Abstract
Injury to lung epithelial cells has a role in multiple lung diseases. We previously identified mitsugumin 53 (MG53) as a component of the cell membrane repair machinery in striated muscle cells. Here we show that MG53 also has a physiological role in the lung and may be used as a treatment in animal models of acute lung injury. Mice lacking MG53 show increased susceptibility to ischemia-reperfusion and over-ventilation induced injury to the lung when compared with wild type mice. Extracellular application of recombinant human MG53 (rhMG53) protein protects cultured lung epithelial cells against anoxia/reoxygenation-induced injuries. Intravenous delivery or inhalation of rhMG53 reduces symptoms in rodent models of acute lung injury and emphysema. Repetitive administration of rhMG53 improves pulmonary structure associated with chronic lung injury in mice. Our data indicate a physiological function for MG53 in the lung and suggest that targeting membrane repair may be an effective means for treatment or prevention of lung diseases.
INTRODUCTION
Living cells in most organs in the human body, including the skin, gastrointestinal tract, striated muscles and lungs are subjected to constant mechanical stress. Plasma membrane disruptions often occur as a result, causing release of intracellular contents and inflammatory mediators and leading to disruption of cellular function and even cell death. In response to loss of cells by these insults, tissues can compensate either by proliferation to replace injured cells or minimize the death of individual cells through repair mechanisms to restore the integrity of cell membrane1-4. Such repair mechanisms are of particular importance to cells with low proliferative capacity. Our previous studies identified MG53, a member of the TRIM family protein, as an essential component of the cell membrane repair machinery in striated muscles5-11. Native MG53 functions in vesicle trafficking and allows for nucleation of intracellular vesicles at sites of membrane disruption5. Knockout mice for mg53 (mg53−/−) display defective membrane repair in striated muscles that leads to progressive skeletal myopathy and increased vulnerability of cardiomyocytes to membrane injury following ischemia reperfusion injury5, 7, 9, 10.
Injury to lung epithelial cells has been implicated in the pathogenesis and progression of multiple lung diseases12, 13. Ischemia-reperfusion (I/R) induced lung injury is a common cause of morbidity and mortality under a wide range of medical conditions, such as lung and/or heart organ transplantation, acute pulmonary embolism, pulmonary thrombosis and cardiopulmonary bypass surgery14-17. Various pathological stresses such as pneumonia, sepsis, trauma and blood transfusion can also result in acute lung injury (ALI) and acute respiratory distress syndrome12, 18. The major clinical manifestations of ALI, including inflammatory cell infiltration, arterial hypoxemia and pulmonary edema, are direct consequences of the disrupted airway and alveolar barrier function. Thus, effective ALI treatment requires resolution of alveolar edema and restoration of epithelial and endothelial barrier integrity. A few approaches for ALI have been developed to address these aspects, including low tidal volume ventilation and fluid-conservative therapy and anti-inflammation13. However, because of the limited knowledge of the cell biology for membrane repair in lung physiology, direct therapeutic approaches for ALI that target membrane repair in airway and alveolar epithelial cells has not been well developed.
While our previously published studies have focused on the cell membrane repair function for MG53 in skeletal and cardiac muscles, here we present evidence that MG53 is also expressed in alveolar cells of the lung tissue. Compared with wild type mice, mg53−/− mice show increased susceptibility to damage following I/R injury and over-ventilation of the lung. We also tested the therapeutic effect of the recombinant human MG53 (rhMG53) protein19 in treating damage to the lung, using in vitro and in vivo models of acute and chronic lung injury. Our data suggest that targeting MG53 function could represent an effective means for restoration of barrier function and integrity of the airway and alveolar epithelial cells during ALI.
RESULTS
MG53 protein is expressed in lung tissue
The mg53 gene was originally cloned from skeletal muscle using an immuno-proteomics approach20. Biochemical studies showed that MG53 protein is enriched in striated muscles5, 7, 21. Here we tested whether MG53 protein is also expressed in the lung. Western blot showed that MG53 could only be detected in lysates of lung tissue derived from the wild type mice, but not in the mg53−/− lung homogenate (Fig. 1A and Supplementary Fig. 1). Quantitative assessment revealed that the level of MG53 protein in the lung tissue is approximately 5% of that in skeletal muscle.
