Abstract
Lysosomes are key cellular organelles that play a crucial role in catabolism by degrading extracellular and intracellular material. It is, therefore, very intriguing that mTORC1 (mechanistic target of rapamycin complex 1), a major promoter of anabolic processes, localizes in its active form to the surface of lysosomes. In recent years, many exciting observations have revealed a tightly regulated crosstalk between mTORC1 activity and lysosomal function. These findings highlight the complex regulatory network that modulates energy metabolism in cells.
Introduction
Target of rapamycin (mTOR) is an evolutionarily conserved serine/threonine kinase that regulates cell growth and division in response to energy levels, growth signals, and nutrients [1]. Control of mTOR activity is critical for the cell since its dysregulation leads to cancer, metabolic disease, and diabetes [2]. In cells, mTOR exists as two structurally distinct complexes termed mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), each one with specificity for different sets of effectors. mTORC1 couples energy and nutrient abundance to cell growth and proliferation by balancing anabolic (protein synthesis and nutrient storage) and catabolic (autophagy and utilization of energy stores) processes. Although different cellular locations for mTORC1 and mTORC2 have been reported (these studies were recently summarized in an excellent review by Betz and Hall [3]), there is a consensus that the localization of mTORC1 to lysosomes is critical for its ability to sense and respond to variations in the levels of amino acids.
When amino acid levels are high, mTORC1 is recruited to the lysosomal surface, where it is activated by the guanosine-5’-triphosphate (GTP)-loaded form of the small GTPase Rheb (Ras homolog enriched in brain) [4,5]. The amino acid-dependent translocation of mTOR to the lysosome requires active Rag GTPases and a Ragulator, a pentameric protein complex that anchors the Rag GTPases to lysosomes [6-8]. The Rag proteins function as heterodimers in which the active complex consists of GTP-bound RagA or B complexed with guanosine diphosphate (GDP)-bound RagC or D. It has been proposed that amino acids trigger the GTP loading of RagA/B proteins, thus promoting the binding to raptor and assembly of an activated mTORC1 complex. However, it is important to mention that this model was recently challenged by a study suggesting that the activation of mTORC1 is not dependent on the Rag GTP charging [9]. Therefore, further studies will be required to solve this apparent discrepancy and fully characterize the mechanism of mTORC1 translocation to lysosomes upon amino acid stimulation. Meanwhile, the activity of Rheb is regulated by a complex consisting of tuberous sclerosis complex 1 (TSC1), TSC2, and TBC1 domain family member 7 (TBC1D7) [10,11]. This complex also localizes to lysosomes and functions as a GTPase-activating protein (GAP) that inhibits the activity of Rheb [12,13]. In the presence of growth factors or insulin, TSC releases its inhibitory activity on Rheb, thus allowing the activation of mTORC1. Therefore, different stimuli must cooperate to ensure proper mTORC1 activation.
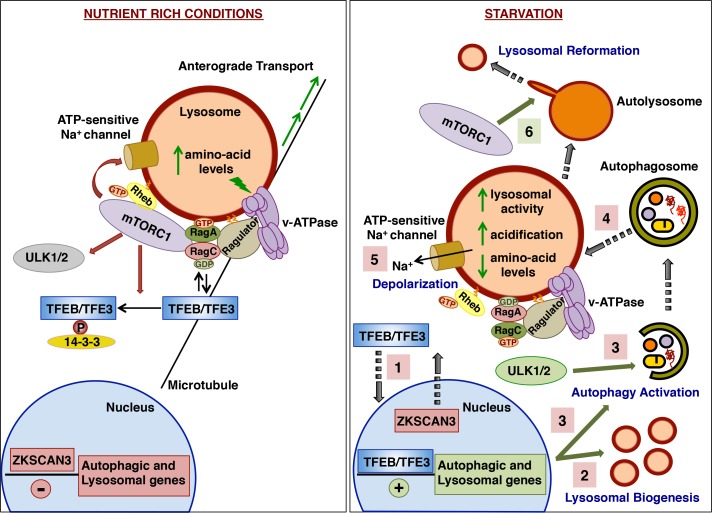
It is important to keep in mind that lysosomes do not simply serve as platforms for the proper assembly of the mTORC1 regulatory pathway. In the last few years, it has become apparent that the activities of mTORC1 and lysosomes are intimately interconnected. The level of amino acids inside the lumen of lysosomes directly modulates mTORC1 activity through the vacuolar H+-adenosine triphosphatase (v-ATPase) [14]. In addition, recent evidence suggests that mTORC1 may play a crucial role in lysosomal function by regulating biogenesis, distribution, and activity of lysosomes (Figure 1).
