Abstract
This study examined the effect of traumatic brain injury (TBI) in young children on sleep problems and the relationship of sleep problems to neuropsychological and psychosocial functioning. Participants were drawn from an ongoing longitudinal study of injury in young children recruited from 3 to 6 years of age. They constituted three groups: orthopedic injury (OI; n=92), complicated mild/moderate TBI (mTBI; n=55); and severe TBI (sTBI; n=20). Caregivers completed the Children's Sleep Habits Questionnaire (CSHQ), as well as ratings of behavioral adjustment, adaptive functioning, and everyday executive function at 1, 6, 12, and 18 months postinjury. Retrospective ratings of preinjury sleep and psychosocial functioning were obtained at the initial assessment. Children completed neuropsychological testing at all occasions. Children with complicated mTBI demonstrated more total sleep problems than children with OI at 6 months postinjury, but not at 12 or 18 months. Children with sTBI displayed more bedtime resistance and shorter sleep duration than those with complicated mTBI or OI at several occasions. Across groups, total sleep problems predicted more emotional and behavioral problems and worse everyday executive function as rated by parents across follow-up occasions. In contrast, sleep problems were generally not related to neuropsychological test performance. The results suggest that young children with TBI demonstrate more sleep problems than children with injuries not involving the head. Sleep problems, in turn, significantly increase the risk of poor psychosocial outcomes across time, but are not associated with worse neuropsychological test performance.
Key words: : behavior, cognitive ability, preschool, sleep, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is the most common cause of death and acquired disability among children and adolescents in the United States.1 Children with TBI often demonstrate a variety of physical, cognitive, and behavioral difficulties.2 Sleep disturbance is one of the more common problems reported after childhood TBI,3 but has been the focus of relatively little research to date.4 A variety of mechanisms have been proposed to explain how TBI might increase the risk for sleep problems, including physical pain, disrupted neural networks, and emotional trauma.4,5
The existing literature on sleep in childhood TBI is equivocal. Several studies have shown more sleep disturbance in children with TBI than in healthy children or in those with orthopedic injuries (OIs).6–10 Sleep problems have been demonstrated in children with both mild (mTBI) and more severe TBI (sTBI), persisting up to 3 years postinjury, as measured by self- and parent ratings as well as by objective sleep-monitoring measures (e.g., actigraphy and polysomnography [PSG]).7,10 However, not all studies have found sleep problems to be associated with TBI or to be related to injury severity. For instance, Milroy and colleagues did not find differences in sleep efficiency or total duration and number of nighttime awakenings among children with mTBI, when compared to children with OI.11
Sleep difficulties may affect children's daytime functioning, including cognition and behavior.12 The cognitive deficits displayed by children with disturbed sleep typically occur within a few specific domains, including attention, memory, and executive functions.13–17 Children with sleep apnea display deficits in executive functions, with moderate-to-large effect sizes.18 Deficits in memory related to sleep deprivation have been less consistent. A recent review found considerable variability in the relationship between sleep-disordered breathing in children and memory deficits across studies.18 To our knowledge, no previous study has examined the relationship of sleep disturbance to cognitive functioning in children with TBI; we cannot be certain that research involving children with sleep-disordered breathing or apnea will generalize to those with TBI, for whom the mechanism of sleep disturbance is quite different.
Sleep problems are also known to be associated with both behavioral and emotional problems.19–21 Children with sleep problems display more symptoms of attention deficit/hyperactivity disorder than typical peers.15,22 Multiple studies have shown that children with sleep-disordered breathing, of all severities, demonstrate increased behavioral deficits.20 Sleep problems not only are related to concurrent behavioral and emotional disorders, but also predict such disorders in the future.23 Among children with TBI, sleep problems have been shown, in a recent study, to predict functional outcomes, including adaptive functioning and social participation.10,24
To summarize, previous research indicates that TBI increases the risk of sleep problems, perhaps regardless of severity of injury. Sleep has also been found to play a role in the development of cognitive deficits, specifically in attention, memory, and executive functions, as well as behavioral difficulties. However, although studies in adults have linked TBI, sleep disturbance, and cognitive and behavioral outcomes,25 these links have not been comprehensively investigated in children with TBI. The most comprehensive previous study, to date, by Tham and colleagues, which also is the only published study that has included young children with TBI, limited the assessment of sleep to a single parent-report question and did not assess cognitive abilities.10
Thus, the aim of the current study was to examine the effect of TBI in young children on sleep problems and the relationship of sleep problems to cognitive and psychosocial functioning. We had three specific hypotheses. First, we predicted that children with TBI would demonstrate more sleep problems than children with OI not involving the head and that the increased problems would persist over time. Second, across both TBI and OI groups, we predicted that children with more sleep problems would demonstrate greater cognitive deficits than children with fewer sleep problems. Finally, among both TBI and OI groups, children with more sleep problems were expected to demonstrate more emotional and behavioral problems, poorer everyday executive functioning, and poorer adaptive functioning than children with fewer sleep problems.
