Abstract
Citrullinated proteins, derived from the conversion of peptidyl-arginine to peptidyl-citrulline, are present in the joints of patients with rheumatoid arthritis (RA), who also uniquely produce high levels of anti-citrullinated protein Abs. Citrullinated fibrinogen (CF) is abundant in rheumatoid synovial tissue, and anti-citrullinated protein Ab-positive RA patients exhibit circulating immune complexes containing CF. Thus, CF is a potential major target of pathogenic autoimmunity in RA. T cells are believed to be involved in this process by initiating, controlling, and driving Ag-specific immune responses in RA. In this study, we isolated a CD4 T cell line specific for CF that produces inflammatory cytokines. When transferred into mice with collagen-induced arthritis (CIA), this T cell line specifically enhanced the severity of autoimmune arthritis. Additionally, pathogenic IgG2a autoantibody levels to mouse type II collagen were increased in mice that received the T cells in CIA, and levels of these T cells were increased in the synovium, suggesting the T cells may have had systemic effects on the B cell response as well as local effects on the inflammatory environment. This work demonstrates that CD4 T cells specific for CF can amplify disease severity after onset of CIA.
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease affecting ~1% of the population, characterized by destruction of the articular cartilage and erosion of the surrounding bone. Anti-citrullinated protein Abs (ACPAs) are a class of autoantibodies that have been shown to be very specific for the diagnosis of RA (1, 2) and also appear in the sera years before disease onset in individuals who eventually develop RA (3–7).
Citrullination, or deimination, is the posttranslational modification of peptidyl-arginine to peptidyl-citrulline, a calcium-dependent process catalyzed by the enzyme peptidyl arginine deiminase (PAD) (8). ACPAs preferentially recognize citrullinated epitopes on specific proteins (9). Although some proteins are citrullinated as part of normal cellular regulatory processes [reviewed in (8)], the presence of high levels of aberrantly citrullinated proteins in the joints of RA patients correlates with disease severity (10). ACPAs have been shown to target citrullinated epithelial (pro) filaggrin (11, 12), fibrin (13, 14), vimentin (15), α-enolase (16), and type II collagen (17). Abs to several of these citrullinated Ags are enriched in the joints of patients with RA (18).
Many articular autoantigens are proposed to play a role in the pathogenesis of disease in RA, including citrullinated fibrinogen (CF). Circulating levels of fibrinogen are increased in inflammatory conditions such as RA [reviewed in (19)], and fibrin deposition in the joint is one of the most consistent features of both RA and animal models of RA (20–22). Citrullinated forms of the α- and β-chains of fibrin have been identified as targets of the autoanti-body response in RA (14). CF is also present in the synovial fluid of patients with RA (23). It was shown that three quarters of ACPA+ RA patients possessed Abs to CF and one half of ACPA+ RA patients exhibited circulating immune complexes containing CF (24). These studies suggest that CF is present in the joint and that autoimmunity targeting this autoantigen may contribute to synovitis in many ACPA+ RA patients.
Epitope spreading occurs, and ACPAs to citrullinated proteins develop in mouse models of RA, including collagen-induced arthritis (CIA), as disease progresses (25, 26). However, T cells specific for citrullinated proteins have not been well characterized. RA-related DRB1 alleles have a common region of highly similar sequence identified as the shared epitope (SE) (27), and, because ACPAs are thought to mediate the association between SE alleles and RA (28, 29), an implied role for citrulline-specific T cells in the pathogenesis of RA is present.
T cell lines and clones have been used as a tool to provide important insight into the mechanisms of development, regulation, and effector function of autoreactive T cells in a wide array of autoimmune diseases. This has been well demonstrated in the NOD mouse model of type 1 diabetes, in which a unique panel of diabetogenic islet-specific CD4 T cell clones has been extensively studied (30). CD4 and CD8 T cell lines and clones have also been used in several experimental models of arthritis, both spontaneous and inducible. These studies have led to many insights with regard to Ag-specific CD4 T cells in the context of the MHC (31), the importance of posttranslationally modified Ags (32), and a variety of protein Ags thought to be involved in the pathogenesis of RA (31, 33–35).
CIA was used in our studies because it is a widely used inducible model of RA, it is MHC restricted, and both B and T cells are required for the manifestation of arthritis in mice [reviewed in (36)], similar to that in human RA. Also, mice with CIA develop circulating Abs reactive with citrullinated epitopes, and arthritis severity has been modulated by citrullinated peptide tolerance and passive transfer methods using mAbs demonstrating ACPA characteristics (25, 26). Additionally, recent studies in our laboratory have shown that the inhibition of PAD resulted in reduced arthritis severity in CIA that is accompanied by decreased generation of synovial citrulline and a decreased Ab response to a subset of autoantigens (37).
Traditionally in mouse models of autoimmune arthritis, isolated T cell clones are reactive to the specific Ag used in the model of interest. In this study, we examined whether T cells specific for citrullinated epitopes had a role in CIA, in which the initial Ag is collagen, but in which epitope spreading to citrullinated proteins occurs. We describe the unique characteristics of a CD4 T cell line specific for CF that produces inflammatory cytokines in response to Ag challenge with both human and mouse CF. We also show that this T cell line is able to significantly amplify arthritis in CIA, traffic to the synovium, and may increase pathogenic IgG2a Ab levels to mouse type II collagen. These results suggest that CF-specific CD4 T cells can play a key role in the pathogenesis of autoimmune arthritis.
Materials and Methods
Mice
DBA/1J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The University of Colorado School of Medicine Institutional Animal Care and Use Committee approved all experiments.
Citrullination (deimination) of fibrinogen
Human fibrinogen (HF) or mouse fibrinogen (MF) (Sigma-Aldrich) was deiminated in vitro, as described previously (26). Fibrinogen was incubated with rabbit type II PAD (Sigma-Aldrich) in 0.1 M Tris-HCl (pH 7.5), 10 mM CaCl2, and 5 mM DTT at a substrate/enzyme ratio of 1 mg:2 U for 2 h at 37°C. The reaction was stopped by the addition of 20 mM EDTA.
Ex vivo analysis of CD4 T cells from draining lymph nodes
CD4 T cell activation marker and cytokine levels were evaluated by ex vivo analysis of T cells from 6- to 8-wk-old mice immunized intradermally with an emulsion of 100 μg human CF (HCF) (citrullinated as above) in CFA (a mixture of 100 μl IFA and 200 μg inactivated Mycobacterium tuberculosis; H37Ra; Difco), mice immunized with CFA alone, or age-matched untreated mice. Draining lymph nodes (LN) were harvested from untreated mice or mice at days 7, 28, and 35 after immunization and stained for activation markers (CD44, CD62L; BD Biosciences) or cultured overnight in Ag assay, followed by intracellular staining to determine cytokine expression on CD4 T cells.
In Ag assays, T cells (2 × 105/ml) were stimulated with freshly isolated DBA/1J peritoneal exudate cells (APCs) at a concentration of 5 × 105/ml in the presence of APCs alone as a negative control, or APCs and Ag (human insulin [HINS] or HCF). After stimulation, cells were incubated with GolgiPlug (BD Biosciences) in the presence or absence of complete media with a mixture of 0.1 μg/ml PMA and 1 μg/ml ionomycin (Sigma-Aldrich) for 4 h, harvested, and then surface stained in staining buffer (0.05% BSA in PBS) containing anti-CD4 Ab (BD Biosciences). Cells were washed and resuspended in permeabilization buffer (0.5% saponin [Sigma-Aldrich] in staining buffer) containing an Ab mix for intracellular staining ( TNF-α, IL-17, IFN-γ, and/or IL-6 from BD Biosciences), and then washed and analyzed on a FACSCalibur flow cytometer.
