Abstract
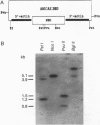
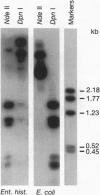
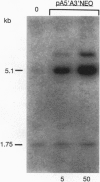
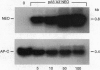
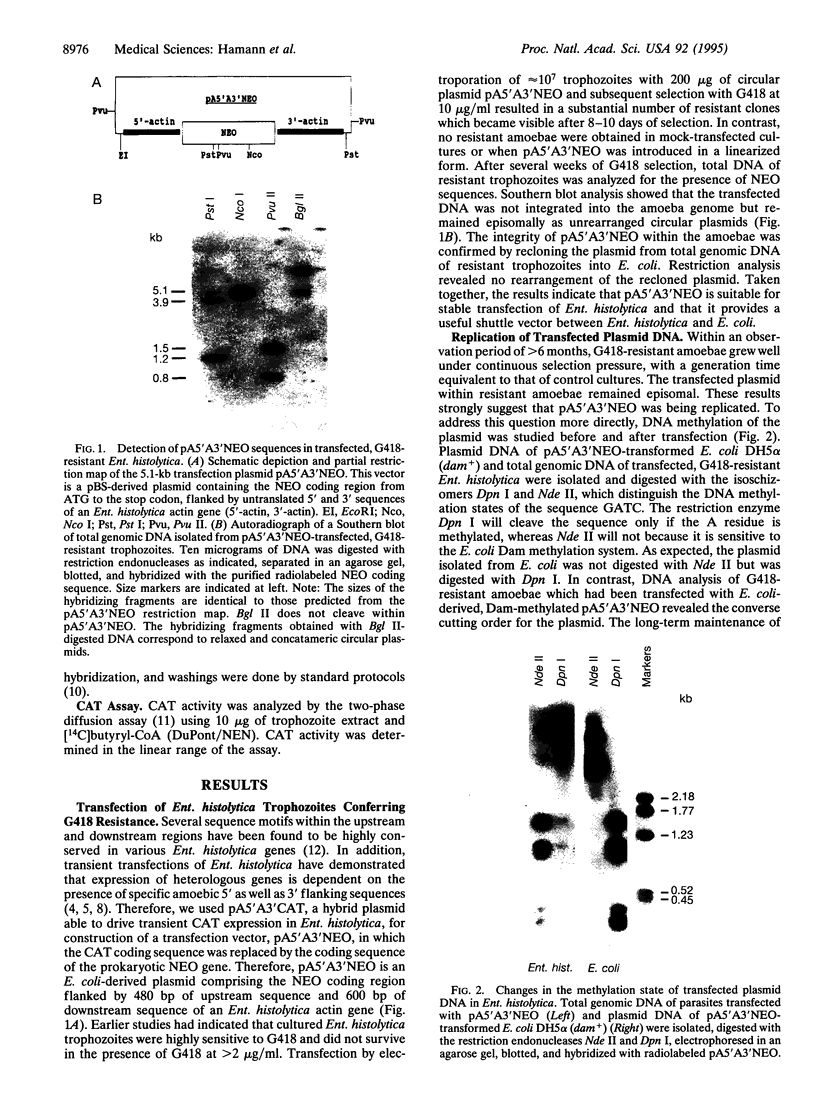
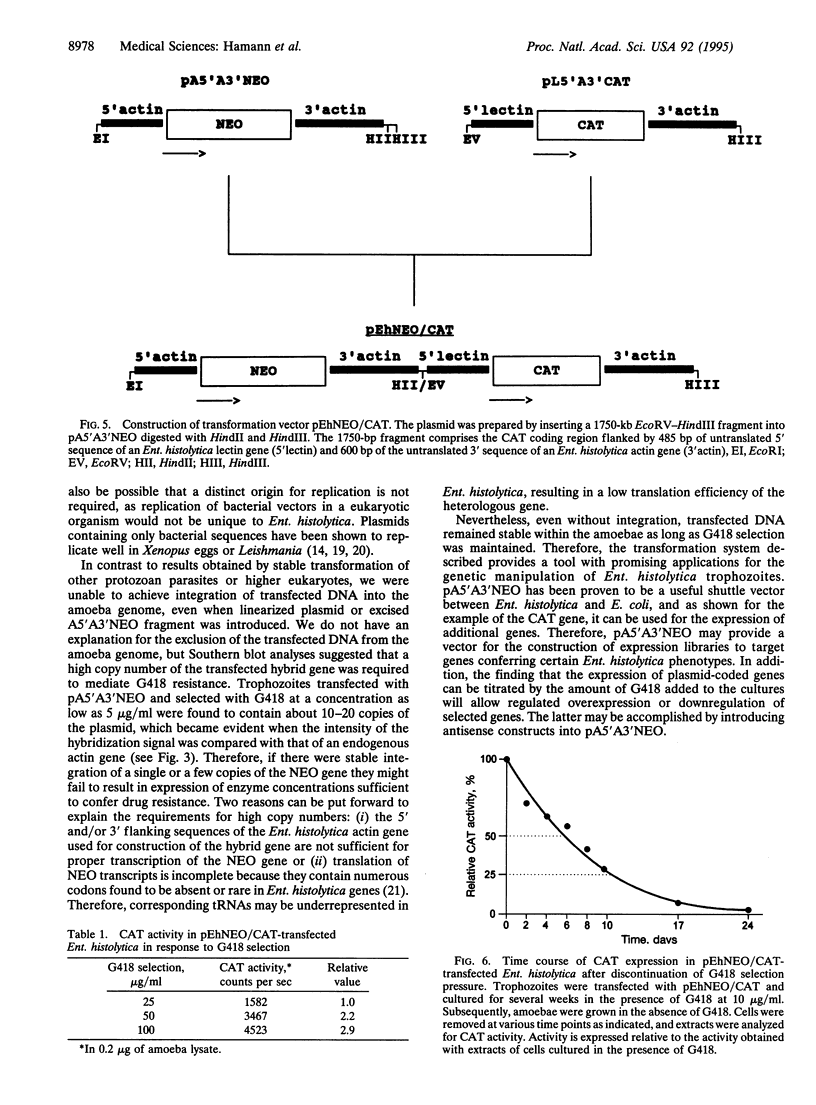
To provide tools for functional molecular genetics of the protozoan parasite Entamoeba histolytica, we investigated the use of the prokaryotic neomycin phosphotransferase (NEO) gene as a selectable marker for the transfection of the parasite. An Escherichia coli-derived plasmid vector was constructed (pA5'A3'NEO) containing the NEO coding region flanked by untranslated 5' and 3' sequences of an Ent. histolytica actin gene. Preceding experiments had revealed that amoebae are highly sensitive to the neomycin analogue G418 and do not survive in the presence of as little as 2 micrograms/ml. Transfection of circular pA5'A3'NEO via electroporation resulted in Ent. histolytica trophozoites resistant to G418 up to 100 micrograms/ml. DNA and RNA analyses of resistant cells indicated that (i) the transfected DNA was not integrated into the amoeba genome but was segregated episomally, (ii) in the amoebae, the plasmid replicated autonomously, (iii) the copy number of the plasmid and the expression of NEO-specific RNA were proportional to the amount of G418 used for selection, and (iv) under continuous selection, the plasmid was propagated over an observation period of 6 months. Moreover, the plasmid could be recloned into E. coli and was found to be unrearranged. To investigate the use of pA5'A3'NEO to coexpress other genes in Ent. histolytica, a second marker, the prokaryotic chloramphenicol acetyltransferase (CAT) gene under control of an Ent. histolytica lectin gene promoter was introduced into the plasmid. Transfection of the amoebae with this construct also conferred G418 resistance and, in addition, allowed continuous expression of CAT