Methods
Participants
Participants were drawn from an ongoing longitudinal study of injury in young children that recruited children between the ages of 3 years, 0 months, and 6 years, 11 months who presented to one of three level 1 trauma centers in Ohio with either a TBI or an OI. Children with TBI were eligible for participation if they sustained a closed-head injury that required overnight hospitalization in association with either a Glasgow Coma Scale (GCS)26 score less than 13 or 13–15 with TBI-related brain abnormalities on computed tomography (CT) or magnetic resonance imaging (MRI). The TBI group was divided into complicated mild/moderate or severe injury. Complicated mTBI was defined by a lowest postresuscitation GCS score of either 9–12 or 13–15 with a trauma-related abnormality on either CT or MRI; sTBI was defined by a GCS score equal to or less than 8. To be included in the OI group, children must have sustained a bone fracture involving any part of the body other than the head that resulted in an overnight hospitalization. Further, children in the OI group could not have had a loss of consciousness or any other findings suggesting brain injury. Exclusion criteria for all groups included a primary language in the home other than English, as well as a history of child abuse, intellectual disability, or preinjury neurological disorder. We did not exclude children with TBI who also had other injuries (61% of all TBI participants). A total of 206 children and their caregivers were enrolled in the larger longitudinal study (23 sTBI, 64 complicated mTBI, and 119 OI). Recruitment rates for families who were successfully contacted were somewhat higher for the TBI group as a whole than for the OI group (53% vs. 35%). However, participants and nonparticipants did not differ on sex, race, age at injury, or census-based estimates of neighborhood income.
Procedure
Participants completed a postacute injury assessment at approximately 1 month after the initial injury (mean [M], 40.59 days; standard deviation [SD], 19.99) and follow-up assessments at 6, 12, and 18 months postinjury. Assessments included direct testing of children's cognitive functioning, during which parents were asked to provide ratings of children's sleep and behavioral adjustment. At the postacute assessment, parents were asked to rate preinjury functioning retrospectively; they rated concurrent, postinjury functioning at all other assessments. Cognitive tests were administered following a fixed order at each assessment. The same cognitive tests were used for all ages, but parents completed different rating scale forms for children 6 years and older versus younger than 6. Attrition was low in the sTBI (5% at 18 months) and complicated mTBI (15%) groups, but higher in the OI group (25%). Attrition was not significantly related to sex, race, or socioeconomic status (SES). Data analyses used mixed models to capitalize on all available data (see below).
Table 1 presents demographics and injury and medical characteristics for the three groups, based on all participants who had sleep ratings available at the initial assessment and at least one of the 6-, 12-, or 18-month follow-up assessments (total n=167). Groups did not differ in sex, race, age at injury, census tract median income, or maternal education. Time between injury and initial assessment was significantly shorter for the OI group than for the TBI groups. This difference likely reflected our willingness to extend recruitment somewhat beyond the desired window (i.e., 3 months postinjury) so as to maximize enrollment of children with TBI. Groups also differed in their mean New Injury Severity Score (NISS),27 defined as the sum of the squares of the Abbreviated Injury Scale (AIS) scores for each child's three most severely injured body regions. Post-hoc tests indicated higher NISS for the sTBI and complicated mTBI groups, compared to the OI group. Groups also differed in mean “non-head-injury” NISS, computed as the NISS minus the AIS for the head region; severity of injuries to regions other than the head was higher in the OI group than in the TBI groups. The mechanism of injury most often involved transportation or falls, consistent with national data on young children.1 Transportation-related injuries were significantly more common in the TBI groups than in the OI group.
Table 1.
Sample Demographic and Clinical Characteristics
| Group | |||
|---|---|---|---|
| Demographics/characteristics | Severe TBI (n=20) | Moderate TBI (n=55) | OI (n=92) |
| Age at injury in years, M (SD) | 4.75 (0.90) | 5.01 (1.19) | 5.15 (1.05) |
| Males, n (%) | 15 (75) | 32 (58) | 47 (51) |
| Nonwhite race, n (%) | 5 (25) | 16 (29) | 23 (25) |
| Census tract median family income in dollars, M (SD) | 55,391 (16,773) | 60,115 (25,010) | 63,993 (23,664) |
| Maternal education, n (%)* | |||
| Less than high school | 5 (25) | 8 (15) | 7 (8) |
| High school degree/GED | 9 (45) | 22 (40) | 32 (35) |
| Partial college | 5 (25) | 10 (18) | 18 (20) |
| College graduate or graduate degree | 1 (5) | 15 (27) | 35 (38) |
| Time since injury to initial assessment in days, M (SD)* | 46.00 (24.01) | 45.96 (24.03) | 34.25 (14.36) |
| External cause of injury, n (%)* | |||
| Transportation | 10 (50) | 20 (36) | 15 (16) |
| Fall | 6 (30) | 29 (53) | 32 (35) |
| Other | 4 (20) | 6 (11) | 45 (49) |
| Lowest GCS score, M (SD)* | 3.95 (1.79) | 13.44 (2.00) | NA |
| Length of hospitalization stay in days, M (SD)* | 11.29 (20.23) | 2.46 (1.86) | 1.40 (1.12) |
| NISS total, M (SD)* | 13.83 (9.04) | 15.28 (7.97) | 7.05 (2.71) |
| NISS non-head-related, M (SD)* | 1.44 (2.23) | 2.60 (5.52) | 7.05 (2.71) |
Group difference significant: p<0.05.