Figure 1. mTORC1 regulates lysosomal function in normal and starvation conditions.
Under nutrient-rich conditions (left panel), active mTORC1 promotes the retention of TFEB and TFE3 in the cytosol as well as inhibition of the ULK1/2 complex and the ATP-sensitive Na+ channel. In addition, lysosomes move toward the periphery of the cell. In contrast, under starvation conditions (right panel), the inactivation of mTORC1 leads to rapid translocation of TFEB and TFE3 to the nucleus (1), induction of lysosomal biogenesis (2), autophagy activation (3), and changes in lysosomal membrane potential (5). Inactivation of mTORC1 might also be required to facilitate the fusion between autophagomes and lysosomes (4). After prolonged periods of starvation, reactivation of mTORC1 is critical to induce autophagic lysosomal reformation (6). Abbreviations: mTORC1, mechanistic target of rapamycin (serine/threonine kinase) complex 1; RHEB, Ras homolog enriched in brain; TFE3, transcription factor binding to IGHM enhancer 3; TFEB, transcription factor EB; ULK, uncoordinated 51-like kinase; v-ATPase, vacuolar H+-adenosine triphosphatase; ZKSCAN3, zinc finger with KRAB and SCAN domains 3.
Lysosomal biogenesis
It was long presumed that the expression of lysosomal genes was constitutive. However, recent evidence revealed that cells constantly monitor lysosomal function and possess the ability to increase the number and activity of lysosomes in response to their energetic or degradative needs. The transcription factor EB (TFEB), a member of the basic helix-loop-helix leucine zipper family of transcription factors, promotes expression of many lysosomal proteins and is considered a master regulator of lysosomal biogenesis. Upon activation, TFEB binds directly to a 10-base pair motif (GTCACGTGAC), known as Coordinated Lysosomal Expression and Regulation (CLEAR) element, enriched in the promoter regions of numerous lysosomal genes, thus promoting their transcription [15]. Interestingly, the activation of TFEB is regulated by mTORC1 [16-18]. In fed cells, active mTORC1 phosphorylates TFEB in several serine residues. mTORC1-dependent phosphorylation of TFEB in serine 211 (S211) is particularly important, as this residue mediates the interaction between TFEB and the cytosolic chaperone 14-3-3 and causes retention of TFEB in the cytosol [16,17]. Following starvation and consequent inactivation of mTORC1, the TFEB/14-3-3 complex dissociates, allowing rapid transport of TFEB to the nucleus and TFEB-regulated expression of lysosomal genes.
Active TFEB also upregulates the expression of critical regulators of the autophagic process, including several proteins implicated in the formation of autophagosomes as well as proteins required for the fusion between autophagosomes and lysosomes [19]. Therefore, TFEB contributes to synchronize the two major cellular degradative systems: autophagy and lysosomes. The ability of mTORC1 to block autophagy initiation was well established but was thought to occur at the protein level. mTORC1 directly phosphorylates and inhibits key components of the uncoordinated 51-like kinase 1/2 (ULK1/ULK2) complex, required for autophagy initiation [20,21]. However, the above-mentioned findings indicate that, by promoting the sequestration of TFEB in the cytosol, mTORC1 also modulates autophagy at the transcriptional level. Finally, TFEB induces expression of critical regulators of lipid metabolism that facilitate the use of energy stores during prolonged periods of starvation and this is a role well conserved during evolution [22-24].
