training, whereas day 2 shows the latencies after a single shock. Dotted lines indicate the 60- and 180-s threshold. Capital letters above groups indicate significant differences assessed by a Mann–Whitney U-test; groups that do not share corresponding letters are significantly different from each other (p≤0.0083). Asterisks (**p≤0.01 and ***p≤0.001) indicate a significant increase in latency from day 1 to 2 within a single treatment. n=the number of fish. (B) Comparison of the percentage of avoider (white), semiavoider (gray), and nonavoider (black) individuals in the different groups on day 2. Capital letters above groups indicate significant differences assessed by a chi-square test (excluding the single semiavoider fish); groups that do not share corresponding letters are significantly different from each other (p≤0.0083). Note that the numbers listed in the white and black part of the bars represent the number of avoider and nonavoider fish in each group and not the percentage of fish.
Except for the 0-V group, median latencies of all groups significantly increased on day 2 compared with day 1: 1 (U=78; p=0.0026), 3 (U=84; p=0.0006), and 9 V (U=19.50; p<0.0001). Median latencies of groups on day 2 ranged from 10 to 180 s and were significantly different between shock conditions [H(3)=20.850; p<0.0001]. Post hoc analysis revealed a significant difference between the 0-V and 9-V groups (p<0.0001). A significant linear trend in median latency was observed across shock intensities (t=2.785; p=0.007).
Avoider and nonavoider fish
We clearly observed behavioral differences between fish regarding their latencies of entering the black compartment. As can be seen in Figure 2A, fish showed latencies either larger than or equal to 180 s, or smaller than 60 s, regardless of treatment. One fish in the 0-V group (latency time of 68 s) was considered a semiavoider and excluded from further analysis.
In the 0-V group we observed four avoider fish (10%). When the intensity of the shock increased from 0 to 9 V, the number of avoider fish increased as well (Fig. 2B). A chi-square analysis revealed significant differences in the ratio of avoider/nonavoider fish across shock conditions (χ2=18.78; df=3; p=0.0003) and we observed a significant difference between the 0 and 9-V groups (χ2=18.42; df=3; p=0.0006). In addition, a chi-square test for trend across groups showed a significant trend across shock intensity (χ2=18.42; df=1; p<0.0001).
Whole-body cortisol content
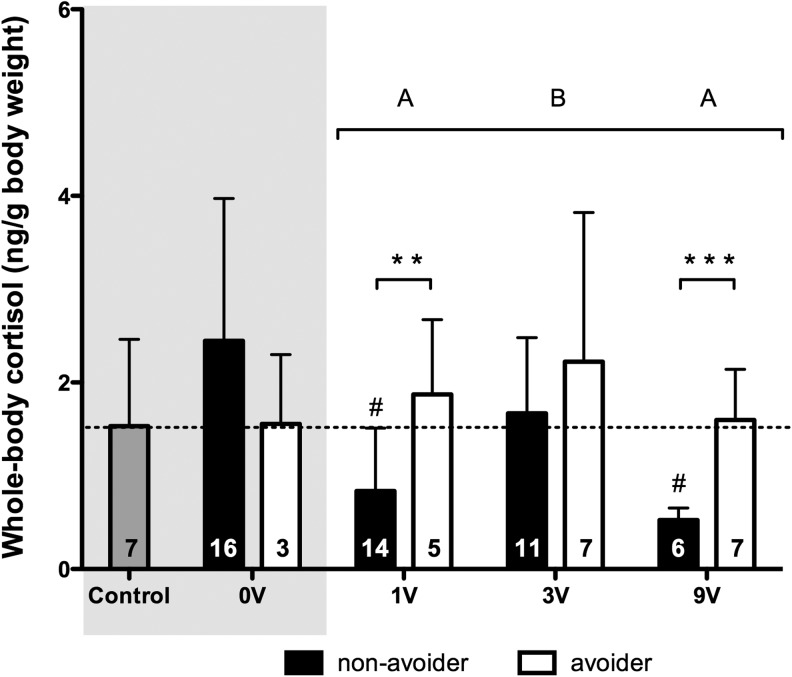
Figure 3 shows the whole-body cortisol content in avoider and nonavoider fish in the different shock groups [the figure excludes two outliers: a 0-V nonavoider (13.73 ng/g body weight) and a 3-V nonavoider (6.41 ng/g body weight)]. Compared with unexposed controls, nonavoider animals in 1- and 9-V groups had significantly lower whole-body cortisol content. Subsequently, a two-way ANOVA (shock intensities and avoider category) revealed no significant effects when the 0-V group was included. Analysis across shock-treated groups (1, 3, and 9 V) revealed significant differences between avoiders and nonavoiders [F(2,44)=4.212; p=0.02]. Post hoc analysis showed that avoiders had a higher whole-body cortisol content than nonavoiders in the 1- and 9-V conditions, but not 3-V condition. We observed a significant effect of shock intensities [F(1,44)=11.84; p=0.001]. Post hoc analysis showed that the 3-V group had a higher whole-body cortisol content than the 1-V group and 9-V group.
