Abstract
Traumatic brain injury (TBI) caused by an explosive blast (blast-TBI) is postulated to result, in part, from transvascular transmission to the brain of a hydrodynamic pulse (a.k.a., volumetric blood surge, ballistic pressure wave, hydrostatic shock, or hydraulic shock) induced in major intrathoracic blood vessels. This mechanism of blast-TBI has not been demonstrated directly. We tested the hypothesis that a blast wave impacting the thorax would induce a hydrodynamic pulse that would cause pathological changes in the brain. We constructed a Thorax-Only Blast Injury Apparatus (TOBIA) and a Jugular-Only Blast Injury Apparatus (JOBIA). TOBIA delivered a collimated blast wave to the right lateral thorax of a rat, precluding direct impact on the cranium. JOBIA delivered a blast wave to the fluid-filled port of an extracorporeal intravenous infusion device whose catheter was inserted retrograde into the jugular vein, precluding lung injury. Long Evans rats were subjected to sublethal injury by TOBIA or JOBIA. Blast injury induced by TOBIA was characterized by apnea and diffuse bilateral hemorrhagic injury to the lungs associated with a transient reduction in pulse oximetry signals. Immunolabeling 24 h after injury by TOBIA showed up-regulation of tumor necrosis factor alpha, ED-1, sulfonylurea receptor 1 (Sur1), and glial fibrillary acidic protein in veins or perivenular tissues and microvessels throughout the brain. The perivenular inflammatory effects induced by TOBIA were prevented by ligating the jugular vein and were reproduced using JOBIA. We conclude that blast injury to the thorax leads to perivenular inflammation, Sur1 up-regulation, and reactive astrocytosis resulting from the induction of a hydrodynamic pulse in the vasculature.
Key words: : blast-TBI; ED-1; GFAP; perivenular inflammation; Sur1, TNF-α
Introduction
Explosive munitions cause more than half of all injuries sustained in military combat and are responsible for an increasing number of civilian casualties related to terrorist acts.1–4 Traumatic brain injury (TBI) caused by an explosive blast (blast-TBI) is one of the most serious wounds suffered by warfighters in modern conflicts. Blast-TBI can range from overt injuries marked by soft tissue damage to the face and scalp complicated by open brain injury, to more insidious injuries with no external physical damage that manifest as persistent neuropsychological or cognitive abnormalities.3–6
In blast-TBI, the brain may be injured directly by the blast wave impacting the cranium or it may be injured indirectly by the transthoracic/transvascular mechanism. With the transthoracic/transvascular mechanism, the brain is said to be injured indirectly by a blast wave that impacts the thorax (or abdomen) and causes a rapid transient pressure wave (a hydrodynamic pulse) to be transmitted to the brain by way of major blood vessels in the neck. Considerable support for this concept has been presented,7–12 but, to our knowledge, all of the supporting evidence has come from experiments in which the entire body of the experimental animal has been exposed to the blast wave. Using this experimental design, it is difficult to distinguish the direct effects on the brain of the blast wave that impacts the cranium13 versus indirect effects mediated by the transthoracic/transvascular mechanism. Understanding the relative contribution of each mechanism is important for designing effective protection.
We sought to test the hypothesis that a blast wave impacting the thorax would induce pathological changes in the brain independent of a direct impact of the blast wave on the cranium. To examine this question, we constructed a Thorax-Only Blast Injury Apparatus (TOBIA) and a Jugular-Only Blast Injury Apparatus (JOBIA). TOBIA delivered a collimated blast wave to the right lateral thorax of a rat with no direct impact on the cranium. JOBIA delivered a blast wave to the fluid-filled port of an extracorporeal intravenous infusion device whose catheter was inserted retrograde into the jugular vein, precluding lung injury. Here, we provide evidence that the transthoracic/transvascular mechanism is indeed an important mechanism of blast-TBI, and that it leads to brain injury that is distinct from that induced by direct exposure of the cranium to blast.
Methods
Thorax-Only Blast Injury Apparatus and Jugular-Only Blast Injury Apparatus
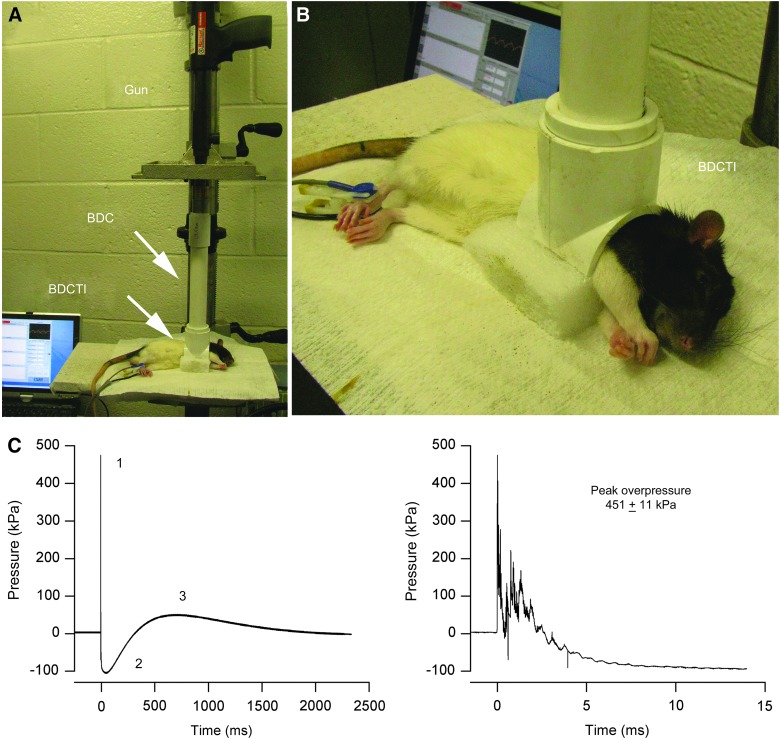
The TOBIA (Fig. 1) is an adaptation of the Cranium Only Blast Injury Apparatus (COBIA), which has been described in detail.13 The blast dissipation chamber (BDC)-cranial interface of COBIA (see Fig. 1 in Kuehn and colleagues13) was replaced with a BDC-thoracic interface, which was constructed from a tee connector for a polyvinyl chloride pipe (inside diameter, 2.54 cm) by removing the opposing ends of the tee and half of the circumference (Fig. 1B). The aperture of the BDC-thoracic interface accommodates the shape of the right lateral thorax of the anesthetized rat when it is placed in a left lateral decubitus position on a foam pillow (Fig. 1A,B). For experiments with the JOBIA, the jugular vein on the right side was catheterized using a fluid-filled vascular access port (MIDA-PU-C30; Instech Soloman Scientific; purchased from Harvard Apparatus, Holliston, MA), and the rubber diaphragm of port was exposed to the blast wave produced by the TOBIA.