Collagen-induced arthritis
Collagen-induced arthritis (CIA), including arthritic scoring and anti-CII ELISA analyses, has been described previously (26); however, the protocol was altered to better observe an amplifying role of T cells in disease. Briefly, at day 0, 6- to 8-wk-old mice were immunized intradermally with an emulsion of CFA (as above) containing 200 μg bovine type II collagen (bCII; Elastin Products). At day 21, mice were immunized as above, but with 200 μg bCII emulsified in 100 μl IFA. Disease incidence in mice was determined by an individual blinded as to the experimental group examining the paw for signs of clinical inflammation. Clinical scores were assigned by giving each paw a number 1–4 based upon visible swelling, with a total possible score of 16.
Culture and expansion of T cell lines
CD4 T cell lines were established in the following manner. Mice were immunized at days 0 and 21 with 100 μg HCF emulsified in CFA, prepared as above. Draining LN were taken from mice at days 7 or 35 and cultured with EL-4 supernatant as a source of IL-2, HCF, and APCs from unimmunized DBA/1J mice in complete medium (CM). For OVA-specific T cell lines, mice were immunized with 100 μg OVA emulsified in CFA, and then LN cells were cultured with OVA instead of HCF, as above. CM is DMEM supplemented with 44 mM sodium bicarbonate, 0.55 mM L-arginine, 0.27 mM L-asparagine, 1.5 mM L-glutamine, 1 mM sodium pyruvate, 50 mg/L gentamicin sulfate, 50 mM 2-ME, 10 mM HEPES, and 10% FCS. T cell lines were restimulated every 2 wk.
CD4 T cell lines were sorted based upon usage of the Vβ of the TCR, and screened for CD4 and Vβ by FLOW cytometry using a TCR Vβ screening panel (BD Pharmingen). Dominant Vβ populations were screened and sorted regularly to confirm and maintain uniform T cell transfers.
T cell numbers were expanded for T cell transfer experiments by subculturing 3–5 × 106 cells in a 5-fold volume of CM and IL-2 4 d after restimulation. T cells were harvested, washed, and resuspended in HBSS for injection into 11- to 13-wk-old DBA/1J recipients at 1 × 107 cells per mouse.
In Vybrant-staining experiments, T cell lines were incubated with Vybrant DiD (Invitrogen) at a concentration of 5 μl cell-labeling solution per 1 × 107 cells for 20 min. T cells were washed and transferred into naive mice or into mice at day 24 of a CIA experiment at 1 × 107 cells per mouse.
Analysis of cytokine production by T cell lines and organs after T cell line injections
CD4 T cell lines were incubated with Ag in Ag assays, and then cytokine production was evaluated by ELISA. Briefly, resting T cells (2 × 105/ml) were stimulated with freshly isolated DBA/1J peritoneal exudate cells (APCs) at a concentration of 5 × 105/ml in the presence of media or APCs alone as a negative control, or Ag (HINS, HCF, mouse CF [MCF], HF, MF, or bCII). Supernatants were collected, and IL-6 and IL-17 cytokine concentrations were determined by specific ELISAs (BD Biosciences). Background concentrations were subtracted from the Ag assays to facilitate interpretation of the results.
For measurement of cytokines by intracellular staining, spleens, LN, and knee synovium were harvested 12 d after mice were transferred with T cells or HBSS and single-cell suspensions were prepared. Cells were stained for intracellular cytokines, as above.
Measurement of Abs
Anti-CII Ab detection
Ninety-six–well plates were coated overnight with 5 mg/ml mouse type II collagen or bCII (Chondrex). Plates were washed with PBS containing 0.5% Tween 20 and then blocked with 10% BSA in PBS. Mouse sera were diluted 1:1000 in PBS and added in duplicate to wells, and plates were incubated. Secondary Abs, HRP-conjugated goat anti-mouse IgG1 or IgG2a (Caltag Laboratories) diluted 1:1000 in PBS, were then added, and then the plates were developed using 1% H2O2, in ABTS solution.
Anti-HCF Ab detection
Ninety-six–well plates were coated with 10 μg/ml HCF in carbonate buffer. Plates were washed as above and blocked with 0.01% BSA in PBS. Mouse sera were diluted 1:50 in PBS and added in duplicate. Secondary Abs, biotin-conjugated goat anti-mouse IgG (Sigma-Aldrich) diluted in blocking buffer containing streptavidin-HRP (Zymax), were added, followed by development of the plate, as above. A standard was obtained by diluting pooled sera from mice immunized with HCF.
Western blot analysis
Tissue samples (LN or knee synovium) from individual mice were isolated at the end point of the experiment and handled, as previously described (37). Tissue samples were resuspended in 50 mM HEPES (pH 7.6), 1.0 mM EDTA, 0.5 mM DTT, and 0.43 mM PMSF. Cell extracts were prepared with a Dounce homogenizer and then clarified by centrifugation. Presence in mouse sera and tissues of MCF was tested by adding 5 μg serum, or 10 μg tissue homogenate per well to Western blots. MCF or MF diluted 1:100 in PBS were used as positive and negative controls, respectively. Blots were washed with PBS containing 0.5% Tween 20 and then blocked with 5% milk in PBS. Blots were incubated with primary Abs that detect CF at 1:2,000 in PBS (Modiquest; MQ13.1), followed by secondary Abs, HRP-conjugated goat anti-mouse IgG (Millipore) diluted 1:10,000 in PBS. To detect the presence of MF, blots were stripped, blocked, and reprobed with primary Abs at 1:500 in PBS (Lifespan Biosciences; LS-C129050) and secondary Abs, HRP-conjugated rabbit anti-mouse IgG (Millipore) diluted 1:10,000 in PBS. Blots were developed using Pierce ECL Substrate (Thermo Scientific).
Statistical analysis
ANOVA with tests for multiple comparisons was used to examine the data for clinical disease activity score in CIA. A comparison of survival curves was used to analyze the percentage of mice with clinical arthritis over time. FLOW cytometry data, mean arthritis prevalence at day 40, the average number of hind paws affected, the percentage of total paws affected, and the anti-CF Ab graphs were analyzed using a two-tailed Student t test. The Pearson correlation coefficient was calculated for comparisons between datasets that were possibly related. The anti-CII Ab graphs were analyzed using a Mann–Whitney U test. All statistical analyses were performed using GraphPad Prism, version 1.0 for Mac (GraphPad, San Diego, CA). Data are expressed as the mean ± SEM, and p values <0.05 were considered significant.