FIG. 3.
Comparison of the whole-body cortisol content in unexposed controls (gray) and in avoider (white) and nonavoider (black) fish in the different groups 2 h postinhibitory avoidance on day 2. The light gray box serves as a quick indicator for groups that did not receive a shock. Bars represent group means plus the standard deviation (SD). Capital letters above groups indicate significant differences assessed by a two-way ANOVA (bracket indicates the groups included in the analysis); groups that not share corresponding letters are significantly different from each other (p≤0.05). Significant differences between non-avoiders and avoiders (Student's T-test) are shown as p≤0.01 (**) and p≤0.001 (***). Significant differences compared with unexposed controls (gray) were assessed by a one-sample Student's t-test and marked by a hash-tag (#p≤0.00625). The numbers listed in the bars represent the number of fish in each group, not the percentage of fish.
Exploratory genetics
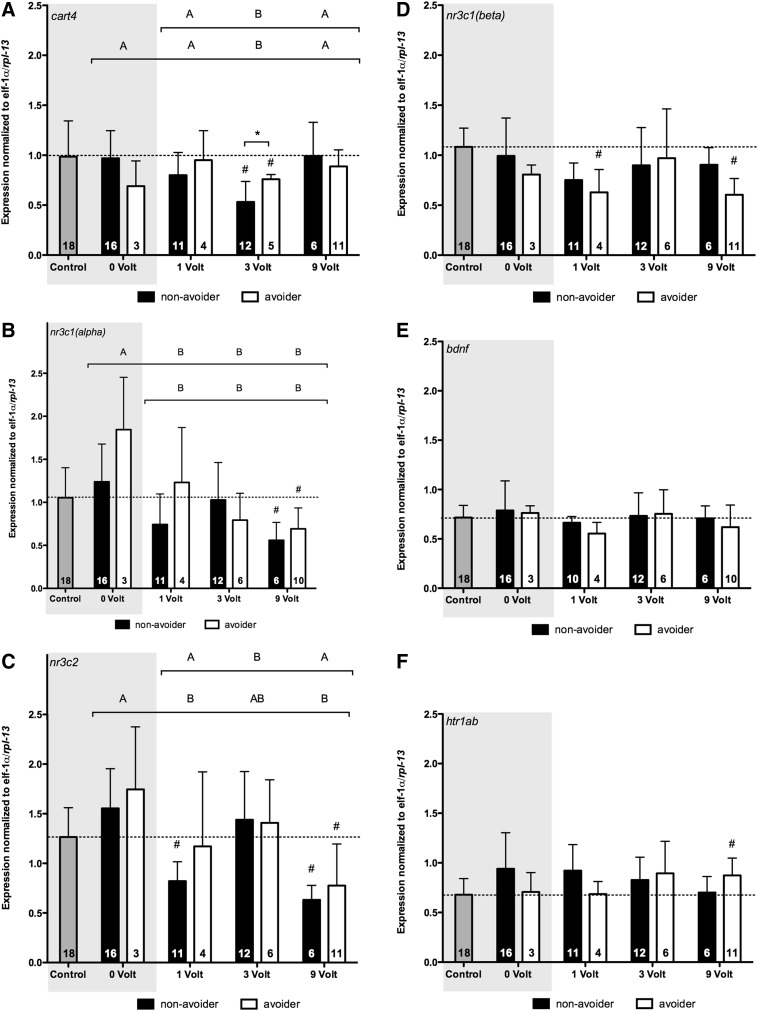
Figure 4 shows the relative expression levels of the different genes, where the figures exclude the following three outliers: for cart4 a 3-V avoider (normalized expression=1.32), for nr3c1(alpha) a 9-V avoider (normalized expression=2.03), and for bdnf a 1-V nonavoider (normalized expression=1.19). Nonavoiders and avoiders in the 3-V group had significantly lower cart4 expression levels compared with the expression level in the unexposed control group (Fig. 4A). Post hoc testing, following a significant main effect for shock intensity [F(3,60)=4.094; p=0.01], showed that the expression level in the 3-V group was lower than the expression levels in the 0-, 1-, and 9-V groups. Further, we found a significant interaction between the avoidance and shock intensity category [F(3,60)=2.889; p=0.04]. Post hoc analysis revealed significant differences between the expression levels in avoiders and nonavoiders in the 3-V group. In addition, post hoc testing following a significant main effect for shock intensity excluding the 0-V group [F(2,43)=7.086; p=0.002] showed that the expression level in the 3-V group was lower than the expression levels in the 1- and 9-V groups. Nonavoiders and avoiders in the 9-V group had significantly lower nr3c1(alpha) expression levels compared with the expression level in the unexposed control group (Fig. 4B). Post hoc testing, following a significant main effect for shock intensity [F(3,60)=10.929; p<0.0001], showed that there were no differences between groups that received a shock (1, 3, and 9 V) and that the expression levels in these groups were lower than the expression level in the 0-V group. We also observed a significant main effect for avoidance category [F(1,60)=5.002; p=0.03] and a significant interaction [F(3,60)=3.047; p=0.04]. Post hoc analysis did not reveal significant differences between avoiders and nonavoiders for any given shock intensity. When excluding the 0-V group from analysis, we observed a significant main effect of shock intensity [F(2,43)=3.773; p=0.03]. Post hoc testing did not reveal significant differences between groups (SNK: p=0.055). In addition, a significant interaction was observed [F(2,43)=3.321; p=0.05]. Post hoc analysis did not reveal significant differences between avoiders and nonavoiders for any given shock intensity. Nonavoiders in the 1-V group and avoiders and non-avoiders in the 9-V group had significantly lower nr3c2 expression levels compared with the unexposed control group (Fig. 4C). Post hoc testing, following a significant main effect for shock intensity [F(3,61)=13.502; p<0.0001], showed that there were no differences between groups that received a shock (1, 3, and 9 V), while the expression levels in the 1- and 9-V groups, but not the 3-V group, were lower than the expression in the 0-V group. In addition, post hoc testing, following a significant main effect for shock intensity excluding the 0-V group [F(2,44)=12.147; p<0.0001], showed that the expression levels in the 1- and 9-V groups were lower than the expression level of the 3-V group. Avoiders in the 1-V group and in the 9-V group had significantly lower nr3c1(beta) expression levels compared with unexposed controls (Fig. 4D). No other significant differences were observed for expression of nr3c1(beta) across shock intensities and avoider category. We observed no significant differences for expression levels of bdnf across shock intensities and avoider category (Fig. 4E). Avoiders in the 9-V group had significantly higher htr1ab expression levels compared with the expression level in the unexposed control group (Fig. 4F). No other statistically significant differences were observed for htr1ab expression across shock intensities and avoider category.
FIG. 4.
(A–F) Comparison of the telencephalic gene expression in unexposed controls (gray) and in avoider (white) and nonavoider (black) fish in the different groups 2 h postinhibitory avoidance on day 2. The light gray box serves as a quick indicator for groups that did not receive a shock. Bars represent group means plus the SD. Capital letters above groups indicate significant differences assessed by a two-way ANOVA (bracket indicates the groups included in the analysis); groups that not share corresponding letters are significantly different from each other (p≤0.05). Significant differences between non-avoiders and avoiders (Student's T-test) are shown as p≤0.05 (*) and p≤0.01 (**). Significant differences compared with unexposed controls (gray) were assessed by a one-sample Student's t-test and marked by a hash-tag (#p≤0.00625). The numbers listed in the bars represent the number of fish in each group, not the percentage of fish.
Principal component analysis
We performed a PCA across groups that received a shock only (1, 3, and 9 V), as the gene expression data in Figure 4 showed the 0-V group to be different from these groups. Although the KMO measure was moderate for this data-set (KMO=0.568), the Bartlett's test of sphericity indicated that the correlations between items were sufficiently large for PCA (χ2=56.484, df=21; p<0.0001). We extracted three factors, explaining 69.64% of variance (Table 2): cart4, nr3c1(alpha), and nr3c2 loaded strongly on Factor 1; bdnf and nr3c1(beta) loaded strongly on Factor 2; and htr1ab and whole-body cortisol content loaded strongly on Factor 3. To assess the overall effect of these factors, we ran two-way ANOVA analyses (with avoidance and shock intensity as categories) across these three different factors. This revealed a significant main effect for shock intensity in Factor 1 [F(1,35)=11.733; p<0.0001]. Post hoc testing revealed that the factor scores of the 1-, 3-, and 9-V groups differed from one another. No statistical difference in the avoider category was observed. For Factor 2 a weak main effect for avoidance category (higher scores for nonavoiders) was observed [F(1,35)=3.461; p=0.07]. No statistical difference was observed for shock intensity. For Factor 3 only a weak main effect for the avoidance category (higher scores for nonavoiders) was observed [F(1,35)=2.773; p=0.10]. No other statistically significant differences were observed.
Table 2.
Variables Loaded onto Factors by a Principal Component Analysis
| Factor 1 | Factor 2 | Factor 3 | |
|---|---|---|---|
| Variable | 28.94% | 26.05% | 14.65% |
| cart4 | −0.753 | −0.334 | 0.069 |
| nr3c1(alpha) | 0.788 | −0.109 | 0.011 |
| nr3c2 | 0.858 | −0.220 | −0.050 |
| nr3c1(beta) | −0.046 | 0.912 | 0.012 |
| bdnf | −0.025 | 0.829 | 0.242 |
| htr1ab | 0.095 | −0.036 | 0.866 |
| Cortisol | 0.093 | −0.280 | −0.586 |
Scores larger than 0.400 or smaller than −0.400 were accepted for loading onto a factor and are shown in bold. This results in the loading of cart4, nr3c1(alpha), and nr3c2 onto Factor 1; the loading of nr3c1(beta) and bdnf onto Factor 2; and the loading of htr1ab and whole-body cortisol content onto Factor 3.
