Abstract
Schizophrenia is a highly disabling disorder whose causes remain to be better understood, and treatments have to be improved. However, several recent advances have been made in diagnosis, etiopathology, and treatment. Whereas reliability of diagnosis has improved with operational criteria, including Diagnostic and Statistical Manual of Mental Disorders, (DSM) Fifth Edition, validity of the disease boundaries remains unclear because of substantive overlaps with other psychotic disorders. Recent emphasis on dimensional approaches and translational bio-behavioral research domain criteria may eventually help move toward a neuroscience-based definition of schizophrenia. The etiology of schizophrenia is now thought to be multifactorial, with multiple small-effect and fewer large-effect susceptibility genes interacting with several environmental factors. These factors may lead to developmentally mediated alterations in neuroplasticity, manifesting in a cascade of neurotransmitter and circuit dysfunctions and impaired connectivity with an onset around early adolescence. Such etiopathological understanding has motivated a renewed search for novel pharmacological as well as psychotherapeutic targets. Addressing the core features of the illness, such as cognitive deficits and negative symptoms, and developing hypothesis-driven early interventions and preventive strategies are high-priority goals for the field. Schizophrenia is a severe, chronic mental disorder and is among the most disabling disorders in all of medicine. It is estimated by the National Institute of Mental Health (NIMH) that 2.4 million people over the age of 18 in the US suffer from schizophrenia. This illness typically begins in adolescence and derails the formative goals of school, family, and work, leading to considerable suffering and disability and reduced life expectancy by about 20 years. Treatment outcomes are variable, and some people are successfully treated and reintegrated (i.e. go back to work). Despite the effort of many experts in the field, however, schizophrenia remains a chronic relapsing and remitting disorder associated with significant impairments in social and vocational functioning and a shortened lifespan. Comprehensive treatment entails a multi-modal approach, including psychopharmacology, psychosocial interventions, and assistance with housing and financial sustenance. Research to date suggests a network of genetic, neural, behavioral, and environmental factors to be responsible for its development and course. This article aims to summarize and explain recent advancements in research on schizophrenia, to suggest how these recent discoveries may lead to a better understanding and possible further development of effective therapies, and to highlight the paradigm shifts that have taken place in our understanding of the diagnosis, etiopathology, and treatment.
Historical background and diagnosis
The concept of schizophrenia as a disease entity has undergone major changes over the past century. Kraepelin distinguished chronic psychoses from functional decline, which he termed dementia praecox, and episodic psychoses, which he called the manic-depressive insanity [1]. Subsequent literature pointed to considerable symptomatic overlap between these disorders, leading to the rather loosely defined, and therefore unreliable, diagnosis of schizoaffective disorder [2]. Over the past century, these categories have—to a large extent—remained the same, as summarized in the current classificatory systems: the DSM and the International Classification of Diseases (ICD) [3].
A noteworthy recent development in psychiatry was the release of the fifth edition of the DSM in 2013. Although no major changes were made to the definitions of schizophrenia and other psychotic disorders, efforts were made to increase simplicity of diagnoses. First, the Kraepelinian subtyping of schizophrenia into paranoid, disorganized, catatonic, and undifferentiated type was eliminated because of a lack of evidence supporting the validity of these distinctions. Second, catatonia was moved to become a specifier across diagnoses rather than a schizophrenia subtype. Third, a more longitudinal approach to this diagnosis was defined. Finally, the previous emphasis on Schneider's [4] “first rank” symptoms (i.e. delusions of thought broadcast and thought insertion) and bizarre delusions was eliminated. Although all this improved the ease of use of the criteria, the validity of these boundaries remained largely in question.
Over the past two decades, it has become increasingly clear that there are neurobiological [5], genetic [6], and treatment response [7] overlaps between these disorders, bringing into question the validity of these categories [8]. A dimensional approach to psychopathology and the view that biological impairments may cut across categories have led to the recent introduction of the research domain criteria (RDoC). RDoC [9] refers to a framework of representing accruing information across molecular, cellular, circuit, and behavioral domains agnostic to symptom-based diagnoses. Although this approach is still in its infancy, it might offer a useful framework for future research that may yield a neuroscience-informed classificatory system [8].
There has also been interest in defining schizophrenia beyond the symptoms listed in the DSM, with recent consideration of cognitive decline as a core feature with psychosis considered by some as a later development [10, 11]. Along these lines, there have also been significant efforts to better understand and characterize schizophrenia at earlier stages of the illness with the concept of schizophrenia prodome, which became of increasing clinical interest [12]. Schizophrenia prodrome, sometimes referred to as ultra-high-risk state or psychosis risk syndrome, is thought to be a spectrum of attenuated positive and negative symptoms that individuals may display several years to months before converting to schizophrenia.
Recent research has begun to elucidate risk factors for conversion to psychosis, including early impaired cognitive (e.g. inattention, concentration difficulties) and social functioning [13]. Even though not all risk factors are currently known, approximately 35% of the individuals with prodromal symptoms convert to schizophrenia [14, 15]. The North American Prodromal Longitudinal Study, a large multicenter study, has begun to demonstrate substantive neuroanatomical [16], neurophysiological [17], neurocognitive [18], and neurohormonal [19] changes during the prodromal phase that may contribute to the risk of schizophrenia [20].
Pathophysiology and etiology
Schizophrenia has a substantial genetic component, with a high heritability (up to 80%), indicating that about 80% of the variation in the trait of schizophrenia may be attributed to genetic factors [21]. Genome-wide association studies (GWASs), which compare the genomes of thousands of healthy and affected individuals, have found several genes associated with increased risk of developing schizophrenia, such as NRGN and ZNF804A [22, 23]. Recent research suggests that genetic risk for schizophrenia is composed of many common genetic alterations, each with a small effect, along with a few uncommon genetic alterations with a larger impact [24]. Additionally, genes that confer risk for schizophrenia may also be associated with bipolar disorder and other psychiatric disorders [25] (Figure 1).
Figure 1. Etiology of schizophrenia.