FIG. 1.
Thorax-Only Blast Injury Apparatus (TOBIA) and the blast wave produced by TOBIA. (A and B) Overview (A) and close-up view (B) of TOBIA with the rat positioned for blast injury. BDC, blast dissipation chamber; BDCTI, blast dissipation chamber thorax interface. (C) Blast wave produced by TOBIA shown at low and high temporal resolution; note that the specific characteristics of the blast wave, including the fast initial peak overpressure (1), the underpressure (2), and the secondary slower overpressure (3), resemble closely the characteristic features of a free-field explosive blast (see Fig. 2 in Ling and colleagues4). Color image is available online at www.liebertpub.com/neu
The blast wave was generated by firing a .22 caliber crimped brass cartridge (power hammer load, “power level” 4, yellow color coding; 179±5 mg of smokeless powder). A BDC (length, 24.5 or 27 cm) was used in the present experiments, yielding peak overpressures of 517 or 462 kPa, respectively,13 when measured at the aperture of the BDC-thoracic interface. New measurements of peak overpressures generated with the 27-cm BDC gave values of 451±11 kPa (Fig. 1C). The BDC also acts as a collimator that shapes the blast wave emerging from the gun. Orienting the principal vector of the collimated blast wave orthogonal to the long axis of the rat minimizes exposure of the cranium to a direct impact from the blast wave.
Blast injury procedure
All procedures were approved by the institutional animal care and use committee of the University of Maryland School of Medicine (Baltimore, MD). This research was conducted in compliance with the Animal Welfare Act Regulations and other federal statutes relating to animals and experiments involving animals and adheres to the principles set forth in the Guide for Care and Use of Laboratory Animals (National Research Council, 1996). Male Long-Evans rats (280–400 g; Harlan Laboratories, Indianapolis, IN) were anesthetized (60 mg/kg of ketamine plus 7.5 mg/kg of xylazine, intraperitoneally [i.p.]) and were allowed to breathe room air spontaneously. Core temperature was maintained at 37°C using an isothermal pad (Deltaphase; Braintree Scientific, Braintree, MA).
For injury by TOBIA, the rat was placed in a left lateral decubitus position with the right lateral thorax positioned in the BDC-thorax interface (Fig. 1A,B). After the blast, the rat was removed from the apparatus and observed for apnea. Rats were nursed on a heating pad until they recovered spontaneous movements.
For sham injury, the above-described procedures were performed, except that the cartridge was not detonated. Two other controls also were studied: 1) blast exposure as described above with TOBIA in rats after ligation of the right internal jugular vein (IJ) and 2) blast exposure with the rat positioned immediately adjacent to the BDC-thorax interface of TOBIA, outside of the trajectory of the collimated blast wave and separated from it by a barrier, so that the rat would not receive a direct impact (nonimpact TOBIA).
For injury by JOBIA, the rat with the catheterized right jugular vein was positioned outside of the trajectory of the collimated blast wave and separated from it by a barrier. Injury was produced by exposing the fluid-filled vascular access port to the blast wave produced by the TOBIA.
Experimental series
We studied 42 rats in four series: series 1 (TOBIA; 2 rats), using peak overpressures of ∼517 kPa (BDC, 24.5 cm); series 2 (TOBIA; 29 rats), using peak overpressures of ∼451 kPa (BDC, 27 cm), including 15 rats with no other procedure, 3 with the vagus nerve sectioned bilaterally, 3 with the right IJ ligated, 4 with the right IJ catheterized for pressure measurements, and 4 with nonimpact exposure to TOBIA; series 3 (TOBIA; 6 rats) with sham injury; and series 4 (JOBIA; 5 rats), using peak overpressures of ∼451 kPa (BDC, 27 cm). The experiments in series 1 with the greater peak overpressure resulted in 100% mortality; these experiments were not pursued. Except for animals with bilateral vagotomies, the experiments in series 2 and 3 resulted in no mortality and are the source of all of the data with TOBIA presented in this report. All rats in series 2–4 were euthanized 24 h after injury for pathological and histological analysis.
Physiological measurements
All rats underwent continuous pulse-oximetry and heart rate recordings with the sensor placed on the hindlimb (Mouse Ox™; STARR Life Sciences Corp., Oakmont, PA). Readings were taken at baseline, immediately after intubation, and then continuously for 30 min after the injury or sham procedure.
In 4 rats, the IJ was catheterized orthograde to record the hydrodynamic pulse originating in the thorax. An adaptor was fabricated to couple the venous catheter to a precision dynamic piezoelectric pressure transducer (150 pC/psi; 0.002–12.0 kHz; Model 100-P; Columbia Research Laboratories, Inc., Woodlyn, PA). Pressures were monitored using a charge amplifier (Model 4601; Columbia Research Laboratories), as previously described.13
Pathology
Necropsies (gross examination of brains and lungs) were performed on all rats; microscopic examinations (hematoxylin and eosin [H&E] sections) of brains and lungs were performed on all rats euthanized at 24 h. Rats were euthanized using a lethal dose of pentobarbital i.p., after which they were perfused with saline, followed by 10% neutral-buffered formalin solution. Brains and lungs were harvested and photographed to document subarachnoid hemorrhages and other abnormalities. Fixed brains were cut sagitally and imaged at high resolution to assess for contusions as well as intraparenchymal or -ventricular hemorrhages. Tissues were then processed for paraffin embedding, sectioned, and stained with H&E for microscopic examination.
Immunohistochemistry
For immunolabeling, perfusion-fixed brains were cryoprotected with 30% sucrose. Cryosections (10 μm) were mounted on slides, blocked in 2% donkey serum with 0.2% Triton X-100 in phosphate-buffered saline (PBS) for 1 h, then incubated overnight with primary antibody (Ab) directed against tumor necrosis factor alpha (TNF-α; 1:100; catalog no.: SC-1350; Santa Cruz Biotechnology, Santa Cruz, CA), laminin (1:400; catalog no.: AT-2404-1; E-Y Labs, San Mateo, CA), ED-1 (1:200; catalog no.: MAB1435; Millipore, Temecula, CA), sulfonylurea receptor 1 (Sur1; 1:200; catalog no.: SC-5789; Santa Cruz Biotechnology), and rat immunoglobulin G (IgG; 1:200; catalog no.: SC-2011; Santa Cruz Biotechnology) at 4°C. For fluorescent secondary labeling, sections were washed three times in PBS, then incubated in the dark with fluorescent-labeled secondary Abs (1:500; Molecular Probes, Invitrogen, Eugene, OR). After 1 h, slides were washed and cover-slipped with Prolong Antifade reagent with 4′,6-diamidino-2-phenylindole (P36931; Invitrogen). Control experiments included the omission of primary Ab.
