Abstract
The Saccharomyces cerevisiae cardiolipin (CL) synthase encoded by the CRD1 gene catalyses the synthesis of CL, which is localized to the inner mitochondrial membrane and plays an important role in mitochondrial function. To investigate how CRD1 expression is regulated, a lacZ reporter gene was placed under control of the CRD1 promoter and the 5′-untranslated region of its mRNA (PCRD1-lacZ). PCRD1-lacZ expression was 2.5 times higher in early stationary phase than in logarithmic phase for glucose grown cells. Non-fermentable growth resulted in a two-fold elevation in expression relative to glucose grown cells. A shift from glycerol to glucose rapidly repressed expression, whereas a shift from glucose to glycerol had the opposite effect. The derepression of PCRD1-lacZ expression by non-fermentable carbon sources was dependent on mitochondrial respiration. These results support a tight coordination between translation and transcription of the CRD1 gene, since similar effects by the above factors on CRD1 mRNA levels have been reported. In glucose-grown cells, PCRD1-lacZ expression was repressed 70% in a pgs1Δ strain (lacks phosphatidylglycerol and CL) compared with wild-type and rho− cells and elevated 2.5-fold in crd1Δ cells, which have increased phosphatidylglycerol levels, suggesting a role for phosphatidylglycerol in regulating CRD1 expression. Addition of inositol to the growth medium had no effect on expression. However, expression was elevated in an ino4Δ mutant but not in ino2Δ cells, suggesting multiple and separate functions for the inositol-responsive INO2/INO4 gene products, which normally function as a dimer in regulating gene function.
Keywords: cardiolipin, Saccharomyces cerevisiae, mitochondria, CRD1 gene expression, β-galactosidase, inositol, phosphatidylglycerol, diauxic shift
Introduction
Cardiolipin (CL) makes up about 15–20% of mitochondrial phospholipids in eukaryotic cells (Hatch, 1996; Jakovcic et al., 1971). As an anionic phospholipid predominantly found in the mitochondrial inner membrane (Gallet et al., 1997; Hatch, 1996), CL plays an essential role in many critical mitochondrial functions, such as solute transport (Battelli et al., 1992; Hoffmann et al., 1994; Mende et al., 1983), protein and phospholipid import (Ardail et al., 1991; Chupin et al., 1995; Eilers et al., 1989; Endo et al., 1989; Shiao et al., 1995), oxidative phosphorylation (Eble et al., 1990; Fry and Green, 1981; Paradies et al., 1997; Petrosillo et al., 2003; Robinson, 1993) and mitochondria-mediated apoptosis (Esposti, 2002; Kirkland et al., 2002; Kriska et al., 2005; McMillin and Dowhan, 2002; Nakagawa, 2004). Much of CL's importance to various mitochondrial processes can be ascribed to its interaction with individual proteins or complexes, which in turn require CL to maintain their structural or functional integrity. For instance, CL is an integral part of the structure of complexes III and IV of the mitochondrial electron transport chain in yeast (Lange et al., 2001) and is required to form a supermolecular complex between these individual complexes that is associated with more efficient growth on non-fermentable carbon sources (Zhang et al., 2002).
In eukaryotes, CL is synthesized from CDP-diacylglycerol (CDP-DAG) through three sequential reactions catalysed by phosphatidylglycerol-P (PG-P) synthase (PGS1 gene product), PG-P phosphatase and CL synthase (CRD1 gene product) in the mitochondrial inner membrane; the above nuclear-encoded genes have been characterized (Chang et al., 1998a,b; Jiang et al., 1997; Tuller et al., 1998). Disruption of the PGS1 gene results in lack of PG and CL as well as growth only on fermentable but not on non-fermentable carbon sources (Chang et al., 1998a), confirming an essential role of PG and/or CL for mitochondrial respiratory function. Compared to pgs1Δ cells, null mutants bearing a disruption of the CRD1 gene have no detectable CL synthesis and are viable on both fermentable and non-fermentable carbon sources, although with a reduced efficiency when using the latter as a carbon source (Chang et al., 1998b; Jiang et al., 1997; Zhang et al., 2002).
Despite the apparent importance of anionic phospholipids in mitochondrial function, regulation of CL synthesis in mitochondrial membranes in response to changes in environment or by factors or mutations affecting mitochondrial functions or general phospholipid biosynthesis has not been completely characterized. Generally, mitochondrial phospholipid biosynthetic activity, as indicated by early genetic and biochemical studies, is subject to regulation by factors affecting mitochondrial development, such as carbon source, growth phase, oxygen, and mutations in mitochondrial DNA (Gaynor et al., 1991), in addition to cross-pathway control by inositol and choline (Greenberg et al., 1988; McGraw and Henry, 1989). Yeast cells grown on non-fermentable carbon sources, entering stationary phase or during aerobic growth have more developed mitochondria and thereby relatively higher CL content in their mitochondrial membranes (Gallet et al., 1997; Gohil et al., 2004; Jakovcic et al., 1971), than cells in early log phase grown on glucose. PG-P synthase activity, the committed step in CL biosynthesis, is regulated in a similar manner by the above factors (Gaynor et al., 1991; Shen and Dowhan, 1998) as well as by inositol or reduced CDP-DAG levels (Shen and Dowhan, 1998). Regulation of PG-P synthase activity by inositol is unique compared with other phospholipid biosynthetic enzymes because an increase in inositol in the media results in a rapid decrease in PG-P synthase activity (Greenberg et al., 1988), which was too fast to be ascribed to repression only in gene expression and was later found due to inactivation of PG-P synthase by phosphorylation (He and Greenberg, 2004). The PG-P phosphatase does not appear to respond to any of the above regulatory factors (Kelly and Greenberg, 1990). The activity of CL synthase, the final step of the CL synthetic pathway, also appears not to be affected by inositol (Tamai and Greenberg, 1990) but is dependent on mitochondrial respiratory chain function (Gohil et al., 2004) and is regulated by mitochondrial development (Jiang et al., 1999).
In this report, the regulation of CRD1 expression by factors affecting mitochondrial development, by inositol and by lesions in other structural genes necessary for CL synthesis, was extended to the translation of gene product. The latter was upregulated with increased PG levels and coordinately regulated with PGS1 gene expression by growth phase, carbon source and mitochondrial respiratory competence, but not by inositol or by the capacity of cells to synthesize CDP-DAG. The magnitude of effects on formation of protein product were in agreement with previously reported effects on mRNA levels, supporting a tight coupling between mRNA levels and final gene product. In addition, evidence is presented for separate and multiple functions of the INO2 and INO4 gene products, normally associated with coordinate regulation of gene expression in response to inositol, in regulating CRD1 expression.
Materials and methods
Materials
All chemicals were reagent grade or better. o-Nitrophenyl β-D-galactopyranoside (ONPG) was purchased from Sigma. Restriction endonucleases were from Promega. Oligonucleotides were commercially prepared by Genosys Biotechnologies. Polymerase chain reaction (PCR) SuperMix, TRIZOL reagent and 5′ RACE system kit were from GibcoBRL. The TOPO™ TA cloning kit was purchased from Invitrogen. Growth media were products of Bio 101 Inc. Yeast nitrogen base without amino acids was from Difco Laboratories. The BCA kit was from Pierce.
Strains, media and growth conditions
Yeast strains used in this study are listed in Table 1. Cells were pre-cultured at 30 °C in a small volume of complete synthetic medium (unless noted otherwise) (Janitor and Subik, 1993) containing either 2% glucose, 2% galactose, 2% sodium lactate or 3% glycerol, with 0.95% ethanol as the carbon source, for 1 or 2 overnights. Aliquots of the overnight cultures were inoculated into 50 ml of the same medium for continued growth, monitored by absorbance at OD600. Cells were harvested by centrifugation at the indicated time period or desired OD600. Where indicated, 10 μm or 70 μM inositol, with or without 1 mM choline, was added to growth media.
Table 1.