Phosphorylation of TFEB by mTORC1 requires the recruitment of TFEB to lysosomes, the compartment where active mTORC1 resides. This recruitment is mediated by the direct interaction between TFEB and active Rag GTPases [25]. Rags also bring follicullin (FLCN) and TSC to lysosomes [13,26-28]. FLCN functions as a RagC/D GAP and is thought to be critical for mTORC1 reactivation when cells change from starvation to nutrient-rich conditions [27]. Therefore, Rags play an important role as docking sites for recruiting mTORC1, mTORC1 effectors (TFEB), and mTORC1 regulators (FLCN and TSC) to the lysosomal surface in a nutrient-dependent manner. Interestingly, TFEB (as well as TFE3; see below) induces the expression of FLCN [26], suggesting the presence of a regulatory loop in which TFEB may contribute to mTORC1 reactivation and therefore its own inhibition.
TFEB is not the only transcription factor implicated in lysosomal biogenesis. TFEB belongs to the MiTF/TFE family of transcription factors that includes MITF, TFE3, and transcription factor EC (TFEC). TFE3 was previously implicated in the development of osteoclasts, activation of the immune system, and control of the allergic response. However, recent evidence showed that TFE3 also induces autophagy and lysosomal biogenesis in starved cells by binding to the CLEAR elements present in the promoter regions of autophagic and lysosomal genes [26]. At the same time, the transcription factor ZKSCAN3 functions as a negative regulator of lysosomal biogenesis and autophagy in fed cells [29]. Interestingly, the nuclear localization of both, TFE3 and ZKSCAN3, is directly regulated by mTORC1 [26,29]. Overall, the emerging picture reveals a close collaboration between mTORC1 and several master regulators of autophagy and lysosomal biogenesis to facilitate an efficient response to the varying energetic demands of the cell.
Lysosomal activity
To maintain an efficient autophagic flux during starvation conditions, cells must couple formation and degradation of autophagosomes. For this, synthesis of autophagosomes ideally should be linked to increased autophagosome-lysosome fusion and increased lysosomal degradative activity. Recent evidence suggests that mTORC1 serves as a negative regulator of lysosomal function. Zhou and colleagues compared lysosomal function between fed and starved cells by measuring lysosomal acidification, cathepsin activity, and rate of proteolysis [30]. They found that the activity of lysosomes was significantly augmented in starved cells, a factor that is probably critical to ensure efficient degradation of the autophagic content. Increased lysosomal activity under starvation conditions required the inactivation of mTORC1, translocation of TFEB to the nucleus, and fusion of autophagosomes with lysosomes. Early investigations revealed that the inhibition of protein synthesis by cycloheximide (CHX) resulted in diminished lysosomal function [31]. Since CHX causes a dramatic increase in the amount of intracellular amino acids, it is possible that the observed effect was due to CHX-mediated activation of mTORC1. The requirement of autophagosome-lysosome fusion for efficient lysosomal degradation is very intriguing and suggests that autophagosomes might contribute important regulators of lysosomal activity. This idea is supported by the finding that TFEB-mediated lysosomal exocytosis is significantly reduced in autophagy-defective cells [32].
The current model suggests that mTORC1 regulates lysosomal function by directly preventing autophagy and TFEB activation. Under starvation, inactivation of mTORC1 releases the inhibition of the ULK1/ULK2 complex, thus promoting formation of autophagosomes. In addition, TFEB is free to translocate to the nucleus and upregulate the expression of critical regulators that further enhance autophagic flux. TFEB also activates expression of genes that directly increase lysosomal activity, including several subunits of the v-ATPase, lysosomal hydrolases, and receptors required for the delivery of those hydrolases to lysosomes [33]. In addition, the inactivation of mTORC1 seems to be required to allow the fusion between autophagosomes and lysosomes. Choi and colleagues (2012) recently identified a synthetic compound, MHY1485, which inhibits lysosomal fusion during starvation-induced autophagy by directly binding and activating mTORC1 [34].
