Abstract
In humans the superfamily of intermediate filament (IF) proteins is encoded by more than 70 different genes, which are expressed in a cell- and tissue-specific manner. IFs assemble into approximately 10 nm-wide filaments that account for the principal structural elements at the nuclear periphery, nucleoplasm, and cytoplasm. They are also required for organizing the microtubule and microfilament networks. In this review, we focus on the dynamics of IFs and how modifications regulate it. We also discuss the role of nuclear IF organization in determining nuclear mechanics as well as that of cytoplasmic IFs organization in maintaining cell stiffness, formation of lamellipodia, regulation of cell migration, and permitting cell adhesion.
Metazoans harbor three filament networks
All metazoan cells contain three distinct filament systems: microtubules, microfilaments, and IFs. Microtubules and microfilaments are built from homogeneous globular proteins, called tubulin and actin, respectively. Being polar, microtubules and microfilaments serve as tracks for molecular motors. In contrast, IFs are apolar and are made of fibrous proteins that are quite diverse (see below). These three cytoskeletal filament systems are in constant and intimate communication with one another. For example, disrupting the IF network organization may disturb the spatial organization of microtubules or microfilaments or both. There is a group of proteins that link the three networks to each other, called cytolinkers [1]. For example, specific binding domains in the different splice variants of plectin and bullous pemphigoid antigen-1 (BPAG1) form cross-bridges between IFs, microtubules, and microfilaments [2,3]. Plectin plays a dominant role in letting IFs execute their functions in skeletal muscle as well as in skin and peripheral nerve [2]. Likewise, the link complex, composed of SUN- and KASH-domain proteins, connects the nuclear IFs, termed lamins, to microtubules, microfilaments, or cytoplasmic IFs [4]. Therefore, exploring the structure and function of each of these cellular networks must consider the possible effects this might have on the other networks.
Intermediate filament proteins represent a complex multi-gene family
The human IF proteins are encoded by over 70 genes. All IF proteins share a common domain organization but have distinct primary sequences [5]. In addition, many of the IF genes give rise to multiple splice variants. Humans have three nuclear IF genes: the LMNA gene encodes the A-type lamins A/C, whereas the LMNB1 and LMNB2 genes encode the B-type lamins B1 and B2, respectively. All cells express at least one B-type lamin, and most differentiated cells express A-type lamins. The cytoplasmic IF proteins are differentially expressed during development and exhibit cell and tissue specificity [5]. For example, epithelial cells express a diverse group of keratins. Mesenchymal, endothelial, and hematopoietic cells express vimentin, muscle cells express desmin, neuronal cells express the neurofilament triplet proteins, neuroglia express GAFP, and so on. A given cell may express four or more different IF proteins, including two or three different lamins and at least two cytoplasmic IF proteins.
Lamins regulate most nuclear activities [6]. They do so by binding to specific partners and chromatin. Indeed, there is an ever-growing number of proteins both at the nuclear periphery and in the nuclear interior that form complexes with lamins in a tissue-specific manner [7]. Hence, studying lamin functions should be done in the context of these specific complexes. In contrast, relatively little is known about the protein complexes that are associated with cytoplasmic IFs. While IFs undergo specific post-translational modifications (including phosphorylation, sumoylation, ubiquitination, glycosylation, and acetylation) that are cell cycle specific or depend on development or disease states, relatively little is known about how these modifications regulate IF dynamics, organization, and activity.
Nuclear intermediate filament dynamics
During interphase, most lamins form highly immobile filaments at the nuclear periphery. In Caenorhabditis elegans, there is also a small fraction of its single lamin protein in the nucleoplasm that appears rather immobile [8]. Nevertheless, these findings do not rule out the possibility of the presence of a mobile fraction of lamin in C. elegans that may be used to repair defective lamin filaments. In mammals, the peripheral lamins are immobile too. However, there is a mobile lamin A fraction in the nucleoplasm whose relative amount depends on cell type, state of differentiation, and probably also the type of stress that is applied to the cell. In fibroblasts, this nucleoplasmic fraction depends on specific lamin-binding partners. For example, loss of the lamin-associated protein 2alpha (LAP2α) causes a significant decrease of the nucleoplasmic A-type lamins and probably an increase in the amount of peripheral lamin A [9,10]. Moreover, it is likely that the fraction of nucleoplasmic lamin A also depends on other lamin A-binding partners — for example, the retinoblastoma protein (pRb) — as well as on proteins that link lamin A to the inner nuclear membrane [11].