Discussion
In this study we show that (1) higher shock intensities increased median latencies and the percentage of avoider fish in a group; (2) whole-body cortisol content and the expression of cart4, nr3c1(alpha), and nr3c2 are associated with shock intensity; and (3) whole-body cortisol content is associated with differences in inhibitory avoidance behavior.
Inhibitory avoidance learning
In line with our prediction, the 0-V shock condition did not lead to an overall increase in the median latency on day 2 compared with day 1. This shows that the handling procedure and exposure to the inhibitory avoidance tank do not contribute to the observed inhibitory avoidance behavior in the other groups (1, 3, and 9 V). Yet, a small percentage of fish in the 0-V group did avoid the black compartment on day 2, even though they had not received a shock on day 1. Possibly these individuals associated netting, a stressful45 and a negative experience that fish will try to avoid,46 with the black compartment and therefore avoided it. In contrast to groups that did receive a shock, no differences between avoiders and nonavoiders were found in whole-body cortisol content and gene expression levels in the 0-V group. Accordingly the behavioral differences between the fish in the 0-V group may be different from the behavioral differences between fish in the groups that received a shock (see “Limitations” heading). Whether these findings in the 0-V group truly reflect behavioral differences under this condition awaits further studies.
In all groups that received a shock we observed a significant increase in median latencies on day 2 compared with day 1. Regarding the effect of 3 V we hereby successfully reproduced results of earlier studies.11–13 Although in all experiments the mean latency to enter the black compartment increased after a single trial using a 3-V shock, these latencies did vary. Whereas Blank et al.,11 Piato et al.,12 and Ng et al.13 reported mean latencies of 150, 50, and 90 s, respectively, we observed a mean latency of 65 s (calculated from data presented in Fig. 2A). These differences in mean latencies may be related to environmental and/or strain differences, as these factors greatly affect the outcome of behavioral studies.47–49
In addition to the replication of earlier findings,11–13 we extend the data of these studies by increasing and decreasing shock intensities and assessing effects on whole-body cortisol content and telencephalic gene expression. As predicted, with increasing shock intensity, the median latencies and the percentage of fish in a group that avoid entering the black compartment on day 2 increased. After the strongest shock (9 V), we still observed fish that did not avoid the black compartment. This observation may be directly related to the impact of the given shock. On-site observations (not further quantified) indicated that 9 V had a strong immediate impact on the fish; some fish, when exposed to the 9-V shock, lost control over their ability to keep equilibrium. We suggest that the loss of equilibrium is a physical result to the 9-V shock, that is, temporary paralysis, as no tonic-clonic cramps followed by a phase of exhaustion were observed, which is indicative for loss of consciousness induced by electricity. When the shock was terminated, fish immediately resumed to swim normally; loss of equilibrium only occurred during the shock, not thereafter. We did not observe any abnormal behavior or freezing on the next day when fish were in the white compartment before the sliding door was opened. Unfortunately, we were unable to link individual responses when receiving the shock to (acquired) avoidance behavior as we were unable to distinguish individual fish. Therefore, we are not able to relate loss of equilibrium during the shock with avoidance behavior on the next day.
While median latencies and the number of avoider fish show a linear increase from 1 to 9 V, neither whole-body cortisol content nor the gene expression levels do follow such a pattern. The expression levels of bdnf, nr3c1(alpha), nr3c1(beta), and htr1ab were not different between groups that received a shock (1, 3, and 9 V). In contrast, expression levels of nr3c2 were higher and of cart4 were lower in the 3-V group compared with the 1- and 9-V groups. Only in the case of nr3c1(alpha) did we see a difference in expression level of all shocked groups compared with the expression levels in the 0-V group. PCA revealed that cart4, nr3c1(alpha), and nr3c2 were loaded onto the same factor. The observed loading of nr3c1(alpha) and nr3c2 onto one factor may be explained by their involvement in learning and memory. Where the MR (Mineralocorticoid Receptor; the product of the nr3c2 gene) is involved in the initial phase of memory, appraisal of information, and response selection, the GR (Glucocorticoid Receptor; the product of the nr3c1 gene) is involved in consolidation.50–53 Under basal conditions the MR is almost fully occupied, as its affinity for cortisol is higher than that of the GR. This could explain why the expression of nr3c2 and the levels of whole-body cortisol content have a similar pattern across groups that received a shock.54 Still, PCA showed that whole-body cortisol content was loaded onto a factor with htr1ab. This could be due to the fact that whole-body cortisol content was also associated with avoidance differences (see “Avoider and nonavoider behavior” heading).
The differences between the 3-V group on one hand and the 1- and 9-V groups on the other may be related to the conflict between innate anxiety (entering the dark compartment) and acquired fear for the dark compartment (avoidance of the dark compartment). While at 1 V the innate response may dominate, as the shock is relatively mild, at 9 V the avoidance response may dominate, as the shock appeared strong. So, in both cases the outcome of the conflict is clear. At 3 V there might be a stronger conflict between the two opposing tendencies, leading to different dynamics in the expression of genes and whole-body cortisol content. As we only measured at a single time point, we can only speculate on this (see “Limitations” heading).