Quantitative immunohistochemistry
Unbiased measurements of specific labeling within regions of interest (ROIs) were obtained using NIS-Elements AR software (Nikon Instruments, Melville, NY) from sections immunolabeled in a single batch, as previously described.14,15 All ROI images for a given signal were captured using uniform parameters of magnification, area, exposure, and gain. The following ROIs were used: 1) For longitudinally oriented penetrating veins in the cortex, a rectangular ROI was defined that had a width twice the largest diameter of the vessel, and a length that encompassed the entire vessel, and 2) for cross-sectioned veins in the hippocampus and hypothalamus, a circular ROI was defined that had twice the largest diameter of the vein. Analysis was performed as follows: For each ROI, a histogram of pixel intensity was constructed to determine the intensity of background labeling. Pixels within the ROI were defined as having specific labeling if their intensity was >1.5×that of the background. The area occupied by pixels with specific labeling was used to calculate the percent of the region of interest (% ROI) with specific labeling.
Statistical analysis
Values are given as mean±standard error. Calculations were performed with OriginPro8 statistical software (OriginLab Corp., Northampton, MA). Statistical comparisons were made using the Student's t-test or a one-way analysis of variance with Fisher's post-hoc comparison, as appropriate. Effects were judged to be statistically significant if p<0.05.
Results
General findings
Apnea and lung injury
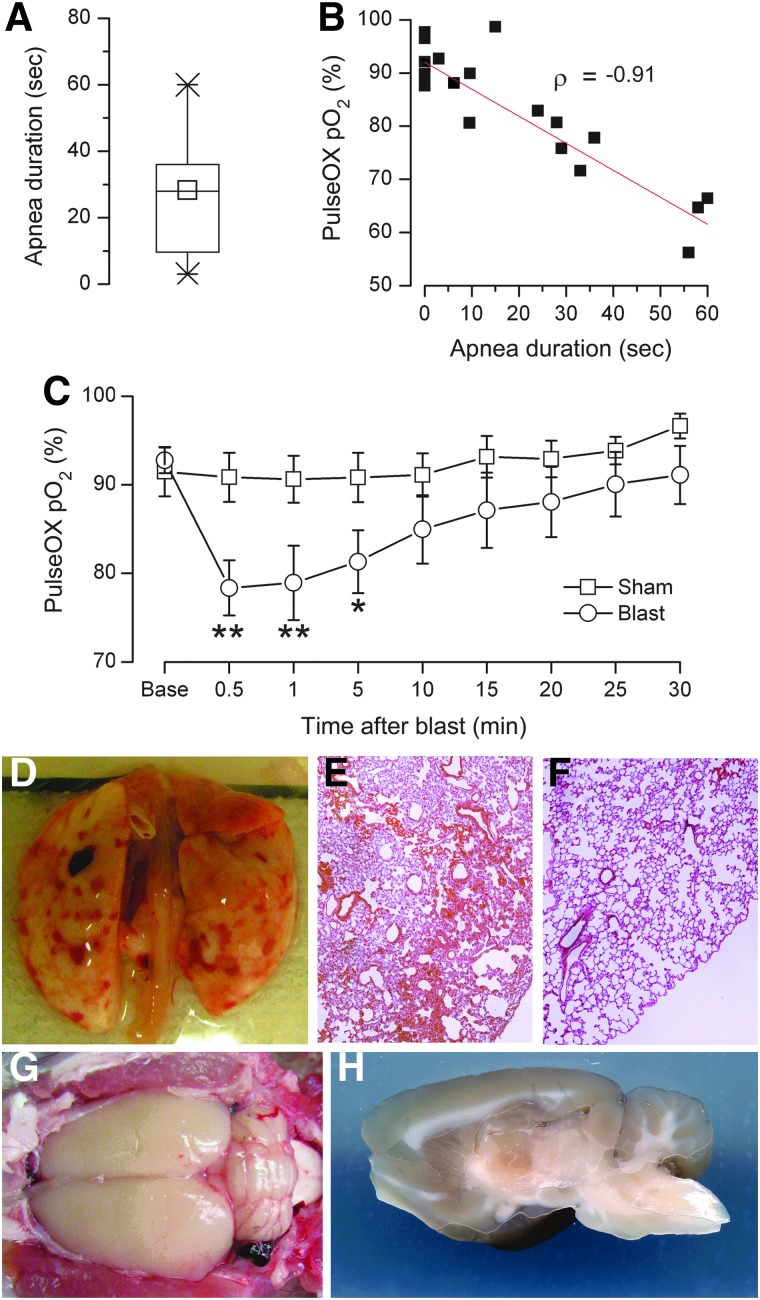
Nonlethal blast injury induced by TOBIA (451±11 kPa) was associated with apnea that lasted up to 60 sec and that was accompanied by a commensurate reduction in O2 saturation (Fig. 2A,B). O2 saturation began to recover after 1 min, but remained depressed up to 30 min, compared to sham-injured rats (Fig. 2C). At 24 h after injury, the lungs showed diffuse patchy hemorrhages bilaterally that were evident on both gross examination and on H&E stained sections (Fig. 2D–F).
FIG. 2.
Blast injury induced by Thorax-Only Blast Injury Apparatus (TOBIA) is associated with pulmonary, but not brain, hemorrhagic injury. (A and B) Box plot of duration of apnea (A) and relationship between apnea duration and O2 saturation, measured by pulse oxymetry, in sham-injured (0 sec apnea) and TOBIA-injured rats 1 min after blast (B); data from 5 and 13 rats in the sham and TOBIA groups, respectively. Box-plot symbols: box, 25th and 75th percentiles;×, 1st and 99th percentiles; line, median; small square, mean; ρ, Pearson's correlation coefficient. (C) Time course of O2 saturation (mean±standard error), measured by pulse oxymetry, in sham-injured (empty squares) and TOBIA-injured rats (empty circles); data from the same rats as in (A). *p<0.05; **p<0.01. (D–F) Images of whole lungs after perfusion (D) and in hematoxylin and eosin sections after blast exposure from TOBIA (E) or after sham injury (F). (G and H) Dorsal (G) and mid-sagittal (H) views of perfused brain after blast exposure from TOBIA. In (D–H), the images shown are representative of findings in the same rats as in (A). Color image is available online at www.liebertpub.com/neu
Brain hemorrhage
Nonlethal blast injury induced by TOBIA was not associated with subarachnoid or intracerebral brain hemorrhages in any of the 12 rats examined (Fig. 2G,H). A small, thin frontal subdural hematoma was identified in 1 of the 12 rats.