TBI, traumatic brain injury; OI, orthopedic injury; M, mean; SD, standard deviation; GED, General Education Diploma; GCS, Glasgow Coma Scale; NISS, New Injury Severity Score; NA, not available.
Measures
Sleep
Sleep problems were assessed using the Children's Sleep Habits Questionnaire (CSHQ), which is a 33-item measure completed by parents that screens for common sleep issues seen in young children.28 The CSHQ provides scores for eight sleep subscales (i.e., bedtime resistance, sleep-onset delay, sleep duration, sleep anxiety, night wakings, parasomnias, sleep-disordered breathing, and daytime sleepiness) and an overall total sleep disturbance score, based on frequency ratings ranging from “never” to “usually.” The CSHQ has shown acceptable reliability and validity.28 For the purposes of the current study, we focused on the total sleep disturbance score as the primary outcome, although we also examined seven of the eight subscales; the sleep-onset delay subscale was excluded from analysis because it consists of a single item. The average within-group correlation for the CSHQ total score ranged from 0.73 to 0.79 across the three postinjury assessments, indicating that the scores were relatively stable across time.
Cognitive functioning
Children completed a battery of cognitive tests to assess overall cognitive ability, language skills, verbal memory, and executive functions. We chose several measures for the current article, based on previous reports showing medium to large effect sizes when comparing the sTBI and OI groups.29 Specific measures included the Differential Ability Scales (DAS),30 which was used to derive the General Conceptual Ability standard score, the NEPSY31 Verbal Fluency and Sentence Repetition subtests, the DAS Recall of Digits subtest, the Woodcock Johnson-III32 Story Recall subtest, and Shape School, which is a Stroop-like measure designed to assess response inhibition and cognitive flexibility.33 On Shape School, children are first taught to name cartoon “pupils” by their shapes or colors. They are then asked to name the color or shapes of certain pupils according to learned rules. Conditions include Simple Naming, Inhibition, Switching, and Both, with the latter involving both inhibition and set switching. An efficiency score was computed for each condition by dividing the number correct by completion time. We used the efficiency score for the Both condition in the current analyses.
Behavioral adjustment
Emotional and behavioral adjustment was assessed using the Child Behavior Checklist (CBCL), which is a widely used parent rating scale.34,35 Two separate age-based forms were used, one for 1.5- to 5-year-old children consisting of 100 items and one for 6- to 18-year-old children consisting of 112 items. We used total T scores for internalizing and externalizing behavior problems in this study.
Everyday executive function
Ratings of children's everyday executive functions were elicited from parents using the Behavior Rating Inventory of Executive Function (BRIEF).36 Two forms were used, one for children 3–5 years of age, containing 63 items, and one for children 6 years of age, containing 86 items. We used the General Executive Composite (GEC) standard score in this study.
Adaptive functioning
Adaptive functioning was assessed using the Adaptive Behavior Assessment System-Second Edition (ABAS),37 using separate forms for children through 5 years of age and 6 or older. We used the Global Adaptive Composite (GAC) standard score in the current study.
Statistical analyses
Analyses were completed using general linear mixed-model analysis to assess sleep and cognitive and behavioral outcomes longitudinally across the postinjury assessments. Mixed-model analyses have a number of advantages, including the ability to use data from all participants, even if they do not complete measures at all time periods, the lack of necessity for equal time periods between assessments, and the ability to utilize both continuous and categorical predictors.
Data analyses were conducted using SAS Proc Mixed statistical software (SAS Institute Inc., Cary, NC) with group (sTBI, complicated mTBI, and OI) included in the models as a categorical predictor. The initial set of analyses examined sleep problems as the dependent variable, to test our first hypothesis regarding group differences in sleep at 6, 12, and 18 months postinjury. In these analyses, the rating of preinjury sleep problems obtained from parents at the postacute assessment was treated as a covariate. The primary analysis focused on the CSHQ total sleep disturbance score, but analyses were also conducted for the seven subscale scores. Analyses included planned, single-degree-of-freedom contrasts comparing the combined TBI groups to the OI group and the mTBI group to the sTBI group.
The second set of analyses examined cognitive and behavioral functioning as dependent variables, with the total sleep disturbance score treated as a time-varying covariate, to test our second and third hypotheses regarding the relationship of sleep to cognitive and behavioral outcomes. A total of 10 analyses were completed, one for each of the six neuropsychological tests and one for each of the four behavior rating measures (CBCL Internalizing, CBCL Externalizing, BRIEF, GEC, ABAS, and GAC). SES, which was defined in terms of a composite of maternal education and median income for the census tract in which the family resided, was treated as a covariate in all analyses, as was race (white vs. nonwhite); retrospective ratings of preinjury behavioral functioning obtained at the postacute assessment were included as covariates in analyses of behavioral outcomes. A dummy variable for test or rating scale version was also included as a covariate in analyses, where appropriate.