Avoider and nonavoider behavior
We also further extended findings of earlier studies by assessing avoider and nonavoider differences in inhibitory avoidance behavior and underlying whole-body cortisol content and gene expression profiles.39 Here, we observed two distinct behavioral types in the groups that received a shock.20 Fish that did not enter the black compartment (latencies ≥180 s), that is, avoider fish, and fish that entered the black compartment (latencies <60 s), that is, nonavoider fish. It is unclear whether such observations were also made by Blank et al.,11 Piato et al.,12 and Ng et al.13 as they did not report on behavioral differences, or the lack thereof, in their papers. However, our observed differences in avoidance behavior are in line with many studies that have shown behavioral differences in relation to performance in challenging tasks for both rodents23,55 and fish.56–60
In the groups that received a shock, avoider fish had overall higher whole-body cortisol content than nonavoider fish (notably in the 1- and 9-V conditions). Classically, differences related to coping styles suggest that reactive subjects (who quickly adjust to new situations through interaction with the environment) have higher levels of cortisol in relation to challenges, while proactive subjects (who slowly adjust to new situations and respond by habits independently of the environment) have lower levels.22,23 Here, adjustments in behavior would amount to overruling innate behavior in favor of a new behavioral response in relation to having received a shock (avoidance learning). From this perspective, avoiders could be considered to be reactive subjects, while nonavoiders could be considered to be proactive subjects. Recently, the role of differences in serotonin (5-HT) levels has become more important in discriminating between reactive and proactive coping styles22,23,61; reactive subjects have higher levels of 5-HT (associated with lower levels of the 5-HT1a receptor) and proactive subjects have lower levels (associated with higher levels of the 5-HT1a receptor). While we have not observed convincing evidence to support this in our data, the fact that whole-body cortisol content and htr1ab load onto the same factor in PCA may suggest the involvement of htr1ab in behavioral differences in zebrafish. Although not significantly, htr1ab showed a weak interaction between the avoidance and shock intensity categories [F(2,44)=2.816; p=0.07], clearly suggesting that future studies addressing this topic seem warranted.
At the level of gene expression, small differences between avoiders and nonavoiders were observed for cart4 (only higher in avoiders in the 3-V group). In rodents upregulation of Cart leads to intensified fear62 and higher levels of Cart have been associated with enhanced learning and memory.30,63 This is similar to what we observed in our avoider fish. In addition, we observed small differences between avoiders and nonavoiders in Factor 2 [nr3c1(beta) and bdnf] from the PCA. That the factor on which bdnf loads is associated with avoidance behavior could be related to the role of BDNF in memory consolidation.64,65 The role of GR-beta in avoidance behavior, learning, and memory is unclear and requires further study.
While it is tempting to relate the avoider and nonavoider differences observed here to reactive and proactive coping styles,21–23 the data do not yet allow for an unequivocal interpretation in that direction. However, when these observations hold, we should find similar results when we select fish on coping style66 before testing them in the inhibitory avoidance paradigm. Future studies in our laboratory are directed at this.
Limitations
A number of limitations of this study need to be discussed. First, we only administered one shock to the fish, thereby effectively studying “one-trial learning,” which may limit the discriminative power of the task. It has been reported that increasing the number of training sessions may improve the performance of zebrafish in learning tasks.67,68 We therefore recently added a second shock to the protocol and observed an increase in learning and higher discriminative power between treatments.20 Second, we did not tag/mark or house fish individually, which hampers correlation between behavior and physiology/gene expression. While housing zebrafish individually is stressful,36 tagging or marking could be an option in future studies.69 Third, we have only used latencies to enter the black compartment as behavioral read-out. However, video recordings of behavior of fish during the 60 s before lifting the sliding door and in the period of choosing to enter the black compartment or not (max. 180 s) allow for more fine-grained behavior analysis afterward. This will more precisely reveal behavioral differences in relation to intensities and between subjects. In our current experiments we are applying this methodology. Fourth, our study did not aim to optimize the time point of sampling to detect effects of inhibitory avoidance learning on whole-body cortisol content and gene expression. We therefore only measured whole-body cortisol and gene expression levels at a single time point, that is, 2 h following inhibitory avoidance. This time window was chosen based on a study by Morsink et al.,24 who showed that between 1 and 3 h following a challenge, difference in a sufficient number of genes can be analyzed. Two hours is beyond the expression of immediate early genes, in which we were not interested in. However, future studies may examine different time points to more precisely assess the dynamics of cortisol and gene expression following behavioral testing. This could also be combined with analyses of different regions in the telencephalon rather than the whole telencephalon.70
Concluding Remarks
We show that an inhibitory avoidance paradigm in combination with the analyses of whole-body cortisol content and the expression of genes in the telencephalon is suitable to study avoidance learning related to intensities (conflict between innate and acquired behavior) and differences between subjects (coping styles). Our data serve as starting point for further unraveling individual differences in brain-behavior relationships underlying (fear avoidance) learning and memory in zebrafish.