Yeast strains and plasmids
| Strains or plasmids | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| DL1 | MATa his3-11, 15 leu2-3, 12 ura3-251, 328, 372 | Van Loon et al. (1983) |
| rho− | MATa rho−, derivative of DL1 | This work |
| YCD4 | MATa pgs1::HIS3, derivative of DL1 | Chang et al. (1998a) |
| YPH98 | MATa ura3-52 lys2-801 ade2-101 leu2Δ1 trp1Δ1 | Sikorski and Hieter (1989) |
| YCD2 | MATa crd1::TRP1, derivative of YPH98 | Chang et al. (1998b) |
| YPH102 | MATα ura3-52 lys2-801 ade2-101 leu2Δ1 his3Δ200 | Sikorski and Hieter (1989) |
| YSD90A | MATα cds1::TRP1, derivative of YPH102 | Shen et al. (1996) |
| SH303 | MATa ino2::TRP1 his3 ura3 leu2 | S. A. Henry |
| SH307 | MATα ino4::LEU2 trp1 his3 ura3 | S. A. Henry |
| SH486 | his3 leu2 trp1 ura3 ino2Δ::TRP1 ino4::LEU2 | S. A. Henry |
| Plasmids | ||
| pMA109 | lacZ URA3 2μ and ColE1 origin | Anderson and Lopes (1996) |
| phCDS1 | PGAL1 –hCDS1 LEU2, 2μ and ColE1 origin | Shen and Dowhan (1997) |
| pSD80 | PDRC1–lacZ, derivative from pMA109 (inverted promoter) | This work |
| pSD90 | PCRD1–lacZ, derivative from pMA109 | This work |
| pSD91 | Derivative of pSD90, a 136 bp sequence including the putative UASINO was deleted from CRD1 promoter | This work |
| pSD92 | Derivative of pSD90, a 126 bp sequence up to the putative UASINO was deleted from CRD1 promoter | This work |
| pSD93 | Derivative of pSD92, a further 107 bp sequence up to the potential TATA box was deleted from CRD1 promoter | This work |
Mapping of the CRD1 gene transcriptional initiation site
The transcriptional initiation site of the CRD1 gene was determined using the method of rapid amplification of cDNA 5′ ends (5′ RACE system) (Frohman et al., 1988). Two gene-specific primers, GSP1 (5′-ATCCATAAAATCAGTGATGCT-3′) and GSP2 (5′-AAACAAACCTAATGCTGGGG TCAA-3′), were utilized in this assay. Total RNA was purified with TRIZOL reagent from yeast cells (Chomczynski, 1993). First strand cDNA was synthesized from total RNA, using the gene-specific primer GSP1. RNase-treated template RNA was used as a control in this step to rule out DNA contamination. After first strand cDNA synthesis, template RNA was removed by treatment with RNase. Synthesized cDNA was then separated, tailed and amplified by nested PCR using a primer against the 5-CAP sequence and gene specific primer GSP2. The PCR product was introduced into the TOPO™ TA cloning vector for subsequent amplification and sequencing.
Plasmid constructions
Plasmid pMA109 (Anderson and Lopes, 1996) that contains the lacZ reporter gene and a URA3 marker was used in this study to generate fusions of the CRD1 promoter with the lacZ gene of E. coli. The 5′ promoter region of the CRD1 gene was amplified from the yeast genome by PCR employing four 5′ primers 5′-AAGGAATTCTGACGAAGGGAGAAGG-3′, 5′-GTCAAGCTTCACTTCACAGTC-ATGTCTTC-3′, 5′-CTCAAGCTTGAAACCATA-TTAAATGTCAA-3′ and 5′-CTTAAGCTTGAGT-ATACAATATTTACAAT-3′ (underlined endonuclease restriction sites were introduced), respectively, with 3′ primer (5′-TAGAATTCCGAAGTAATGC-GGAGC-3′). They were synthesized according to the DNA sequence surrounding the CRD1 gene (Chang et al., 1998b). The 5′ primers were targeted to the sequence starting from the 338th, 191st, 216th and 96th bp, respectively, upstream of the CRD1 start codon, and the 3′ primer ends at the 44th bp in the CRD1 open reading frame (see Figure 1). The PCR-amplified 5′ promoter regions of the CRD1 gene were then ligated into plasmid pMA109 individually, using restriction sites introduced onto each pair of primers, generating plasmids pSD90, pSD91, pSD92 and pSD93, respectively. The final plasmids include a DNA fragment encoding the first 15 amino acids of the CRD1 gene product, fused in-frame with the lacZ gene. All plasmids were confirmed by sequencing. The plasmid pSD80, in which the CRD1 promoter was inverted as opposed to plasmid pSD90, was used as a control. Plasmids were introduced into yeast cells by transformation of CaCl2-treated cells (Shen et al., 1996). Transformants were selected by growth in the absence of uracil.
Figure 1.
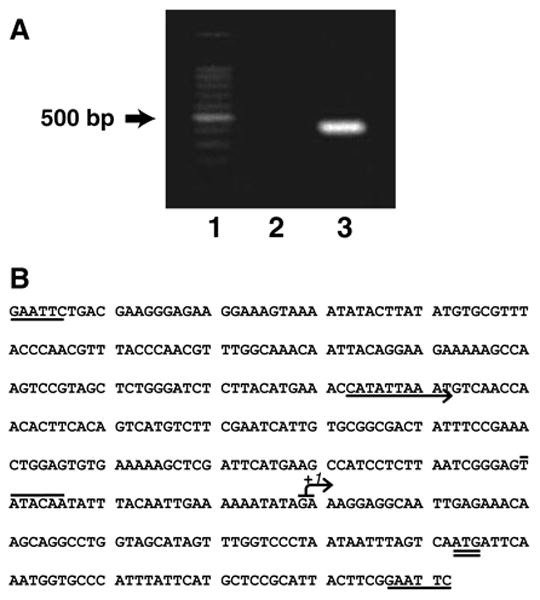
Mapping of CRD1 gene transcription initiation site and the CRD1 promoter sequence. (A) DNA fragment containing the end of the 5′-untranslated region of the CRD1 gene. Lane 1, Promega 100 bp step marker. Lane 2, sample mRNA treated with RNase prior to the RACE reaction. Lane 3, 5′ RACE product from CRD1 mRNA. (B) Diagram of CRD1 promoter. EcoRI sites (single underline) used for the construction of gene fusions, transcription initiation start sites (arrow with +I), a potential UASINO (arrow), TATA box of putative promoter (overline) and the putative translation initiation codon (double underline)
Isolation of rho− mutants
Isolation of ethidium bromide-induced rho− mutants was performed as described previously (Shen and Dowhan, 1998). Rho− mutants were verified by their inability to grow on a non-fermentable carbon source.
Preparation of cell extracts and enzyme assays
Preparation of cell extracts was carried out at 4°C, as previously described (Shen and Dowhan, 1998). In summary, yeast cells were harvested, washed and disrupted using glass beads and a mini bead-beater. The supernatant, after centrifugation at 1500 × g for 10 min, was used for β-galactosidase activity assays. β-galactosidase activities were expressed as Miller units (380 × optical density at 420 nm produced per min per mg of total protein in cell extracts). Protein concentration in each cell extract was determined using a BCA protein assay kit.
DNA gel electrophoresis mobility shift assay
Yeast extracts containing both the soluble cytosolic and nuclear fractions were prepared as reported with modifications (Biswas and Biswas, 1990). Cells grown in 1% yeast extract, 2% peptone and 2% glucose (YPD) were harvested at an OD600 of 1.0, washed with and resuspended in 1/30 volume extraction buffer (200 mM Tris–HCl, pH 8.0, 400 mM ammonium sulphate, 10 mM MgCl2, 1 mM EDTA, 10% glycerol, 7 mM β-mercaptoethanol, 1 mM phenylmethylsulphonylfluoride, 1 μg/ml leupepsin and 1 μg/ml pepstatin). Cells were disrupted in a Mini-beadbeater™ and, following incubation at 0°C for 30 min, unlysed cells and cell debris were removed by centrifugation at 10000 × g for 1 h at 4°C. Protein in the resulting supernatant was precipitated by the addition of 100% ammonium sulphate in protein buffer (10 mM HEPES, pH 8.0, 5 mM EDTA) to a final concentration of 40%. Following incubation for 30 min at 4 °C with gentle agitation, protein was collected by centrifugation at 25 000 × g for 10 min and the pellet was resuspended in protein buffer with 7 mM β-mercaptoethanol, 1 mM phenylmethylsulphonylfluoride, 1 μg/ml leupepsin, 1 μg/ml pepstatin and 20% glycerol. The soluble protein extract was then desalted with a PD-10 Sephadex G25M column. The 95 bp DNA template for the gel mobility shift assay was amplified by PCR from the CRD1 promoter, using primers GEL5 (5′-CACTTCACAGTCATGTCTTCGA-3′) and GEL3 (5′-CCGATTAAGAGGATGCTTCAT-3′). Synthesized DNA was labelled at the 5′ terminus with [γ-P32]ATP by T4 polynucleotide kinase. Binding reactions were carried out in 20 μl binding buffer containing 4 mM Tris-HCl, pH 8.0, 40 mM NaCl, 4 mM MgCl2, 5% glycerol, 0.5 ng radio-labelled DNA probe and 10 μg yeast extract for 25 min at 23 °C. After binding, the reaction mixture was loaded immediately onto a 4% polyacrylamide gel and electrophoresed at 25 mA at room temperature. Following electrophoresis, the gel was dried and exposed to X-ray film overnight.