Cytoplasmic intermediate filament dynamics is regulated by phosphorylation and sumoylation
For many years, IFs were considered to be static elements of the cell cytoskeleton, primarily based upon the findings that (a) they could be isolated intact as 10 nm filaments [12], and (b) there was little evidence for soluble pools of IF subunits [13]. Indeed, this view is consistent with in vitro studies which have demonstrated that there is hardly any exchange of subunits among filaments, even after several days of incubation [14]. These early studies suggested that the steady state for IFs was regulated mainly by protein synthesis and degradation or post-translational modifications. This behavior strongly contrasts with that found for microtubules and microfilaments, which depend on large pools of soluble subunits that exchange at their ends in the course of their disassembly/reassembly. On the other hand, microinjection of soluble IF protein into live cells led to its incorporation into the endogenous IF cytoskeleton within minutes, documenting that in situ IFs behave dynamically [15-17]. Further insights into the dynamic properties of IFs were derived from fluorescence recovery after photobleaching (FRAP) analyses [15,18].
During mitosis, the disassembly of vimentin IFs in fibroblasts is mediated by phosphorylation of vimentin at Ser55 by the cyclin-dependent kinase 1 (cdk1) [19,20]. Furthermore, the integrity of the IF cytoskeleton in interphase cells requires protein phosphatase activity [21]. A combination of in vivo and in vitro experiments have identified a large number of kinases that phosphorylate one or more of the 41 sites in vimentin identified to date [20,22]. The kinases/phosphatases responsible for phosphorylation/dephosphorylation of vimentin are highly regulated and very sensitive to chemical and mechanical stimuli such as growth factors and shear stress [15]. Another regulator of IF dynamics appears to be sumoylation, a reversible post-translational modification that plays roles in many processes, including cell division, transcriptional regulation, chromosome integrity, and the DNA damage response. In C. elegans, SUMO, a small ubiquitin-like protein modifier, regulates the assembly of the cytoplasmic IF protein IFB-1 by maintaining a cytoplasmic pool of soluble sumoylated IFB-1 [23]. In the absence of SUMO, IFB-1 forms ectopic filaments and aggregates in the lateral epidermis of the worm. Also, depletion of SUMO or mutation of the SUMO acceptor site on IFB-1 results in a reduction of the soluble IFB-1 pool. The generality of reversible post-translational modifications such as phosphorylation, sumoylation, and others to regulate IF dynamics remains to be seen.
Lamins are master regulators of nuclear mechanics and mechanotransduction
The role of lamins in maintaining nuclear shape is best demonstrated in C. elegans embryos where downregulation of the single lamin gene causes rapid changes in nuclear shape in the context of the whole embryo (Figure 1A) [24]. Changes in nuclear shape such as lobulation and membrane invaginations are hallmarks of mutations in the LMNA gene and are thought to be involved in the etiology of the diseases caused by mutations in this gene, termed laminopathic diseases [25]. Indeed, one of the hypotheses that tries to explain laminopathies, termed the “mechanical hypothesis” (the other two being called the “gene expression” and the “proliferation/differentiation hypothesis” [26]), suggests that mutations in LMNA alter its structure and thereby lead to nuclear fragility. This, in turn, results in cell death and progressive failure in muscle tissues where nuclei get broken down following exposure to repetitive mechanical stress [27].
Figure 1. Lamin affects nuclear shape and the cell differentiated state.
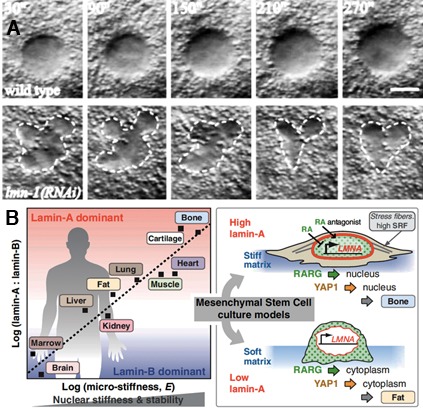
(A) Nuclear morphology dramatically changes in Caenorhabditis elegans nuclei downregulated for lamin. Five consecutive images of the AB cell nucleus in a two-cell C. elegans embryo were taken at 1-minute intervals by using four-dimensional time-lapse recording. The central focal plane for each nucleus is shown. The top panels are from a wildtype embryo, and the bottom panels are from the downregulated embryo, with an outline of the nucleus in dotted line. Bar represents 5 mm. Frame (A) was reproduced with permission from the American Society for Cell Biology [24]. (B) Tissue micromechanics correlate with abundance of collagens and nuclear lamins, which influence cell differentiation. (Left) Collagen and lamin-A levels scale with elasticity (E), consistent with matching tissue stress to nuclear mechanics. (Right) Matrix stiffness in tissue culture increases cell tension and stabilizes lamin A, regulating its own transcription and that of stress fiber genes, enhancing differentiation. RARG, YAP1, and SRF are transcription factors. Frame (B) was reproduced with permission from the American Association for the Advancement of Science [30]. RA, retinoic acid (vitamin A). (http://www.sciencemag.org/content/341/6149/1240104.abstract).