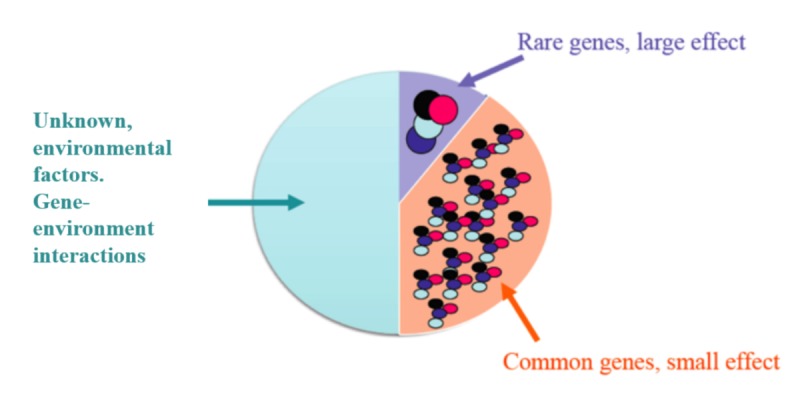
The multifactorial etiology of schizophrenia including a) rare genes that have a large effect, b) common genes that have a small effect, and c) the environmental factors and gene-environmental interactions that confer risk for schizophrenia.
Various environmental factors may interact with susceptibility genes to increase the risk of schizophrenia; these interactions are the focus of an emerging area of investigation called epigenetics. One of the few replicated findings in this relatively new field is an interaction between cannabis use and the AKT1 gene on the risk of psychosis [26, 27]. Other findings in epigenetics that have not yet been replicated include interactions between a history of fetal hypoxia and hypoxia-related genes on volume of the hippocampus [28] and interactions between childhood trauma and variants of the serotonin transporter [29] and COMT gene [27] on cognitive functioning. Although epigenetics research has the potential to greatly impact clinical practice, few studies have attempted to replicate findings. Among those studies attempting replication, few have been able to confirm previous findings, implying possible publication bias and a need for larger sample sizes in this type of research [30].
Although early neurobiological theories of schizophrenia largely focused on excessive dopamine, more recent research reflects a more nuanced role of dopamine and has pointed to the importance of other neurotransmitters such as GABA and glutamate. Recent animal models [31] and genetic studies of humans [32] suggest that hypofunction of the N-methyl-D-aspartate (NMDA) glutamatergic receptor may underlie schizophrenia. Glutamate models of schizophrenia may provide an additional explanation to the dopaminergic models for the cognitive symptoms of this illness and may ultimately yield novel pharmacological treatment approaches [33].
Neuropathology research indicates that schizophrenia is characterized by abnormal maturation of prefrontal networks during late adolescence and early adulthood, likely due to excessive pruning of synapses and dendritic spines [34, 35]. Pre- and post-synaptic abnormalities in inhibitory neurons such as the parvalbumin interneuron may disturb these critical neurodevelopmental processes [36]. Recent research using optogenetics indicates that parvalbumin interneurons may influence gamma oscillations, which in turn are associated with cognitive function [37]. Myelination (another critical neurodevelopmental process) is also abnormal in schizophrenia, as shown by post-mortem studies demonstrating reduced expression of myelin basic protein in cortical regions [38]. Disturbances in both myelination and the inhibitory control of synaptic pruning may contribute to cognitive deficits in schizophrenia [39]. These recent advances in neuropathology build upon previous post-mortem research demonstrating reduced neuropil, but not a reduced number of neurons, in the brains of adults with schizophrenia [40].
Recent studies have shown compelling evidence that neuropathological changes in schizophrenia might set in during the critical period of adolescence, proximal to the onset of psychosis. Gray-matter declines appear to occur in the early phase of schizophrenia and may be related to poorer outcomes. The early phase of psychosis may also be associated with elevations in presynaptic dopamine turnover [41] as well as increases in glutamatergic activity [42]. These observations highlight the importance of early recognition and intervention targeted to the pathophysiological processes close to the onset of psychosis.
In the past several years, inflammation and oxidative stress have re-emerged as potentially important aspects of pathophysiology in a subset of affected individuals. Multiple studies have demonstrated elevated levels of cytokines and other signs of immune system activation in individuals with psychosis [43, 44], and genetic studies have reported correlations between schizophrenia and genes involved in the immune response [23]. Recent rodent work has observed that exposure to infectious or inflammatory agents in utero can lead to behavioral and neurobiological alterations resembling those seen in schizophrenia [45]. Oxidative stress, which is associated with inflammation, may also be elevated in schizophrenia. For example, a recent meta-analysis of studies on oxidative stress markers observed reduced levels of the anti-oxidant red blood cell superoxide dismutase in schizophrenia [46].
Additionally, some recent work has focused on autoimmune dysfunction as a cause of psychosis. For example, anti-NMDA-receptor encephalitis is a potentially treatable but under-diagnosed cause of psychosis that results from the production of antibodies against NMDA receptors [47]. A number of young individuals presenting with their first episode of psychosis may have detectable auto-antibodies against this receptor or other neuronal proteins, such as voltage-gated potassium channels [48]. In addition, epidemiological data suggest a bi-directional association between psychosis and some common autoimmune diseases: individuals with one type of illness are at greater risk of developing the other type [49]. The potential therapeutic applications of these findings are being actively explored.
Various forms of imaging research have been critical in advancing our understanding of the neurobiology of schizophrenia. Studies have reported subtle structural alterations, including enlargement of the third and lateral ventricles, slight reductions in whole-brain gray matter volume, and slight reductions in the volumes of temporal, frontal, and limbic regions [50, 51]. Functional imaging studies have observed reduced activation of the dorsolateral prefrontal cortex during tasks of executive function [52] and abnormalities of limbic system activation during tasks involving emotional stimuli [53]. In addition, studies using diffusion tensor imaging, a method of visualizing white matter, have found evidence of white matter changes in frontal and temporal lobes that would imply decreased connectivity among these regions [54]. Together, these findings support the conceptualization of schizophrenia as a disorder of brain connectivity [55]. Studies in the past few years have used various forms of network analysis to uncover decreased regional connectivity in both first-episode schizophrenia [56] and the broader psychotic spectrum.
The next major challenge is to translate neuroimaging findings into the clinical setting. Consequently, recent research has started to integrate various imaging modalities with genetic, electrophysiological, and clinical data to identify biomarkers, which may eventually be relevant for clinical diagnosis and management.
Pharmacological treatments in schizophrenia
Antipsychotic drugs have been the mainstay of schizophrenia treatment since the introduction of chlorpromazine, focusing on decreasing the frequency and severity of psychotic episodes as well as improving the functional capacity of individuals with schizophrenia [57]. However, adverse effects and suboptimal outcomes associated with first-generation antipsychotics (FGAs) led to the development of second-generation antipsychotics (SGAs), which due to their 5HT-2A antagonism are generally associated with reduced extrapyramidal symptoms (EPSs) as compared with FGAs [58]. However, there is controversy concerning the categorization of FGA and SGAs; some literature differentiates them based on their ability to cause EPSs, whereas other studies base it on their antagonism of the dopamine D-2 receptors [59, 60]. The first and most efficacious SGA for the treatment of refractory schizophrenia, clozapine, is limited by the risk of agranulocytosis, which necessitates the use of periodic monitoring of blood cell counts. EPSs are lowest with clozapine and highest with haloperidol. All drugs except haloperidol, ziprasidone, and lurasidone produce more weight gain, with olanzapine and clozapine producing the greatest weight gain. Prolactin elevation is highest with risperidone and paliperidone. All drugs, except for amisulpride, paliperidone, sertindole, and iloperidone, are significantly more sedating than placebo [61].