Results
Transcriptional initiation of CL synthase gene
The transcriptional initiation site for the CRD1 gene has not been reported. In order to learn the properties and determine an appropriate length of the CRD1 promoter to be used in this study, we used the 5′ RACE method to map the transcriptional initiation site of the CRD1 gene. Total cellular RNA was isolated and used to generate a cDNA (Figure 1A) from the CRD1 mRNA, as described in Materials and methods. The cDNA product was then placed into the TOPO™ TA cloning vector and sequenced. The sequencing data showed that transcription of the CRD1 gene initiates from either the 63rd or 64th base upstream of the start codon (Figure 1B). However, because the cDNA was capped with polycytidine after reverse transcription, we cannot distinguish between an initiation site at the 63rd base adenosine or the 64th base guanosine. Relative to the transcription start site, a putative TATA box was also predicted, and is indicated in Figure 1B.
Regulation of CRD1 gene expression by growth phase and carbon source
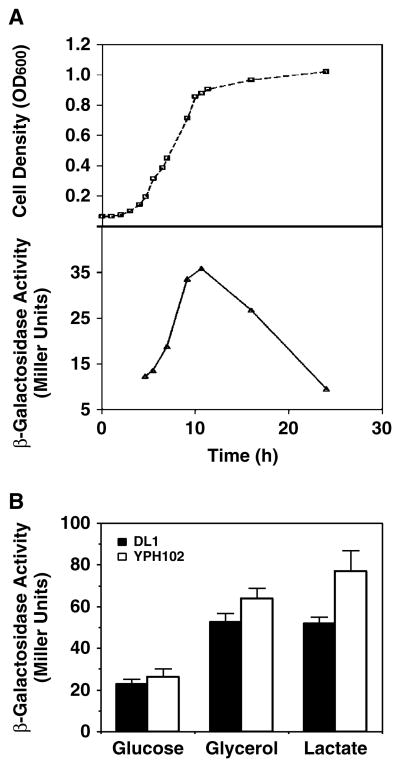
Growth phase and carbon source can affect CL content as a result of altered mitochondrial development and expression of PG-P synthase activity (Gallet et al., 1997; Jakovcic et al., 1971). Therefore, the expression of the terminal enzyme, CL synthase, in the CL biosynthetic pathway may also be subject to growth phase and carbon source regulation in coordination with expression of PG-P synthase. Coordinate transcriptional and translational regulation of the CL synthase expression was monitored by β-galactosidase activity, resulting from a CRD1 gene promoter–lacZ reporter gene fusion (plasmid pSD90). PCRD1–lacZ gene expression normalized to total protein (Figure 2A) increased with the extent of cell growth, reaching a maximum at early stationary phase and dropping significantly in late stationary phase. Since cellular CL also increases during growth and reaches a maximum in stationary phase (Jakovcic et al., 1971), comparisons were made using mid-log cells in all subsequent studies, unless specified otherwise.
Figure 2.
PCRD1 –lacZ expression in response to growth phase and carbon source. (A) Wild-type strain DL1 carrying plasmid pSD90 (PCRD1 –lacZ) was inoculated into inositol-free medium containing 2% glucose. Cell growth was monitored by OD600 and β-galactosidase activity was determined at the indicated times. (B) Wild-type strains DL1 and YPH102, all bearing plasmid pSD90 (PCRD1 –lacZ), were grown to mid-log phase in an inositol-free medium containing 2% of different carbon sources. Cells were harvested and β-galactosidase activity was determined in whole-cell extracts. Each value represents the mean of at least three experiments
Reporter gene expression in response to carbon sources was tested using two unrelated wild-type yeast strains with different genetic backgrounds (YPH102 and DL1; see Table 1) in order to limit background effects. PCRD1–lacZ gene expression was repressed when cells were grown in glucose medium as compared to the expression in non-fermentable media (lactate or glycerol/ethanol as a carbon source) (Figure 2B). Although strain YPH102 exhibited 10–20% higher β-galactosidase activity than strain DL1, the overall dependence of PCRD1–lacZ gene expression on a carbon source was the same in both genetic backgrounds. The increase in PCRD1–lacZ expression induced by growth on a non-fermentable is in line with the increase in CL content of cells grown on a non-fermentable carbon source (Jakovcic et al., 1971).
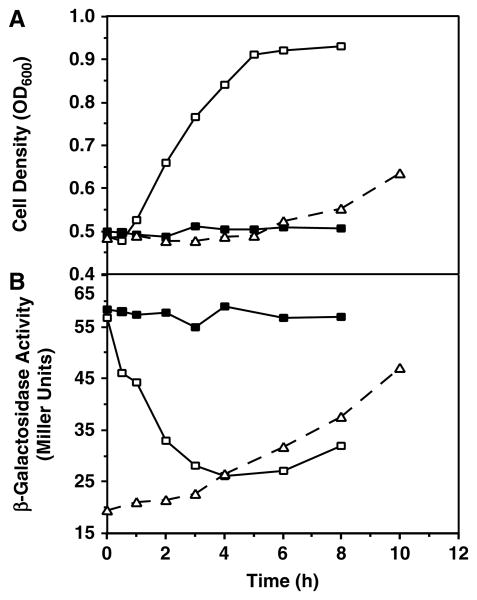
Yeast cells grown in glucose have two phases of growth (Gallet et al., 1997), the first of which is characterized by catabolite repression of expression of mitochondrial proteins (Perlman and Mahler, 1974). Before all the glucose in the media is depleted, cells reach stationary phase, where they adaptively increase mitochondrial function in order to metabolize the alcohol accumulated as a byproduct of glycolysis (Hajek and Bedwell, 1994). To investigate the effect of oxidative phosphorylation-dependent growth on PCRD1–lacZ expression, a switch of cells from a fermentable to a non-fermentable carbon source (diauxic shift) was carried out (Figure 3, dashed lines). After a shift of mid-log glucose-grown cells to a non-fermentable carbon source, growth was arrested for the first 5 h, during which a slow increase (∼40%) in β-galactosidase activity was observed. The activity increased more rapidly when cell growth resumed after the lag phase and reached a level of 2.3-fold as high as that in cells remaining in glucose, indicating that the metabolism of glycerol/ethanol was important for the rapid derepression of PCRD1–lacZ expression. This result also agrees with the 2–2.5-fold increase in β-galactosidase activity for cells grown on a non-fermentable carbon source compared with those grown in glucose.
Figure 3.