activity in quantities corresponding to the amount of G418 used for selection. When selection was discontinued, transfected plasmids were lost as indicated by an exponential decline of CAT activity in trophozoite extracts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruchhaus I., Leippe M., Lioutas C., Tannich E. Unusual gene organization in the protozoan parasite Entamoeba histolytica. DNA Cell Biol. 1993 Dec;12(10):925–933. doi: 10.1089/dna.1993.12.925. [DOI] [PubMed] [Google Scholar]
- Dhar S. K., Choudhury N. R., Bhattacharaya A., Bhattacharya S. A multitude of circular DNAs exist in the nucleus of Entamoeba histolytica. Mol Biochem Parasitol. 1995 Mar;70(1-2):203–206. doi: 10.1016/0166-6851(95)00008-o. [DOI] [PubMed] [Google Scholar]
- Diamond L. S., Harlow D. R., Cunnick C. C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Horstmann R. D., Leippe M., Tannich E. Recent progress in the molecular biology of Entamoeba histolytica. Trop Med Parasitol. 1992 Dec;43(4):213–218. [PubMed] [Google Scholar]
- Huber M., Koller B., Gitler C., Mirelman D., Revel M., Rozenblatt S., Garfinkel L. Entamoeba histolytica ribosomal RNA genes are carried on palindromic circular DNA molecules. Mol Biochem Parasitol. 1989 Jan 15;32(2-3):285–296. doi: 10.1016/0166-6851(89)90077-7. [DOI] [PubMed] [Google Scholar]
- Hyrien O., Méchali M. Plasmid replication in Xenopus eggs and egg extracts: a 2D gel electrophoretic analysis. Nucleic Acids Res. 1992 Apr 11;20(7):1463–1469. doi: 10.1093/nar/20.7.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioutas C., Schmetz C., Tannich E. Identification of various circular DNA molecules in Entamoeba histolytica. Exp Parasitol. 1995 Mar;80(2):349–352. doi: 10.1006/expr.1995.1045. [DOI] [PubMed] [Google Scholar]