Several large-scale investigations suggest no clear superiority of SGAs over FGAs among first-episode patients [62] or chronic patients [63] in regard to positive, cognitive or social outcomes [64]. Furthermore, both FGAs and SGAs do not sufficiently target negative symptoms (with only olanzapine and asenapine showing moderate effects [65-67]) and sometimes insufficiently treat positive symptoms [68, 69]. Clozapine seems to be the most effective medicine, whereas the effectiveness of olanzapine and risperidone over other antipsychotics remains controversial [61, 70-72]. In terms of relapse prevention, SGAs have a modest benefit compared with FGAs [73]. Newer antipsychotics such as asenapine, iloperidone, lurasidone, and paliperidone do not seem to be significantly better than haloperidol [61], and for the treatment of refractory schizophrenia, clozapine has been shown to be significantly more efficacious than other agents [74-78]. However, several studies suggest that olanzapine and risperidone have greater efficacy over other antipsychotics, but this remains controversial and more research is necessary in this area. Additionally, amisulpride, olanzapine, clozapine, paliperidone, and risperidone show significantly lower all-cause discontinuation than several other agents [61, 79-81]. The Schizophrenia Patient Outcome Research Team (PORT) summarized strong empirical support for both FGAs and SGAs in acute and maintenance treatment of schizophrenia and for the use of clozapine for treatment-resistant positive symptoms, hostility, and suicidal behaviors [82].
Research over the past decade further investigated agents that stimulate the NMDA glutamate receptor, including partial and full agonists at the glycine site and glutamate 2/3 receptor agonists, and found that they may ameliorate negative symptoms with some success if used in conjunction with antipsychotics [83]. The PORT concludes, however, that there is still limited information on the use of adjunctive pharmacological agents as well as the treatment of co-occurring substance abuse [82]. None of the pharmacological agents to date effectively ameliorate cognitive deficits, which are a core feature of schizophrenia; larger and more rigorous studies are needed to examine the potential pro-cognitive effects of medications that impact dopaminergic, nicotinergic, glutamatergic, GABAergic, and other novel targets [84].
Long-acting injectable antipsychotics are helpful in treating patients with poor medication adherence and have more controlled distributions in the body; for these reasons, they are superior to oral antipsychotics in preventing hospitalizations [85] and may foster a better therapeutic alliance. Both FGA (haloperidol and fluphenazine) and SGA (risperidone, paliperidone, olanzapine, and aripiprazole) medications are now available as long-acting preparations. Recent preliminary investigations suggest a therapeutically beneficial response to dose reduction and alternate day dosing [86] in the early stages of remitted first-episode psychosis. However, more research is needed to confirm these observations [87].
Recent advances in the understanding of schizophrenia have restored interest in inflammatory and oxidative stress pathways as the pathogenesis for a subset of patients. Research on human and animal models supports this new insight [88-93]. In a review by Sommer et al., 26 double-blind randomized controlled trials reported on the efficacy of anti-inflammatory agents such as aspirin, celecoxib, davunetide, fatty acids (eicosapentaenoic acids and docosahexaenoic acids), estrogens, minocycline, and antioxidants such as N-acetylcysteine (NAC) as treatment augmentation for schizophrenia [94]. Aspirin, estrogens, and NAC showed significant effects; in contrast, celecoxib, minocycline, davunetide, and fatty acids showed no significant difference [95]. Whereas more research is needed to investigate the therapeutic effects of both current and novel anti-inflammatory agents, current evidence does suggest a benefit in treating inflammation.
Pharmacogenomics is a growing field in the treatment of schizophrenia and can bring the field of psychiatry closer to achieving evidence-based personalized medicine with the goals of predicting better treatment response and reducing medication-induced side effects. For example, polymorphisms in the serotonergic system are associated with the efficacy of clozapine and risperidone, dopamine D3 receptor polymorphisms are associated with response to clozapine and olanzapine, and D2 variants are associated with the efficacy of risperidone [96]. As for side effects, the serotonergic system (HTR2C) and hypothalamic leptin-melanocortin genes (MC4R) can predict antipsychotic-induced weight gain [97, 98], cytochrome P450 (CYP2D6) and dopamine receptor variants are associated with tardive dyskinesia [96, 99], and major histocompatibility complex (human leukocyte antigen [HLA]) markers have been consistently found to be associated with clozapine-induced agranulocytosis. However, despite progress made in pharmacogenomics, the field has encountered obstacles such as replication inconsistencies, small study sizes, and lack of randomized control trials.
Psychosocial treatments
Antipsychotic medications are a necessary but not sufficient treatment for schizophrenia. The broad objectives of treatment should be reducing the frequency and severity of episodes of psychotic exacerbation as well as improving the functional capacity and quality of lives of the individuals afflicted with the illness. Thus, in tandem with research over the past decades, the urgently needed multimodal care has continued to evolve. Psychoanalytic treatments beginning in the early 20th century seemed to insufficiently address the burden caused by the illness. In the early ’60s, major role therapy and family psychoeducation were introduced based on the interpersonal and family theories of psychosis. With further research in the field and increased knowledge about the nature of the specific deficits (cognitive, social, and affective), more disease-specific psychotherapies started to develop in the ’80s and ’90s. These therapies target both pathophysiology and other core manifestations of the disease.
The PORT recently provided an extensive summary of the current evidence-based psychosocial treatment [100]. Cognitive behavioral therapy (CBT) is based on the theory that the way we interpret events has cognitive, emotional, and behavioral consequences, which lead to the creation and maintenance of unhelpful responses. CBT has been a successful approach for other mental illnesses such as depression [101] and anxiety [102]. More recently, CBT has been applied to the treatment of positive as well as negative symptoms. Research shows that CBT can improve positive symptoms [103, 104] but is less consistent with the improvement of negative symptoms [105, 106]. A recent review shows that CBT may mostly be efficient in the short term (i.e. more than 12 months) [107].