Perivenular neuroinflammation
Tumor necrosis factor alpha
Immunolabeling was performed 24 h after nonlethal blast exposure from TOBIA. Veins were identified as structures >20 μm in diameter that immunolabeled for laminin, were alkaline phosphatase negative,16 and had a single layer of cells in their wall. Coimmunolabeling showed prominent up-regulation of TNF-α in perivenular tissues throughout the brain, compared to sham control (Fig. 3A–C). We quantified the abundance of TNF-α in perivenular tissues. Analyzing veins in the cortex, hippocampus, and hypothalamus showed significant up-regulation of TNF-α in each location, compared to sham controls (Fig. 3D).
FIG. 3.
Perivenular neuroinflammation induced by Thorax-Only Blast Injury Apparatus (TOBIA). (A–C) Coimmunolabeling for TNF-α (green) and laminin (red) showing up-regulation of TNF-α in perivenular tissues after blast injury induced by TOBIA in hippocampus (HC) (A) and in hypothalamus (HT) (B), but not in a sham-injured rat (C). (D) Bar graph showing the abundance of perivenular TNF-α in cortex (CTX), hippocampus, and hypothalamus, compared to sham (sham data from three regions combined). All bars, 50 μm; % ROI, percent region of interest. **p<0.01; five veins per region from 5 rats in the sham and TOBIA groups. (E–G) Coimmunolabeling for TNF-α (green) and ED-1 (red) showing up-regulation of ED-1 in the wall of vessels that also display abundant TNF-α up-regulation after blast injury induced by TOBIA. Arrows point to ED-1-positive cells outside of the endothelial layer, including one with a thin process typical of microglia (G). Data shown are representative of findings in the same rats as in (A–D). TNF-α, tumor necrosis factor alpha. Color image is available online at www.liebertpub.com/neu
ED-1
We immunolabeled for ED-1, which identifies macrophages as well as activated microglia. In many, but not all, cases, veins that exhibited prominent up-regulation of TNF-α also showed ED-1-positive cells in the vessel wall outside of the endothelium (Fig. 3E–G). In some instances, the morphology of the ED-1-positive cells was more consistent with that of activated microglia than that of invading macrophages (Fig. 3G).
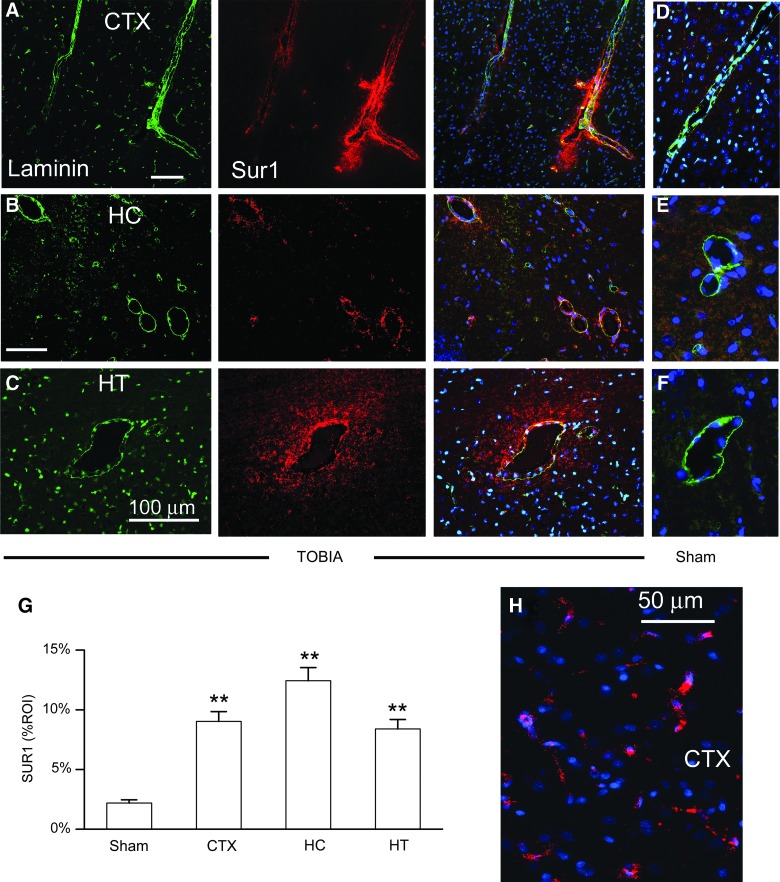
Sulfonylurea receptor 1
We immunolabeled for Sur1, which is up-regulated in response to various central nervous system (CNS) injuries, including inflammation.17–20 Sur1 was strongly up-regulated in vascular tissues throughout the brain (Fig. 4). Laminin-positive veins from TOBIA-injured rats showed prominent up-regulation of Sur1 in endothelial cells (ECs) as well as in perivenular areas (Fig. 4A–C), compared to sham-injured controls (Fig. 4D–F). Quantification of venular and perivenular Sur1 in cortex, hippocampus, and hypothalamus showed significant up-regulation, compared to sham controls (Fig. 4G). Microvessels in the cortex, thalamus, and hippocampus also showed up-regulation of Sur1 (Fig. 4H). Larger arteries at the base of the brain (anterior, middle, and posterior cerebral arteries) showed little or no up-regulation of Sur1 (not shown).
FIG. 4.
Perivenular neuroinflammation induced by Thorax-Only Blast Injury Apparatus (TOBIA) is associated with up-regulation of Sur1. (A–C) Coimmunolabeling for laminin (green) and Sur1 (red) along with superimposed images (right), showing up-regulation of Sur1 in veins and perivenular tissues in cortex (CTX) (A), hippocampus (HC) (B), and hypothalamus (HT) (C) after blast injury induced by TOBIA. (D–F) Superimposed images of sections coimmunolabeled for laminin (green) and Sur1 (red) showing absence of Sur1 in perivenular tissues in cortex (D), hippocampus (E), and hypothalamus (F) after sham injury. (G) Bar graph showing the abundance of Sur1 in perivenular tissues of the regions indicated after blast injury induced by TOBIA versus sham injury (sham data from three regions combined); 5 veins per region from 5 and 8 rats in the sham and TOBIA groups, respectively. % ROI, percent region of interest. **p<0.01. (H) Section of cortex immunolabeled for Sur1 showing Sur1 up-regulation in elongated structures consistent with microvessels. Data shown in (H) are representative of findings in 3 rats. Sur1, sulfonylurea receptor 1. Color image is available online at www.liebertpub.com/neu
We also examined Sur1 expression in rats subjected to nonimpact TOBIA, wherein the rat was positioned immediately adjacent to the BDC-thorax interface of TOBIA, outside of the trajectory of the collimated blast wave. As with sham injured rats, Sur1 expression was minimal in the hippocampus of rats with nonimpact blast exposure (not shown), confirming that direct impact of the blast wave on the thorax was required.