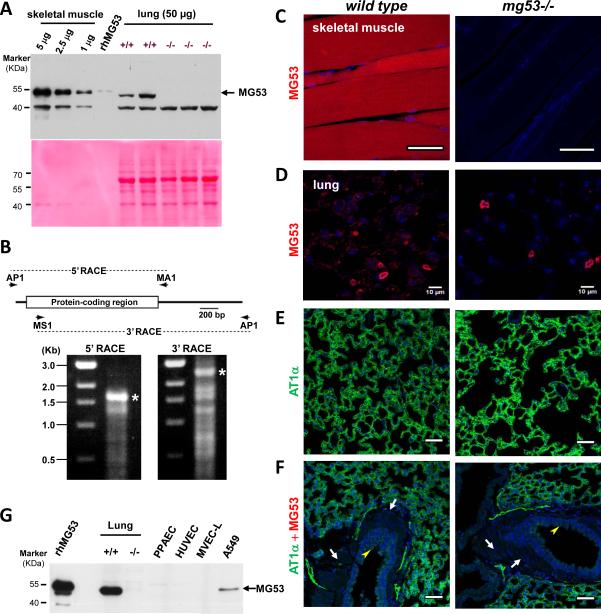
Figure 1.
Expression of MG53 in lung tissue. A. Homogenates of lung tissue derived from the wild type and mg53−/− mice were used for western blot for detection of MG53. 0.1 ng rhMG53 was used as positive control. For comparative purpose, the content of MG53 in skeletal muscle was assayed at different concentrations of muscle tissues. Ponceau S staining reveals differential loading of the skeletal muscle and lung tissues. A nonspecific 40 kDa protein was also recognized by our custom-made anti-MG53 antibody. The uncropped western blots images are shown on Supplementary Fig 1. B. Characterization of lung mg53 transcripts by RACE cDNA amplification. The cDNA amplification strategy is illustrated in the upper panel. A mouse lung cDNA preparation was amplified using AP1 and MA1 primers in the 5’-RACE reaction or using MS1 and AP1 primers in the 3’-RACE reaction. Amplified cDNA products in each RACE reaction were analyzed in agarose electrophoresis as shown in the lower panel. Putative full-length cDNAs marked with asterisks were extracted from agarose gels and subcloned into a plasmid vector for sequencing. The protein-coding sequence of the amplified lung mg53 cDNAs was identical to that of muscle mg53 cDNAs determined in our previous study. C. IHC staining with an anti-MG53 antibody revealed high level of MG53 in wild type skeletal muscle, which is absent in the mg53−/−muscle. D-F. IHC staining of lung tissues derived from wild type (left panels) or mg53−/− mice (right panels). Compared with skeletal muscle, low level of MG53 could be detected in lung (D). Background staining in the mg53−/− lung likely reflects auto fluorescence of capillary cells or non-specific activity of the anti-MG53 antibody (see the 40 kDa band in panel A). The MG53 expression pattern in wild type lung matches to that of AT1α, a type I alveolar cell marker (E). The MG53 and AT1α (type 1a angiotensin II receptor) (anti-AT1α, Novus Biologicals NB600-1015) stainings (D and E) were shown separately to better indicate the localization of MG53 due to its low expression in alveolar cells. The cross section of bronchioles revealed negative staining for MG53 in both airway smooth muscle layer (arrow) and the neighboring ciliated endothelial lining of the airway lumen (arrow head) (F). All scale bars represent 50 μm. G. Immunoblotting of MG53 with lysates from mouse lung tissues, and cells derived from A549, PPAEC, MVEC-L and HUVEC (50 μg/lane). 1 ng rhMG53 served as a positive control for the immunoblotting.
We consistently observed a slight mobility shift of MG53 from the lung tissue compared with that in the muscle tissue and the rhMG53 protein obtained from E. coli fermentation19 (Fig. 1A). To investigate whether this is due to splice variation among different tissues, Rapid amplification of cDNA end (RACE) analysis was performed with a mouse lung cDNA library. Both 5’-RACE and 3’-RACE produced cDNA coding sequences for MG53 (Fig. 1B). Sequencing of multiple clones from the 5’-RACE and 3’-RACE reactions revealed identical mg53 sequences in lung and muscle tissue (Supplementary Table 1). These studies indicate that the full length mRNA for MG53 is expressed in the lung, thus potential posttranslational modification/processing mechanisms may be responsible for the mobility shift for MG53 observed in the lung tissue. Further studies will be required to understand this mechanism.