Social skills training (SST) is based on a behavioral model that targets the improvement of a person's ability to function skillfully in social situations (i.e. interactions). SST has emerged among the possible treatments for schizophrenia [108] to address social skill deficits primary to developmental derailments but also secondary due to both positive and negative symptoms [109]. SST has been found to improve both positive and negative symptoms [106], and some of the improvements may persist at follow-up [110].
Family therapy is based on a model that suggests that problematic behaviors are maintained and created by patterns in systems (i.e. proximal or distal family) [111]. System theory underlies the multifamily treatment approach that includes coping recommendations, problem solving, crisis intervention, reduction of pathogenic interactions such as high “expressed emotions” (e.g. criticism, hostility), and (in its core) psychoeducation. Psychoeducation enables not only the patient but also the family and others to recognize early warning signs, which is particularly important in an illness that shows such high vulnerability to stress.
Assertive community treatment (ACT) offers a multidisciplinary approach that is usually combined with SST, CBT, or any personal support. Teams include peer support specialists and practitioners with expertise in psychiatry, substance abuse treatment, and employment. Although ACT reduces time in the hospital for mental illnesses in general [112], it seems specifically to improve housing stability [113] and reduce hospitalization rates, especially in patients with higher baseline hospitalization rates [113].
Personal therapy was developed by Hogarty et al. [114] on the basis of supportive psychotherapy. It is one of the few approaches that was designed specifically for people suffering from schizophrenia and combines SST with some common elements of CBT. Personal therapy is modeled to the phases of recovery; thus, it is a long-term endeavor and seems to decrease the probability of relapse [115].
Cognitive remediation therapy (CRT) is a computer-based intervention that was originally designed to improve deficient cognitive abilities (e.g. attention, memory, and executive function) in people with traumatic brain injury [116] but since has proven to help in people with depression [117], eating disorders [118], and schizophrenia [117]. Whereas CRT by itself has no effect on improving negative symptoms [119], the combination of CRT with SST, groups, and problem solving has been found to be promising [120]. Although preliminary results showed improvements in speed of processing, attention, working memory, executive function, and social cognition in a cognitive enhancement therapy (CET) compared with a personal therapy group, rigorous validation is needed, and the durability of these improvements remains to be investigated.
For many patients the ability to resume work or school is the ultimate goal. Thus, supported employment interventions are of significant importance – the most commonly studied being the Individual Placement and Support (IPS) [121] model. An important principle of the IPS model is that minimal pre-vocational training is provided, and the job itself becomes the primary training environment. There is clear evidence that supported employment strategies help return people with schizophrenia to work [122], even for young people with their first episode of psychosis [123]. A recent meta-analysis of a total of 11 studies found that competitive employment rates were 61% for patients and 23% for controls and that about 30% out of the 61% worked more than 20 hours weekly. Supported employment further increased the duration of employment (47% of the 52-week year) and the time of onset of employment (approximately 10 weeks earlier than controls). In conclusion, the effect sizes support the effectiveness of evidence-based supported employment as one of the most robust interventions.
Given the clinical heterogeneity of schizophrenia, it is important to choose the right treatment for the right patient; thus, although supportive treatment might benefit all symptom domains, CBT may be particularly beneficial for those with residual psychotic symptoms and cognitive remediation, SST for those with cognitive or social cognition deficits or both, and ACT for those at risk for frequent hospitalizations or those who have had recent homelessness. There is increasing emphasis on tailoring psychotherapeutic interventions to the phase of the illness (e.g. personal therapy), since the primary goals of intervention might vary across phases. More research is needed, however, to examine active ingredients of the therapeutic modalities that work and to identify the synergistic effects of combinations of interventions that are hypothesis-driven and cost-effective.
Conclusions and Future directions
In summary, although our understanding of the causes and treatments of schizophrenia remains limited, several important paradigm shifts have occurred. The diagnosis of schizophrenia is still symptom-based, but increasing amounts of data point to the large genetic and neurobiological overlaps between psychotic, affective, and developmental disorders, suggesting that future classifications of these illnesses need to move toward more evidence-based, valid, and biologically based categories and dimensions. Pathophysiology is now seen as developmentally mediated alterations in neuroplasticity, manifesting in a cascade of neurotransmitter and circuit dysfunctions setting in around adolescence. Etiology is now seen as an interaction between multiple genes of small-effect and some rare large-effect genes and unknown environmental factors. These observations may help therapeutic interventions move beyond the current sole focus on dopamine toward novel therapeutic targets such as glutamate, GABA, and calcium channels. The focus of intervention has expanded beyond relief of psychotic symptoms alone toward restoring functionality by targeting dimensions such as cognitive deficits and negative symptoms. Research is intensifying on the possible utility of several evidence-based psychotherapy modalities in combination with pharmacological approaches. Treatments are still based on serendipitous discoveries from decades ago, and the urgent need is to discover novel interventions based on etiopathology. Finally, recent incremental advances in understanding the etiopathology have motivated vigorous prevention approaches in early phases of the illness and early interventions with novel pharmacological targets and plasticity-based treatments such as cognitive remediation.
Acknowledgments
We would like to thank all the patients that allow us to learn from them, all the collaborators who are doing terrific clinical work, and all the researchers who help us gain better insight.
Abbreviations
- ACT
assertive community treatment
- CBT
cognitive behavioral therapy
- CRT
cognitive remediation therapy
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- EPS
extrapyramidal symptom
- FGA
first-generation antipsychotic
- IPS
Individual Placement and Support
- NAC
N-acetylcysteine
- NMDA
N-methyl-D-aspartate
- PORT
Schizophrenia Patient Outcome Research Team
- RDoC
research domain criteria
- SGA
second-generation antipsychotic
- SST
social skills training
Disclosures
This work was supported in part by National Institutes of Health grants MH 64023, 60902, 78113, and 92440 (MSK).