Immunoglobulin G
We used endogenous IgG as a measure of “leakiness” of the blood–brain barrier (BBB). When examined at 24 h, extravascular IgG was not identified in any region in any rat (n=5; not shown).
Protection by jugular ligation, but not by vagal sectioning
Sectioning the cervical vagus bilaterally in rabbits has been reported to protect from blast-TBI when tissues were examined 30 min after blast exposure.21 We attempted to replicate this experiment, but in 3 rats, sectioning the cervical vagus bilaterally, either without (n=2) or with (n=1) blast exposure, was fatal within a few hours, as previously reported.22 As neuroinflammation at 24 h could not be evaluated, these experiments were not pursued.
We recorded the hydrodynamic pulse in the IJ that is hypothesized to be induced by blast exposure of the thorax. The IJ was catheterized orthograde and we used a high-bandwidth pressure recording device to record the pressure wave emanating from the thorax. In 4 rats, complex pressure transients were recorded (Fig. 5A) that had the general features of the blast wave recorded in air (Fig. 1C), including a fast initial overpressure, but that also had a strong slower component of negative pressure. These recordings demonstrated that a blast wave impacting the thorax could induce a hydrodynamic pulse within the thoracic vasculature that could travel retrograde within the venous system and ascend into the brain.
FIG. 5.
Perivenular neuroinflammation induced by Thorax-Only Blast Injury Apparatus (TOBIA) requires patency of the internal jugular vein (IJ). (A) Hydrodynamic pulse recorded in the jugular vein produced by TOBIA shown at low and high temporal resolution; note the fast pressure transient with general features similar to those of the blast wave recorded in air (see Fig. 1C). (B and C) Coimmunolabeling for GFAP (green) and Sur1 (red), showing prominent up-regulation of Sur1 in veins, and up-regulation of GFAP in perivenular tissues of the hippocampus on the side with a patent IJ (B), compared to weak expression on the side with a ligated IJ, after blast induced by TOBIA; asterisks denote veins. (D) Bar graph showing a quantitative analysis of Sur1 and GFAP in or around hippocampal veins from the side of the patent (P-IJ) versus the ligated (L-IJ) internal jugular. **p<0.01; ***p<0.001; 21 and 30 veins from patent-IJ versus ligated-IJ sides, respectively, in 3 rats. GFAP, glial fibrillary acidic protein; Sur1, sulfonylurea receptor 1. Color image is available online at www.liebertpub.com/neu
To further examine the role of the jugular vein in conveying the hydrodynamic pulse from the thorax to the brain, we performed experiments in which one jugular vein was ligated. We compared Sur1 expression in the hippocampus on the ligated side to that on the nonligated side. As shown above, Sur1 was prominently up-regulated in hippocampal veins on the nonligated side (Fig. 5B). By contrast, Sur1 expression was minimal in hippocampal veins on the side with the ligated IJ (Fig. 5C). Quantification of Sur1 expression confirmed significantly less up-regulation on the side with the ligated IJ (Fig. 5D).
The same tissues were coimmunolabeled for glial fibrillary acidic protein (GFAP), a marker of reactive astrocytosis. As with Sur1 expression, up-regulation of GFAP was significantly more prominent on the nonligated side, compared to the ligated side (Fig. 5B–D).
These data suggested that the cervical veins served as major conduits conveying the hydrodynamic pulse from the thorax to the brain to produce perivenular neuroinflammation.
Replication by Jugular-Only Blast Injury Apparatus
The findings above showing protection from TOBIA-induced blast-TBI with jugular ligation suggested that a hydrodynamic pulse ascending toward the brain would be sufficient, without lung injury, to cause perivenular neuroinflammation. To explore this hypothesis, we utilized JOBIA, in which the jugular vein was catheterized retrograde using a fluid-filled vascular access port, and the port—instead of the thorax—was exposed to the blast wave produced by the TOBIA (Fig. 6). This method allowed us to generate a hydrodynamic pulse outside of the body and deliver it to the vascular system of the brain, separating effects of the blast wave itself on the body from effects of the hydrodynamic pulse generated by the blast.
FIG. 6.
Jugular-Only Blast Injury Apparatus (JOBIA). (A and B) Views of the fluid-filled vascular access port used for retrograde catheterization of the internal jugular vein. When the port—instead of the thorax—is exposed to a blast from Thorax-Only Blast Injury Apparatus (TOBIA), the catheter delivers a hydrodynamic pulse to the internal jugular vein without injuring the lungs. (C) Overview of JOBIA with the rat positioned behind a barrier to protect it from direct blast exposure. BDC, blast dissipation chamber; VP, vascular port. (D) Hydrodynamic pulse recorded from the end of the catheter of the vascular access port when the port is exposed to a blast, shown at low and high temporal resolution; note the fast pressure transient with general features similar to those of the hydrodynamic pulse recorded in the internal jugular vein with blast exposure of the thorax (see Fig. 5A). Color image is available online at www.liebertpub.com/neu
Sur1 was prominently up-regulated in hippocampal veins on the side exposed to JOBIA, but not on the contralateral side (Fig. 7A,B). Similarly, TNF-α overexpression and GFAP up-regulation were prominent in hippocampal veins on the side exposed to JOBIA, but not on the contralateral side (Fig. 7C–E). Quantification of Sur1 and TNF-α expression confirmed significantly greater up-regulation on the side exposed to JOBIA (Fig. 7F).
FIG. 7.