There is increasing evidence that the nucleus is a mechanosensor of both external and internal forces [28]. The nuclear rigidity and its ability to sense mechanical stress require the A-type lamins. Indeed, loss of A-type lamins leads to both mechanical weakness and defects in mechanical stress-dependent gene expression in vivo [29]. In mammals, applying an external force may alter intranuclear protein mobility. Similarly, exposing isolated nuclei to shear stress leads to exposure of a buried cysteine within the lamin Ig fold which, in turn, may trigger further—yet unidentified—signaling events [30]. Mammalian B-type lamins are probably dispensable for maintaining nuclear rigidity and mechanosensing. In contrast, the C. elegans lamin, which is classified as a B-type lamin with many characteristics of A-type lamins, is required for nuclear rigidity and probably for mechanosensing as well [24,31]. Interestingly, cells lacking the lamin-binding protein emerin exhibit abnormal shape and impaired activation of mechanosensitive genes but normal nuclear mechanics [32].
Lamin A expression is controlled by tissue stiffness and cell matrix rigidity
A recent study reveals a direct correlation between an increased lamin A expression and increased stiffness of the tissue (Figure 1B) [30]. This observation suggests that the ability of a tissue to withstand physical insults requires an increased expression of lamin A. For example, soft tissues such as brain, kidney, and fat exhibit a low lamin A-to-lamin B ratio, whereas stiff tissues such as cartilage and bone reveal a high lamin A-to-lamin B ratio, with muscle showing an intermediate ratio. The lamin A-to-lamin B ratio also correlates well with the amount of collagen that the corresponding cells express. The expression of lamin A as well as the differentiation capability of a given cell depends, at least in part, on the elasticity of the matrix it is growing on. For example, cells expressing low amounts of lamin A when grown on a soft matrix tend to undergo adipogenesis, whereas the same cells grown on a stiff matrix show increased lamin A expression and undergo osteogenesis. Similarly, the ability of human mesenchymal stem cells to differentiate requires a soft matrix to grow on. Taken together, tissue stiffness and matrix softness lead to changes in the levels of lamin A expression which, in turn, can help stabilize the nucleus and thereby contribute to lineage determination.
Keratin, vimentin, and desmin intermediate filaments are major determinants of cell stiffness
Whereas the involvement of microfilaments and microtubules in the mechanical behavior of cells has been investigated in great detail, comparably little has been published about the contribution of IFs to cell and tissue mechanics. In the past, the mechanical role of IFs has been inferred mostly from measurements performed on individual IFs or IF gels in vitro [33-37]. Recently, by performing active micro-rheology using optical tweezers, it was demonstrated that although vimentin IF networks contribute little to the stiffness of a cell's cortex, they are a critical regulator of intracellular cell mechanics [38,39]. More specifically, these studies showed that in mouse embryonic fibroblasts the vimentin IF network both increases the mechanical integrity of the cells and localizes/stabilizes intracellular organelles.
Without causing major rearrangements of the microfilament and microtubule cytoskeleton, the vimentin IF network of rat fibroblasts can specifically be targeted and modulated by transfecting the cells with mutant desmin (Des) variants. Depending on the variant, transfectants appear either softer or stiffer than untransfected fibroblasts by nano-indenting the cells with an atomic force microscopy (AFM) tip [40]. For example, expression of the non-filament-forming DesL345P mutant led to a collapse of the endogenous vimentin IF network in the perinuclear region that, in turn, was accompanied by stiffening of the perinuclear cell space. More generally, by measuring mechanical changes caused via IF rearrangements in intact cells, it was shown that IFs play a crucial role in the mechanical behavior of cells not only at large deformations but also in the nanomechanical response of individual cells [41].