mTORC1 might also regulate lysosomal function by directly modulating the activity of key lysosomal proteins. The v-ATPase is a multisubunit proton pump composed of a V1 complex that catalyzes ATP hydrolysis and a transmembrane VO complex that rotated upon ATP hydrolysis. The best-characterized role of v-ATPase is to pump protons inside the lumen of the lysosome, thus promoting lysosomal acidification. However, recent evidence suggests a novel function of v-ATPase in nutrient sensing [14]. When the level of amino acids in the lumen of lysosomes is low, the v-ATPase interacts with the Ragulator and prevents the activation of Rag GTPases. In contrast, when amino acids are abundant, the v-ATPase undergoes conformational changes that release the guanine nucleotide exchange factor (GEF) activity of Ragulator, leading to the activation of Rag heterodimers and the recruitment of mTORC1 to lysosomes. Although it is still undetermined whether the v-ATPase is a direct sensor of amino acid levels or additional proteins are implicated in this process, it is clear that v-ATPase plays a critical role linking amino acid levels to mTORC1 activation. Open questions that remain unanswered include how the structural rearrangements undergone by the v-ATPase in different nutrient conditions affect lysosomal acidification and whether mTORC1 directly modulates v-ATPase activity as part of a regulatory feedback loop. In this regard, it was recently shown that mTORC1 is involved in controlling v-ATPase assembly in dendritic cells [35].
Another interesting possibility was recently suggested by Cang and colleagues [36]. Under nutrient- and ATP-rich conditions, mTORC1 binds and inhibits the activity of an endolysosomal ATP-sensitive Na+ channel formed by the two-pore channels TPC1 and TPC2. When the levels of ATP are reduced, something that occurs under starvation and other stress conditions like hypoxia, ischemia, or hyperosmotic stress, mTORC1 redistributes to the cytosol, allowing opening of the channel and the release of Na+ and other ions from the lumen of the lysosome to the cytosol. The depolarization of the lysosomal membrane may potentially affect many different lysosomal parameters, including the luminal pH, fusion of lysosomes with autophagosomes, or transport of nutrients.
Lysosomal positioning
In the presence of amino acids and growth factor, lysosomes tend to localize closer to the plasma membrane. It has been suggested that lysosomal positioning may have an important impact on mTOR activity by regulating the proximity of the kinase to upstream signals. In contrast, the retrograde transport of lysosomes to the perinuclear area under starvation conditions is thought to be critical to facilitate fusion with autophagosomes. The Rubinsztein group recently used different approaches to alter the distribution of lysosomes within cells [37]. As expected, the localization of lysosomes in the periphery correlated with increased mTOR activity, whereas the inhibition of lysosomal scattering resulted in diminished mTOR activity and, consequently, increased number of autophagosomes. The Rubinsztein group observed that, under starvation conditions, certain proteins implicated in the anterograde movement of lysosomes, such as kinesin KIF2 (kinesin heavy chain member 2) and ARL8B (ADP-ribosylation factor-like 8B), dissociate from lysosomal membranes, thus facilitating the movement of lysosomes towards the center of the cell. The mechanism by which nutrients regulate lysosomal distribution is currently unknown, but, as mentioned in the previous section, it is possible that nutrient-dependent changes in lysosomal membrane potential or mTORC1 activity regulate the association of lysosomes with microtubules or specific motors.