Adaptation to non-fermentative and fermentative growth during switch of carbon source. Mid-log phase cells of wild-type strain DL1 carrying plasmid pSD90 (PCRD1 –lacZ) growing in an inositol-free medium with either 2% glucose or 3% glycerol/ethanol were collected, washed with an inositol-free medium without carbon source, and at time zero switched to medium with the opposite carbon source, respectively [either from glucose to glycerol/ethanol (dashed lines) or from glycerol/ethanol to glucose (solid lines)]; in the latter case, the glucose medium was supplemented either with (closed symbols) or without (open symbols) 10 mM cycloheximide. Cells were sampled at the indicated time points. (A) Cell density was measured by OD600 and (B) β-galactosidase activity was determined in whole cell extracts. Values are representative of three experiments
Next, adaptation of expression of CL synthase to catabolite repression was studied during the reverse process from non-fermentative to fermentative growth (Figure 3, solid lines). A rapid decrease in β-galactosidase activity and little lag in cell growth were observed as soon as the cells were shifted to glucose medium (open square), consistent with catabolite repression by glucose; 4 h after the shift, a steady state was reached of ∼50% of the derepressed level. This is in agreement with Figure 2B, indicating the same level of repression in medium containing glucose as opposed to glycerol/ethanol. A slight increase in β-galactosidase activity was observed after 6 h, when cells began to enter stationary phase, consistent with the observation that CRD1 gene expression is induced when glucose-grown cells enter stationary phase (Figure 2A). Treatment of the cells with an inhibitor of nuclear-encoded protein synthesis (10 mM cycloheximide) during the shift from glycerol/ethanol to glucose totally blocked the cell growth, as well as changes in β-galactosidase activity, regardless of the presence of glucose in the media (solid square). These data indicate that the rapid decrease in β-galactosidase in the absence of cycloheximide in the media is due to a dilution effect caused by a rapid cell growth and at the same time significant repression of Pcrd1-lacZ expression.
Effect of mitochondrial dysfunction on PCRD1 –lacZ expression
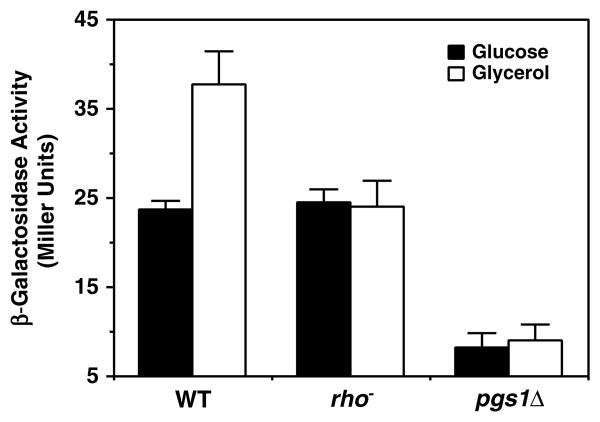
The above results indicate that carbon source regulation of CL synthase activity might be closely related to mitochondrial biosynthesis. This raises the question of how gene expression would respond in cells with respiratory-deficient mitochondria. This question was addressed using two respiratory deficient strains, rho− (DL1 rho) and pgs1Δ (YCD4), both derived from the same wild-type parent strain, DL1. Rho− mutants have extensive lesions in mitochondrial DNA and are unable to respire on a non-fermentable carbon source, due to the lack of several mitochondrial-encoded proteins that are critical for oxidative phosphorylation. Similarly, the pgs1Δ mutant strain has severe defects in mitochondrial function (Chang et al., 1998a; Janitor and Subik, 1993) and, like rho− cells, cannot grow on non-fermentable carbon sources (Chang et al., 1998a). Pgs1Δ cells also exhibit a petite lethal phenotype, initially characterized by incompatibility with extensive mutations in mitochondrial DNA (Janitor and Subik, 1993) but later ascribed to incompatibility with growth on ethidium (usually used to generate petite mutants) because of a defect in cell wall synthesis that is associated with lack of mitochondrial anionic phospholipids (Zhong et al., 2005). In addition, cells carrying this mutation cannot efficiently maintain mitochondrial DNA and eventually become rho− mutants (Zhong et al., 2005). Therefore, the pgs1Δ mutation displays a rho− phenotype. Unlike the wild-type respiratory-competent strain DL1, there was no increase in β-galactosidase activity when its pgs1Δ or rho− derivatives were shifted from glucose to glycerol/ethanol (Figure 4). Interestingly, in glucose-grown mid-log cells in which mitochondrial function is significantly repressed, expression of PCRD1–lacZ was the same in the rho+ and rho− cells but significantly reduced in pgs1Δ cells, which, in addition to being respiratory-incompetent, also lack the CL precursor PG.
Figure 4.
PCRD1 –lacZ expression in response to mitochondrial function. The parental strain DL1, a rho− mutant and pgs1A mutant (YCD4) all bearing plasmid pSD90 (PCRD1 –lacZ) were grown to mid-log phase in an inositol-free medium containing 2% glucose. Then half of each culture was maintained in glucose medium while the other half was washed and shifted to 3% glycerol/ethanol medium. Cells were harvested after 6 h and β-galactosidase activity was determined in whole cell extracts. Each value represents the mean of three experiments
Expression of PCRD1 –lacZ in crd1 Δ and cds1 Δ mutants
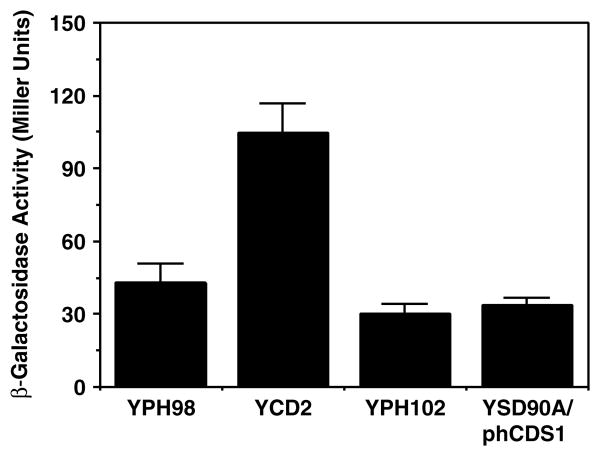
The crd1 Δ mutant can grow on both fermentable and non-fermentable carbon sources, although it is somewhat compromised when utilizing the latter as substrate (Chang et al., 1998b; Gohil et al., 2004; Jiang et al., 1997; Zhang et al., 2003). In cells lacking the terminal product of the CL biosynthetic pathway, PG accumulates (Chang et al., 1998b). Since experiments with pgs1Δ cells suggested a possible role of decreased PG level in downregulating CL synthase expression, the possibility that an elevated level of PG might induce an increased amount of CL synthase expression was investigated. The PCRD1–lacZ reporter gene was introduced into a crd1 Δ mutant strain and cells were cultured in galactose-containing media to prevent glucose repression. The level of β-galactosidase in the crd1 Δ mutant strain YCD2 was 2.5-fold higher than that in the parental strain YPH98 (Figure 5).
Figure 5.
PCRD1–lacZ expression in crd1Δ and cds1Δ mutant strains. The parental strain (YPH98) and crd1Δ mutant (YCD2) with plasmid pSD90 (Pcrd1–lacZ) were grown to mid-log in a minimal medium with 2% galactose. β-galactosidase activity of the mutant was compared with that of the wild-type. The cds1Δ mutant YSD90A carrying a human cDNA encoding a CDP-DAG synthase (plasmid phCDS) under the control of PGAL1 promoter was transformed with plasmid pSD90 and cells were grown to mid-log phase in minimal medium with 2% galactose (to induce the plasmid-borne copy of the hCDS1 gene). β-galactosidase activity was assayed in the whole cell extracts and compared with that of the parental wild-type strain YPH102 (CDS) grown in the same medium. Each value represents the mean of three experiments
CDP-DAG is an important intermediate for glycerol-phospholipid biosynthesis in yeast, from which three branches of de novo phospholipid biosynthetic pathways diverge (Carman and Henry, 1989). Therefore, CDP-DAG is the precursor for the synthesis of several major phospholipids, including phosphatidylserine, phosphatidylethanolamine, phosphatidylcholine, phosphatidylinositol, PG and CL. The formation of CDP-DAG is catalysed by CDP-DAG synthase encoded by the CDS1 gene, which occupies a central position in phospholipid metabolism (Shen et al., 1996). Yeast mutants with decreased CDP-DAG synthase activity exhibit a pleiotropic phenotype (Shen and Dowhan, 1997). Reduction of cellular CDP-DAG synthase activity results in elevated inositol 1-P synthase, phosphatidylserine synthase and PG-P synthase levels via transcriptional regulation and a decrease in PI synthase levels through post-translational events (Shen and Dowhan, 1997, 1998). The precise mechanism by which the level of CDP-DAG or the ability of cells to synthesize CDP-DAG affects the levels of these synthases has not been elucidated, but this regulation differs from the regulation of phospholipid metabolism by inositol, in that the former is independent of the INO2, INO4 and OPI1 regulatory circuit. Since CL synthase functions at the end-point of the CL synthetic pathway downstream of CDP-DAG biosynthesis and directly utilizes a second molecule of CDP-DAG as substrate, we investigated whether CL synthesis is subject to regulation by CDP-DAG levels. As mentioned earlier, the cds1Δ mutation is lethal unless complemented by the expression of the human CDP-DAG synthase induced from the PGAL1 promoter. Even when grown in galactose-containing medium, the complemented mutant has reduced levels of CDP-DAG synthase and exhibits all of the phenotypes associated with low levels of CDP-DAG synthesis (Shen and Dowhan, 1997). Unlike expression of other phospholipid biosynthetic activities, PCRD1–lacZ expression in the cds1Δ null mutant strain complemented by the low levels of CDP-DAG synthase (supplied by plasmid phCDS1) was similar to that in the wild-type strain YPH102 with normal levels of CDP-DAG synthase (Figure 5).