While keratin IFs form cytoskeletal networks that are essential for the structural integrity of epithelial cells, these keratin IF networks also act as an intracellular scaffold that significantly contributes to cell stiffness, thereby helping epithelial cells to withstand external mechanical forces [42]. Furthermore, keratin IFs have a critical impact on the invasive behavior of epithelial cells [43], whereas keratin-free cells show about a 60% higher deformability, and actin depolymerization by latrunculin A yields a significantly less pronounced softening of the same cells. Also, it has been demonstrated that the mechanical resilience of keratinocytes is critically determined by their keratin network system with far-reaching implications for epithelial pathophysiology [44]. For example, in various human epithelial cancer cell lines, the observed rearrangements of the keratin IF network were hypothesized to result in significant changes of the cells' visco-elastic properties [45].
Vimentin organization modulates lamellipodia formation and cell migration
While vimentin IF networks extend throughout the rear and perinuclear space of migrating fibroblasts, in lamellipodial regions only non-filamentous vimentin particles are observed. In contrast, in serum-starved or non-motile fibroblasts, vimentin IF networks extend to the entire cell periphery. Upon serum addition or activation of Rac1, vimentin IFs become rapidly phosphorylated, coincident with vimentin IF disassembly and retraction from the cell periphery where lamellipodia form. Furthermore, when vimentin organization in motile fibroblasts is disrupted by a dominant-negative mutant or by vimentin gene silencing, there is a loss of cell polarity, as evidenced by the formation of lamellipodia encircling the entire cell, as well as reduced cell migration. These findings demonstrate an antagonistic relationship between the existence of a peripheral vimentin IF network and the formation of a lamellipodium, the locomotory structure residing at the leading edge of migrating mesenchymal cells [46].
Vimentin and cell adhesion
Despite the wide use of vimentin as a marker of the epithelial-mesenchymal transition (EMT) that occurs during embryogenesis and metastasis, the functional implications of vimentin expression during EMT are only poorly understood. It has now been shown that following the microinjection of vimentin or transfection with vimentin cDNA, epithelial cells rapidly adopt mesenchymal shapes coincident with vimentin IF assembly [47]. Concomitant with these shape transitions, a loss of desmosomal contacts, an increase in cell motility, and a significant increase in focal adhesion (FA) dynamics are observed. These findings demonstrate that vimentin IFs play a critical role in the cell shape, FA, and motility changes that occur during EMT.
Recently, another hitherto unrecognized regulatory role of vimentin IFs has been described that involves their interaction with FAs: cytoskeleton-regulated mechanosensing through FA-anchored (via plectin) vimentin IFs [48]. Excitingly, this work has identified a novel mechanistic link between vimentin IF targeting to FAs and integrin-mediated FA kinase ([FAK] – the major mechanosensor molecule) signaling to regulate cell migration. These findings provide important new insights into the molecular mechanisms underlying IF-regulated mechanosensing, a feature that is fundamental for controlled cell movement and tumor progression.
For cells to develop long-range forces and transport cargos to the periphery, the microtubule- and organelle-rich region at the center of the cell, the endoplasm, needs to spread toward the cell edge. Depletion of the actin cross-linking protein filamin A (FlnA) causes a collapse of the endoplasm into a sphere around the nucleus of fibroblasts and disruption of FAs, indicating that FlnA is involved in endoplasmic spreading and FA growth [49]. Because focal contact size and adhesion involve the vimentin IF network [50], the role of vimentin IFs in endoplasmic spreading was examined [51]. Accordingly, wildtype cells expressing a vimentin variant unable to polymerize show deficient endoplasmic spreading as well as defects in FA growth. Moreover, treatment of these cells with the calpain inhibitor 1, ALLN, restores FA growth despite the lack of vimentin IFs, but does not restore endoplasmic spreading, implying that vimentin IFs are needed for endoplasm spreading. Consistent with that hypothesis, vimentin IFs are always displaced from FAs when the endoplasm does not spread. Taken together, these and other findings suggest that endoplasmic spreading requires the coalescence of vimentin IFs at force-bearing FAs [51].
Conclusions
It is clear that IFs are essential components of cell architecture. Studies of their roles in cell and nuclear mechanics and cell migration and differentiation are now being facilitated through insights into the mechanisms that lead to human diseases, including cancer.