Initial models included all interactions involving group, any covariates, and time since injury. Subjects were considered a random effect, so that each subject was initially modeled with independent slopes and intercepts with respect to time. After fitting an initial model, we reduced model complexity to achieve the most parsimonious model. We followed an iterative process, eliminating predictors for which the F tests for fixed effects were not significant, starting with three-way interactions, and then re-estimating the model before examining lower-level interactions, and, finally, main effects. For any significant interaction, all of the main effects and lower-level interactions upon which the significant interaction was based were retained in the model.
Results
Group differences in sleep
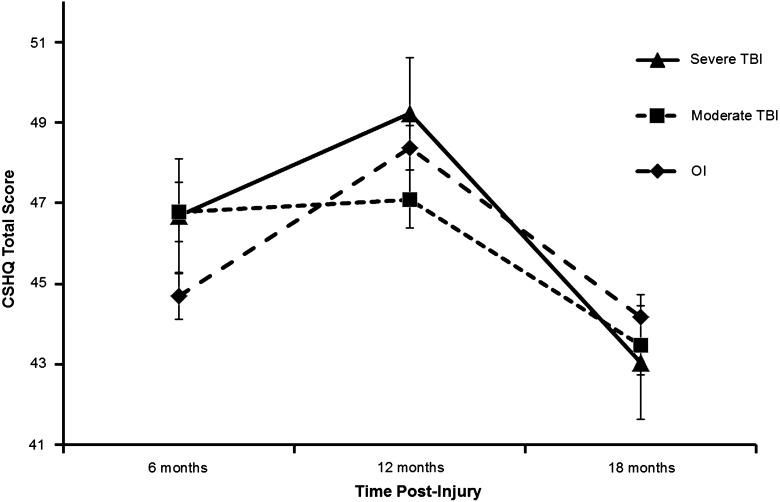
The CSHQ total sleep problems score was significantly higher among children with TBI, as reflected in a significant main effect for group (F(2, 207)=3.71; p=0.026), although the magnitude of group differences varied over time, as reflected in a significant group×time interaction (F(2, 105)=4.93; p=0.009; see Fig. 1). The planned contrast between the combined TBI groups and the OI group was marginally significant at 6 months postinjury (t(274)=1.78; p=0.077; Cohen's d=.22), but not significant at 12 or 18 months. None of the contrasts comparing the mTBI and sTBI groups was significant for the total sleep problems score. Post-hoc analyses showed that parents of children with complicated mTBI reported significantly more sleep problems at 6 months than they did for the OI group (t(303)=2.39; p=0.017; Cohen's d=0.29). The difference between the sTBI group and the OI group at 6 months was of a similar magnitude, but not statistically significant (t(353)=1.32; p=0.187; Cohen's d=0.27); this likely reflects the smaller sample size and larger standard error among the sTBI group, compared to the complicated mTBI and OI groups.
FIG. 1.
Total sleep disturbance by group across time (estimated mean and standard error). CSHQ, Children's Sleep Habits Questionnaire; TBI, traumatic brain injury; OI, orthopedic injury.
Analyses of the CSHQ subscales (see Table 2) revealed a significant group×time interaction for parasomnias (F(2, 158)=3.28; p=0.040) and a trend toward a significant group×time interaction for bedtime resistance (F(2, 162)=2.89; p=0.059). For parasomnias, none of the planned contrasts between the combined TBI groups and the OI group or between the mild/moderate and severe TBI groups were significant. For bedtime resistance, the planned contrast between the combined TBI groups and the OI group was marginally significant at 6 months postinjury (t(283)=1.67; p=0.096; Cohen's d=0.19), but not significant at 12 or 18 months. The sTBI group had significantly higher bedtime resistance than the mTBI group at 6 (t(286)=2.14; p=0.033; Cohen's d=0.40), 12 (t(158)=2.85; p=0.005; Cohen's d=0.44), and 18 months (t(259)=2.50; p=0.013; Cohen's d=0.44). Moreover, post-hoc analyses showed that the sTBI group had significantly higher ratings for bedtime resistance than the OI group at the 6-month assessment (t(383)=2.69; p=0.008; Cohen's d=0.51). Neither the group main effect nor the group×time interaction was significant for the remaining subscales (sleep duration, sleep anxiety, night wakings, sleep-disordered breathing, and daytime sleepiness). However, planned contrasts showed that the combined TBI groups had significantly shorter sleep duration than the OI group at 6 (t(326)=2.35; p=0.035; Cohen's d=0.27) and 12 months (t(171)=2.20; p=0.029; Cohen's d=0.22). Moreover, the sTBI group had significantly shorter sleep duration than the mTBI group at 18 months (t(310)=2.35; p=0.019; Cohen's d=0.49).
Table 2.