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/m/6/57
References
- 1.Kraepelin E. Manic depressive insanity and paranoia. The Journal of Nervous and Mental Disease. 1921;53:350. doi: 10.1097/00005053-192104000-00057. [DOI] [Google Scholar]
- 2.Heckers S. Is schizoaffective disorder a useful diagnosis? Curr Psychiatry Rep. 2009;11:332–7. doi: 10.1007/s11920-009-0048-3. [DOI] [PubMed] [Google Scholar]
- 3.Feighner JP, Robins E, Guze SB, Woodruff RA, Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972;26:57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- 4.Schneider K. Clinical Psychopathology New York, NY: Grune & Stratton. City: Inc; 1959. [Google Scholar]
- 5.Rimol LM, Hartberg CB, Nesvåg R, Fennema-Notestine C, Hagler DJ, Pung CJ, Jennings RG, Haukvik UK, Lange E, Nakstad PH, Melle I, Andreassen OA, Dale AM, Agartz I. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/9098956
- 6.Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1161613
- 7.Ivleva EI, Morris DW, Moates AF, Suppes T, Thaker GK, Tamminga CA. Genetics and intermediate phenotypes of the schizophrenia--bipolar disorder boundary. Neurosci Biobehav Rev. 2010;34:897–921. doi: 10.1016/j.neubiorev.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Keshavan MS, Clementz BA, Pearlson GD, Sweeney JA, Tamminga CA. Reimagining psychoses: an agnostic approach to diagnosis. Schizophr Res. 2013;146:10–6. doi: 10.1016/j.schres.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718434848
- 10.Kahn RS, Keefe Richard S E. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–12. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- 11.Keshavan MS, Kulkarni S, Bhojraj T, Francis A, Diwadkar V, Montrose DM, Seidman LJ, Sweeney J. Premorbid cognitive deficits in young relatives of schizophrenia patients. Front Hum Neurosci. 2010;3:62. doi: 10.3389/neuro.09.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 2013;37:1680–91. doi: 10.1016/j.neubiorev.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornblatt BA, Carrión RE, Addington J, Seidman L, Walker EF, Cannon TD, Cadenhead KS, McGlashan TH, Perkins DO, Tsuang MT, Woods SW, Heinssen R, Lencz T. Risk factors for psychosis: impaired social and role functioning. Schizophr Bull. 2012;38:1247–57. doi: 10.1093/schbul/sbr136. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718434865
- 14.Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718434893
- 15.Ruhrmann S, Schultze-Lutter F, Salokangas Raimo K R, Heinimaa M, Linszen D, Dingemans P, Birchwood M, Patterson P, Juckel G, Heinz A, Morrison A, Lewis S, von Reventlow Heinrich Graf, Klosterkötter J. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67:241–51. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718434894
- 16.Brent BK, Thermenos HW, Keshavan MS, Seidman LJ. Gray matter alterations in schizophrenia high-risk youth and early-onset schizophrenia: a review of structural MRI findings. Child Adolesc Psychiatr Clin N Am. 2013;22:689–714. doi: 10.1016/j.chc.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Tricht Mirjam J, Ruhrmann S, Arns M, Müller R, Bodatsch M, Velthorst E, Koelman Johannes H T M, Lo Bour J, Zurek K, Schultze-Lutter F, Klosterkötter J, Linszen DH, Haan L de, Brockhaus-Dumke A, Nieman DH. Can quantitative EEG measures predict clinical outcome in subjects at Clinical High Risk for psychosis? A prospective multicenter study. Schizophr Res. 2014;153:42–7. doi: 10.1016/j.schres.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Woodberry KA, Seidman LJ, Giuliano AJ, Verdi MB, Cook WL, McFarlane WR. Neuropsychological profiles in individuals at clinical high risk for psychosis: relationship to psychosis and intelligence. Schizophr Res. 2010;123:188–98. doi: 10.1016/j.schres.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, Heinssen R, Mathalon DH, Perkins DO, Seidman LJ, Tsuang MT, Cannon TD, McGlashan TH, Woods SW. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatry. 2013;74:410–7. doi: 10.1016/j.biopsych.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Addington J, Cadenhead KS, Cannon TD, Cornblatt B, McGlashan TH, Perkins DO, Seidman LJ, Tsuang M, Walker EF, Woods SW, Heinssen R. North American Prodrome Longitudinal Study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull. 2007;33:665–72. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718434895
- 21.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–92. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718434915
- 22.Williams HJ, Norton N, Dwyer S, Moskvina V, Nikolov I, Carroll L, Georgieva L, Williams NM, Morris DW, Quinn EM, Giegling I, Ikeda M, Wood J, Lencz T, Hultman C, Lichtenstein P, Thiselton D, Maher BS, Malhotra AK, Riley B, Kendler KS, Gill M, Sullivan P, Sklar P, Purcell S, Nimgaonkar VL, Kirov G, Holmans P, Corvin A, Rujescu D, et al. Fine mapping of ZNF804A and genome-wide significant evidence for its involvement in schizophrenia and bipolar disorder. Mol Psychiatry. 2011;16:429–41. doi: 10.1038/mp.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietiläinen Olli P H, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Børglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Böttcher Y, Olesen J, Breuer R, Möller H, Giegling I, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–7. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/2136989
- 24.Doherty JL, O'Donovan MC, Owen MJ. Recent genomic advances in schizophrenia. Clin Genet. 2012;81:103–9. doi: 10.1111/j.1399-0004.2011.01773.x. [DOI] [PubMed] [Google Scholar]
- 25.Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–9. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717986067
- 26.Di Forti M, Iyegbe C, Sallis H, Kolliakou A, Falcone MA, Paparelli A, Sirianni M, La Cascia C, Stilo SA, Marques TR, Handley R, Mondelli V, Dazzan P, Pariante C, David AS, Morgan C, Powell J, Murray RM. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry. 2012;72:811–6. doi: 10.1016/j.biopsych.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Alemany S, Goldberg X, van Winkel R, Gastó C, Peralta V, Fañanás L. Childhood adversity and psychosis: examining whether the association is due to genetic confounding using a monozygotic twin differences approach. Eur Psychiatry. 2013;28:207–12. doi: 10.1016/j.eurpsy.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Haukvik UK, Saetre P, McNeil T, Bjerkan PS, Andreassen OA, Werge T, Jönsson EG, Agartz I. An exploratory model for G x E interaction on hippocampal volume in schizophrenia; obstetric complications and hypoxia-related genes. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1259–65. doi: 10.1016/j.pnpbp.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Aas M, Djurovic S, Athanasiu L, Steen NE, Agartz I, Lorentzen S, Sundet K, Andreassen OA, Melle I. Serotonin transporter gene polymorphism, childhood trauma, and cognition in patients with psychotic disorders. Schizophr Bull. 2012;38:15–22. doi: 10.1093/schbul/sbr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–9. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, Bolshakov VY, Coyle JT. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc Natl Acad Sci USA. 2013;110:E2400–9. doi: 10.1073/pnas.1304308110. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718434917