Many interesting properties of the IFs remain to be determined. These include the structure of the different types of protein assemblies both within the nucleus and throughout the cytoplasm, the roles of post-translational modifications in regulating their dynamics, and the protein complexes they form and their precise roles in development and differentiation.
Acknowledgments
We gratefully acknowledge funding from the Morasha Legacy 1798/10, the Muscular Dystrophy Association, the Israeli Science Foundation, the Binational Israel-USA Science Foundation (BSF 2007215), and the COST NANONET (BM1002).
Abbreviations
- EMT
epithelial-mesenchymal transition
- FA
focal adhesion
- FlnA
filamin A
- IF
intermediate filament
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/6/54
Contributor Information
Yosef Gruenbaum, Email: gru@vms.huji.ac.il.
Ueli Aebi, Email: ueli.aebi@unibas.ch.
References
- 1.Chang L, Goldman RD. Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol. 2004;5:601–13. doi: 10.1038/nrm1438. [DOI] [PubMed] [Google Scholar]
- 2.Castañón MJ, Walko G, Winter L, Wiche G. Plectin-intermediate filament partnership in skin, skeletal muscle, and peripheral nerve. Histochem Cell Biol. 2013;140:33–53. doi: 10.1007/s00418-013-1102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718450238
- 3.Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res. 2007;313:2189–203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Fridkin A, Penkner A, Jantsch V, Gruenbaum Y. SUN-domain and KASH-domain proteins during development, meiosis and disease. Cell Mol Life Sci. 2009;66:1518–33. doi: 10.1007/s00018-008-8713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrmann H, Bär H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Cell Mol Biol. 2007;8:562–73. doi: 10.1038/nrm2197. [DOI] [PubMed] [Google Scholar]
- 6.Prokocimer M, Davidovich M, Nissim-Rafinia M, Wiesel-Motiuk N, Bar D, Barkan R, Meshorer E, Gruenbaum Y. Nuclear lamins: key regulators of nuclear structure and activities. J Cell Mol Med. 2009;13:1059–85. doi: 10.1111/j.1582-4934.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Las Heras JI, Meinke P, Batrakou DG, Srsen V, Zuleger N, Kerr AR, Schirmer EC. Tissue specificity in the nuclear envelope supports its functional complexity. Nucleus. 2013;4:460–77. doi: 10.4161/nucl.26872. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718450239
- 8.Wiesel N, Mattout A, Melcer S, Melamed-Book N, Herrmann H, Medalia O, Aebi U, Gruenbaum Y. Laminopathic mutations interfere with the assembly, localization and dynamics of nuclear lamins. Proc Natl Acad Sci USA. 2008;105:180–5. doi: 10.1073/pnas.0708974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moir RD, Spann TP, Lopez-Soler RI, Yoon M, Goldman AE, Khuon S, Goldman RD. The dynamics of the nuclear lamins during the cell cycle-relationship between structure and function. J Struct Biol. 2000;129:324–34. doi: 10.1006/jsbi.2000.4251. [DOI] [PubMed] [Google Scholar]
- 10.Naetar N, Foisner R. Lamin complexes in the nuclear interior control progenitor cell proliferation and tissue homeostasis. Cell Cycle. 2009;8:1488–93. doi: 10.4161/cc.8.10.8499. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718450244
- 11.Pekovic V, Harborth J, Broers JL, Ramaekers FC, van Engelen B, Lammens M, von Zglinicki T, Foisner R, Hutchison C, Markiewicz E. Nucleoplasmic LAP2alpha-lamin A complexes are required to maintain a proliferative state in human fibroblasts. J Cell Biol. 2007;176:163–72. doi: 10.1083/jcb.200606139. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1061842
- 12.Starger JM, Brown WE, Goldman AE, Goldman RD. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978;78:93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soellner P, Quinlan RA, Franke WW. Identification of a distinct soluble subunit of an intermediate filament protein: tetrameric vimentin from living cells. Proc Natl Acad Sci USA. 1985;82:7929–33. doi: 10.1073/pnas.82.23.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winheim S, Hieb AR, Silbermann M, Surmann EM, Wedig T, Herrmann H, Langowski J. Deconstructing the late phase of vimentin assembly by total internal reflection fluorescence microscopy (TIRFM) PLoS ONE. 2011;6:e19202. doi: 10.1371/journal.pone.0019202. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718450245