Acknowledgments
The authors thank Dr. Lars Ebbesson for helpful comments and suggestions during experimental design. Mr. Dirk Burggraaf who provided the electrodes for the inhibitory avoidance tank and Mr. F.A. Tom Spanings who organized fish husbandry and offered technical assistance are hereby acknowledged. We also like to thank two anonymous reviewers for their critical review, which very much improved this article. This study was sponsored by NWO (program: value of animal welfare; project No. 827.09.040). In addition, G.F. and M.G. were financially supported by the European Union's Seventh Framework Programme (FP7/2010–2014) project “COPEWELL” (grant agreement No. 265957).
Disclosure Statement
No competing financial interests exist.
References
- 1.Miklósi Á, Andrew RJ. The zebrafish as a model for behavioral studies. Zebrafish 2006;3:227–234 [DOI] [PubMed] [Google Scholar]
- 2.Gerlai R. High-throughput behavioral screens: the first step towards finding genes involved in vertebrate brain function using zebrafish. Molecules 2010;15:2609–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buccafusco JJ, Levin ED, Cerutti DT. Behavioral Neuroscience of Zebrafish, 2nd ed. Boca Raton, FL: CRC Press, 2009 [PubMed] [Google Scholar]
- 4.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet 2007;8:353–367 [DOI] [PubMed] [Google Scholar]
- 5.Howe K, Clark MD, Torroja CF, et al. . The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013;496:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jekosch K. The zebrafish genome project: sequence analysis and annotation. Zebrafish 2004;77:225–239 [DOI] [PubMed] [Google Scholar]
- 7.Mathavan S, Lee S, Mak A, et al. . Transcriptome analysis of zebrafish embryogenesis using microarrays. PLoS Genet 2005;1:260–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucitt MB, Price TS, Pizarro A, et al. . Analysis of the zebrafish proteome during embryonic development. Mol Cell Proteomics 2008;7:981–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart A, Gaikwad S, Kyzar E, Green J, Roth A, Kalueff AV. Modeling anxiety using adult zebrafish: a conceptual review. Neuropharmacology 2012;62:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalueff AV, Stewart AM, Kyzar EJ, et al. . Time to recognize zebrafish “affective” behavior. Behaviour 2012;149:1019–1036 [Google Scholar]
- 11.Blank M, Guerim LD, Cordeiro RF, Vianna MRM. A one-trial inhibitory avoidance task to zebrafish: rapid acquisition of an NMDA-dependent long-term memory. Neurobiol Learn Mem 2009;92:529–534 [DOI] [PubMed] [Google Scholar]
- 12.Piato ÂL, Capiotti KM, Tamborski AR, Oses JP. Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog NeuroPsychopharmacol Biol Psychiatry 2011;35:561–567 [DOI] [PubMed] [Google Scholar]
- 13.Ng M-C, Hsu C-P, Wu Y-J, Wu S-Y, Yang Y-L, Lu K-T. Effect of MK-801-induced impairment of inhibitory avoidance learning in zebrafish via inactivation of extracellular signal-regulated kinase (ERK) in telencephalon. Fish Physiol Biochem 2012;38:1099–1106 [DOI] [PubMed] [Google Scholar]
- 14.Maximino C, de Brito TM, da Silva Batista AW. Measuring anxiety in zebrafish: a critical review. Behav Brain Res 2010;214:157–171 [DOI] [PubMed] [Google Scholar]
- 15.Sison M, Gerlai R. Behavioral performance altering effects of MK-801 in zebrafish (Danio rerio). Behav Brain Res 2011;220:331–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pradel G, Schachner M, Schmidt R. Inhibition of memory consolidation by antibodies against cell adhesion molecules after active avoidance conditioning in zebrafish. J Neurobiol 1999;39:197–206 [PubMed] [Google Scholar]
- 17.Agranoff BW, Davis RE, Casola L, Lim R. Actinomycin D blocks formation of memory of shock-avoidance in goldfish. Science 1967;158:1600–1601 [DOI] [PubMed] [Google Scholar]
- 18.Champagne DL, Hoefnagels CCM, de Kloet RE, Richardson MK. Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research. Behav Brain Res 2010;214:332–342 [DOI] [PubMed] [Google Scholar]
- 19.Stephenson JF, Whitlock KE, Partridge JC. Zebrafish preference for light or dark is dependent on ambient light levels and olfactory stimulation. Zebrafish 2011;8:17–22 [DOI] [PubMed] [Google Scholar]
- 20.Manuel R. Abstract Book. Lunteren, Netherlands: 11th ENP Meeting, 2013 [Google Scholar]
- 21.Coppens CM, de Boer SF, Koolhaas JM. Coping styles and behavioural flexibility: towards underlying mechanisms. Phil Trans R Soc B 2010;365:4021–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol 2010;31:307–321 [DOI] [PubMed] [Google Scholar]