Regulation of CRD1 expression by inositol
Inositol is a major regulator of PC and PI biosynthesis (Carman and Henry, 1989; Greenberg and Lopes, 1996). Inositol also appears to regulate CL biosynthesis by repressing the expression of PG-P synthase activity (Greenberg et al., 1988; Shen and Dowhan, 1998) but not the level of PG-P phosphatase or CL synthase activities (Tamai and Greenberg, 1990). A consensus UASINO element (untranslated activation sequence responsive to inositol), which could serve as a cis-acting site for trans-acting factors encoded by the INO2-INO4-OPI1 regulatory genes in inositol-dependent regulation (Carman and Henry, 1989), is not present near the CRD1 gene. However, a potential UASINO-like element is found in the promoter region, which is the same as the consensus UASINO element in eight of its 10 bases (Figure 1B). PCRD1–lacZ expression was examined in the presence of different inositol/choline concentrations in both glucose- and glycerol/ethanol-containing media. Inositol and/or choline had no effect on the fusion gene expression (data not shown), consistent with the previous report of the lack of an effect on CL synthase activity levels (Tamai and Greenberg, 1990). Since the difference between the potential UASINO-like element and a consensus one lies in the first six bp, which is an E-box motif (Hoshizaki et al., 1990; Nikoloff et al., 1992) critical for the ino2p and ino4p heterodimer binding, it may explain the lack of response of the CL synthase gene to inositol.
Next, we investigated whether fusion gene expression is affected in strains carrying null alleles of the INO2 or INO4 regulatory gene. Because these mutants are inositol auxotrophs and cannot grow in inositol-free media, 70 μm inositol was added to the growth media to support cell growth. Compared to the wild-type strain DL1, PCRD1–lacZ expression (pSD90) was unaffected in ino2Δ and ino2Δ/ino4Δ strains but was ∼2.5-fold higher in an ino4Δ strain (Figure 6A), regardless of the level of inositol and/or choline present in the medium (data not shown). This observation was unexpected, since the INO2 and INO4 gene products generally act as a heterodimeric activator (Schwank et al., 1995), and strains null in the INO2 or INO4 genes usually express reduced levels of gene products responsible for phospholipid biosynthesis (Bailis et al., 1987; Hirsch and Henry, 1986). To further investigate this effect, a series of deletions in the CRD1 promoter region were created and the resulting plasmids were transformed into wild-type, ino2Δ, ino4Δ and ino2Δ/ino4Δ strains to assess PCRD1–lacZ expression. Compared with plasmid pSD90 (full length upstream of the CRD1 promoter), a four- to five-fold increase in β-galactosidase activity was observed in wild-type, ino2Δ and ino2Δ/ino4Δ cells and a more than 20-fold increase in β-galactosidase activity was observed in a ino4Δ mutant when an 136 bp upstream sequence including the UASINO-like element was deleted (plasmid pSD91). These data suggested that the 136 bp sequence upstream of the CRD1 promoter functions as a general repressing element whose deletion enhances CRD1 expression, particularly in the absence of the INO4 gene product. To determine whether the UASINO-like element contributes to this effect, strains carrying plasmid pSD92 (126 bp deletion upstream of the UASINO-like element) were examined. A slight increase in β-galactosidase level was observed in wild-type, ino2Δ and ino2Δ/ino4Δ strains and a small drop was observed in ino4Δ cells when compared to plasmid pSD91, but the ino4Δ mutant still showed the greatest increase. This result confirmed that the UASINO-like element plays a limited role in regulating CRD1 gene expression and the sequence downstream from the UASINO-like element alone is sufficient for mediating CRD1 gene expression and responding to regulatory actions by the INO2/INO4 regulatory genes. When this downstream sequence was deleted (plasmid pSD93), the increased reporter expression in ino4Δ cells was abolished. Therefore, the cis-acting element responsive in ino4Δ cells lies between the UASINO-like element and the putative TATA site of the CRD1 gene.
Figure 6.
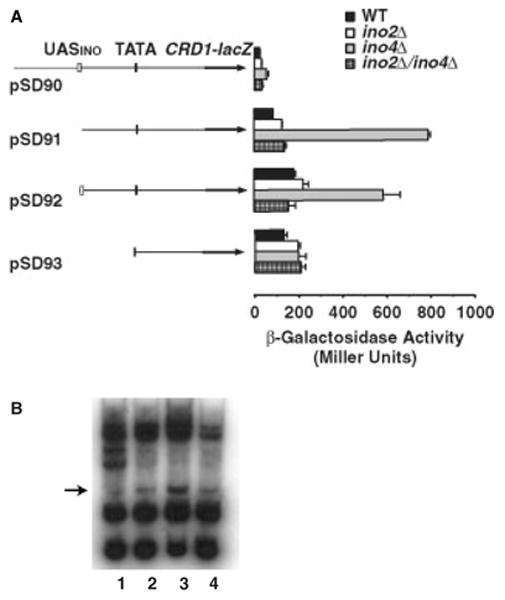
PCRD1–lacZ gene expression in ino2Δ and ino4Δ mutant strains. (A) Wild-type strain DL1, the ino2Δ mutant SH303, the ino4Δ mutant SH307 and the ino2Δ/ino4Δ double mutant SH486, all bearing either plasmid pSD90, pSD91, pSD92 or pSD93, were grown to mid-log phase in a minimal medium containing 2% glucose supplemented with 70 μM inositol. β-galactosidase activity was determined in whole cell extracts. Each value represents the mean of three experiments. The diagram illustrates the relevant genetic components carried by each plasmid. (B) DNA gel electrophoresis of mobility shift assay. A 95 bp CRD1 promoter sequence representing the cis-element upstream of the CRD1 promoter was used as a probe and EMSA was performed using this element and cell extracts isolated from the indicated cells. Lane 1, DL1; lane 2, ino2Δ; lane 3, ino4Δ; and lane 4, ino2Δ/ino4Δ
The above results suggest a possible mechanism by which the cis-element may play a role in control of CRD1 gene expression, i.e. by differential binding to transcriptional factors in the wild-type, ino2Δ, ino4Δ and ino2Δ/ino4Δ strain backgrounds. To test this hypothesis, gel electrophoresis mobility shift assay (EMSA) was used to determine whether extracts isolated from these strains interact differently with a synthesized 95 bp DNA probe representing the cis-element. The result in Figure 6B showed that a few band shifts were detected when using extracts isolated from these strains, two of which appear to exist commonly among all strains except one that occurs predominately in ino4Δ cells. Because equal amounts of protein extract and DNA probe were used in this experiment and similar amounts of other band shifts were observed in all strains, the dominant band shift with ino4Δ extracts is due to transcriptional factors that can bind to the cis-element and exist predominately in the ino4Δ background.