- 15.Goldman RD, Cleland MM, Murthy P, Mahammad S, Kuczmarski ER. Inroads into the structure and function of intermediate filament networks. J Struct Biol. 2012;177:14–23. doi: 10.1016/j.jsb.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller RK, Vikstrom KL, Goldman RD. Keratin incorporation into intermediate filament networks is a rapid process. J Cell Biol. 1991;113:843–55. doi: 10.1083/jcb.113.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vikstrom KL, Borisy GG, Goldman RD. Dynamic aspects of intermediate filament networks in BHK-21 cells. Proc Natl Acad Sci USA. 1989;86:549–53. doi: 10.1073/pnas.86.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vikstrom KL, Lim SS, Goldman RD, Borisy GG. Steady state dynamics of intermediate filament networks. J Cell Biol. 1992;118:121–9. doi: 10.1083/jcb.118.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou YH, Bischoff JR, Beach D, Goldman RD. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990;62:1063–71. doi: 10.1016/0092-8674(90)90384-Q. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson JE, He T, Trejo-Skalli AV, Härmälä-Brasken AS, Hellman J, Chou YH, Goldman RD. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci. 2004;117:919–32. doi: 10.1242/jcs.00906. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718450246
- 21.Eriksson JE, Brautigan DL, Vallee R, Olmstead J, Fujiki H, Goldman RD. Cytoskeletal integrity in interphase cells requires protein phosphatase activity. Proc Natl Acad Sci USA. 1992;89:11093–7. doi: 10.1073/pnas.89.22.11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant H. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res. 2007;313:2098–109. doi: 10.1016/j.yexcr.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaminsky R, Denison C, Bening-Abu-Shach U, Chisholm AD, Gygi SP, Broday L. SUMO regulates the assembly and function of a cytoplasmic intermediate filament protein in C. elegans. Dev Cell. 2009;17:724–35. doi: 10.1016/j.devcel.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1282028
- 24.Liu J, Rolef-Ben Shahar T, Riemer D, Spann P, Treinin M, Weber K, Fire A, Gruenbaum Y. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell. 2000;11:3937–47. doi: 10.1091/mbc.11.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreiber KH, Kennedy BK. When lamins go bad: nuclear structure and disease. Cell. 2013;152:1365–75. doi: 10.1016/j.cell.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foisner R, Aebi U, Bonne G, Gruenbaum Y, Novelli G. Nuclear envelope-linked rare human diseases: From molecular pathophysiology towards clinical applications. Neuromuscular Disorders. 2007;17:655–60. doi: 10.1016/j.nmd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Gruenbaum Y, Wilson KL, Harel A, Goldberg M, Cohen M. Nuclear Lamins - Structural Proteins with Fundamental Functions. J Struct Biol. 2000;129:313–23. doi: 10.1006/jsbi.2000.4216. [DOI] [PubMed] [Google Scholar]
- 28.Rowat AC, Lammerding J, Herrmann H, Aebi U. Towards an integrated understanding of the structure and mechanics of the cell nucleus. BioEssays. 2008;30:226–36. doi: 10.1002/bies.20720. [DOI] [PubMed] [Google Scholar]
- 29.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RS. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–8. doi: 10.1172/JCI200419670. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718039243
- 30.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718089920
- 31.Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, Lee RT. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem. 2006;281:25768–80. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718039235
- 32.Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, Lee RT. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170:781–91. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718039244
- 33.Muecke N, Kreplak L, Kirmse R, Wedig T, Herrmann H, Langowski J, Aebi U. Assessing the Flexibility of Intermediate Filaments by Atomic Force Microscopy. J Mol Biol. 2004;335:1241–50. doi: 10.1016/j.jmb.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 34.Kreplak L, Baer H, Leterrier JF, Herrmann H, Aebi U. Exploring the Mechanical Behavior of Single Intermediate Filaments. J Mol Biol. 2005;354:569–77. doi: 10.1016/j.jmb.2005.09.092. [DOI] [PubMed] [Google Scholar]