Perivenular neuroinflammation induced by Thorax-Only Blast Injury Apparatus (TOBIA) is reproduced by Jugular-Only Blast Injury Apparatus (JOBIA). (A and B) Hippocampal sections immunolabeled for Sur1 showing up-regulation of Sur1 in veins of the perforant pathway by the hydrodynamic pulse induced by JOBIA (A), but not on the contralateral side (B). Arrows point to veins in the perforant pathway. DG, dentate gyrus; CA3, cornu ammonis region 3. (C–E) Superimposed images of sections coimmunolabeled for laminin or Sur1 (green), and TNF-α or GFAP (red), as indicated, of hippocampal tissues ipsilateral (C and D) and contralateral (E) to the hydrodynamic pulse induced by JOBIA, showing prominent neuroinflammation induced by the hydrodynamic pulse. (F) Bar graph showing the abundance of GFAP and TNF-α in perivenular tissues of the perforant pathway associated with the hydrodynamic pulse induced by JOBIA. % ROI, percent region of interest; 15 contralateral and nine ipsilateral veins from 3 rats. **p<0.01; ***p<0.001. Sur1, sulfonylurea receptor 1; GFAP, glial fibrillary acidic protein; TNF-α, tumor necrosis factor alpha. Color image is available online at www.liebertpub.com/neu
Discussion
The principal manifestation of brain injury observed with TOBIA consisted of perivascular changes consistent with neuroinflammation, with findings predominantly in perivenular, rather than periarterial, tissues. The inflammatory marker, TNF-α, was significantly up-regulated in perivenular tissues, and, in many instances, this was accompanied by the finding of ED-1-positive cells (macrophages or activated microglia) around veins. TNF-α up-regulation and microglial activation have been reported previously with whole-body blast exposure,23–25 but not predominantly in perivenular locations. Sur1 also was prominently up-regulated in veins, perivenular tissues, and in microvessels. Recent studies have shown that Sur1 is a sensitive marker in response to neuroinflammatory and other injurious stimuli in the brain and spinal cord.17–19
A second important finding of the present study is that the injury induced by exposure of the thorax to a sublethal explosive blast appears to be attributable to a hydrodynamic pulse that originates in the thorax and propagates into the brain through the IJs (Fig. 8). Several concordant findings support this hypothesis: 1) Direct measurements using a high-bandwidth pressure recording device positioned in the jugular vein showed a fast transient surge of blood pressure (a hydrodynamic pulse) induced by blast exposure of the thorax; 2) ligating one IJ essentially prevented perivenular neuroinflammation ipsilateral, but not contralateral, to the ligated vein; 3) perivenular neuroinflammation was reproduced by a similar hydrodynamic pulse generated outside of the body and delivered to the brain through an internal jugular catheter. To our knowledge, these data are the first to provide direct evidence that a blast wave impacting the thorax can induce a hydrodynamic pulse within the vasculature that affects the brain.
FIG. 8.
Summary hypothesis of events induced by Thorax-Only Blast Injury Apparatus (TOBIA) and Jugular-Only Blast Injury Apparatus (JOBIA) that result in cerebral perivenular inflammation. The blast waves produced by cartridge detonation with both TOBIA and JOBIA are identical (black arrows). The blast wave in air impacts a fluid-filled structure (gray shading) that is oriented orthogonally—the superior (sup.) vena cava in TOBIA and the access port in JOBIA—thereby generating a hydrodynamic pulse (green arrows) that propagates rostrally and can be monitored in the jugular (jug.) vein by a high-frequency pressure transducer. The waveforms actually recorded with TOBIA and JOBIA are shown at identical scales (the first 1000 msec and peak overpressures of 50–60 mm Hg). TOBIA and JOBIA produce similar fast initial peak overpressures, but only TOBIA generates a strong negative underpressure, possibly as a result of the downward thrust of the diaphragm (small black arrows). The peak overpressures, which are similar with TOBIA and JOBIA, are believed to be responsible for depositing kinetic energy (small red arrows) in cerebral veins that results in the similar biological responses of perivenular inflammation with both devices. Color image is available online at www.liebertpub.com/neu
The hydrodynamic pulse recorded in the jugular vein after blast exposure by TOBIA and JOBIA was complex. The initial fast overpressure was similar in magnitude and duration in both cases (Figs. 5A, 6D, and 8), consistent with both being induced by the same blast wave (Fig. 1C, recorded in air) impacting a column of fluid (superior vena cava or access port) that was oriented orthogonally, and that led to the high-frequency pressure transducer positioned within the jugular vein (Fig. 8). The similarity in the initial overpressures suggests that the air-filled thorax is largely transparent to the blast wave because it propagates through air, both outside and inside the thorax. The initial fast overpressure was followed by a slower underpressure that differed greatly in magnitude with the two devices (Figs. 5A, 6D, and 8). With TOBIA, but not with JOBIA, a large underpressure was observed that may have been caused by a strong negative pressure in the thorax created by a downward thrust of the diaphragm caused by the blast. The similar peak overpressures observed with TOBIA and JOBIA, rather than the dissimilar underpressures, seem more likely to be responsible for the similar biological responses observed with the two devices. Additional work will be required to validate these and other details of our hypothesis of the events induced by TOBIA and JOBIA that result in cerebral perivenular inflammation (Fig. 8).
The idea that a pressure wave generated by a blast can be transmitted inside the body has met with some controversy. Early studies by Cernak and colleagues7,21,26 indicated that local exposure of the thorax yields similar biological effects in the CNS as whole-body exposure, from which the researchers concluded that kinetic energy was being transferred from the thorax to the brain. On the other hand, Säljö et al.27 reported that when the abdomen is exposed to a blast, the maximal peak overpressure recorded in the brain is only 3% of that in the abdomen, giving little support to the idea of significant transmission of pressure within the body. In their study, Säljö et al.27 showed dramatic reductions in the high-frequency components of the blast wave (“filtering”), reflecting attenuation by anisotropic body structures, including brain parenchyma. However, these researchers recorded pressures within brain parenchyma, not in the venous system, which is situated early in the path of the hydrodynamic pulse, before filtering by brain parenchyma can take place. Our recordings show that the uniform column of fluid within large veins appears to maintain the integrity of the high-frequency components of the hydrodynamic pulse, as we recorded in the jugular vein. Together, these observations suggest that the cerebral venous system, rather than the brain parenchyma itself, may be vulnerable to a blast wave impacting the thorax or abdomen.
The transthoracic/transvascular mechanism of brain injury demonstrated here was postulated first by Dr. Oliver Garai during World War II28 and since has received extensive support from current thinking and from modern experiments.7,9,10,12 When a blast wave impacts the thorax (or abdomen), a sudden increase in pressure on the walls of large blood vessels accelerates the incompressible column of fluid, creating a rapid transient displacement of blood—a hydrodynamic pulse (Fig. 8). This phenomenon has received several designations in the literature, including “volumetric blood surge,”12 “ballistic pressure wave,”29 “hydrostatic shock,”8,9 and “hydraulic shock”11 (the latter two designations sometimes are used to refer to cases induced by a penetrating projectile). The hydrodynamic pulse radiates away from its site of origin, traveling most readily within the vasculature, rushing to distant organs. It ascends easily into the vasculature of the brain, because there are no valves to impede its transmission. In its most energetic form, the hydrodynamic pulse may rupture distant blood vessels, resulting in immediate distant hemorrhages, including in the brain.8,29 If the hydrodynamic pulse is not sufficiently energetic to rupture distant vessels, it may still have enough energy to injure endothelial and other vascular cells, resulting in effects similar to those observed in a “hypertensive crisis” (e.g., formation of cerebral edema).30 A natural extension of this concept is that the hydrodynamic pulse also may activate mechanosensitive signaling pathways in ECs that results in up-regulation of adaptive genes in the endothelium that predispose to delayed micro- and perivascular dysfunction.