Lysosomal reformation
The catabolic activity of autophagy not only is critical to ensure cell survival when nutrients are limited but also regulates autophagy termination and lysosomal homeostasis. The Lenardo group found that, after long periods of starvation, mTORC1 is reactivated [38]. This is probably due to the degradation of the autophagosome content and consequent increase in the level of amino acids inside lysosomes. In agreement with this idea, the inhibition of lysosomal degradation was sufficient to suppress mTORC1 reactivation. Additional factors may contribute to regulate mTORC1 reactivation. For example, the depletion of spinster, a late endosomal/lysosomal sugar transporter, prevented mTORC1 reactivation and caused the accumulation of enlarged autolysosomes [39]. The role of spinster in lysosomal homeostasis was further supported by a recent study showing that this protein regulates fertility and fat content of lipid droplets in Caenorhabditis elegans by modulating lysosomal function and morphology [40]. Importantly, mTORC1 reactivation is required for the formation of nascent lysosomes from autophagosomes, a process known as autophagic lysosomal reformation (ALR). In this process, long and stable tubules emanate from autolysosomes and eventually pinch off to form nascent lysosomes. Over time, these proto-lysosomes become acidic and acquire degradative capacity, thus becoming mature lysosomes. The mechanism that orchestrates ALR is very complex and requires the synthesis and accumulation of specific phosphatidylinositols in certain regions of the reformation tubules as well as recruitment of clathrin and clathrin adaptors AP2 and AP4 [41,42]. Although it is still unclear how mTORC1 reactivation promotes ALR, these findings reveal an important role of mTORC1 in the recycling of critical lysosomal components for which synthesis and transport are energetically demanding. Reactivation of mTORC1 may also halt or at least slow down autophagy, thus preventing autophagic cell death [43,44]. The participation of mTORC1 in ALR may seem contradictory with its inhibitory role in lysosomal biogenesis and function. However, these observations probably reflect a broader role of mTORC1 in coordinating autophagy termination. It is also worth mentioning that ALR was observed when autophagy was induced by serum and glutamine starvation. Further studies will have to determine whether ALR also occurs under other forms of starvation (for example, glucose or amino acid starvation).
The function of mTORC1 in lysosomal reformation is conserved during evolution. In yeast, TORC1 has been shown to mediate vacuolar fission (but not fusion) [45]. Under nutrient restriction, TORC1 inactivation could alter the fusion-fission equilibrium, resulting in an increase in the size and a reduction in the number of vacuolar (lysosomal) structures, as it has been observed in both yeast and mammalian cells.
mTORC1 activity is also critical for fission of phagosomes and entotic vesicles [46]. Internalization of pathogens or live cells by phagocytosis or entosis, respectively, results in the formation of large macroendocytic vacuoles that fuse with lysosomes. Upon degradation, nutrients and vacuolar components are recycled back to the lysosomal network by the mTORC1-regulated fission of the phagosome/entotic vesicle. Although this recycling closely resembles ALR, it is important to point out that there are important differences between the two processes. The two most remarkable are that the vesicles produced by phagosome and entotic vacuole fission contain luminal components and that inhibitors of mTORC1 do not completely block tubulation (although the vesicle shrinkage is significantly delayed). Overall, the aforementioned studies indicate an essential role of mTORC1 in lysosome reformation.
Concluding remarks
mTORC1 stimulates important anabolic processes, such as protein synthesis and the accumulation of energy stores, whereas lysosomes are critical mediators of catabolism. Therefore, it is not surprising that the activities of mTORC1 and lysosomes are deeply interconnected. In the last few years, we have begun discerning the molecular mechanism that governs this connection. The emerging picture points to lysosomes as key regulators of nutrient signaling and energy homeostasis. However, many exciting questions still await clarification, including how the whole cell adapts to starvation conditions, the identification of novel regulators of the lysosomal/mTORC1 pathway, and the interplay between nutrient sensing and disease.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Heart, Lung, and Blood Institute.