Results
CF and Abs to CF are present in tissues and sera of DBA/1J mice with CIA
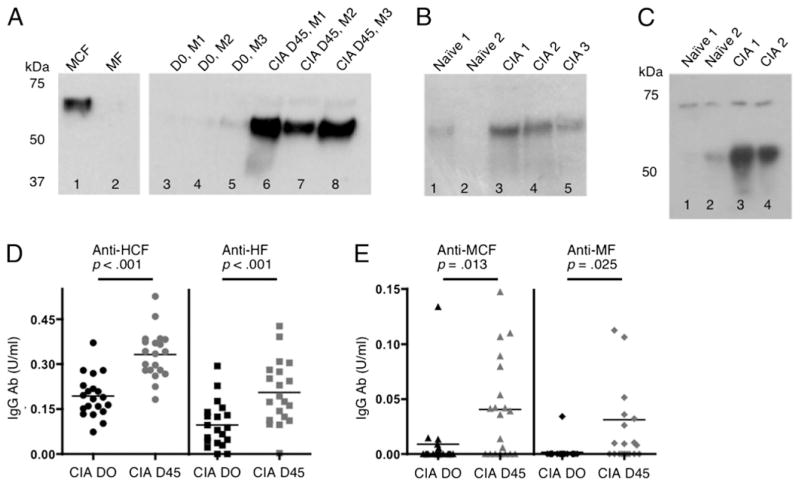
Previously, we found that DBA/1J mice with CIA exhibited a higher total citrulline content in the synovium than mice without disease (37). Also, by Western blot analysis, day 35 sera of mice with CIA contained Abs to CF (26). To determine whether CF was specifically present and/or upregulated in the tissues of mice with CIA, Western blots were performed using an Ab recognizing MCF. All blots were also probed with an Ab recognizing native fibrinogen to confirm equivalent loading of gels and fibrinogen band location (data not shown). Fibrinogen is a soluble 340-kDa glycoprotein composed of three nonidentical polypeptide chains, Aα (63.5 kDa), Bβ (56 kDa), and γ (47 kDa) [reviewed in (38)]. Our data indicate that circulating levels of MCF were higher in the sera of mice with CIA than in the same mice before immunization with CIA (Fig. 1A). Although baseline levels were present, mice with CIA also demonstrated higher levels than naive mice of MCF in the synovial tissue (Fig. 1B) and LN (Fig. 1C). LN tissue from naive mice and mice with CIA exhibits two visible CF bands, most likely the α- and β-chains (Fig. 1C). Whereas the upper band remained the same intensity in naive mice or mice with CIA, the lower m.w. band tended to increase in intensity as disease progressed. These results are similar to human studies wherein α-and β-chains display the highest levels of citrullination, and the γ-chain and fibrinogen degradation products and/or peptides are weakly citrullinated (14, 23, 39–41).
FIGURE 1.
MCF and Abs to CF are present in tissues and sera of mice with CIA. Sera (5 μg) or tissue homogenates (10 μg) from synovium or LN of naive mice or mice immunized with bCII were loaded per well onto Western blots and probed with a mAb detecting MCF in (A)–(C). (A) Lane 1 is MCF, loaded as a positive control, and lane 2 is MF as a negative control. Lanes 3–5 of the same gel are serum samples from three individual mice (M1–3) prior to immunization with bCII, and lanes 6–8 are samples from the same mice at day 45 of CIA. (B) Lanes 1 and 2 are synovial samples from unimmunized mice, and lanes 3–5 are samples from mice with CIA. (C) Lanes 1 and 2 are LN samples from unimmunized mice, and lanes 3 and 4 are LN samples from mice with CIA. In (D) and (E), sera from individual mice from day 0 or 45 of CIA were incubated on a 96-well plate coated with HCF or HF (D) and MCF or MF (E), and the presence of mouse IgG Abs was detected by ELISA. The bar represents the mean for each time point, and n = 21 mice.
In addition to elevated levels of CF, serum Ab levels to HCF, HF, MCF, and MF were analyzed by ELISA and showed increased levels present at day 45 of CIA compared with those at day 0 in the same mice (Fig. 1D, 1E). Overall, these results are consistent with our previous findings of citrullinated Ags in the serum, LN, and synovium of mice with CIA, and identify CF as an Ag that increases in the tissues of mice with CIA.
Immunization with HCF produces activated CD4 T cells
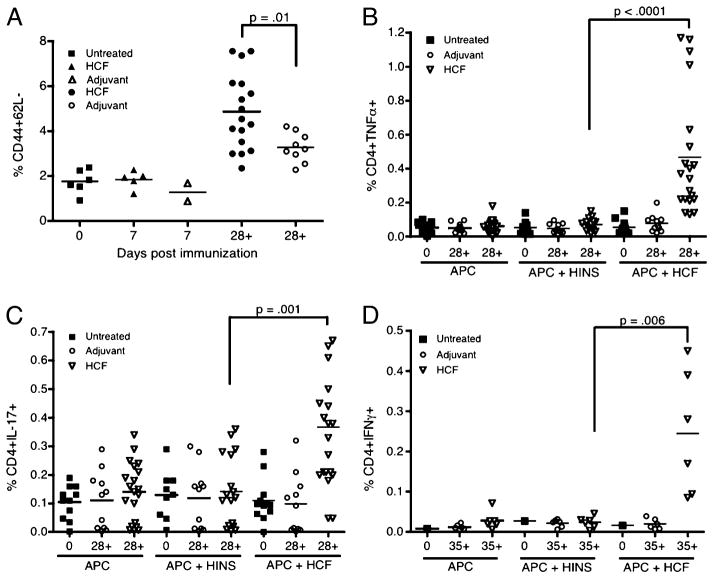
To determine whether immunization with HCF led to increases in activated CD4 T cells, DBA/1J mice were immunized at days 0 and 21 with HCF, and, at days 7, 28, or 35, single-cell suspensions were prepared from the draining LN. Levels of CD4+ cells expressing CD44high+CD62L− increased after the second immunization with HCF and were found to be significantly higher than in adjuvant-immunized mice (Fig. 2A, Supplemental Fig. 1A). These data indicate that immunization with HCF drives CD4 T cells to assume an activated, effector memory phenotype.
FIGURE 2.
HCF-immunized mice develop CD4 T cells with an activated effector phenotype (CD44high+CD62L−) and the ability to produce proinflammatory cytokines. DBA/1J mice, aged 6–8 wk, were untreated (Untreated), or immunized on days 0 and 21 with CFA (Adjuvant) or HCF in CFA (HCF), and draining LN were harvested at various time points (days 7, 28, or 35). (A) Surface staining was performed, followed by flow cytometric analysis, in which CD4+ cells were gated from live cells. The percentage of CD44high+ 62L− CD4 T cells is quantified for each group of mice with each shape representing one mouse. In (B)–(D), LN cell suspensions were stimulated with APCs alone as a negative control, APCs and irrelevant Ag (HINS), or APCs and HCF. After stimulation, cells were incubated with GolgiPlug for 4 h and then stained for intracellular cytokines. The percentage of (B) TNF-α+ CD4 T cells, (C) IL-17+ CD4 T cells, and (D) IFN-γ+ CD4 T cells is quantified for each group of mice. Relevant statistically significant differences are shown, and the bar represents the mean for each group at the respective time point.
CD4 T cells produce proinflammatory cytokines upon stimulation with HCF
As there were higher levels of CD4 T cells with a more activated phenotype in mice immunized with HCF than in mice receiving adjuvant only, we investigated cytokine production in response to Ag. Mice were immunized with HCF, and cells from the draining LN were incubated with PMA-ionomycin as a positive control, APCs alone as a negative control, APCs and HINS as an irrelevant Ag, or APCs and HCF, and then stained for intracellular cytokines. CD4+ T cells from mice immunized with HCF produced TNF-α in response to challenge with HCF after day 28 (Fig. 2B, Supplemental Fig. 1B). CD4+ T cells from mice immunized with HCF also produced IL-17 after day 28 (Fig. 2C, Supplemental Fig. 1C) and IFN-γ after day 35 (Fig. 2D). As detected by ELISA, low levels of IL-6 were also secreted by CD4 T cells (data not shown). We conclude that IL-17, TNF-α, and IFN-γ are produced by CD4 T cells after immunization with HCF, suggesting that a proinflammatory memory-type response is produced.