Discussion
CL is a phospholipid predominantly located in the mitochondrial inner membrane. Therefore, its levels may be regulated by factors affecting mitochondrial membrane development. This study clearly demonstrated that CL synthesis, as indicated by expression of lacZ from CRD1 promoter–gene fusions, was elevated in cells grown in or shifted to non-fermentable carbon sources and in early stationary phase where alcohol accumulates; normal mitochondrial respiratory function or metabolism of non-fermentable carbon source by mitochondria was a prerequisite for this induction. These results extend similar conclusions reached by monitoring mRNA levels by Northern blot analysis, in that they eliminate translational regulation as a means of controlling CL synthase levels and hence CL content of cells. Such information is relevant in light of the fact that for many genes of S. cerevisiae there is little correlation in absolute amounts or in changes in mRNA levels with the amount of the respective protein products, making measurement of mRNA levels only a poor predictor of protein functional level (Gygi et al., 1999). Currently, however, it is not clear how growth phase or carbon sources regulate CRD1 gene expression. Interestingly, CL, the lipid product of the gene product of CRD1, participates in oxidative phosphorylation and is essential for functions of many proteins/complexes in the electron transfer chain located in the inner mitochondrial membrane (Haines and Dencher, 2002; Koshkin and Greenberg, 2000; Rusnak et al., 1997). Therefore, for cells growing in a non-fermentable carbon source, accelerated synthesis of CL would be important for the biogenesis of respiratory-competent mitochondria. The observed lag in growth with increasing β-galactosidase expression during this transition may represent a period when cells are adapting themselves in response to metabolic changes by increasing CL content in the membrane, and may also be related to the need for CL as a structural component of individual components of the electron transport chain (Eble et al., 1990; Fry and Green, 1981; Robinson et al., 1990) or organization of these components into higher-order complexes (Zhang et al., 2002, 2005). These data, taken together with other reports (Jiang et al., 1999), establish unequivocally that factors affecting mitochondrial biogenesis, such as carbon source, growth phase or mitochondrial mutations (discussed below), affect CRD1 gene expression at the level of transcription and that there appears to be no additional regulation at the translational level as there is for many other genes of yeast.
The lack of derepression of β-galactosidase production in respiratory-compromised cells by exposure to a non-fermentable carbon source argues that the presence of respiratory-competent mitochondria, cell growth and/or metabolism of a non-fermentable carbon source, not simply a non-fermentable carbon source, is critical for derepressed CRD1 gene expression. Similar to data obtained here, it was also reported that CRD1 mRNA derepression cannot be induced by growth phase in rho− cells, in contrast to their isogenic wild-type strains (Jiang et al., 1999), again supporting the existence of functional mitochondria as important to CRD1 gene expression which may be coordinately regulated with the biogenesis of mitochondria.
Although both rho− and pgs1Δ cells are respiratory-deficient and are deficient in mitochondrial DNA (Zhong et al., 2005), an additional drop in the expression from the CRD1 promoter was observed in pgs1Δ cells, which cannot be ascribed solely to mitochondrial dysfunction, but instead could be caused by the lack of PG in the mitochondrial membranes. The lack of the precursor to CL synthesis in mitochondrial membranes may serve as a regulatory signal to repress CL synthase expression, via a mechanism currently not understood. It should be noted that lack of PG also severely represses cytoplasmic translation of COX4 mRNA without significantly affecting mRNA levels (Ostrander et al., 2001; Su and Dowhan, 2005), resulting in lack of this subunit of the mitochondrial cytochrome c oxidase. The increase in PCRD1–lacZ gene expression in a crd1Δ mutant, which has highly elevated PG levels, also supports a role for PG levels in regulating expression of CL synthase. This result is also in agreement with the report that there was a three-fold increase of a truncated CRD1 mRNA in crd1Δ cells compared with that in wild-type cells, as determined by Northern blotting analysis (Jiang et al., 1999). However, expression of CL synthase was found to be independent of the capacity of cells to synthesize its common substrate and precursor, CDP-DAG.
Current and previous results indicate that both CRD1 gene transcription and CL synthase activity in yeast are insensitive to cross-pathway control by the inositol regulatory system. However, in this study, CRD1 gene expression was increased 2.5-fold in ino4Δ cells compared with wild-type cells. This result differs from a previous study, which reported there were no difference in CRD1 mRNA level between wild-type, ino2Δ and ino4Δ cells (Jiang et al., 1999). The basis for the difference between these two studies is not clear, although the methods of analysis were different. Since the increase in lacZ activity occurred only in ino4Δ cells but not in wild-type or ino2Δ cells, Ino4p may be a negative regulator of CRD1 gene expression. Additionally, expression of lacZ was restored to the wild-type level when INO2 was deleted in ino4Δ cells, suggesting that Ino2p is critical for the elevated expression of the CRD1 gene. Ino2p and Ino4p are members of the basic helix–loop–helix (bHLH) family of DNA-binding proteins that bind the consensus canonical bHLH site 5′-CANNTG-3′, also known as E-box (Hoshizaki et al., 1990; Nikoloff et al., 1992). Binding of Ino2p/Ino4p to UASINO is required for activation of many phospholipid synthetic genes (Hirsch and Henry, 1986; Loewy and Henry, 1984). Heterodimerization between Ino2p and Ino4p through their bHLH domains forms a functional heterodimeric activator in which Ino2p provides two separate domains for transactivation, and its dimerization with Ino4p determines their binding specificity for UASINO (Schwank et al., 1995). Because of the different nature of the INO2 and INO4 gene products, it is possible that they may play separate roles in the regulation of phospholipid metabolism. This was first suggested by Morlock et al. (1988) in the study of regulation of PA phosphatase by inositol in S. cerevisiae. Compared to wild-type cells supplemented with inositol, PA phosphatase activity was reduced in an ino2Δ mutant but was not affected in an ino4Δ mutant. The PIS1 gene is another example in which its expression was increased only in an ino2Δ but not in an ino4Δ cells (Anderson and Lopes, 1996). In addition, the DNA-binding region of Ino2p shares a high homology with the mammalian Myc family of proteins (Nikoloff et al., 1992), which have the ability to form multiple heterodimers with different partners (Amati and Land, 1994). In fact, Ino2p has been implicated in interacting with other factors (Block-Alper et al., 2002). Based on available evidence, the deletion analysis results in Figure 6A may be explained by the following model. In wild-type cells, Ino2p dimerizes preferentially with Ino4p. However, because there is no functional UASINO sequence in the CRD1 promoter, the dimeric activator cannot bind to DNA in the vicinity of the CRD1 gene and, as a result, CRD1 gene expression is not affected. In contrast, in the absence of Ino4p, Ino2p may potentially interact with perhaps another bHLH transcriptional factor. Binding to this factor results in specific binding of the dimeric complex to a currently unidentified site between the UASINO-like element and the TATA site, activating downstream gene transcription. In the absence of Ino2p, such as in ino2Δ and ino2Δ/ino4Δ cells, or in the absence of the cis-acting element, the DNA–dimeric activator complex cannot be formed and therefore a high level of transcription cannot be induced. The DNA gel mobility shift assay with the CRD1 promoter and yeast lysates supports this hypothesis by showing complex formation predominately in ino4Δ cells. Our observation coupled with the above results for PIS gene expression and PA phosphatase levels suggests a more widespread and new role for INO2 and/or INO4 in regulation of phospholipid biosynthesis in yeast.
Acknowledgments
This work was supported by NIH grant GM56389 to W.D.