Abbreviations
- ALR
autophagic lysosomal reformation
- CLEAR
Coordinated Lysosomal Expression and Regulation
- CHX
cycloheximide
- FLCN
folliculin
- GAP
GTPase-activating protein
- MITF
microphthalmia-associated transcription factor
- mTORC1
mechanistic target of rapamycin (serine/threonine kinase) complex 1
- RHEB
Ras homolog enriched in brain
- TFE3
transcription factor binding to IGHM enhancer 3
- TFEB
transcription factor EB
- TSC1
tuberous sclerosis 1
- TSC2
tuberous sclerosis 2
- ULK1
uncoordinated 51-like kinase 1
- v-ATPase
vacuolar H+-adenosine triphosphatase
- ZKSCAN3
zinc finger with KRAB and SCAN domains 3
Disclosures
The author declares that she has no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/6/52
References
- 1.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol. 2013;203:563–74. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718290684
- 4.Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–71. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1013733
- 5.Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–65. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718454652
- 6.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/2986956
- 7.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1116121
- 8.Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717956431
- 9.Oshiro N1, Rapley J, Avruch J. Amino acids activate mammalian target of rapamycin (mTOR) complex 1 without changing Rag GTPase guanyl nucleotide charging. J Biol Chem. 2014;289:2658–74. doi: 10.1074/jbc.M113.528505. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718206553
- 10.Huang J, Dibble CC, Matsukazi M, Manning BD. The TSC1-TSC2 complex is required for proper activation of the mTOR complex 2. Mol Cell Biol. 2008;28:4104–15. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1115144
- 11.Dibble CC, Elis W, Menon S, Qin W, Klekota J, Asara JM, Finan PM, Kwiatkowski DJ, Murphy LO, Manning BD. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell. 2012;47:535–46. doi: 10.1016/j.molcel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718454680
- 12.Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, Cantley LC, Manning BD. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–85. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718278268
- 13.Demetriades C, Doumpas N, Teleman AA. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell. 2014;156:786–99. doi: 10.1016/j.cell.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718278267
- 14.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–83. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13364989
- 15.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–7. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1164676
- 16.Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–14. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci Signal. 2012;5:ra42. doi: 10.1126/scisignal.2002790. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718454838
- 18.Settembre C, Zoncu R, Medina D.L, Vetrini F, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard M.C, Facchinetti V, Sabatini D.M, Ballabio A. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012;31:1095–108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718454844
- 19.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–33. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/11545957
- 20.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–91. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718454852
- 21.Hosokawa N, Sasaki T, Iemura S, Natsume T, Hara T, Mizushima N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy. 2009;5:973–79. doi: 10.4161/auto.5.7.9296. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718454853
- 22.Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, Huynh T, Carissimo A, Palmer D, Klisch TJ, Wollenberg AC, Di Bernardo D, Chan L, Irazoqui JE, Ballabio A. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013;15:647–58. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718403997
- 23.Grove CA, De Masi F, Barrasa MI, Newburger DE, Alkema MJ, Bulyk ML, Walhout AJ. A multiparameter network reveals extensive divergence between C. elegans bHLH transcription factors. Cell. 2009;138:314–27. doi: 10.1016/j.cell.2009.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1162875
- 24.O'Rourke EJ, Ruvkun G. MXL-3 and HLH-30 transcriptionally link lipolysis and autophagy to nutrient availability. Nat Cell Biol. 2013;15:668–76. doi: 10.1038/ncb2741. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718455002
- 25.Martina JA, Puertollano R. Rag GTPases mediate amino acid-dependent recruitment of TFEB and MITF to lysosomes. J Cell Biol. 2013;200:475–91. doi: 10.1083/jcb.201209135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martina JA, Diab HI, Lishu L, Jeong-A L, Patange S, Raben N, Puertollano R. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci Signal. 2014;7(309):ra9. doi: 10.1126/scisignal.2004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsun ZY, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, Spooner E, Sabatini DM. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell. 2013;52:495–505. doi: 10.1016/j.molcel.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718133757