- Mahbubani H. M., Paull T., Elder J. K., Blow J. J. DNA replication initiates at multiple sites on plasmid DNA in Xenopus egg extracts. Nucleic Acids Res. 1992 Apr 11;20(7):1457–1462. doi: 10.1093/nar/20.7.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel R., Tannich E. Transfection and transient expression of chloramphenicol acetyltransferase gene in the protozoan parasite Entamoeba histolytica. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7095–7098. doi: 10.1073/pnas.91.15.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou B., Roy G., Ouellette M. Autonomous replication of bacterial DNA plasmid oligomers in Leishmania. Mol Biochem Parasitol. 1994 May;65(1):39–49. doi: 10.1016/0166-6851(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Patnaik P. K., Kulkarni S. K., Cross G. A. Autonomously replicating single-copy episomes in Trypanosoma brucei show unusual stability. EMBO J. 1993 Jun;12(6):2529–2538. doi: 10.1002/j.1460-2075.1993.tb05908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy J. E., Mann B. J., Pho L. T., Petri W. A., Jr Transient transfection of the enteric parasite Entamoeba histolytica and expression of firefly luciferase. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7099–7103. doi: 10.1073/pnas.91.15.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I. Immunobiology of human infection by Entamoeba histolytica. Pathol Immunopathol Res. 1989;8(3-4):179–205. doi: 10.1159/000157148. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tannich E., Horstmann R. D. Codon usage in pathogenic Entamoeba histolytica. J Mol Evol. 1992 Mar;34(3):272–273. doi: 10.1007/BF00162976. [DOI] [PubMed] [Google Scholar]
- Tannich E., Horstmann R. D., Knobloch J., Arnold H. H. Genomic DNA differences between pathogenic and nonpathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5118–5122. doi: 10.1073/pnas.86.13.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. A. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986 Mar-Apr;8(2):228–238. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]