References
- Amati B, Land H. Myc–Max–Mad: a transcription factor network controlling cell cycle progression, differentiation and death. Curr Opin Genet Dev. 1994;4:102–108. doi: 10.1016/0959-437x(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Lopes JM. Carbon source regulation of PIS1 gene expression in Saccharomyces cerevisiae involves the MCM1 gene and the two-component regulatory gene, SLN1. J Biol Chem. 1996;271:26 596–26 601. doi: 10.1074/jbc.271.43.26596. [DOI] [PubMed] [Google Scholar]
- Ardail D, Lerme F, Louisot P. Involvement of contact sites in phosphatidylserine import into liver mitochondria. J Biol Chem. 1991;266:7978–7981. [PubMed] [Google Scholar]
- Bailis AM, Poole MA, Carman GM, Henry SA. The membrane-associated enzyme phosphatidylserine synthase is regulated at the level of mRNA abundance. Mol Cell Biol. 1987;7:167–176. doi: 10.1128/mcb.7.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli D, Bellei M, Arrigoni-Martelli E, Muscatello U, Bobyleva V. Interaction of carnitine with mitochondrial cardiolipin. Biochim Biophys Acta. 1992;1117:33–36. doi: 10.1016/0304-4165(92)90158-q. [DOI] [PubMed] [Google Scholar]
- Biswas SB, Biswas EE. ARS binding factor I of the yeast Saccharomyces cerevisiae binds to sequences in telomeric and nontelomeric autonomously replicating sequences. Mol Cell Biol. 1990;10:810–815. doi: 10.1128/mcb.10.2.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block-Alper L, Webster P, Zhou X, et al. IN02, a positive regulator of lipid biosynthesis, is essential for the formation of inducible membranes in yeast. Mol Biol Cell. 2002;13:40–51. doi: 10.1091/mbc.01-07-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman GM, Henry SA. Phospholipid biosynthesis in yeast. Annu Rev Biochem. 1989;58:635–669. doi: 10.1146/annurev.bi.58.070189.003223. [DOI] [PubMed] [Google Scholar]
- Chang SC, Heacock PN, Clancey CJ, Dowhan W. The PEL1 gene (renamed PGS1) encodes the phosphatidylglycerol-phosphate synthase of Saccharomyces cerevisiae. J Biol Chem. 1998a;273:9829–9836. doi: 10.1074/jbc.273.16.9829. [DOI] [PubMed] [Google Scholar]
- Chang SC, Heacock PN, Mileykovskaya E, Voelker DR, Dowhan W. Isolation and characterization of the gene (CLS1) encoding cardiolipin synthase in Saccharomyces cerevisiae. J Biol Chem. 1998b;273:14 933–14 941. doi: 10.1074/jbc.273.24.14933. [DOI] [PubMed] [Google Scholar]
- Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–534. 536–537. [PubMed] [Google Scholar]
- Chupin V, Leenhouts JM, de Kroon AI, de Kruijff B. Cardiolipin modulates the secondary structure of the presequence peptide of cytochrome oxidase subunit IV: a 2D 1H-NMR study. FEBS Lett. 1995;373:239–244. doi: 10.1016/0014-5793(95)01054-i. [DOI] [PubMed] [Google Scholar]
- Eble KS, Coleman WB, Hantgan RR, Cunningham CC. Tightly associated cardiolipin in the bovine heart mitochondrial ATP synthase as analyzed by 31P nuclear magnetic resonance spectroscopy. J Biol Chem. 1990;265:19 434–19 440. [PubMed] [Google Scholar]
- Eilers M, Endo T, Schatz G. Adriamycin, a drug interacting with acidic phospholipids, blocks import of precursor proteins by isolated yeast mitochondria. J Biol Chem. 1989;264:2945–2950. [PubMed] [Google Scholar]
- Endo T, Eilers M, Schatz G. Binding of a tightly folded artificial mitochondrial precursor protein to the mitochondrial outer membrane involves a lipid-mediated conformational change. J Biol Chem. 1989;264:2951–2956. [PubMed] [Google Scholar]
- Esposti MD. Lipids, cardiolipin and apoptosis: a greasy licence to kill. Cell Death Diff. 2002;9:234–236. doi: 10.1038/sj.cdd.4400997. [DOI] [PubMed] [Google Scholar]
- Frohman MA, Dush MK, Martin GR. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA. 1988;85:8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry M, Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981;256:1874–1880. [PubMed] [Google Scholar]
- Gallet PF, Petit JM, Maftah A, Zachowski A, Julien R. Asymmetrical distribution of cardiolipin in yeast inner mitochondrial membrane triggered by carbon catabolite repression. Biochem J. 1997;324:627–634. doi: 10.1042/bj3240627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor PM, Hubbell S, Schmidt AJ, et al. Regulation of phosphatidylglycerolphosphate synthase in Saccharomyces cerevisiae by factors affecting mitochondrial development. J Bacteriol. 1991;173:6124–6131. doi: 10.1128/jb.173.19.6124-6131.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohil VM, Hayes P, Matsuyama S, et al. Cardiolipin biosynthesis and mitochondrial respiratory chain function are interdependent. J Biol Chem. 2004;279:42 612–42 618. doi: 10.1074/jbc.M402545200. [DOI] [PubMed] [Google Scholar]
- Greenberg ML, Hubbell S, Lam C. Inositol regulates phosphatidylglycerolphosphate synthase expression in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:4773–4779. doi: 10.1128/mcb.8.11.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ML, Lopes JM. Genetic regulation of phospholipid biosynthesis in Saccharomyces cerevisiae. Microbiol Rev. 1996;60:1–20. doi: 10.1128/mr.60.1.1-20.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines TH, Dencher NA. Cardiolipin: a proton trap for oxidative phosphorylation. FEBS Lett. 2002;528:35–39. doi: 10.1016/s0014-5793(02)03292-1. [DOI] [PubMed] [Google Scholar]
- Hajek P, Bedwell DM. Characterization of the mitochondrial binding and import properties of purified yeast F1-ATPase beta subunit precursor. Import requires external ATP. J Biol Chem. 1994;269:7192–7200. [PubMed] [Google Scholar]
- Hatch GM. Regulation of cardiolipin biosynthesis in the heart. Mol Cell Biol. 1996;159:139–148. doi: 10.1007/BF00420916. [DOI] [PubMed] [Google Scholar]
- He Q, Greenberg ML. Post-translational regulation of phosphatidylglycerolphosphate synthase in response to inositol. Mol Microbiol. 2004;53:1243–1249. doi: 10.1111/j.1365-2958.2004.04202.x. [DOI] [PubMed] [Google Scholar]
- Hirsch JP, Henry SA. Expression of the Saccharomyces cerevisiae inositol-1-phosphate synthase (INO1) gene is regulated by factors that affect phospholipid synthesis. Mol Cell Biol. 1986;6:3320–3328. doi: 10.1128/mcb.6.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Stockl A, Schlame M, Beyer K, Klingenberg M. The reconstituted ADP/ATP carrier activity has an absolute requirement for cardiolipin as shown in cysteine mutants. J Biol Chem. 1994;269:1940–1944. [PubMed] [Google Scholar]
- Hoshizaki DK, Hill JE, Henry SA. The Saccharomyces cerevisiae INO4 gene encodes a small, highly basic protein required for derepression of phospholipid biosynthetic enzymes. J Biol Chem. 1990;265:4736–4745. [PubMed] [Google Scholar]
- Jakovcic S, Getz GS, Rabinowitz M, Jakob H, Swift H. Cardiolipin content of wild-type and mutant yeasts in relation to mitochondrial function and development. J Cell Biol. 1971;48:490–502. doi: 10.1083/jcb.48.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janitor M, Subik J. Molecular cloning of the PEL1 gene of Saccharomyces cerevisiae that is essential for the viability of petite mutants. Curr Genet. 1993;24:307–312. doi: 10.1007/BF00336781. [DOI] [PubMed] [Google Scholar]
- Jiang F, Gu Z, Granger JM, Greenberg ML. Cardiolipin synthase expression is essential for growth at elevated temperature and is regulated by factors affecting mitochondrial development. Mol Microbiol. 1999;31:373–379. doi: 10.1046/j.1365-2958.1999.01181.x. [DOI] [PubMed] [Google Scholar]
- Jiang F, Rizavi HS, Greenberg ML. Cardiolipin is not essential for the growth of Saccharomyces cerevisiae on fermentable or non-fermentable carbon sources. Mol Microbiol. 1997;26:481–491. doi: 10.1046/j.1365-2958.1997.5841950.x. [DOI] [PubMed] [Google Scholar]