The HCF4Vβ7+ line responds in an Ag-specific manner to CF by producing IL-6 and IL-17
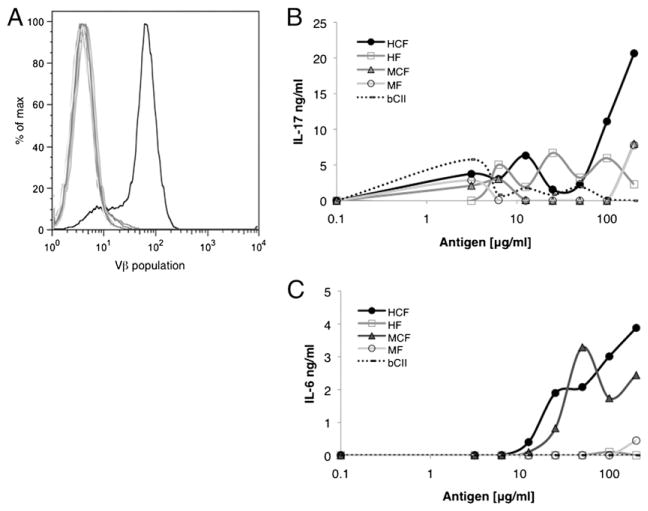
To establish CD4 T cell lines specific for CF, cells from draining LN were isolated. Lines were cultured long-term with HCF, IL-2, and APCs, and Ag assays were used to examine each line for Ag specificity. One line, designated HCF4, was characterized as a CD4+ T cell line that secreted IL-6 and IL-17 in response to challenge with HCF and MCF, but not with unmodified HF or MF (data not shown). To determine the heterogeneity of the HCF4 line, TCR gene usage was screened by FLOW cytometry. Several dominant Vβ populations were present, and results from sorting and Ag assay experiments showed that T cells with reactivity to CF could be segregated into a few Vβ populations. One population, termed HCF4Vβ7+, was characterized as a uniform population with a single defined Vβ7 peak of CD4 T cells (Fig. 3A). This line was maintained thereafter by regular resorting. Upon stimulation with Ag (HCF, HF, MCF, or MF), the HCF4Vβ7+ CD4 T cell line responded only to HCF to produce IL-17 (Fig. 3B) and to HCF and MCF to produce IL-6 (Fig. 3C). Although high concentrations of Ag were required to see an IL-17 response by the HCF4Vβ7 line, the response was consistent and was not observed at any concentration with the control T cell line or in the absence of Ag. Also, APCs most likely process whole protein inefficiently, resulting in the need for an increased Ag concentration. We also confirmed that this line did not respond to bCII (data not shown). Together, these results indicate that the HCF4Vβ7+ CD4 T cell line is a uniform population of cells that produces inflammatory cytokines in response to CF.
FIGURE 3.
The HCF4Vβ7+ line is specific for CF. DBA/1J mice, aged 6–8 wk, were immunized on days 0 and 21 with HCF in CFA, and draining LN were harvested at day 35. Cell lines from LN were cultured long-term with HCF and IL-2. The HCF4 line was sorted by the Vβ of the TCR. (A) Vβ expression of HCF4Vβ7 was analyzed by FLOW cytometry and is displayed in a histogram. The black line represents the Vβ7 population, and gray lines represent all other Vβ populations tested. To evaluate Ag specificity, HCF4Vβ7 was stimulated with APCs alone as a negative control, APCs and bCII as an irrelevant control, or APCs and HCF, HF, MCF, or MF. Supernatants were harvested from the line at 48 h after stimulation with Ag, and the presence of IL-17 (B) and IL-6 (C) was assayed by ELISA. Data are displayed as the mean ± SEM and are representative of at least two experiments. Background concentrations were subtracted from Ag assays.
The HCF4Vβ7+ CD4 T cell line enhances the severity of arthritis and increases the cumulative incidence over time of CIA
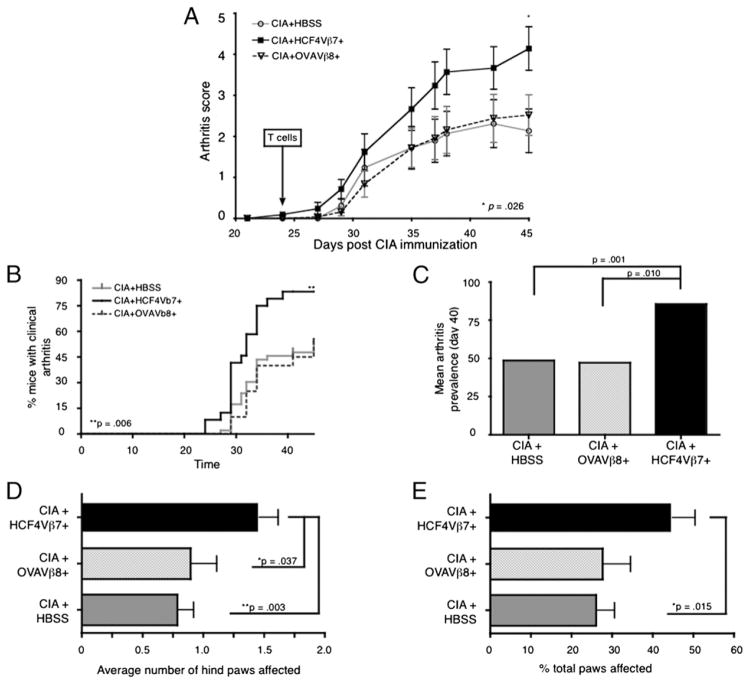
As there were increased levels of CF and production of ACPAs in CIA as disease progressed (Fig. 1), we asked whether CD4 T cells specific for CF could exacerbate autoimmune arthritis in mice immunized with bCII in CFA and then in IFA to produce only an intermediate level of arthritis. As a control, we derived and sorted a CD4 T cell line, designated OVAVβ8+, that produced IL-17 and IL-6 in response only to OVA (data not shown). Either HCF4Vβ7+ or OVAVβ8+ T cells were administered to mice 3 d after the second immunization (day 24) and before signs of clinical disease. Before T cell transfers, lines were activated for 4 d by restimulation with Ag and APCs and then further expanded for 3 d with IL-2 alone. Clinical disease activity was assessed in mice three times weekly from day 21 until the experimental end point, day 45. The control OVAVβ8+ CD4 T cell line did not increase disease over the HBSS-injected control group in CIA (Fig. 4A). However, the HCF4Vβ7+ T cell line increased the clinical disease activity in mice after day 30 of the experiment, and this difference became statistically significant by the experimental end point at day 45. In mice receiving the HCF4Vβ7+ T cell line, disease incidence increased to >80%, as compared with 50–60% in the control groups by the end point of the experiment (Fig. 4B).
FIGURE 4.