Immunohistochemical (IHC) staining with lung tissues derived from wild type and mg53−/− mice was used to probe the cell-type specific expression for MG53 in the lung. As shown in Fig. 1C, high level expression of MG53 was detected from the wild type skeletal muscle but not from the mg53−/− skeletal muscle. Compared with skeletal muscle, lower level expression of MG53 was present in the alveolar epithelial cells (Fig. 1D). The distribution of MG53 was similar to that of AT1α, a specific cell marker for type I alveolar epithelial cells (Fig. 1E). These histological studies also indicated that MG53 is absent from smooth muscle and vascular endothelial cells (Fig. 1F). Because of the difficulty of isolating primary cultured type I and type II alveolar epithelial cells from mice, we performed immunoblot with cultured adenocarcinoma human type II alveolar epithelial cells (A549), human lung microvascular endothelial cells (MVEC-L), human pulmonary artery endothelial cells (PPAEC) and human umbilical vein endothelial cells (HUVEC). As shown in Fig. 1G, endogenous MG53 could be detected in A549 cells, but not in endothelial cells. In a small portion of IHC staining with the pulmonary vessel wall, we observed a strong striation pattern of MG53 signal which is present in the wild type lung and absent from the mg53−/− lung (see Supplementary Fig. 2). Such MG53 signal likely originated from potential lung-resident cardiomyocytes, as a recent report by Krachlauer et al showed that the mouse and rat lung tissues contain unique patterns of venous cardiomyocytes22. Taken together, our data suggest that MG53 is present in both type I and type II alveolar epithelial cells, but absent from endothelial cells.
Knockout of MG53 exacerbates stress-induced lung injury
The presence of MG53 in the lung tissue suggests the possibility of a lung phenotype in the mg53−/−mice. Histological analysis revealed pathological changes with lung derived from the mg53−/− mice under normal conditions. Hematoxylin-Eosin (HE) staining showed enlarged alveolar space with the mg53−/−lung when compared with the wild type littermates (Fig. 2A). This finding is consistent with the expression of MG53 in the alveolar epithelial cells, as the absence of MG53 may lead to defective alveolar structure in the mutant mice.
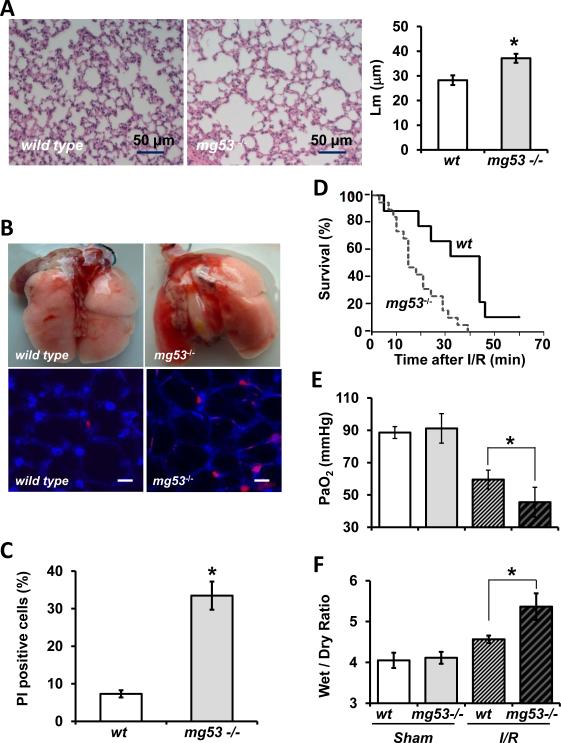
Figure 2.