- 35.Kreplak L, Herrmann H, Aebi U. Tensile Properties of Single Desmin Intermediate Filaments. Biophys J. 2008;94:2790–9. doi: 10.1529/biophysj.107.119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasza KE, Rowat AC, Liu J, Angelini TE, Brangwynne CP, Koenderink GH, Weitz DA. The Cell as a Material. Curr Opin Cell Biol. 2007;19:101–7. doi: 10.1016/j.ceb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Koester S, Lin YC, Herrmann H, Weitz DA. Nanomechanics of Vimentin Intermediate Filament Networks. Soft Matter. 2010;6:1910–4. doi: 10.1039/c000113a. [DOI] [Google Scholar]
- 38.Buehler MJ. Mechanical players – The role of intermediate filaments in cell mechanics and organization. Biophys J. 2013;105:1733–4. doi: 10.1016/j.bpj.2013.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo M, Ehrlicher AJ, Mahammad S, Fabich H, Jensen MH, Moore JR, Freedberg JJ, Goldman RD, Weitz DA. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys J. 2013;105:1562–8. doi: 10.1016/j.bpj.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718134027
- 40.Plodinec M, Loparic M, Suetterlin R, Herrmann H, Aebi U, Schoenenberger CA. The nanomechanical properties of rat fibroblasts are modulated by interfering with the vimentin intermediate filament system. J Struct Biol. 2011;174:476–84. doi: 10.1016/j.jsb.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Herrmann H, Strelkov SV, Burkhard P, Aebi U. Intermediate filaments: primary determinants of cell architecture and plasticity. J Cli Invest. 2009;119:1772–83. doi: 10.1172/JCI38214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sivaramakrishnan S, DeGiulio JV, Lorand L, Goldman RD, Ridge KM. Micromechanical properties of keratin intermediate filament networks. Proc Natl Acad Sci USA. 2008;105:889–94. doi: 10.1073/pnas.0710728105. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718450250
- 43.Seltmann K, Fritsch AW, Kaes JA, Magin TM. Keratins significantly contribute to cell stiffness and impact invasive behavior. Proc Natl Acad Sci USA. 2013;110:18507–12. doi: 10.1073/pnas.1310493110. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718156864
- 44.Ramms L, Fabris G, Windoffer R, Schwarz N, Springer R, Zhou C, Lazar J, Stiefel S, Hersch N, Schnakenberg U, Magin TM, Leube RE, Merkel R, Hoffmann B. Keratins as the main component for the mechanical integrity of keratinocytes. Proc Natl Acad Sci USA. 2013;110:18513–8. doi: 10.1073/pnas.1313491110. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718156891
- 45.Beil M, Micoulet A, von Wichert G, Paschke S, Walther P, Omary MB, Van Veldhoven PP, Gern U, Wolff-Hieber E, Eggermann J, Waltenberger J, Adler G, Spatz J, Seufferlein T. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nat Cell Biol. 2003;5:803–11. doi: 10.1038/ncb1037. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1016450
- 46.Helfand BT, Mendez MG, Murthy SNP, Shumaker DK, Grin B, Mahammad S, Aebi U, Wedig T, Wu YI, Hahn KM, Inagaki M, Herrmann H, Goldman RD. Vimentin organization modulates the formation of lamellipodia. Mol Biol Cell. 2011;22:1274–89. doi: 10.1091/mbc.E10-08-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mendez MG, Kojima SI, Goldman RD. Vimentin induces changes in cell shape, motility and adhesion during epithelial to mesenchymal transition. FASEB J. 2010;24:1838–51. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gregor M, Osmanagic-Myers S, Burgstaller G, Wolfram M, Fischer I, Walko G, Resch GP, Joergl A, Herrmann H, Wiche G. Mechanosensing through focal adhesion-anchored intermediate filaments. FASEB J. 2014;28:715–29. doi: 10.1096/fj.13-231829. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718210066
- 49.Lynch CD, Gauthier NC, Biais N, Lazar AM, Roca-Cusachs P, Yu CH, Sheets MP. Filamin depletion blocks endoplasmic spreading and destabilizes force-bearing adhesions. Mol Biol Cell. 2011;22:1263–73. doi: 10.1091/mbc.E10-08-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718450251
- 50.Tsuruta D. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci. 2003;116:4977–84. doi: 10.1242/jcs.00823. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1017144
- 51.Lynch CD, Lazar AM, Iskratsch T, Zhang X, Sheets MP. Endoplasmic spreading requires coalescence of vimentin intermediate filaments at force-bearing adhesions. Mol Biol Cell. 2013;24:21–30. doi: 10.1091/mbc.E12-05-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718450252