CSHQ Subscale Estimated Means and Standard Errors by Group and Assessment Occasion
| OI | Moderate TBI | Severe TBI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 months | 12 months | 18 months | 6 months | 12 months | 18 months | 6 months | 12 months | 18 months | |
| Sleep duration | 3.59 (0.10) | 3.88 (0.11) | 3.61 (0.11) | 3.92 (0.14) | 3.80 (0.14) | 3.55 (0.14) | 4.04 (0.26) | 4.38 (0.24) | 4.05 (0.23) |
| Sleep anxiety | 5.62 (0.16) | 5.64 (0.17) | 5.46 (0.17) | 5.66 (0.21) | 5.71 (0.21) | 5.41 (0.21) | 6.06 (0.38) | 5.57 (0.36) | 5.39 (0.35) |
| Night waking | 4.07 (0.12) | 4.05 (0.12) | 3.91 (0.12) | 4.34 (0.16) | 3.95 (0.16) | 3.93 (0.16) | 4.14 (0.30) | 4.09 (0.26) | 3.53 (0.26) |
| Sleep-disordered breathing | 3.42 (0.07) | 3.46 (0.08) | 3.53 (0.08) | 3.41 (0.10) | 3.43 (0.10) | 3.45 (0.10) | 3.56 (0.18) | 3.64 (0.17) | 3.46 (0.17) |
| Daytime sleepiness | 12.95 (0.26) | 13.16 (0.28) | 12.90 (0.28) | 13.58 (0.36) | 12.76 (0.36) | 12.72 (0.37) | 12.66 (0.66) | 12.79 (0.61) | 12.59 (0.60) |
| Parasomnias | 8.80 (0.14) | 8.84 (0.15) | 8.63 (0.15) | 9.21 (0.19) | 8.79 (0.19) | 8.77 (0.19) | 9.19 (0.36) | 9.09 (0.33) | 8.10 (0.32) |
| Bedtime resistance | 8.12 (0.18) | 8.13 (0.19) | 8.03 (0.19) | 8.16 (0.24) | 7.62 (0.24) | 7.23 (0.25) | 9.44 (0.46) | 8.94 (0.43) | 8.62 (0.41) |
CSHQ, Children's Sleep Habits Questionnaire;TBI, traumatic brain injury; OI, orthopedic injury.
Sleep and cognitive functioning
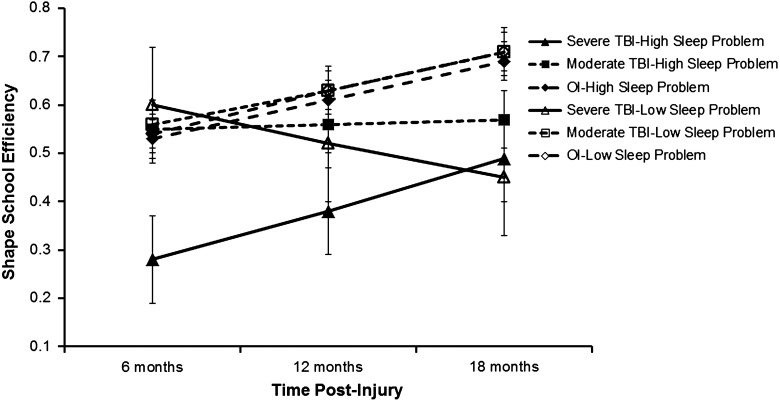
Analyses generally failed to reveal significant relationships between sleep disturbance and neuropsychological test performance, with the possible exception of the Shape School task. A three-way interaction involving group, sleep disturbance, and time since injury on the Shape School task approached significance (F(2, 174)=3.15; p=0.054). Follow-up analyses revealed that children in the sTBI group who also experienced a higher level of sleep disturbance (as estimated at 1 SD above the overall group mean) performed significantly more poorly than all other groups at 6 and 12 months postinjury, but by 18 months, all children with sTBI performed below the other two groups, irrespective of level of sleep disturbance (see Fig. 2).
FIG. 2.
Shape school performance by group and level of sleep disturbance (estimated mean and standard error). Means were estimated at +1 and −1 standard deviation above the overall group mean for CSHQ total sleep disturbance score. CSHQ total score was treated as a time-varying covariate. CSHQ, Children's Sleep Habits Questionnaire; TBI, traumatic brain injury; OI, orthopedic injury.
Sleep and psychosocial functioning
Sleep disturbance was not related to adaptive functioning, as rated on the ABAS. However, sleep disturbance was positively related to emotional and behavioral problems, as rated on the CBCL, as well as to deficits in everyday executive function, as rated on the BRIEF. The relationships held across both groups; no group×sleep interactions were significant. Specifically, children with greater sleep disturbance were rated as displaying more internalizing problems, irrespective of group (F(1, 377)=7.81; p=0.006). Similarly, children with greater sleep problems were also rated as exhibiting more externalizing problems (F(1, 374)=14.30; p=0.0002). Parents also rated children with more sleep problems as demonstrating greater executive function impairments (F(1, 263)=6.88; p=0.009). The average within-group correlation between the CSHQ total score and the CBCL problem scores at each assessment ranged from 0.28 to 0.55. The average within-group correlation between the CSHQ total score and the BRIEF total score at each assessment ranged from 0.38 to 0.42.