- 32.Timms AE, Dorschner MO, Wechsler J, Choi KY, Kirkwood R, Girirajan S, Baker C, Eichler EE, Korvatska O, Roche KW, Horwitz MS, Tsuang DW. Support for the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia from exome sequencing in multiplex families. JAMA Psychiatry. 2013;70:582–90. doi: 10.1001/jamapsychiatry.2013.1195. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718434918
- 33.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–34. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 35.Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–65. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 36.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1161515
- 38.Matthews PR, Eastwood SL, Harrison PJ. Reduced myelin basic protein and actin-related gene expression in visual cortex in schizophrenia. PLoS ONE. 2012;7:e38211. doi: 10.1371/journal.pone.0038211. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718434924
- 39.Catts VS, Fung SJ, Long LE, Joshi D, Vercammen A, Allen KM, Fillman SG, Rothmond DA, Sinclair D, Tiwari Y, Tsai S, Weickert TW, Shannon Weickert C. Rethinking schizophrenia in the context of normal neurodevelopment. Front Cell Neurosci. 2013;7:60. doi: 10.3389/fncel.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 41.Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D, Valmaggia L, Allen P, Murray R, McGuire P. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry. 2011;16:885–6. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/11936959
- 42.Tandon N, Bolo NR, Sanghavi K, Mathew IT, Francis AN, Stanley JA, Keshavan MS. Brain metabolite alterations in young adults at familial high risk for schizophrenia using proton magnetic resonance spectroscopy. Schizophr Res. 2013;148:59–66. doi: 10.1016/j.schres.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 43.Song X, Fan X, Song X, Zhang J, Zhang W, Li X, Gao J, Harrington A, Ziedonis D, Lv L. Elevated levels of adiponectin and other cytokines in drug naïve, first episode schizophrenia patients with normal weight. Schizophr Res. 2013;150:269–73. doi: 10.1016/j.schres.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 44.Bergink V, Gibney SM, Drexhage HA. Autoimmunity, inflammation, and psychosis: a search for peripheral markers. Biol Psychiatry. 2014;75:324–31. doi: 10.1016/j.biopsych.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 45.Smyth AM, Lawrie SM. The Neuroimmunology of Schizophrenia. Clin Psychopharmacol Neurosci. 2013;11:107–17. doi: 10.9758/cpn.2013.11.3.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–9. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718074530
- 47.Finke C, Kopp UA, Prüss H, Dalmau J, Wandinger K, Ploner CJ. Cognitive deficits following anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatr. 2012;83:195–8. doi: 10.1136/jnnp-2011-300411. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718434946
- 48.Zandi MS, Irani SR, Lang B, Waters P, Jones PB, McKenna P, Coles AJ, Vincent A, Lennox BR. Disease-relevant autoantibodies in first episode schizophrenia. J Neurol. 2011;258:686–8. doi: 10.1007/s00415-010-5788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benros ME, Mortensen PB, Eaton WW. Autoimmune diseases and infections as risk factors for schizophrenia. Ann N Y Acad Sci. 2012;1262:56–66. doi: 10.1111/j.1749-6632.2012.06638.x. [DOI] [PubMed] [Google Scholar]
- 50.Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–56. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718434954
- 51.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–45. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718434955
- 52.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–22. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K, Wolf DH, Bilker WB, Gur RC. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64:1356–66. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- 54.Yao L, Lui S, Liao Y, Du M, Hu N, Thomas JA, Gong Q. White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:100–6. doi: 10.1016/j.pnpbp.2013.04.019. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718068882
- 55.Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44:953–61. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang R, Wei Q, Kang Z, Zalesky A, Li M, Xu Y, Li L, Wang J, Zheng L, Wang B, Zhao J, Zhang J, Huang R. Disrupted brain anatomical connectivity in medication-naïve patients with first-episode schizophrenia. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0706-z. [DOI] [PubMed] [Google Scholar]
- 57.DELAY J, LAINE B, BUISSON JF. Note concernant l'action de l'isonicotinyl-hydrazide utilisé dans le traitement des états dépressifs. Ann Med Psychol (Paris) 1952;110:689–92. [PubMed] [Google Scholar]
- 58.Miyamoto S, Wolfgang W. 5 Pharmacologic Treatment of. Comprehensive Care of Schizophrenia: A Textbook of Clinical Management. 2012::4. [Google Scholar]
- 59.Johnstone EC, Crow TJ, Frith CD, Carney MW, Price JS. Mechanism of the antipsychotic effect in the treatment of acute schizophrenia. Lancet. 1978;1:848–51. doi: 10.1016/S0140-6736(78)90193-9. [DOI] [PubMed] [Google Scholar]
- 60.Kapur S, Remington G. Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Annu Rev Med. 2001;52:503–17. doi: 10.1146/annurev.med.52.1.503. [DOI] [PubMed] [Google Scholar]
- 61.Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–62. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718025121
- 62.Davidson M, Galderisi S, Weiser M, Werbeloff N, Fleischhacker WW, Keefe RS, Boter H, Keet Ireneus P M, Prelipceanu D, Rybakowski JK, Libiger J, Hummer M, Dollfus S, López-Ibor JJ, Hranov LG, Gaebel W, Peuskens J, Lindefors N, Riecher-Rössler A, Kahn RS. Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: a randomized, open-label clinical trial (EUFEST) Am J Psychiatry. 2009;166:675–82. doi: 10.1176/appi.ajp.2008.08060806. [DOI] [PubMed] [Google Scholar]
- 63.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe Richard S E, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 64.Swartz MS, Perkins DO, Stroup TS, Davis SM, Capuano G, Rosenheck RA, Reimherr F, McGee MF, Keefe Richard S E, McEvoy JP, Hsiao JK, Lieberman JA. Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am J Psychiatry. 2007;164:428–36. doi: 10.1176/appi.ajp.164.3.428. [DOI] [PubMed] [Google Scholar]