The HCF4Vβ7+ T cell line increases disease activity in CIA. Arthritis was induced by an immunization of bCII in CFA at day 0, followed by bCII in IFA at day 21. Mice were transferred with 1 × 107 T cells from the HCF4Vβ7+ or the control OVAVβ8+ T cell lines or injected with HBSS as a negative control on day 24 of the protocol, and clinical disease activity was assessed three times weekly thereafter. (A) Mice receiving the HCF4Vβ7+ T cell line display increased clinical disease activity over mice in control groups. (B) Mice receiving the HCF4Vβ7+ T cell line have significantly increased cumulative incidence over time as compared with control groups. (C) Transfer of the HCF4Vβ7+ T cell line significantly increased arthritis prevalence over control groups. (D) The average number of hind paws and (E) the percentage of total paws affected by arthritis were both increased in mice transferred with HCF4Vβ7+ T cells over control groups. Data in (A) and (C)–(E) are expressed as the mean ± SEM, and relevant statistical significance is displayed on each graph. For all experiments, n = 29 mice injected with HBSS, n = 21 mice injected with the HCF4Vβ7+ line, and n = 25 mice injected with the OVAVβ8+ line.
At day 40, the mean arthritis prevalence was also significantly increased (Fig. 4C), the average number of affected hind paws was significantly greater (Fig. 4D), and the total number of paws affected was increased in mice injected with the HCF4Vβ7+ T cell line (Fig. 4E). These results suggest that mice injected with T cells specific for CF develop arthritis faster and more mice develop arthritis than control groups. Furthermore, more hind paws were affected per mouse and more paws per mouse were affected (Fig. 4E), indicating that a more systemic effect on arthritis is induced by the addition of HCF4Vβ7+ T cells.
Autoantibody levels to CII are increased in mice immunized with bCII and injected with the HCF4Vβ7+ line
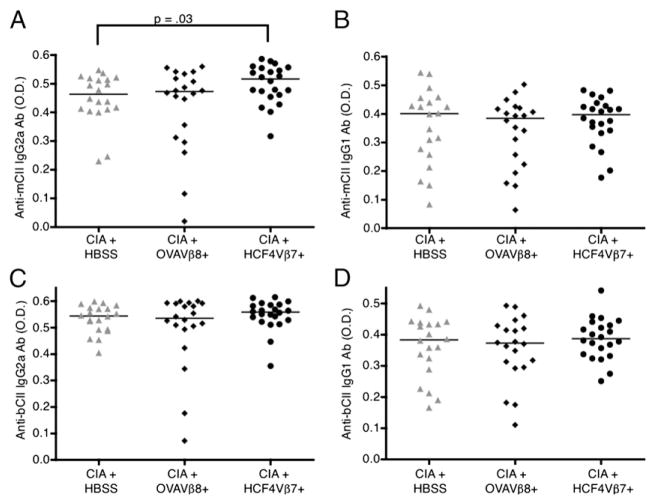
To determine whether the anti-CII autoantibody response was affected by the injection of the HCF4Vβ7+ T cell line, Ab levels were analyzed at the end point of the experiment in mice injected with the HCF4Vβ7+ T cell line compared with mice in the control groups. In mice transferred with HCF4Vβ7+, IgG2a anti-mouse CII Ab levels were significantly increased over levels of Ab in HBSS-injected control mice and tended to be increased over levels of Ab in OVAVβ8-injected control mice, although not significantly (Fig. 5A). IgG2a anti-bovine CII Ab levels and IgG1 anti-mouse and anti-bovine Ab levels were similar in all groups (Fig. 5B–D). These data indicate that T cells specific for CF may have an effect on the autoantibody response in CIA.
FIGURE 5.
Autoantibody levels to CII are increased in mice with CIA transferred with the HCF4Vβ7+ T cell line. Arthritis was induced and T cells were transferred at day 24, as in Fig. 4. Sera from individual mice of each group were incubated on a 96-well plate coated with mouse (A, B) or bovine (C, D) CII and detected with an anti-mouse IgG1 or IgG2a Ab. All results display the difference in OD of day 45 levels minus day 0 levels. The bar indicates the median for each group.
In vivo, IL-17–producing HCF4Vβ7 CD4 T cells are increased in the joints
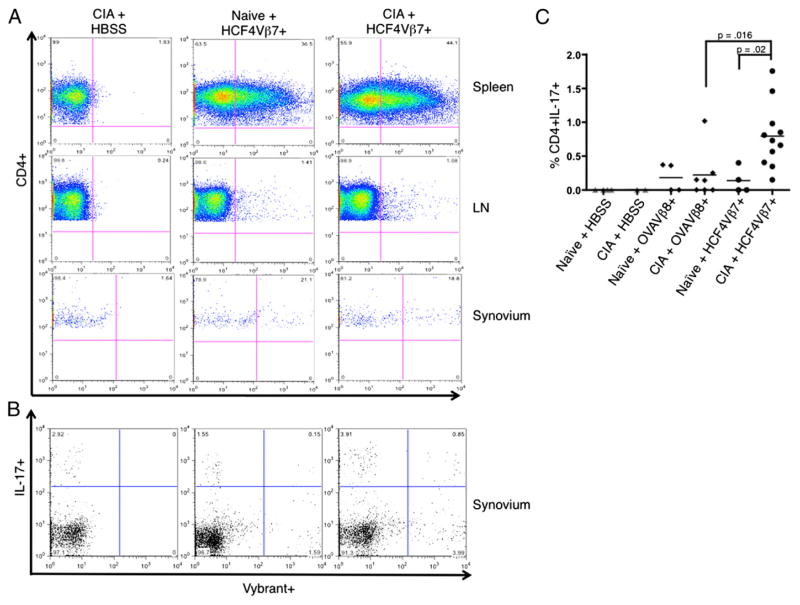
To examine whether the HCF4Vβ7+ T cell line would migrate to the synovium where it could increase damage locally, T cells were stained with Vybrant, a fluorescent dye that integrates into the cell membrane, prior to transfer. Stained cells were injected into naive mice or mice with CIA at day 24. Twelve days later, mice were sacrificed, and spleens, LN, and synovium from the knees were isolated and analyzed by flow cytometry for Vybrant expression. Vybrant-stained HCF4Vβ7+ T cells migrated to the spleen in mice regardless of whether the mice were immunized with bCII (Fig. 6A, top panels); they also migrated to the LN in the same manner, although there were substantially fewer Vybrant+ T cells in the LN (Fig. 6A, middle panels). Interestingly, the cells migrated to the knee synovium in both naive mice and mice immunized with bCII (Fig. 6A, bottom panels). Similar levels of the OVAVβ8+ CD4 T cell line were also detected in the spleen, LN, and synovium of naive mice or mice immunized with bCII (data not shown). Upon PMA-ionomycin stimulation of cells ex vivo, CD4 T cells from the spleen and LN of mice injected with either the HCF4Vβ7+ or OVAVβ8+ line produced similar levels of both IL-17 and TNF-α (data not shown). Low numbers of Vybrant+ CD4+ cell were also present in the synovium and produced a background level of IL-17 in all groups of mice injected with T cells. However, there was a clear significant increase in levels of Vybrant+IL-17+ CD4 T cells in mice receiving HCF4Vβ7+ over all control groups (Fig. 6B, upper right-hand gate, quantified in Fig. 6C). Thus, although both the HCF4Vβ7+ and OVAVβ8+ T cell lines migrate to the spleen, LN, and synovium of mice, these results indicate that more IL-17–producing HCF4Vβ7+ cells are present in the synovium of mice with CIA.