- 23.Koolhaas JM, de Boer SF, Buwalda B, van Reenen K. Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain Behav Evol 2007;70:218–226 [DOI] [PubMed] [Google Scholar]
- 24.Morsink MC, Steenbergen PJ, Vos JB, et al. . Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J Neuroendocrinol 2006;18:239–252 [DOI] [PubMed] [Google Scholar]
- 25.Koot S, Baars A, Hesseling P, van den Bos R, Joëls M. Time-dependent effects of corticosterone on reward-based decision-making in a rodent model of the Iowa Gambling Task. Neuropharmacology 2013;70:306–315 [DOI] [PubMed] [Google Scholar]
- 26.de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci 1999;22:422–426 [DOI] [PubMed] [Google Scholar]
- 27.Flik G, Klaren PHM, Van den Burg EH, Metz JR, Huising MO. CRF and stress in fish. Gen Comp Endocrinol 2006;146:36–44 [DOI] [PubMed] [Google Scholar]
- 28.Liu IYC. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. J Neurosci 2004;24:7958–7963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maximino C, Puty B, Benzecry R, et al. . Role of serotonin in zebrafish (Danio rerio) anxiety: relationship with serotonin levels and effect of buspirone, WAY 100635, SB 224289, fluoxetine and para-chlorophenylalanine (pCPA) in two behavioral models. Neuropharmacology 2013;71:83–97 [DOI] [PubMed] [Google Scholar]
- 30.Upadhya MA, Nakhate KT, Kokare DM, Singru PS. Cocaine- and amphetamine-regulated transcript peptide increases spatial learning and memory in rats. Life Sci 2011;88:322–324 [DOI] [PubMed] [Google Scholar]
- 31.Upadhya MA, Kokare DM, Subhedar NK. Cocaine- and amphetamine-regulated transcript peptide (CART) in the central nucleus of amygdala potentiates behavioral and hormonal responses of the rat exposed to its predator. Behav Brain Res 2013;243:129–137 [DOI] [PubMed] [Google Scholar]
- 32.Akash G, Kaniganti T, Tiwari NK, Subhedar NK, Ghose A. Differential distribution and energy status–dependent regulation of the four CART neuropeptide genes in the zebrafish brain. J Comp Neurol 2014;522:2266–2285 [DOI] [PubMed] [Google Scholar]
- 33.Broglio C, Gomez A, Duran E, Ocana FM. Hallmarks of a common forebrain vertebrate plan: specialized pallial areas for spatial, temporal and emotional memory in actinopterygian fish. Brain Res Bull 2005;66:277–281 [DOI] [PubMed] [Google Scholar]
- 34.Mueller T, Dong Z, Berberoglu MA, Guo S. The dorsal pallium in zebrafish, Danio rerio (Cyprinidae, Teleostei). Brain Res 2011;1381:95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebbesson L, Braithwaite VA. Environmental effects on fish neural plasticity and cognition. J Fish Biol 2012;81:2151–2174 [DOI] [PubMed] [Google Scholar]
- 36.Parker MO, Millington ME, Combe FJ, Brennan CH. Housing conditions differentially affect physiological and behavioural stress responses of zebrafish, as well as the response to anxiolytics. PLoS One 2012;7:e34992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsunaga W, Watanabe E. Habituation of medaka (Oryzias latipes) demonstrated by open-field testing. Behav Proc 2010;85:142–150 [DOI] [PubMed] [Google Scholar]
- 38.Wong K, Elegante M, Bartels B, et al. . Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav Brain Res 2010;208:450–457 [DOI] [PubMed] [Google Scholar]
- 39.MacKenzie S, Ribas L, Pilarczyk M, Capdevila DM, Kadri S, Huntingford FA. Screening for coping style increases the power of gene expression studies. PLoS One 2009;4:e5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canavello PR, Cachat JM, Beeson EC, et al. . Measuring endocrine (cortisol) responses of zebrafish to stress. In: Zebrafish Neurobehavioral Protocols, Vol 51 Neuromethods. Totowa, NJ: Humana Press, pp.135–142, 2011 [Google Scholar]
- 41.Gorissen M, Bernier NJ, Manuel R, et al. . Recombinant human leptin attenuates stress axis activity in common carp (Cyprinus carpio L.). Gen Comp Endocrinol 2012;178:75–81 [DOI] [PubMed] [Google Scholar]
- 42.Vandesompele J, De Preter K, Pattyn F, et al. . Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002;3:RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferguson GA. Statistical Analysis in Psychology and Education, 5 ed. Singapore: McGraw Hill Book Company, 1981 [Google Scholar]
- 44.Kalueff AV, Gebhardt M, Stewart AM, et al. . Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013;10:70–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huising MO, Metz JR, van Schooten C, et al. . Structural characterisation of a cyprinid (Cyprinus carpio L.) CRH, CRH-BP and CRH-R1, and the role of these proteins in the acute stress response. J Mol Endocrinol 2004;32:627–648 Available at www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15171705 [DOI] [PubMed]