Increased susceptibility of the mg53−/− mice to over-ventilation and I/R induced lung injury. A. H/E staining of lung section derived from wild type and mg53−/− mice under basal conditions. Mean linear intercept measurements (Lm) of alveolar spaces reveal significant difference between the wild type and mg53−/− lung. B. Representative images of wild type and mg53−/− lung subjected to over-ventilation (upper panels). The subpleural lung images of wild type and mg53−/− mice show PI+ alveolus cells after over-ventilation (lower panels). Scale bar = 10 μm. C. Quantification of PI+ cells in the subplueral lung area after over-ventilation (n=4 for wild type; n=5 for mg53−/−, *P<0.05, Student t-test). D. Survival rate of mg53−/− and wild type mice was recorded after I/R. The product limit (Kaplan-Meier) estimate of the cumulative survival was assessed with the log-rank test to evaluate for significant differences in survival (*P<0.05, n=19 for mg53−/− mice and n=9 for wild type mice, Kaplan-Meier survival analysis (mean ± SEM). E. Arterial blood samples were drawn from individual mice, the plasma PaO2 concentrations were measured (*P<0.05 vs. wild type, n=6 in each group, ANOVA, mean ± SEM). F. Lung edema was measured as the wet/dry weight ratio of the excised lung from mice (*P<0.05 vs. wild type, n=5, ANOVA). Animals receiving normal low-tidal ventilation without I/R were used as sham controls.
We evaluated the susceptibility of these animals to over-ventilation induced lung injury. For this purpose, wild type and mg53−/− mice were ventilated at 30 ml/kg (tidal volume/body weight) to introduce acute stress to lung resident cells. Propidium iodide (PI) was injected into the right ventricle immediately after removal of injurious stress for labeling of necrotic cells. Wounded subpleural alveolus resident cells were then visualized by confocal microscopy23, 24. As shown in Fig. 2B, 30-min over ventilation did not produce measurable injury or changes in gross morphology to the wild type lungs. In contrast, the same conditions in mg53−/− lungs resulted in large areas of hemorrhage and atelectasis, indicating increased susceptibility to injury of the lung tissue in the absence of MG53. Confocal imaging showed that a small percentage of alveolus resident cells were labeled by PI in the wild type lung following over-ventilation (Fig. 2B, lower left), which was consistent with previous studies by Gajic et al23. In the mg53−/− mice, the subpleural area of the lung displayed more PI-positive cells (Fig. 2B, lower right), indicating the possibility of defective repair in these cells. Statistical analysis showed that following over-ventilation significantly more PI-positive cells appear in the mg53−/− lung compared to the wild type lung (Fig. 2C).
We also tested whether the absence of MG53 could alter the response of the lung following ischemia/reperfusion (I/R) induced injury. For this purpose, littermates of wild type and mg53−/− mice were subjected to 1 h ischemia/1 h reperfusion by clamping of the left pulmonary artery and hilum to induce complete ischemia and anoxia of left lung. Compared with the wild type controls, the mg53−/−mice had lower survival rate following I/R injury (Fig. 2D). Pulmonary function assessed by measurement of arterial blood gas showed that the PaO2 in wild type mice measured at 59.5 ± 5.8 mmHg, while it dropped to 45.5 ± 9.2 mmHg in mg53−/− mice (n=6 in each, p value < 0.05, ANOVA) after I/R induced lung injury (Fig. 2E). The wet/dry ratio of lungs harvested from wild type mice was 4.56 ± 0.09, whereas that in the mg53−/− mice was 5.37 ± 0.33 (n=6 in each, p < 0.05, ANOVA) (Fig. 2F). These data suggest that systemic ablation of MG53 leads to increased susceptibility of the mice to I/R-induced injury to the lung.
MG53 protects against injury in cultured lung epithelial cells
We used cultured rat lung epithelial (RLE) cells to investigate the extent that MG53 participates in membrane repair of lung resident cells. The RLE cells were transfected with a fusion protein containing the green fluorescent protein (GFP) linked to the amino-terminal end of the mouse MG53 (GFP-MG53). GFP-MG53 in RLE cells localized primarily to the cytosol and plasma membrane (Fig. 3A, left), a subcellular distribution similar to that observed in striated muscle5 and other non-muscle cell types19. In response to injury caused by penetration of a micro-electrode into the plasma membrane, rapid translocation of GFP-MG53 labeled intracellular vesicles toward the acute injury site was observed (Fig. 3A, right). This GFP-MG53 translocation to membrane injury sites in RLE cells is similar to those observed in C2C12, HEK293 and other cell types5, 19.