FIGURE 6.
In vivo, IL-17–producing HCF4Vβ7+ CD4 T cells migrate to joints. Naive mice or mice with CIA, as described in Materials and Methods, were injected with 1 × 107 T cells stained with Vybrant at day 24 of the protocol, and spleens, LN, and knee synovium were harvested 12 d later. The negative control for these studies was a mouse with CIA injected with HBSS (CIA + HBSS). (A) Plots show representative examples of expression of Vybrant-stained HCF4Vβ7+ T cells analyzed by flow cytometry in naive mice or mice immunized with bCII. Cells from the spleen, LN, and synovium are displayed. (B) Inflammatory cytokine expression was analyzed by intracellular staining, following incubation with PMA-ionomycin and Golgi plug. Plots show representative examples of IL-17 expression by Vybrant-stained HCF4Vβ7+ T cells from knee synovium in naive mice or mice immunized with bCII. (C) The percentage of Vybrant+IL-17+ CD4 T cells in the synovium (upper right quadrant in B) was quantified for each group of mice, as well as in naive mice and mice with CIA injected with the OVAVβ8 line. Each bar indicates the mean, and the relevant statistical significance is displayed. n = 2 mice injected with HBSS, n = 11 mice injected with the HCF4Vβ7+ line, and n = 7 mice injected with the OVAVβ8+ line.
Discussion
In this study, we demonstrate a role for T cells specific for CF in the development of inflammatory arthritis in CIA. We first established that both increased circulating CF and increased Ab levels to CF are present in mice after immunization with bCII. This observation led us to hypothesize that CF-specific T cells might accelerate or otherwise modify disease in this model. Data from ex vivo experiments confirmed that activated CD4 T cells producing proinflammatory cytokines could be isolated from LN of mice immunized with HCF, and cultured long-term in the form of T cell lines. The HCF4Vβ7+ CD4 T cell line produced proinflammatory cytokines in response to challenge with CF, and, when transferred in vivo, primed HCF4Vβ7 CD4 T cells exacerbated CIA in mice. Additionally, mice transferred with HCF4Vβ7+ displayed increased circulating IgG2a anti-mouse CII Ab levels and synovial HCF4Vβ7+ IL-17+ CD4 T cells.
That the HCF4Vβ7 CD4 T cell line exacerbated CIA in mice is an important finding in several respects. First, fibrinogen deposition in the joint is a hallmark of inflammatory autoimmune arthritis, and CF is a major autoantigen in RA. Increased amounts of CF were present in synovial tissue of mice with CIA, indicating that local APCs could present CF to transferred Ag-specific IL-17–secreting CD4 T cells in an inflammatory environment such as that induced by CIA (42). Second, the T cell line responds only to citrullinated forms of fibrinogen, and yet, it increases clinical disease in a mouse model in which B and T cell reactivity to bCII is necessary for disease induction. To our knowledge, these are the first studies testing a T cell line specific for an Ag other than CII in CIA, which is, in effect, testing the hypothesis that both epitope spreading to CF and posttranslationally modified epitopes are important in T cell–driven disease. Epitope spreading of autoreactive T cell responses to new Ags has not been tested in animal models of RA, although it has been described in experimental autoimmune encephalomyelitis (43). Furthermore, posttranslational modifications of CII have been shown to create novel T cell determinants that are important in disease induction and/or exacerbation of CIA, but other modified Ags have not been tested in CIA (32, 44).
Our research implicates reactivity to citrullinated proteins. We and others have hypothesized that anti-citrulline reactivity is not responsible for the initial break in tolerance in CIA or in experimental autoimmune encephalomyelitis, but that it amplifies disease severity after onset (26, 37, 45). This study provides additional evidence that in autoimmune arthritis, anti-citrulline reactivity represents a “second hit” after some initial inflammatory event is triggered in a genetically susceptible individual. We have not yet observed arthritis in naive mice injected with only the HCF4Vβ7+ T cell line. However, it has been suggested that in the absence of immunization with Ag, arthritogenic Th17 cells are insufficient to produce arthritis and self-reactive T cells will only differentiate into Th17 cells and persist in a cytokine environment that facilitates this differentiation, such as innate immunity induced by microbial products (46). This may indicate that a stimulus such as CIA induction could be necessary for CF-specific T cells to enhance arthritis.
A third important aspect of this research is that a Th17 line that retains its characteristics in culture is a unique finding in itself. Th1 and Th17 cells have been shown to colocalize in regions of inflammation and may require each other for recruitment (47–49), suggesting that both subsets may be relevant to disease pathogenesis. Th17 cells are important in inflammatory responses in autoimmune diseases such as RA (50), multiple sclerosis (51), and psoriasis (52), but Th17 T cell lines have not been well studied or well characterized. The cytokines secreted most consistently by the HCF4Vβ7 line upon stimulation with Ag were IL-6 and IL-17, both integral to pathogenesis of RA and CIA. Although the HCF4Vβ7 CD4 T cell line produced both IL-6 and IL-17 in response to HCF, IL-6 was also produced in response to MCF, which could drive Th17 cell production in vivo by inhibiting TGF-β–induced T regulatory differentiation (53–55). Upon ex vivo analysis of Vybrant-stained HCF4Vβ7+ T cells in CIA, intracellular staining showed the production of TNF-α as well as IL-17. Under the right experimental conditions, the line may produce IFN-γ by intracellular staining as well. We are curious to take a more in-depth approach to determine whether these transferred T cells shift to a Th1 phenotype in vivo, such as was the case in animal models of diabetes and multiple sclerosis (47, 56, 57). Furthermore, we are attempting to isolate a Th1 line to compare with the HCF4Vβ7+ line.
Finally of note, although the question of whether Ag-specific T cells exert their effects locally or from secondary lymphoid organs (58, 59) has been debated, our results suggest that the HCF4Vβ7+ T cell line may have affected disease from both locations. The cumulative incidence over time, the total number of paws affected, and mean arthritis prevalence were all increased in mice transferred with HCF4Vβ7, indicating a systemic effect of CF-specific T cells. Furthermore, IL-6 production by HCF4Vβ7 in vivo could have increased circulating IgG2a autoantibody levels to CII in mice with CIA, as in RA, in which IL-6–stimulated Th17 cells can induce B cell differentiation and Ab production via secretion of IL-21 (reviewed in Ref. 60). In addition, although we saw similar levels of IL-17 in LN and spleens of mice with CIA whether or not they were transferred with the HCF4Vβ7 line, more IL-17–producing HCF4Vβ7+ cells were present in the synovium of mice with CIA, indicating a local effect of the cells in an already inflamed environment. An alternative explanation for enhanced arthritis in this study is that HCF4Vβ7+ CD4 T cells act as a local source of IL-17 in the joint, as reported previously (61). However, in our studies, OVA-specific CD4 T cells also produced IL-17 in response to OVA and trafficked to spleens, LN, and synovium when transferred into mice with CIA, but arthritis was not increased in these animals. Finally, the amplifying effect could be due to enhanced production of pathogenic anti-bCII Abs in mice receiving the HCF4Vβ7+ T cell line.