Discussion
In support of our first hypothesis, children with TBI showed more sleep problems than children with OI. Children with a complicated mTBI demonstrated increased total sleep problem scores 6 months postinjury, compared to children who experienced an OI; children with sTBI also demonstrated more sleep problems than children with OI at 6 months, but the difference was not statistically significant, likely because of the smaller size of the sTBI group. In contrast, at 12 and 18 months postinjury, neither TBI group in the current study demonstrated a significant increase in overall sleep problems, relative to the OI group. However, the sTBI group showed significantly greater bedtime resistance, as compared to the complicated mTBI group at all occasions, and to the OI group at 6 months. The combined TBI groups showed shorter sleep duration than the OI group at 6 and 12 months, and the sTBI group displayed significantly shorter sleep duration than the complicated mTBI group at 18 months. Although the clinical significance of the findings is uncertain, given the modest size of the group differences, the results are consistent with previous research documenting increased sleep problems after pediatric TBI up to 3 years postinjury.6,9,10 The relationship of sleep problems to TBI severity was not entirely clear, however, and further research is needed to determine the pathophysiological mechanisms that account for increased sleep disturbance after TBI.
Contrary to our second hypothesis, we found little support for a relationship between sleep problems and neuropsychological deficits. One possible exception was found involving the Shape School task. On that task, children with sTBI who experienced a higher level of sleep disturbance performed worse than all other children on the Shape School task at both 6 and 12 months postinjury. By 18 months, the effect of sleep disturbance as a moderator of outcome among the sTBI group was no longer apparent. Thus, children who experience sleep problems in association with a sTBI may be at greater risk for difficulties with executive functions, at least during the first year postinjury. However, this finding must be regarded cautiously, given both that the interaction was only marginally significant and that sleep problems were not associated with scores on other individually administered tests of overall cognitive ability, language skills, or verbal memory in this age range.
Our third hypothesis was supported by evidence that children who experience more sleep problems are at an increased risk for internalizing and externalizing problems, as well as for deficits in everyday executive functions. These findings are consistent with a recent study showing that sleep problems in children with TBI predict deficits in everyday functioning, as reflected in deficits in certain aspects of adaptive functioning and social participation.10 More broadly, the findings are consistent with an extensive body of literature that documents behavioral and functional consequences of sleep problems in children.12,18,38 Indeed, the current findings held true for both the TBI and OI groups, suggesting that sleep problems likely have effects on psychosocial functioning independent of TBI.
A major limitation of the current study is the reliance on parent report of sleep disturbance. A related limitation is that parents were asked to retrospectively recall their child's sleep status before injury; their ratings may have been biased by the children's acute postinjury outcomes. Future longitudinal studies that prospectively recruit children with TBI and OI should consider assessing preinjury sleep problems at the time of admission to the hospital, rather than relying on retrospective ratings made longer after the injury. Incorporation of objective measures of sleep disturbance, such as PSG and actigraphy, in addition to parent-report measures, would also be useful.7,9,11
Another limitation of the study pertains to the measurement of sleep disturbance. Although the CSHQ has been validated in several different populations, it is not necessarily predictive of specific sleep disorders.39,40 Notably, this limitation extends to virtually all current questionnaires and rating scales designed to assess sleep problems. A related limitation is the reliance on parent report of emotional and behavioral functioning and everyday executive function, introducing shared rater variance into the assessment of sleep and functional outcomes. Finally, the CBCL Internalizing scale includes several sleep-related items, so the relationship of the CSHQ to the CBCL Internalizing scale is somewhat confounded by overlapping content.