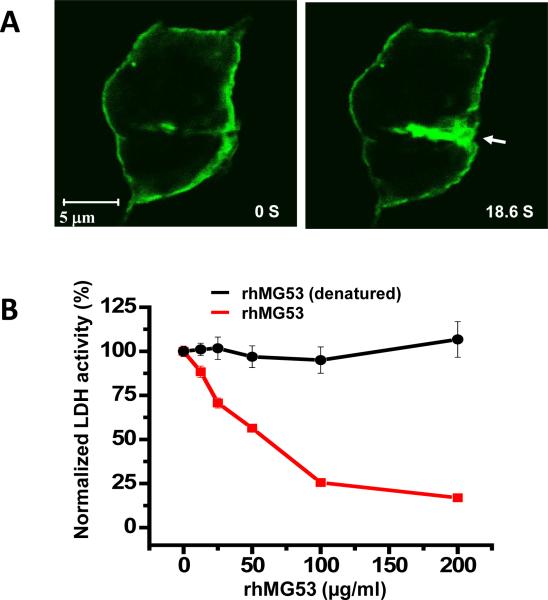
Figure 3.
In vitro assay with MG53 protection against injury to cultured RLE cells. A. RLE cell transfected with GFP-MG53 show rapid translocation of GFP-MG53 containing intracellular vesicles toward the acute plasma membrane injury site following penetration of a microelectrode. Left panel – cell image taken immediately after injury, right pane – image taken 18.6 s after injury. Arrow shows the microelectrode injury site. Visualization of live cell imaging of the GFP-MG53 movement process can be found in Supplemental Movie 1. B. RLE cells were treated with external rhMG53 or boiled rhMG53 (denatured control protein) in the cell culture medium and then exposed to mechanical membrane damage by glass beads. Membrane damage is measured by LDH release from cells. rhMG53 reduced LDH release due to mechanical damage and this protective effect is dose dependent, n=9-12 for each data point (mean ± SEM).
We previously showed that the rhMG53 protein can protect various cell types against cell membrane disruption when applied to the extracellular solution19. Here we examined if rhMG53 was effective in protecting RLE cells from mechanical injuries. As shown in Fig. 3B, application of rhMG53 to the extracellular solution before RLE cells were injured with glass micro-beads8, 11, 19 reduced the amount of intracellular enzyme, lactate dehydrogenase (LDH), released from the cells into the culture medium in a dose dependent fashion. Reduced LDH release from RLE cells indicates that there was decreased membrane disruption. As control, denatured rhMG53 protein proved to be ineffective at minimizing membrane damage in this assay.
Our previous study demonstrated that the membrane repair function for rhMG53 was mediated by interaction with exposed phosphatidylserine at the injury sites19. To test whether this also occurs in the lung epithelial cells, we used the A549 human lung epithelial cells that were subjected to treatment with anoxia/reoxygenation (A/R). As shown in Fig. 4A, in A549 cells without treatment of A/R, the endogenous MG53 protein was mostly distributed in the cytosol (left panel); after A/R treatment more MG53 was distributed at the cell surface membrane (right panel). The exogenously applied rhMG53 could concentrate at the cell surface membrane only in A/R-treated A549 cells but not in untreated cells (Fig. 4B). This membrane localization pattern appeared to be specific for rhMG53, as labeled bovine serum albumin (BSA) applied to the cells could not translocate to the injured membrane of A/R-treated A549 cells. Strong co-localization of labeled rhMG53 (Rhodamine red fluorescence) and Annexin V (FITC green fluorescence) at the surface membrane of A/R treated A549 cells could be detected (Fig. 4C), indicating that the membrane repair effect of rhMG53 in lung epithelial cells is likely mediated through binding to phosphatidylserine at the injured surface membrane.
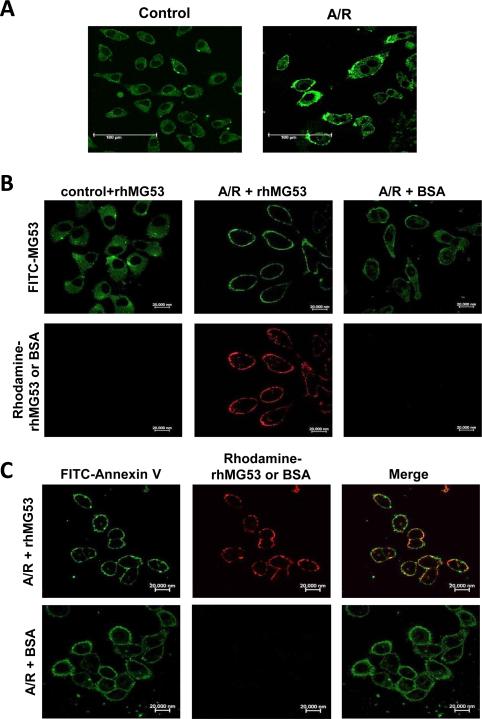
Figure 4.