- 46.Ramsay JM, Feist GW, Varga ZM, Westerfield M, Kent ML, Schreck CB. Whole-body cortisol response of zebrafish to acute net handling stress. Aquaculture 2009;297:157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandillo S, Tucci V, Hoelter SM, et al. . Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol Genomics 2008;34:243–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richter SH, Garner JP, Wuerbel H. Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nat Methods 2009;6:257–261 [DOI] [PubMed] [Google Scholar]
- 49.van der Staay FJ, Steckler T. The fallacy of behavioral phenotyping without standardisation. Genes Brain Behav 2002;1:9–13 [DOI] [PubMed] [Google Scholar]
- 50.Cerqueira JJ, Catania C, Sotiropoulos I. Corticosteroid status influences the volume of the rat cingulate cortex—a magnetic resonance imaging study. J Psychiatr Res 2005;39:451–460 [DOI] [PubMed] [Google Scholar]
- 51.Lupien S, McEwen B. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev 1997;24:1–27 [DOI] [PubMed] [Google Scholar]
- 52.Zhou M, Bakker EHM, Velzing EH, et al. . Both mineralocorticoid and glucocorticoid receptors regulate emotional memory in mice. Neurobiol Learn Mem 2010;94:530–537 [DOI] [PubMed] [Google Scholar]
- 53.Horst Ter JP, Van Der Mark MH, Arp M, Berger S. Stress or no stress: mineralocorticoid receptors in the forebrain regulate behavioral adaptation. Neurobiol Learn Mem 2012;98:33–40 [DOI] [PubMed] [Google Scholar]
- 54.Reul JMHM, De Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 1985;117:2505–2511 [DOI] [PubMed] [Google Scholar]
- 55.Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev 2005;29:3–38 [DOI] [PubMed] [Google Scholar]
- 56.Brelin D, Petersson E, Winberg S. Divergent stress coping styles in juvenile brown trout (Salmo trutta). Ann N Y Acad Sci 2006;1040:239–245 [DOI] [PubMed] [Google Scholar]
- 57.Øverli Ø, Sørensen C, Pulman KGT, et al. . Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci Biobehav Rev 2007;31:396–412 [DOI] [PubMed] [Google Scholar]
- 58.Thomson JS, Watts PC, Pottinger TG, Sneddon LU. Physiological and genetic correlates of boldness: characterising the mechanisms of behavioural variation in rainbow trout, Oncorhynchus mykiss. Horm Behav 2011;59:67–74 [DOI] [PubMed] [Google Scholar]
- 59.Conrad JL, Weinersmith KL, Brodin T, Saltz JB, Sih A. Behavioural syndromes in fishes: a review with implications for ecology and fisheries management. J Fish Biol 2011;78:395–435 [DOI] [PubMed] [Google Scholar]
- 60.Huntingford FA, Andrew G, Mackenzie S, et al. . Coping strategies in a strongly schooling fish, the common carp Cyprinus carpio. J Fish Biol 2010;76:1576–1591 [DOI] [PubMed] [Google Scholar]
- 61.Øverli Ø, Winberg S, Pottinger TG. Behavioral and neuroendocrine correlates of selection for stress responsiveness in rainbow trout—a review. Integr Comp Biol 2005;45:463–474 [DOI] [PubMed] [Google Scholar]
- 62.Kask A, Schiöth HB, Mutulis F, Wikberg J, Rägo L. Anorexigenic cocaine- and amphetamine-regulated transcript peptide intensifies fear reactions in rats. Brain Res 2000;857:283–285 [DOI] [PubMed] [Google Scholar]
- 63.Yermolaieva O, Chen J, Couceyro PR, Hoshi T. Cocaine- and amphetamine-regulated transcript peptide modulation of voltage-gated Ca2+ signaling in hippocampal neurons. J Neurosci 2001;21:7474–7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monfils M-H, Cowansage KK, LeDoux JE. Brain-derived neurotrophic factor: linking fear learning to memory consolidation. Mol Pharmacol 2007;72:235–237 [DOI] [PubMed] [Google Scholar]
- 65.Schulz-Klaus B, Lessmann V. BDNF-dependent consolidation of fear memories in the perirhinal cortex. Front Behav Neurosci 2013;7:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tudorache C, Schaaf MJM, Slabbekoorn H. Covariation between behaviour and physiology indicators of coping style in zebrafish (Danio rerio). J Endocrinol 2013;219:251–258 [DOI] [PubMed] [Google Scholar]
- 67.Arthur D, Levin E. Spatial and non-spatial visual discrimination learning in zebrafish (Danio rerio). Anim Cogn 2001;4:125–131 [Google Scholar]
- 68.Williams FE, White D, Messer WS. A simple spatial alternation task for assessing memory function in zebrafish. Behav Proc 2002;58:125–132 [DOI] [PubMed] [Google Scholar]
- 69.Cousin X, Daouk T, Péan S, Lyphout L, Schwartz M-E, Bégout M-L. Electronic individual identification of zebrafish using radio frequency identification (RFID) microtags. J Exp Biol 2012;215:2729–2734 [DOI] [PubMed] [Google Scholar]
- 70.Aoki T, Kinoshita M, Aoki R, et al. . Imaging of neural ensemble for the retrieval of a learned behavioral program. Neuron 2013;78:881–894 [DOI] [PubMed] [Google Scholar]