We did not identify the mechanism by which the hydrodynamic pulse triggered perivenular inflammation, Sur1 up-regulation, and reactive astrocytosis, but we suspect that a transient opening of the BBB may be responsible. When extravasated, serum-born substances, such as fibrinogen, are well known to initiate an inflammatory response.31 Immunolabeling showed that another endogenous serum protein, IgG, which is five times more abundant in serum than fibrinogen, could not be detected in perivascular tissues at 24 h. However, extravasation of serum IgG after whole-body blast exposure has been reported to be transient, peaking at 3 h and declining thereafter.24 In our experiment, serum proteins extravasated as a result of opening of the BBB may have been degraded by 24 h. Alternatively, at least part of the response that we documented involving TNF-α and Sur1 could have been the result of direct mechanical activation of endothelial transcription by the hydrodynamic pulse. Sp1 and nuclear factor kappa B are both mechanosensitive transcription factors32–36 that are known to up-regulate transcription of TNF-α as well as Sur1.18,19,37
The pattern of injury observed in our experiments with TOBIA was very different from the pattern of injury we reported in experiments that utilized a COBIA.13 With COBIA, subarachnoid hemorrhage was an invariant finding, whereas with TOBIA, subarachnoid hemorrhage was never observed. Also, with COBIA, neurons in many regions were affected, whereas with TOBIA, direct pathological involvement of neurons was not apparent. It is important to stress that our studies with COBIA versus TOBIA are not sufficiently advanced to make categorical distinctions about patterns of injury with cranium-only versus thorax-only blast impact, and that much work remains to be done to examine the effect of these different mechanisms of blast-TBI, both in isolation and in combination. However, the pattern suggested by the present experiments seems logical, in that indirect injury through the transthoracic/transvascular mechanism would be predicted to predispose primarily to vascular/perivascular injury. It will be of interest to determine the long-term consequences of perivenular inflammation, Sur1 up-regulation, and reactive astrocytosis that we observed in this rat model of blast-TBI. Similarly, magnetic resonance imaging (MRI) examination of humans exposed to explosive blast may be warranted, because abnormalities of perivascular spaces apparently serve as an MRI marker of inflammatory activity in the brain.38,39
Acknowledgments
The authors are grateful to Dr. Ibolja Cernak for exceedingly informative discussions on mechanisms of blast-TBI. This work was supported by grants (to J.M.S.) from the Veterans Administration (Baltimore, MD; BX001629) and from the Department of the Army (W81XWH-08-2-0157). This article was presented, in part, at the Military Health Systems Research Symposium, Ft. Lauderdale, Florida, August 13–16, 2012.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Coupland R. M., and Meddings D.R. (1999). Mortality associated with use of weapons in armed conflicts, wartime atrocities, and civilian mass shootings: literature review. BMJ 319, 407–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coupland R. M., and Samnegaard H.O. (1999). Effect of type and transfer of conventional weapons on civilian injuries: retrospective analysis of prospective data from Red Cross hospitals. BMJ 319, 410–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belanger H. G., Kretzmer T., Yoash-Gantz R., Pickett T., and Tupler L.A. (2009). Cognitive sequelae of blast-related versus other mechanisms of brain trauma. J. Int. Neuropsychol. Soc. 15, 1–8 [DOI] [PubMed] [Google Scholar]
- 4.Ling G., Bandak F., Armonda R., Grant G., and Ecklund J. (2009). Explosive blast neurotrauma. J. Neurotrauma 26, 815–825 [DOI] [PubMed] [Google Scholar]
- 5.Clemedson J. C. (1956). Blast injury. Physiol. Rev. 36, 336–354 [DOI] [PubMed] [Google Scholar]
- 6.Cernak I., Savic J., Zunic G., Pejnovic N., Jovanikic O., and Stepic V. (1999). Recognizing, scoring, and predicting blast injuries. World J. Surg. 23, 44–53 [DOI] [PubMed] [Google Scholar]
- 7.Cernak I., Wang Z., Jiang J., Bian X., and Savic J. (2001). Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J. Trauma 50, 695–706 [DOI] [PubMed] [Google Scholar]
- 8.Courtney M., and Courtney A. (2008). Scientific evidence for hydrostatic shock. Available at: http://arxiv.org/abs/0803.3051v1 (Last accessed December24, 2013)
- 9.Courtney A. C., and Courtney M.W. (2009). A thoracic mechanism of mild traumatic brain injury due to blast pressure waves. Med. Hypotheses 72, 76–83 [DOI] [PubMed] [Google Scholar]
- 10.Long J. B., Bentley T.L., Wessner K.A., Cerone C., Sweeney S., and Bauman R.A. (2009). Blast overpressure in rats: recreating a battlefield injury in the laboratory. J. Neurotrauma 26, 827–840 [DOI] [PubMed] [Google Scholar]
- 11.Cernak I. (2010). The importance of systemic response in the pathobiology of blast-induced neurotrauma. Front. Neurol. 1, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y., and Huang W. (2011). Non-impact, blast-induced mild TBI and PTSD: concepts and caveats. Brain Inj. 25, 641–650 [DOI] [PubMed] [Google Scholar]
- 13.Kuehn R., Simard P.F., Driscoll I., Keledjian K., Ivanova S., Tosun C., Williams A., Bochicchio G., Gerzanich V., and Simard J.M. (2011). Rodent model of direct cranial blast injury. J. Neurotrauma 28, 2155–2169 [DOI] [PubMed] [Google Scholar]
- 14.Gerzanich V., Ivanov A., Ivanova S., Yang J.B., Zhou H., Dong Y., and Simard J.M. (2003). Alternative splicing of cGMP-dependent protein kinase I in angiotensin-hypertension: novel mechanism for nitrate tolerance in vascular smooth muscle. Circ. Res. 93, 805–812 [DOI] [PubMed] [Google Scholar]