- Kelly BL, Greenberg ML. Characterization and regulation of phosphatidylglycerolphosphate phosphatase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1990;1046:144–150. doi: 10.1016/0005-2760(90)90181-v. [DOI] [PubMed] [Google Scholar]
- Kirkland RA, Adibhatla RM, Hatcher JF, Franklin JL. Loss of cardiolipin and mitochondria during programmed neuronal death: evidence of a role for lipid peroxidation and autophagy. Neuroscience. 2002;115:587–602. doi: 10.1016/s0306-4522(02)00512-2. [DOI] [PubMed] [Google Scholar]
- Koshkin V, Greenberg ML. Oxidative phosphorylation in cardiolipin-lacking yeast mitochondria. Biochem J. 2000;347:687–691. [PMC free article] [PubMed] [Google Scholar]
- Kriska T, Korytowski W, Girotti AW. Role of mitochondrial cardiolipin peroxidation in apoptotic photokilling of 5-aminolevulinate-treated tumor cells. Arch Biochem Biophys. 2005;433:435–446. doi: 10.1016/j.abb.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Lange C, Nett JH, Trumpower BL, Hunte C. Specific roles of protein–phospholipid interactions in the yeast cytochrome bc1 complex structure. EMBO J. 2001;20:6591–6600. doi: 10.1093/emboj/20.23.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy BS, Henry SA. The INO2 and INO4 loci of Saccharomyces cerevisiae are pleiotropic regulatory genes. Mol Cell Biol. 1984;4:2479–2485. doi: 10.1128/mcb.4.11.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw P, Henry SA. Mutations in the Saccharomyces cerevisiae opi3 gene: effects on phospholipid methylation, growth and cross-pathway regulation of inositol synthesis. Genetics. 1989;122:317–330. doi: 10.1093/genetics/122.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin JB, Dowhan W. Cardiolipin and apoptosis. Biochim Biophys Acta. 2002;1585:97–107. doi: 10.1016/s1388-1981(02)00329-3. [DOI] [PubMed] [Google Scholar]
- Mende P, Huther FJ, Kadenbach B. Specific and reversible activation and inactivation of the mitochondrial phosphate carrier by cardiolipin and nonionic detergents, respectively. FEBS Lett. 1983;158:331–334. doi: 10.1016/0014-5793(83)80607-3. [DOI] [PubMed] [Google Scholar]
- Morlock KR, Lin YP, Carman GM. Regulation of phosphatidate phosphatase activity by inositol in Saccharomyces cerevisiae. J Bacteriol. 1988;170:3561–3566. doi: 10.1128/jb.170.8.3561-3566.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y. Initiation of apoptotic signal by the peroxidation of cardiolipin of mitochondria. Ann N Y Acad Sci. 2004;1011:177–184. doi: 10.1007/978-3-662-41088-2_18. [DOI] [PubMed] [Google Scholar]
- Nikoloff DM, McGraw P, Henry SA. The INO2 gene of Saccharomyces cerevisiae encodes a helix–loop–helix protein that is required for activation of phospholipid synthesis. Nucleic Acids Res. 1992;20:3253. doi: 10.1093/nar/20.12.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander DB, Zhang M, Mileykovskaya E, Rho M, Dowhan W. Lack of mitochondrial anionic phospholipids causes an inhibition of translation of protein components of the electron transport chain. A yeast genetic model system for the study of anionic phospholipid function in mitochondria. J Biol Chem. 2001;276:25 262–25 272. doi: 10.1074/jbc.M103689200. [DOI] [PubMed] [Google Scholar]
- Paradies G, Petrosillo G, Ruggiero FM. Cardiolipin-dependent decrease of cytochrome c oxidase activity in heart mitochondria from hypothyroid rats. Biochim Biophys Acta. 1997;1319:5–8. doi: 10.1016/s0005-2728(97)00012-1. [DOI] [PubMed] [Google Scholar]
- Perlman PS, Mahler HR. Derepression of mitochondria and their enzymes in yeast: regulatory aspects. Arch Biochem Biophys. 1974;162:248–271. doi: 10.1016/0003-9861(74)90125-8. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Ruggiero FM, Di Venosa N, Paradies G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FA S E B J. 2003;17:714–716. doi: 10.1096/fj.02-0729fje. [DOI] [PubMed] [Google Scholar]
- Robinson NC. Functional binding of cardiolipin to cytochrome c oxidase. J Bioenerg Biomembr. 1993;25:153–163. doi: 10.1007/BF00762857. [DOI] [PubMed] [Google Scholar]
- Robinson NC, Zborowski J, Talbert LH. Cardiolipin-depleted bovine heart cytochrome c oxidase: binding stoichiometry and affinity for cardiolipin derivatives. Biochemistry. 1990;29:8962–8969. doi: 10.1021/bi00490a012. [DOI] [PubMed] [Google Scholar]
- Rusnak A, Mangat R, Xu F, McClarty G, Hatch GM. Cardiolipin remodeling in a Chinese hamster lung fibroblast cell line deficient in oxidative energy production. J Bioenerg Biomembr. 1997;29:291–298. doi: 10.1023/a:1022418328922. [DOI] [PubMed] [Google Scholar]
- Schwank S, Ebbert R, Rautenstrauss K, Schweizer E, Schuller HJ. Yeast transcriptional activator INO2 interacts as an Ino2p/Ino4p basic helix–loop–helix heteromeric complex with the inositol/choline-responsive element necessary for expression of phospholipid biosynthetic genes in Saccharomyces cerevisiae. Nucleic Acids Res. 1995;23:230–237. doi: 10.1093/nar/23.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Dowhan W. Regulation of phospholipid biosynthetic enzymes by the level of CDP-diacylglycerol synthase activity. J Biol Chem. 1997;272:11 215–11 220. doi: 10.1074/jbc.272.17.11215. [DOI] [PubMed] [Google Scholar]
- Shen H, Dowhan W. Regulation of phosphatidylglycerophosphate synthase levels in Saccharomyces cerevisiae. J Biol Chem. 1998;273:11 638–11 642. doi: 10.1074/jbc.273.19.11638. [DOI] [PubMed] [Google Scholar]
- Shen H, Heacock PN, Clancey CJ, Dowhan W. The CDS1 gene encoding CDP-diacylglycerol synthase in Saccharomyces cerevisiae is essential for cell growth. J Biol Chem. 1996;271:789–795. doi: 10.1074/jbc.271.2.789. [DOI] [PubMed] [Google Scholar]
- Shiao YJ, Lupo G, Vance JE. Evidence that phosphatidylserine is imported into mitochondria via a mitochondria-associated membrane and that the majority of mitochondrial phosphatidylethanolamine is derived from decarboxylation of phosphatidylserine. J Biol Chem. 1995;270:11 190–11 198. doi: 10.1074/jbc.270.19.11190. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Dowhan W. Translational regulation of nuclear gene COX4 expression by mitochondrial content of phosphatidylglycerol and cardiolipin in Saccharomyces cerevisiae. Mol Cell Biol. 2005 doi: 10.1128/MCB.26.3.743-753.2006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai KT, Greenberg ML. Biochemical characterization and regulation of cardiolipin synthase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1990;1046:214–222. doi: 10.1016/0005-2760(90)90192-z. [DOI] [PubMed] [Google Scholar]
- Tuller G, Hrastnik C, Achleitner G, et al. YDL142c encodes cardiolipin synthase (Cls1p) and is non-essential for aerobic growth of Saccharomyces cerevisiae. FEBS Lett. 1998;421:15–18. doi: 10.1016/s0014-5793(97)01525-1. [DOI] [PubMed] [Google Scholar]
- Van Loon APGM, Van Eijk E, Grivell LA. Biosynthesis of the ubiquinol–cytochrome c reductase complex in yeast. Discoordinate synthesis of the 11 kDa subunit in response to increased gene copy number. EMBO J. 1983;2:1765–1770. doi: 10.1002/j.1460-2075.1983.tb01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem. 2002;277:43 553–43 556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mileykovskaya E, Dowhan W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J Biol Chem. 2005;280:29 403–29 408. doi: 10.1074/jbc.M504955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Su X, Mileykovskaya E, Amoscato AA, Dowhan W. Cardiolipin is not required to maintain mitochondrial DNA stability or cell viability for Saccharomyces cerevisiae grown at elevated temperatures. J Biol Chem. 2003;278:35 204–35 210. doi: 10.1074/jbc.M306729200. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Gvozdenovic-Jeremic J, Webster P, Zhou J, Greenberg ML. Loss of function of KRE5 suppresses temperature sensitivity of mutants lacking mitochondrial anionic lipids. Mol Biol Cell. 2005;16:665–675. doi: 10.1091/mbc.E04-09-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]