- 28.Petit CS, Roczniak-Ferguson A, Ferguson SM. Recruitment of folliculin to lysosomes supports the amino acid-dependent activation of Rag GTPases. J Cell Biol. 2013;202:1107–22. doi: 10.1083/jcb.201307084. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718128637
- 29.Chauhan S, Goodwin JG, Manyam G, Wang J, Kamat AM, Boyd DD. ZKSCAN3 is a master transcriptional repressor of autophagy. Mol Cell. 2013;50:16–28. doi: 10.1016/j.molcel.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717980675
- 30.Zhou J, Tan SH, Nicolas V, Bauvy C, Yang ND, Zhang J, Xue Y, Codogno P, Shen HM. Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res. 2013;23:508–23. doi: 10.1038/cr.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717996262
- 31.Thoene JG, Lemons R, Boskovich S, Borysko K. Inhibitors of protein synthesis also inhibit lysosomal proteolysis. Studies using cystinotic fibroblasts. J Clin Invest. 1985;75:370–6. doi: 10.1172/JCI111709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spampanato C, Feeney E, Li L, Cardone M, Lim JA, Annunziata F, Zare H, Polishchuk R, Puertollano R, Parenti G, Ballabio A, Raben N. Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol Med. 2013;5:691–706. doi: 10.1002/emmm.201202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmieri M, Impey S, Kang H, di Ronza A, Pelz C, Sardiello M, Ballabio A. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum Mol Genet. 2011;20:3852–66. doi: 10.1093/hmg/ddr306. [DOI] [PubMed] [Google Scholar]
- 34.Choi YJ1, Park YJ, Park JY, Jeong HO, Kim DH, Ha YM, Kim JM, Song YM, Heo HS, Yu BP, Chun P, Moon HR, Chung HY. Inhibitory effect of mTOR activator MHY1485 on autophagy: suppression of lysosomal fusion. PLoS One. 2012;7:e43418. doi: 10.1371/journal.pone.0043418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liberman R1, Bond S, Shainheit MG, Stadecker MJ, Forgac M. Regulated assembly of vacuolar ATPase is increased during cluster disruption-induced maturation of dendritic cells through a phosphatidylinositol 3-kinase/mTOR-dependent pathway. J Biol Chem. 2014;289:1355–63. doi: 10.1074/jbc.M113.524561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cang C1, Zhou Y, Navarro B, Seo YJ, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell. 2013;152:778–90. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/717988236
- 37.Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M, Menzies FM, O'Kane CJ, Deretic V, Rubinsztein DC. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011;13:453–60. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/10821956
- 38.Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–6. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/3833956
- 39.Rong Y, McPhee CK, Deng S, Huang L, Chen L, Liu M, Tracy K, Baehrecke EH, Yu L, Lenardo MJ. Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proc Natl Acad Sci USA. 2011;108:7826–31. doi: 10.1073/pnas.1013800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han M1, Chang H, Zhang P, Chen T, Zhao Y, Zhang Y, Liu P, Xu T, Xu P. C13C4.5/Spinster, an evolutionarily conserved protein that regulates fertility in C. elegans through a lysosome-mediated lipid metabolism process. Prot Cell. 2013;4:364–72. doi: 10.1007/s13238-013-3015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718002385
- 41.Rong Y, Liu M, Ma L, Du W, Zhang H, Tian Y, Cao Z, Li Y, Ren H, Zhang C, Li L, Chen S, Xi J, Yu L. Clathrin and phosphatidylinositol-4,5-bisphosphate regulate autophagic lysosome reformation. Nat Cell Biol. 2012;14:924–34. doi: 10.1038/ncb2557. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717959007
- 42.Sridhar S, Patel B, Aphkhazava D, Macian F, Santambrogio L, Shields D, Cuervo AM. The lipid kinase PI4KIIIβ preserves lysosomal identity. EMBO J. 2013;32:324–39. doi: 10.1038/emboj.2012.341. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717982099
- 43.Yu L, Strandberg L, Lenardo MJ. The selectivity of autophagy and its role in cell death and survival. Autophagy. 2008;4:567–73. doi: 10.4161/auto.5902. [DOI] [PubMed] [Google Scholar]
- 44.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–2. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]; f1000.com/prime/1018916
- 45.Michaillat L1, Baars TL, Mayer A. Cell-free reconstitution of vacuole membrane fragmentation reveals regulation of vacuole size and number by TORC1. Mol Biol Cell. 2012;23:881–95. doi: 10.1091/mbc.E11-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krajcovic M1, Krishna S, Akkari L, Joyce JA, Overholtzer M. mTOR regulates phagosome and entotic vacuole fission. Mol Biol Cell. 2013;24:3736–45. doi: 10.1091/mbc.E13-07-040. [DOI] [PMC free article] [PubMed] [Google Scholar]