In sum, our data demonstrated that T cells specific for CF have a role in the development and exacerbation of mouse autoimmune arthritis. Because CF is a major autoantigen in RA, these studies are relevant to the human disease process. Notably, recent studies have demonstrated the presence of citrullinated vimentin peptide-specific T cells in patients with RA (62). Furthermore, T cells from SE+ patients with RA produced proinflammatory cytokines, including IL-6, in response to citrullinated self Ag (63). These data suggest that CF-specific T cells may have a role in the development of human RA, and that modulation of these cells may ameliorate arthritis.
Supplementary Material
Acknowledgments
We thank Jonathan Hannan and William Arend for assistance with manuscript preparation and Jane Buckner for many useful experimental suggestions.
Abbreviations used in this article
- ACPA
anti-citrullinated protein Ab
- bCII
bovine type II collagen
- CF
citrullinated fibrinogen
- CIA
collagen-induced arthritis
- CM
complete medium
- HCF
human CF
- HF
human fibrinogen
- HINS
human insulin
- LN
lymph node
- MCF
mouse CF
- MF
mouse fibrinogen
- PAD
peptidyl arginine deiminase
- RA
rheumatoid arthritis
- SE
shared epitope
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Goldbach-Mansky R, Lee J, McCoy A, Hoxworth J, Yarboro C, Smolen JS, Steiner G, Rosen A, Zhang C, Ménard HA, et al. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000;2:236–243. doi: 10.1186/ar93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bizzaro N, Mazzanti G, Tonutti E, Villalta D, Tozzoli R. Diagnostic accuracy of the anti-citrulline antibody assay for rheumatoid arthritis. Clin Chem. 2001;47:1089–1093. [PubMed] [Google Scholar]
- 3.Majka DS, V, Holers M. Can we accurately predict the development of rheumatoid arthritis in the preclinical phase? Arthritis Rheum. 2003;48:2701–2705. doi: 10.1002/art.11224. [DOI] [PubMed] [Google Scholar]
- 4.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 5.Rantapää-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 6.Deane KD, O’Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, Gilliland WR, Edison JD, Norris JM, Robinson WH, Holers VM. The number of elevated cytokines and chemokines in pre-clinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum. 2010;62:3161–3172. doi: 10.1002/art.27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majka DS, Deane KD, Parrish LA, Lazar AA, Barón AE, Walker CW, Rubertone MV, Gilliland WR, Norris JM, Holers VM. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis. 2008;67:801–807. doi: 10.1136/ard.2007.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Venrooij WJ, Pruijn GJ. Citrullination: a small change for a protein with great consequences for rheumatoid arthritis. Arthritis Res. 2000;2:249–251. doi: 10.1186/ar95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundberg K, Nijenhuis S, Vossenaar ER, Palmblad K, van Venrooij WJ, Klareskog L, Zendman AJ, Harris HE. Citrullinated proteins have increased immunogenicity and arthritogenicity and their presence in arthritic joints correlates with disease severity. Arthritis Res Ther. 2005;7:R458–R467. doi: 10.1186/ar1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoet RM, Voorsmit RA, Van Venrooij WJ. The perinuclear factor, a rheumatoid arthritis-specific autoantigen, is not present in keratohyalin granules of cultured buccal mucosa cells. Clin Exp Immunol. 1991;84:59–65. doi: 10.1111/j.1365-2249.1991.tb08124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon M, Girbal E, Sebbag M, Gomès-Daudrix V, Vincent C, Salama G, Serre G. The cytokeratin filament-aggregating protein filaggrin is the target of the so-called “antikeratin antibodies,” autoantibodies specific for rheumatoid arthritis. J Clin Invest. 1993;92:1387–1393. doi: 10.1172/JCI116713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapuy-Regaud S, Sebbag M, Baeten D, Clavel C, Foulquier C, De Keyser F, Serre G. Fibrin deimination in synovial tissue is not specific for rheumatoid arthritis but commonly occurs during synovitides. J Immunol. 2005;174:5057–5064. doi: 10.4049/jimmunol.174.8.5057. [DOI] [PubMed] [Google Scholar]
- 14.Masson-Bessière C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, Serre G. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha-and beta-chains of fibrin. J Immunol. 2001;166:4177–4184. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 15.Vossenaar ER, Després N, Lapointe E, van der Heijden A, Lora M, Senshu T, van Venrooij WJ, Ménard HA. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004;6:R142–R150. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinloch A, Tatzer V, Wait R, Peston D, Lundberg K, Donatien P, Moyes D, Taylor PC, Venables PJ. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1421–R1429. doi: 10.1186/ar1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkhardt H, Sehnert B, Bockermann R, Engström A, Kalden JR, Holmdahl R. Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis. Eur J Immunol. 2005;35:1643–1652. doi: 10.1002/eji.200526000. [DOI] [PubMed] [Google Scholar]
- 18.Snir O, Widhe M, Hermansson M, von Spee C, Lindberg J, Hensen S, Lundberg K, Engström A, Venables PJ, Toes RE, et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 2010;62:44–52. doi: 10.1002/art.25036. [DOI] [PubMed] [Google Scholar]
- 19.Schultz DR, Arnold PI. Properties of four acute phase proteins: C-reactive protein, serum amyloid A protein, alpha 1-acid glycoprotein, and fibrinogen. Semin Arthritis Rheum. 1990;20:129–147. doi: 10.1016/0049-0172(90)90055-k. [DOI] [PubMed] [Google Scholar]
- 20.Marty I, Péclat V, Kirdaite G, Salvi R, So A, Busso N. Amelioration of collagen-induced arthritis by thrombin inhibition. J Clin Invest. 2001;107:631–640. doi: 10.1172/JCI11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang YH, Carmeliet P, Hamilton JA. Tissue-type plasminogen activator deficiency exacerbates arthritis. J Immunol. 2001;167:1047–1052. doi: 10.4049/jimmunol.167.2.1047. [DOI] [PubMed] [Google Scholar]
- 22.Zacharski LR, Brown FE, Memoli VA, Kisiel W, Kudryk BJ, Rousseau SM, Hunt JA, Dunwiddie C, Nutt EM. Pathways of coagulation activation in situ in rheumatoid synovial tissue. Clin Immunol Immunopathol. 1992;63:155–162. doi: 10.1016/0090-1229(92)90008-c. [DOI] [PubMed] [Google Scholar]
- 23.Takizawa Y, Suzuki A, Sawada T, Ohsaka M, Inoue T, Yamada R, Yamamoto K. Citrullinated fibrinogen detected as a soluble citrullinated autoantigen in rheumatoid arthritis synovial fluids. Ann Rheum Dis. 2006;65:1013–1020. doi: 10.1136/ard.2005.044743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X, Okeke NL, Sharpe O, Batliwalla FM, Lee AT, Ho PP, Tomooka BH, Gregersen PK, Robinson WH. Circulating immune complexes contain citrullinated fibrinogen in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R94. doi: 10.1186/ar2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd BA, Ho PP, Sharpe O, Zhao X, Tomooka BH, Kanter JL, Steinman L, Robinson WH. Epitope spreading to citrullinated antigens in mouse models of autoimmune arthritis and demyelination. Arthritis Res Ther. 2008;10:R119. doi: 10.1186/ar2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, Holers VM. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116:961–973. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis: an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 28.Kaltenhäuser S, Pierer M, Arnold S, Kamprad M, Baerwald C, Häntzschel H, Wagner U. Antibodies against cyclic citrullinated peptide are associated with the DRB1 shared epitope and predict joint erosion in rheumatoid arthritis. Rheumatology. 2007;46:100–104. doi: 10.1093/rheumatology/kel052. [DOI] [PubMed] [Google Scholar]