- 65.Breier A, Schreiber JL, Dyer J, Pickar D. National Institute of Mental Health longitudinal study of chronic schizophrenia. Prognosis and predictors of outcome. Arch Gen Psychiatry. 1991;48:239–46. doi: 10.1001/archpsyc.1991.01810270051007. [DOI] [PubMed] [Google Scholar]
- 66.Tandon R, Ribeiro SC, DeQuardo JR, Goldman RS, Goodson J, Greden JF. Covariance of positive and negative symptoms during neuroleptic treatment in schizophrenia: a replication. Biol Psychiatry. 1993;34:495–7. doi: 10.1016/0006-3223(93)90242-6. [DOI] [PubMed] [Google Scholar]
- 67.Buchanan RW, Panagides J, Zhao J, Phiri P, den Hollander W, Ha X, Kouassi A, Alphs L, Schooler N, Szegedi A, Cazorla P. Asenapine versus olanzapine in people with persistent negative symptoms of schizophrenia. J Clin Psychopharmacol. 2012;32:36–45. doi: 10.1097/JCP.0b013e31823f880a. [DOI] [PubMed] [Google Scholar]
- 68.Mazure CM, Nelson JC, Jatlow PI, Bowers MB. Drug-responsive symptoms during early neuroleptic treatment. Psychiatry Res. 1992;41:147–54. doi: 10.1016/0165-1781(92)90106-D. [DOI] [PubMed] [Google Scholar]
- 69.Leucht S, Arbter D, Engel RR, Kissling W, Davis JM. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14:429–47. doi: 10.1038/sj.mp.4002136. [DOI] [PubMed] [Google Scholar]
- 70.Davis JM, Chen N, Glick ID. A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry. 2003;60:553–64. doi: 10.1001/archpsyc.60.6.553. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718434969
- 71.Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1144912
- 72.Glick ID, Correll CU, Altamura AC, Marder SR, Csernansky JG, Weiden PJ, Leucht S, Davis JM. Mid-term and long-term efficacy and effectiveness of antipsychotic medications for schizophrenia: a data-driven, personalized clinical approach. J Clin Psychiatry. 2011;72:1616–27. doi: 10.4088/JCP.11r06927. [DOI] [PubMed] [Google Scholar]
- 73.Kishimoto T, Agarwal V, Kishi T, Leucht S, Kane JM, Correll CU. Relapse prevention in schizophrenia: a systematic review and meta-analysis of second-generation antipsychotics versus first-generation antipsychotics. Mol Psychiatry. 2013;18:53–66. doi: 10.1038/mp.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wahlbeck K, Cheine M, Essali A, Adams C. Evidence of clozapine's effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156:990–9. doi: 10.1176/ajp.156.7.990. [DOI] [PubMed] [Google Scholar]
- 75.Chakos M, Lieberman J, Hoffman E, Bradford D, Sheitman B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry. 2001;158:518–26. doi: 10.1176/appi.ajp.158.4.518. [DOI] [PubMed] [Google Scholar]
- 76.Lewis SW, Barnes Thomas R E, Davies L, Murray RM, Dunn G, Hayhurst KP, Markwick A, Lloyd H, Jones PB. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull. 2006;32:715–23. doi: 10.1093/schbul/sbj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, Swartz MS, Perkins DO, Keefe Richard S E, Davis CE, Severe J, Hsiao JK. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163:600–10. doi: 10.1176/appi.ajp.163.4.600. [DOI] [PubMed] [Google Scholar]
- 78.Souza JS, Kayo M, Tassell I, Martins CB, Elkis H. Efficacy of olanzapine in comparison with clozapine for treatment-resistant schizophrenia: evidence from a systematic review and meta-analyses. CNS Spectr. 2013;18:82–9. doi: 10.1017/S1092852912000806. [DOI] [PubMed] [Google Scholar]
- 79.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe Richard S E, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 80.Johnsen E, Jørgensen HA. Effectiveness of second generation antipsychotics: a systematic review of randomized trials. BMC Psychiatry. 2008;8:31. doi: 10.1186/1471-244X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, Asenjo Lobos C, Schwarz S, Davis JM. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166:152–63. doi: 10.1176/appi.ajp.2008.08030368. [DOI] [PubMed] [Google Scholar]
- 82.Kreyenbuhl J, Buchanan R, Kelly D, Noel J, Boggs D, Fischer B, Himelhoch S, Fang B, Peterson E, Aquino P. The 2009 schizophrenia patient outcomes research team (PORT) psychopharmacological treatment recommendations. International Clinical Psychopharmacology. 2011;26:e54–e55. doi: 10.1097/01.yic.0000405726.61759.a4. [DOI] [Google Scholar]
- 83.Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–7. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 84.Keefe Richard S E, Buchanan RW, Marder SR, Schooler NR, Dugar A, Zivkov M, Stewart M. Clinical trials of potential cognitive-enhancing drugs in schizophrenia: what have we learned so far? Schizophr Bull. 2013;39:417–35. doi: 10.1093/schbul/sbr153. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717984633
- 85.Kishimoto T, Nitta M, Borenstein M, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74:957–65. doi: 10.4088/JCP.13r08440. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718422822
- 86.Remington G, Seeman P, Feingold A, Mann S, Shammi C, Kapur S. “Extended” antipsychotic dosing in the maintenance treatment of schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72:1042–8. doi: 10.4088/JCP.09m05866yel. [DOI] [PubMed] [Google Scholar]
- 87.Wunderink L, Nieboer RM, Wiersma D, Sytema S, Nienhuis FJ. Recovery in remitted first-episode psychosis at 7 years of follow-up of an early dose reduction/discontinuation or maintenance treatment strategy: long-term follow-up of a 2-year randomized clinical trial. JAMA Psychiatry. 2013;70:913–20. doi: 10.1001/jamapsychiatry.2013.19. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718039850
- 88.Hanson DR, Gottesman II. Theories of schizophrenia: a genetic-inflammatory-vascular synthesis. BMC Med Genet. 2005;6:7. doi: 10.1186/1471-2350-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M, Yamauchi Y, Yamada S, Kanba S. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115–21. doi: 10.1016/j.pnpbp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 90.Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–8. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 91.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sørensen HJ, Nielsen PR, Pedersen CB, Benros ME, Nordentoft M, Mortensen PB. Population impact of familial and environmental risk factors for schizophrenia: A nationwide study. Schizophr Res. 2014;153:214–9. doi: 10.1016/j.schres.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 94.Sommer IE, Neggers Sebastian F W. Repetitive transcranial magnetic stimulation as a treatment for auditory hallucinations. Neuropsychopharmacology. 2014;39:239–40. doi: 10.1038/npp.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718435008