Further limitations include the difficulty of assessing neuropsychological deficits in preschoolers. The young age of the children in the study may have precluded the detection of subtle differences as a function of sleep problems, given the relative insensitivity of neuropsychological measures at that age.18 The process of standardized, one-on-one cognitive assessment may also compensate for sleep-related deficits in sustained attention, motivation, or emotion regulation that are more evident in less-structured, real-world settings.12,18 On the other hand, we have previously demonstrated significant differences on these tests as a function of TBI itself, suggesting that the tests are sensitive to significant disruptions in cognitive abilities, at least those associated with brain trauma.29,41
Despite these limitations, the current study provides support for the notion that sleep problems occur more often after TBI than after other injuries, and that sleep problems are related to the day-to-day functioning of young children. The findings have important clinical implications, the most important being that a thorough evaluation of young children with TBI should include screening questions about sleep disturbance. If sleep problems are reported, more detailed assessment can be conducted, including objective measures collected in a sleep laboratory, to facilitate the diagnosis of specific sleep disorders.4 Sleep disruption could potentially hamper both school performance and psychosocial adjustment in young children. However, many sleep problems are treatable, with the preponderance of evidence supporting behavioral and cognitive-behavioral interventions, and the successful treatment of sleep disorders after TBI will likely yield demonstrable improvements in children's daily functioning.38
Acknowledgments
The research reported here was supported by grant R01 HD42729 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (to Dr. Shari Wade). The authors acknowledge the contributions of Christine Abraham, Andrea Beebe, Lori Bernard, Anne Birnbaum, Beth Bishop, Tammy Matecun, Karen Oberjohn, Elizabeth Roth, and Elizabeth Shaver in data collection and coding. The Cincinnati Children's Medical Center Trauma Registry, Rainbow Pediatric Trauma Center, Rainbow Babies and Children's Hospital, Nationwide Children's Hospital Trauma Program, and MetroHealth Center Department of Pediatrics and Trauma Registry provided assistance with recruitment.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths 2002–2006. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention: Atlanta, GA [Google Scholar]
- 2.Yeates K.O. (2010). Traumatic brain injury, in: Pediatric Neuropsychology: Research, Theory, and Practice, 2nd ed. Yeates K.O., Ris M.D., Taylor H.G., and Pennington B.F. (eds.). New York: Guilford Press, pps. 112–146 [Google Scholar]
- 3.Hooper S.R., Alexander J., Moore D., Sasser H.C., Laurent S., King J., Bartel S., and Callahan B. (2004). Caregiver reports of common symptoms in children following a traumatic brain injury. NeuroRehabilitation 19, 175–189 [PubMed] [Google Scholar]
- 4.Stores G., and Stores R. (2013). Sleep disorders in children with traumatic brain injury: a case of serious neglect. Dev. Med. Child Neurol. 55, 797–805 [DOI] [PubMed] [Google Scholar]
- 5.Thaxton L., and Myers M.A. (2002). Sleep disturbances and their management in patients with brain injury. J. Head Trauma Rehabil. 17, 335–348 [DOI] [PubMed] [Google Scholar]
- 6.Beebe D.W., Krivitzky L., Wells C.T., Wade S.L., Taylor H.G., and Yeates K.O. (2007). Brief report: parental report of sleep behaviors following moderate or severe pediatric traumatic brain injury. J. Pediatr. Psychol. 32, 845–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufman Y., Tzischinsky O., Epstein R., Etzioni A., Lavie P., and Pillar G. (2001). Long-term sleep disturbances in adolescents after minor head injury. Pediatr. Neurol. 24, 129–134 [DOI] [PubMed] [Google Scholar]
- 8.Pillar G., Averbooch E., Katz N., Peled N., Kaufman Y., and Shahar E. (2003). Prevalence and risk of sleep disturbances in adolescents after minor head injury. Pediatr. Neurol. 29, 131–135 [DOI] [PubMed] [Google Scholar]
- 9.Sumpter R.E., Dorris L., Kelly T., and McMillan T.M. (2013). Pediatric sleep difficulties after moderate-severe traumatic brain injury. J. Int. Neuropsychol. Soc. 19, 829–834 [DOI] [PubMed] [Google Scholar]
- 10.Tham S.W., Palermo T.M., Vavilala M.D., Wang J., Jaffe K.M., Koepsell T.D., Dorsch A., Temkin N., Durbin D., and Rivara F.P. (2012). The longitudinal course, risk factors and impact of sleep disturbances in children with traumatic brain injury. J. Neurotrauma 29, 154–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milroy G., Dorris L., and McMillan T.M. (2008). Brief report: sleep disturbances following mild traumatic brain injury. J. Pediatr. Psychol. 33, 242–247 [DOI] [PubMed] [Google Scholar]
- 12.Beebe D.W. (2011). Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatr. Clin. N. Am. 58, 649–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali N.J., Pitzon D., and Stradling J.R. (1996). Sleep disordered breathing: effects of adenotonsillectomy on behaviour and psychological functioning. Eur. J. Pediatr. 155, 56–62 [DOI] [PubMed] [Google Scholar]
- 14.Archbold K.H., Giordani B., Ruzicka D., and Chervin R. (2004). Cognitive executive dysfunction in children with mild sleep-disordered breathing. Biol. Res. Nurs. 5, 168–176 [DOI] [PubMed] [Google Scholar]