- 29.van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Huizinga TW, Toes RE, de Vries RR. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 2006;54:1117–1121. doi: 10.1002/art.21739. [DOI] [PubMed] [Google Scholar]
- 30.Haskins K. Pathogenic T-cell clones in autoimmune diabetes: more lessons from the NOD mouse. Adv Immunol. 2005;87:123–162. doi: 10.1016/S0065-2776(05)87004-X. [DOI] [PubMed] [Google Scholar]
- 31.Holmdahl R, Klareskog L, Rubin K, Larsson E, Wigzell H. T lymphocytes in collagen II-induced arthritis in mice: characterization of arthritogenic collagen II-specific T-cell lines and clones. Scand J Immunol. 1985;22:295–306. doi: 10.1111/j.1365-3083.1985.tb01884.x. [DOI] [PubMed] [Google Scholar]
- 32.Corthay A, Bäcklund J, Broddefalk J, Michaëlsson E, Goldschmidt TJ, Kihlberg J, Holmdahl R. Epitope glycosylation plays a critical role for T cell recognition of type II collagen in collagen-induced arthritis. Eur J Immunol. 1998;28:2580–2590. doi: 10.1002/(SICI)1521-4141(199808)28:08<2580::AID-IMMU2580>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Buzás EI, Brennan FR, Mikecz K, Garzó M, Negroiu G, Holló K, Cs-Szabó G, Pintye E, Glant TT. A proteoglycan (aggrecan)-specific T cell hybridoma induces arthritis in BALB/c mice. J Immunol. 1995;155:2679–2687. [PubMed] [Google Scholar]
- 34.Chiocchia G, Boissier MC, Ronziere MC, Herbage D, Fournier C. T cell regulation of collagen-induced arthritis in mice. I. Isolation of type II collagen-reactive T cell hybridomas with specific cytotoxic function. J Immunol. 1990;145:519–525. [PubMed] [Google Scholar]
- 35.Wakasa-Morimoto C, Toyosaki-Maeda T, Matsutani T, Yoshida R, Nakamura-Kikuoka S, Maeda-Tanimura M, Yoshitomi H, Hirota K, Hashimoto M, Masaki H, et al. Arthritis and pneumonitis produced by the same T cell clones from mice with spontaneous autoimmune arthritis. Int Immunol. 2008;20:1331–1342. doi: 10.1093/intimm/dxn091. [DOI] [PubMed] [Google Scholar]
- 36.Kannan K, Ortmann RA, Kimpel D. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology. 2005;12:167–181. doi: 10.1016/j.pathophys.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, Luo Y, Levitt B, Glogowska M, Chandra P, et al. N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186:4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams RA, Schachtrup C, Davalos D, Tsigelny I, Akassoglou K. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: lessons from multiple sclerosis. Curr Med Chem. 2007;14:2925–2936. doi: 10.2174/092986707782360015. [DOI] [PubMed] [Google Scholar]
- 39.Okumura N, Haneishi A, Terasawa F. Citrullinated fibrinogen shows defects in FPA and FPB release and fibrin polymerization catalyzed by thrombin. Clin Chim Acta. 2009;401:119–123. doi: 10.1016/j.cca.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 40.van Beers JJ, Raijmakers R, Alexander LE, Stammen-Vogelzangs J, Lokate AM, Heck AJ, Schasfoort RB, Pruijn GJ. Mapping of citrullinated fibrinogen B-cell epitopes in rheumatoid arthritis by imaging surface plasmon resonance. Arthritis Res Ther. 2010;12:R219. doi: 10.1186/ar3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Tian X, Li Z. Prevalence and clinical significance of antibodies to citrullinated fibrinogen (ACF) in Chinese patients with rheumatoid arthritis. Clin Rheumatol. 2007;26:1505–1512. doi: 10.1007/s10067-007-0544-y. [DOI] [PubMed] [Google Scholar]
- 42.Fox DA, Gizinski A, Morgan R, Lundy SK. Cell-cell interactions in rheumatoid arthritis synovium. Rheum Dis Clin North Am. 2010;36:311–323. doi: 10.1016/j.rdc.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 44.Dzhambazov B, Lindh I, Engström A, Holmdahl R. Tissue trans-glutaminase enhances collagen type II-induced arthritis and modifies the immunodominant T-cell epitope CII260-270. Eur J Immunol. 2009;39:2412–2423. doi: 10.1002/eji.200939438. [DOI] [PubMed] [Google Scholar]
- 45.Carrillo-Vico A, Leech MD, Anderton SM. Contribution of myelin autoantigen citrullination to T cell autoaggression in the central nervous system. J Immunol. 2010;184:2839–2846. doi: 10.4049/jimmunol.0903639. [DOI] [PubMed] [Google Scholar]
- 46.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Connor RA, Prendergast CT, Sabatos CA, Lau CW, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181:3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Bäckström BT, Sobel RA, Wucherpfennig KW, Strom TB, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, Shnier R, Portek IJ. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54:1122–1131. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 51.Matusevicius D, Kivisäkk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 52.Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, Dooley LT, Lebwohl M CNTO 1275 Psoriasis Study Group. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 53.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 54.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 55.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Bending D, De la Peña H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angyal A, Egelston C, Kobezda T, Olasz K, László A, Glant TT, Mikecz K. Development of proteoglycan-induced arthritis depends on T cell-supported autoantibody production, but does not involve significant influx of T cells into the joints. Arthritis Res Ther. 2010;12:R44. doi: 10.1186/ar2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rankin AL, Reed AJ, Oh S, Cozzo Picca C, Guay HM, Larkin J, III, Panarey L, Aitken MK, Koeberlein B, Lipsky PE, et al. CD4+ T cells recognizing a single self-peptide expressed by APCs induce spontaneous auto-immune arthritis. J Immunol. 2008;180:833–841. doi: 10.4049/jimmunol.180.2.833. [DOI] [PubMed] [Google Scholar]
- 60.Ferraccioli G, Zizzo G. The potential role of Th17 in mediating the transition from acute to chronic autoimmune inflammation: rheumatoid arthritis as a model. Discov Med. 2011;11:413–424. [PubMed] [Google Scholar]
- 61.Murakami M, Okuyama Y, Ogura H, Asano S, Arima Y, Tsuruoka M, Harada M, Kanamoto M, Sawa Y, Iwakura Y, et al. Local microbleeding facilitates IL-6- and IL-17-dependent arthritis in the absence of tissue antigen recognition by activated T cells. J Exp Med. 2011;208:103–114. doi: 10.1084/jem.20100900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snir O, Rieck M, Gebe JA, Yue BB, Rawlings CA, Nepom G, Malmström V, Buckner JH. Identification and functional characterization of T cells reactive to citrullinated vimentin in HLA-DRB1*0401-positive humanized mice and rheumatoid arthritis patients. Arthritis Rheum. 2011;63:2873–2883. doi: 10.1002/art.30445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Law SC, Street S, Yu CH, Capini C, Ramnoruth S, Nel HJ, van Gorp E, Hyde C, Lau K, Pahau H, et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res Ther. 2012;14:R118. doi: 10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.