- 95.Sommer IE, van Westrhenen R, Begemann Marieke J H, de Witte Lot D, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40:181–91. doi: 10.1093/schbul/sbt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arranz MJ, Rivera M, Munro JC. Pharmacogenetics of response to antipsychotics in patients with schizophrenia. CNS Drugs. 2011;25:933–69. doi: 10.2165/11595380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 97.Zhang J, Malhotra AK. Pharmacogenetics of antipsychotics: recent progress and methodological issues. Expert Opin Drug Metab Toxicol. 2013;9:183–91. doi: 10.1517/17425255.2013.736964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Müller DJ, Chowdhury NI, Zai CC. The pharmacogenetics of antipsychotic-induced adverse events. Curr Opin Psychiatry. 2013;26:144–50. doi: 10.1097/YCO.0b013e32835dc9da. [DOI] [PubMed] [Google Scholar]
- 99.Ravyn D, Ravyn V, Lowney R, Nasrallah HA. CYP450 pharmacogenetic treatment strategies for antipsychotics: a review of the evidence. Schizophr Res. 2013;149:1–14. doi: 10.1016/j.schres.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 100.Dixon LB, Dickerson F, Bellack AS, Bennett M, Dickinson D, Goldberg RW, Lehman A, Tenhula WN, Calmes C, Pasillas RM, Peer J, Kreyenbuhl J. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophr Bull. 2010;36:48–70. doi: 10.1093/schbul/sbp115. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13847960
- 101.Thase ME. Comparative effectiveness of psychodynamic psychotherapy and cognitive-behavioral therapy: it's about time, and what's next? Am J Psychiatry. 2013;170:953–6. doi: 10.1176/appi.ajp.2013.13060839. [DOI] [PubMed] [Google Scholar]
- 102.Goldin PR, Ziv M, Jazaieri H, Werner K, Kraemer H, Heimberg RG, Gross JJ. Cognitive reappraisal self-efficacy mediates the effects of individual cognitive-behavioral therapy for social anxiety disorder. J Consult Clin Psychol. 2012;80:1034–40. doi: 10.1037/a0028555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kern RS, Glynn SM, Horan WP, Marder SR. Psychosocial treatments to promote functional recovery in schizophrenia. Schizophr Bull. 2009;35:347–61. doi: 10.1093/schbul/sbn177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull. 2008;34:523–37. doi: 10.1093/schbul/sbm114. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13853958
- 105.Grant PM, Reisweber J, Luther L, Brinen AP, Beck AT. Successfully Breaking a 20-Year Cycle of Hospitalizations With Recovery-Oriented Cognitive Therapy for Schizophrenia. Psychol Serv. 2013 doi: 10.1037/a0033912. [DOI] [PubMed] [Google Scholar]
- 106.Elis O, Caponigro JM, Kring AM. Psychosocial treatments for negative symptoms in schizophrenia: current practices and future directions. Clin Psychol Rev. 2013;33:914–28. doi: 10.1016/j.cpr.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cormac I, Jones C, Campbell C, Silveira dMNJ. Cognitive behaviour therapy for schizophrenia. The Cochrane Library. 2004 doi: 10.1002/14651858.CD000524.pub2. [DOI] [PubMed] [Google Scholar]
- 108.Horan WP, Kern RS, Tripp C, Hellemann G, Wynn JK, Bell M, Marder SR, Green MF. Efficacy and specificity of social cognitive skills training for outpatients with psychotic disorders. J Psychiatr Res. 2011;45:1113–22. doi: 10.1016/j.jpsychires.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dickinson D, Bellack AS, Gold JM. Social/communication skills, cognition, and vocational functioning in schizophrenia. Schizophr Bull. 2007;33:1213–20. doi: 10.1093/schbul/sbl067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rus-Calafell M, Gutiérrez-Maldonado J, Ribas-Sabaté J. A virtual reality-integrated program for improving social skills in patients with schizophrenia: a pilot study. J Behav Ther Exp Psychiatry. 2014;45:81–9. doi: 10.1016/j.jbtep.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 111.Malik N. Family Systems Theory. Encyclopedia of Behavioral Medicine. 2013:774–75. [Google Scholar]
- 112.Burns T, Catty J, Dash M, Roberts C, Lockwood A, Marshall M. Use of intensive case management to reduce time in hospital in people with severe mental illness: systematic review and meta-regression. BMJ. 2007;335:336. doi: 10.1136/bmj.39251.599259.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Remington G, Foussias G, Agid O. Progress in defining optimal treatment outcome in schizophrenia. CNS Drugs. 2010;24:9–20. doi: 10.2165/11530250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 114.Hogarty GE, Kornblith SJ, Greenwald D, DiBarry AL, Cooley S, Flesher S, Reiss D, Carter M, Ulrich R. Personal therapy: a disorder-relevant psychotherapy for schizophrenia. Schizophr Bull. 1995;21:379–93. doi: 10.1093/schbul/21.3.379. [DOI] [PubMed] [Google Scholar]
- 115.Hogarty GE, Kornblith SJ, Greenwald D, DiBarry AL, Cooley S, Ulrich RF, Carter M, Flesher S. Three-year trials of personal therapy among schizophrenic patients living with or independent of family I: description of study and effects on relapse rates. FOCUS: The Journal of Lifelong Learning in Psychiatry. 2004;2:146–157. doi: 10.1176/ajp.154.11.1504. [DOI] [PubMed] [Google Scholar]
- 116.Kay T. Neuropsychological treatment of mild traumatic brain injury. The Journal of Head Trauma Rehabilitation. 1993;8:74–85. doi: 10.1097/00001199-199309000-00009. [DOI] [Google Scholar]
- 117.Elgamal S, McKinnon MC, Ramakrishnan K, Joffe RT, MacQueen G. Successful computer-assisted cognitive remediation therapy in patients with unipolar depression: a proof of principle study. Psychol Med. 2007;37:1229–38. doi: 10.1017/S0033291707001110. [DOI] [PubMed] [Google Scholar]
- 118.Lask B. Cognitive Remediation Therapy. Eating Disorders in Childhood and Adolescence. 2013:301. [Google Scholar]
- 119.Hodge Marie Antoinette Redoblado, Siciliano D, Withey P, Moss B, Moore G, Judd G, Shores EA, Harris A. A randomized controlled trial of cognitive remediation in schizophrenia. Schizophr Bull. 2010;36:419–27. doi: 10.1093/schbul/sbn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eack SM, Mesholam-Gately RI, Greenwald DP, Hogarty SS, Keshavan MS. Negative symptom improvement during cognitive rehabilitation: results from a 2-year trial of Cognitive Enhancement Therapy. Psychiatry Res. 2013;209:21–6. doi: 10.1016/j.psychres.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bond GR. Principles of the Individual Placement and Support model: Empirical support. Psychiatr Rehabil J. 1998;22:11. doi: 10.1037/h0095271. [DOI] [Google Scholar]
- 122.Bond GR, Drake RE, Becker DR. An update on randomized controlled trials of evidence-based supported employment. Psychiatr Rehabil J. 2008;31:280–90. doi: 10.2975/31.4.2008.280.290. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718434989
- 123.Killackey E, Jackson HJ, McGorry PD. Vocational intervention in first-episode psychosis: individual placement and support v. treatment as usual. The British journal of psychiatry. 2008;193:114–120. doi: 10.1192/bjp.bp.107.04310. [DOI] [PubMed] [Google Scholar]