- 15.Chervin R.D., Archbold K.H., Dillon J.E., Panahi P., Pituch K.J., Dahl R.E., and Guilleminault C. (2002). Inattention, hyperactivity, and symptoms of sleep disordered breathing. Pediatrics 109, 449–456 [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb D. J., Chase C., Vezina R. M., Heeren T. C., Corwin M. J., Auerbach S. H., Weese-Mayer D.E., and Lesko S.M. (2004). Sleep disordered breathing symptoms are associated with poorer cognitive function in 5 year old children. J. Pediatr. 145, 458–464 [DOI] [PubMed] [Google Scholar]
- 17.Kheirandish L., and Gozal D. (2006). Neurocognitive dysfunction in children with sleep disorders. Dev. Sci. 9, 388–399 [DOI] [PubMed] [Google Scholar]
- 18.Beebe D.W. (2006). Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep 29, 1115–1134 [DOI] [PubMed] [Google Scholar]
- 19.Aronen E., Paavonen J., Fjallberg M., Soininen M., and Torronen J. (2000). Sleep and psychiatric symptoms in school-age children. J. Am. Acad. Child Adolesc. Psychiatry 39, 502–508 [DOI] [PubMed] [Google Scholar]
- 20.Bourke R.S., Anderson V., Yang J.S.C., Jackman A.R., Killedar A., Nixon G. M., Davey M.J., Walker A.M., Trinder J., and Horne R.S.C. (2011). Neurobehavioral function is impaired in children with all severities of sleep disordered breathing. Sleep Med. 12, 222–229 [DOI] [PubMed] [Google Scholar]
- 21.Johnson E., Chilcoat H., and Breslau N. (2000). Trouble sleeping and anxiety/depression in childhood. Psychiatry Res. 94, 93–102 [DOI] [PubMed] [Google Scholar]
- 22.Picchietti D.L., Underwood D.J., Farris W.A., Walters A.S., Shah M.M., Dahl R.E., Trubnick L.J., Bertocci M.A., Wagner M., and Hening W.A. (1999). Further studies on periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. Mov. Disord. 14, 1000–1007 [DOI] [PubMed] [Google Scholar]
- 23.Gregory A.M., and O'Connor T. G. (2002). Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. J. Am. Acad. Child Adolesc. Psychiatry 41, 964–971 [DOI] [PubMed] [Google Scholar]
- 24.Osorio M.B., Kurowski B.G., Beebe D., Taylor H.G., Brown T.M., Kirkwood M. W., and Wade S.L. (2013). Association of daytime somnolence with executive functioning in the first six months after adolescent traumatic brain injury. PM&R 5, 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmood O., Rapport L.J., Hanks R.A., and Fichtenberg N.L. (2004). Neuropsychological performance and sleep disturbance following traumatic brain injury. J. Head Trauma Rehabil. 19, 378–390 [DOI] [PubMed] [Google Scholar]
- 26.Teasdale G., and Jennett B. (1974). Assessment of coma and impaired consciousness: a practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 27.Osler T., Bakker S.P., and Long W.A. (1997). A modification of the injury severity score that both improves accuracy and simplifies scoring. J. Trauma 43, 922–925 [DOI] [PubMed] [Google Scholar]
- 28.Owens J.A., Spirito A., and McGuinn M. (2000). The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep 23, 1–9 [PubMed] [Google Scholar]
- 29.Gerrard-Morris A., Taylor H.G., Yeates K.O., Walz N.C., Stancin T., Minich N., and Wade S.L. (2010). Cognitive development after traumatic brain Injury in young children. J. Int. Neuropsychol. Soc. 16, 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott C. (1990). Differential Ability Scales: Introductory and Technical Handbook. The Psychological Corporation: San Antonio, TX [Google Scholar]
- 31.Korkman M., Kirk U., and Kemp S. (1998). NEPSY: A Developmental Neuropsychological Assessment. The Psychological Corporation San Antonio, TX [Google Scholar]
- 32.Woodcock R.W., McGrew K., and Mather N. (2001). Woodcock-Johnson III Tests of Achievement. Riverside: Itasca, IL [Google Scholar]
- 33.Espy K.A. (1997). The Shape School: assessing executive function in preschool children. Dev. Neuropsychol. 13, 495–499 [Google Scholar]
- 34.Achenbach T.M., and Rescorla L.A. (2000). Manual for the ASEBA Preschool Forms and Profiles. University of Vermont, Research Center for Children, Youth, and Families: Burlington, VT [Google Scholar]
- 35.Achenbach T.M., and Rescorla L.A. (2001). Manual for the ASEBA School-Age Forms and Profiles. ASEBA: Burlington, VT [Google Scholar]
- 36.Gioia G.A., Isquith P.K., Guy S.C., and Kenworthy L. (2000). Behavior Rating of Executive Function: Professional Manual. Psychological Assessment: Lutz, FL [Google Scholar]
- 37.Harrison P.L., and Oakland T. (2003). Adaptive Behavior Assessment System Manual—Second Edition. Western Psychological Services: Los Angeles, CA [Google Scholar]
- 38.Beebe D.W. (2012). A brief primer on sleep for pediatric and child clinical neuropsychologists. Child Neuropsychol. 18, 313–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luginbuehl M., and Kohler W.C. (2009). Screening and evaluation of sleep disorders in children and adolescents. Child Adolesc. Psychiatr. Clin. N. Am. 18, 825–838 [DOI] [PubMed] [Google Scholar]
- 40.Spruyt K., and Gozal D. (2011). Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep Med. Rev. 15, 19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor H.G., Swartwout M.D., Yeates K.O., Walz N.C., Stancin T., and Wade S.L. (2008). Traumatic brain injury in young children: Post-acute effects on cognitive and school readiness skills. J. Int. Neuropsychol. Soc. 7, 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]