- 15.Patel A. D., Gerzanich V., Geng Z., and Simard J.M. (2010). Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J. Neuropathol. Exp. Neurol. 69, 1177–1190 [DOI] [PubMed] [Google Scholar]
- 16.Ghazi-Birry H. S., Brown W.R., Moody D.M., Challa V.R., Block S.M., and Reboussin D.M. (1997). Human germinal matrix: venous origin of hemorrhage and vascular characteristics. AJNR Am. J. Neuroradiol. 18, 219–229 [PMC free article] [PubMed] [Google Scholar]
- 17.Ortega F. J., Gimeno-Bayon J., Espinosa-Parrilla J.F., Carrasco J.L., Batlle M., Pugliese M., Mahy N., and Rodriguez M.J. (2012). ATP-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia-ischemia in rats. Exp. Neurol. 235, 282–296 [DOI] [PubMed] [Google Scholar]
- 18.Simard J. M., Geng Z., Woo S.K., Ivanova S., Tosun C., Melnichenko L., and Gerzanich V. (2009). Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 29, 317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simard J. M., Woo S.K., Schwartzbauer G.T., and Gerzanich V. (2012). Sulfonylurea receptor 1 in central nervous system injury: a focused review. J. Cereb. Blood Flow Metab. 32, 1699–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tosun C., Kurland D.B., Mehta R., Castellani R.J., Dejong J.L., Kwon M.S., Woo S.K., Gerzanich V., and Simard J.M. (2013). Inhibition of the Sur1-Trpm4 channel reduces neuroinflammation and cognitive impairment in subarachnoid hemorrhage. Stroke 44, 3522–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cernak I., Savic J., Malicevic Z., Zunic G., Radosevic P., Ivanovic I., and Davidovic L. (1996). Involvement of the central nervous system in the general response to pulmonary blast injury. J. Trauma 40, S100–S104 [DOI] [PubMed] [Google Scholar]
- 22.Shay H., Komarov S.A., and Gruenstein M. (1949). Effects of vagotomy in the rat. Arch. Surg. 59, 210–226 [DOI] [PubMed] [Google Scholar]
- 23.Dalle Lucca J. J., Chavko M., Dubick M.A., Adeeb S., Falabella M.J., Slack J.L., McCarron R., and Li Y. (2012). Blast-induced moderate neurotrauma (BINT) elicits early complement activation and tumor necrosis factor alpha (TNFalpha) release in a rat brain. J. Neurol. Sci. 318, 146–154 [DOI] [PubMed] [Google Scholar]
- 24.Readnower R. D., Chavko M., Adeeb S., Conroy M.D., Pauly J.R., McCarron R.M., and Sullivan P.G. (2010). Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res. 88, 3530–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kochanek P. M., Dixon C.E., Shellington D.K., Shin S.S., Bayir H., Jackson E., Kagan V., Yan H.Q., Swauger P.V., Parks S., Ritzel D.V., Bauman R.A., Clark R., Garman R.H., Bandak F., Ling G.S., and Jenkins L.W. (2013). Screening of biochemical and molecular mechanisms of secondary injury and repair in the brain after experimental blast-induced traumatic brain injury in rats. J. Neurotrauma 30, 920–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cernak I., Radosevic P., Malicevic Z., and Savic J. (1995). Experimental magnesium depletion in adult rabbits caused by blast overpressure. Magnes. Res. 8, 249–259 [PubMed] [Google Scholar]
- 27.Säljö A., Arrhén F., Bolouri H., Mayorga M., and Hamberger A. (2008). Neuropathology and pressure in the pig brain resulting from low-impulse noise exposure. J. Neurotrauma 25, 1397–1406 [DOI] [PubMed] [Google Scholar]
- 28.Garai O. (1944). Blast injury—non-fatal case with neurological signs. Lancet 243, 788–789 [Google Scholar]
- 29.Courtney M., and Courtney A. (2011). History and evidence regarding hydrostatic shock. Neurosurgery 68, E596–E597 [DOI] [PubMed] [Google Scholar]
- 30.Gardner C. J., and Lee K. (2007). Hyperperfusion syndromes: insight into the pathophysiology and treatment of hypertensive encephalopathy. CNS Spectr. 12, 35–42 [DOI] [PubMed] [Google Scholar]
- 31.Davalos D., and Akassoglou K. (2012). Fibrinogen as a key regulator of inflammation in disease. Semin. Immunopathol. 34, 43–62 [DOI] [PubMed] [Google Scholar]
- 32.Davis M. E., Grumbach I.M., Fukai T., Cutchins A., and Harrison D.G. (2004). Shear stress regulates endothelial nitric-oxide synthase promoter activity through nuclear factor kappaB binding. J. Biol. Chem. 279, 163–168 [DOI] [PubMed] [Google Scholar]
- 33.Verstraeten S. V., Mackenzie G.G., and Oteiza P.I. (2010). The plasma membrane plays a central role in cells response to mechanical stress. Biochim. Biophys. Acta 1798, 1739–1749 [DOI] [PubMed] [Google Scholar]
- 34.Yun S., Dardik A., Haga M., Yamashita A., Yamaguchi S., Koh Y., Madri J.A., and Sumpio B.E. (2002). Transcription factor Sp1 phosphorylation induced by shear stress inhibits membrane type 1-matrix metalloproteinase expression in endothelium. J. Biol. Chem. 277, 34808–34814 [DOI] [PubMed] [Google Scholar]
- 35.Abumiya T., Sasaguri T., Taba Y., Miwa Y., and Miyagi M. (2002). Shear stress induces expression of vascular endothelial growth factor receptor Flk-1/KDR through the CT-rich Sp1 binding site. Arterioscler. Thromb. Vasc. Biol. 22, 907–913 [DOI] [PubMed] [Google Scholar]
- 36.Korenaga R., Yamamoto K., Ohura N., Sokabe T., Kamiya A., and Ando J. (2001). Sp1-mediated downregulation of P2X4 receptor gene transcription in endothelial cells exposed to shear stress. Am. J. Physiol. Heart Circ. Physiol. 280, H2214–H2221 [DOI] [PubMed] [Google Scholar]
- 37.Falvo J. V., Tsytsykova A.V., and Goldfeld A.E. (2010). Transcriptional control of the TNF gene. Curr. Dir. Autoimmun. 11, 27–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wuerfel J., Haertle M., Waiczies H., Tysiak E., Bechmann I., Wernecke K.D., Zipp F., and Paul F. (2008). Perivascular spaces—MRI marker of inflammatory activity in the brain? Brain 131, 2332–2340 [DOI] [PubMed] [Google Scholar]
- 39.Ge Y., Law M., Herbert J., and Grossman R.I. (2005). Prominent perivenular spaces in multiple sclerosis as a sign of perivascular inflammation in primary demyelination. AJNR Am. J. Neuroradiol. 26, 2316–2319 [PMC free article] [PubMed] [Google Scholar]