Localization of MG53 to the surface membrane of A549 cells after treatment with anoxia and reoxygenation (A/R). A. Immunofluorescence staining of endogenous MG53 in A549 cells. Control cells show cytosolic distribution of MG53 and A/R treated cells display membrane localization of MG53. B. Top panels show FITC-staining of endogenous MG53 in control cells (left), A/R treated cells (middle and right). Bottom panels show Rhodamine-labeled rhMG53 added to control cells (left) and A/R treated cells (middle), or Rhodamine-labeled BSA added to A/R treated A549 cells (right). C. Left panels show FITC-labeling of Annexin V in A/R treated A549 cells following incubation with rhMG53 (top) or BSA as control (bottom). Middle panels show Rhodamine-labeling of rhMG53 (top) or lack of Rhodamine-labeling of BSA (bottom) in A/R treated A549 cells. Right panels show colocalization of Annexin V and rhMG53 (top) and lack of colocalization between Annexin V and BSA (bottom) in A/R treated A549 cells. The images shown were representative of > 104 cells in at least four separate experiments.
Intravenous delivery of rhMG53 ameliorates I/R induced lung injury
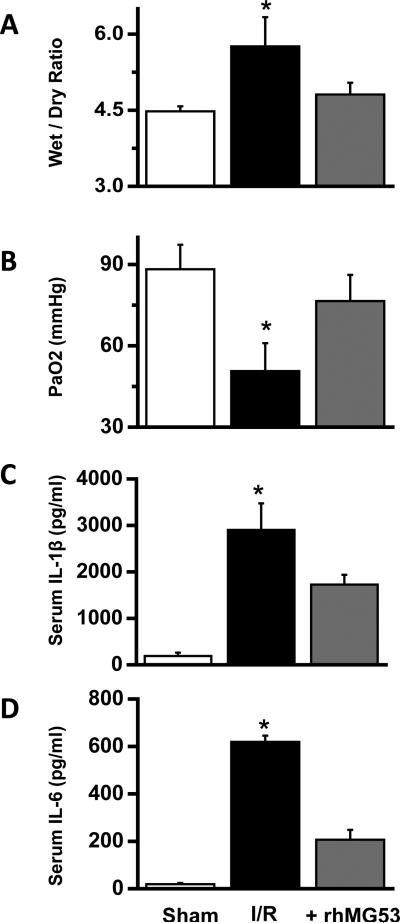
Given the protective effects seen with rhMG53 in RLE and A549 cells, we tested if systemic application of rhMG53 can protect against ischemia-reperfusion (I/R) induced lung injury in vivo. Based on the in vitro assay using rhMG53 in RLE cells (Fig. 3B), we determined a dose of 6 mg rhMG53 protein/kg body weight for experiments with the rat model. This dose was applied by intravenous (IV) injection through the tail vein 30 min before the animals were subjected to I/R induced lung injury. To quantify the effect of rhMG53 on I/R-induced lung edema, the wet/dry ratio was measured (Fig. 5A). Clearly, rhMG53 application produced a significant reduction in edema compared to the control group. Hypoxemia is another pathologic change after lung I/R injury. Arterial blood gas analysis showed that PaO2 was decreased in the I/R-treated group relative to the sham operated group, and application of rhMG53 significantly improved PaO2 function in rats subjected to I/R lung injury (Fig 5B). While the concentration of pro-inflammatory cytokines (IL-1β and IL-6) in the serum were elevated in rats subjected to I/R lung injury, the application of rhMG53 led to significant reduction in these pro-inflammatory factors (Fig. 5C and 5D).
Figure 5.
Protective effect of rhMG53 against I/R induced lung injury in rats. A. Effect of rhMG53 precondition on edema in lung from I/R rats. Lung edema was measured as the wet/dry weight ratio of the excised lung tissue from rats (*P<0.05 vs. others, n=5, ANOVA, mean ± SEM). B. Effect of rhMG53 precondition on gas exchange after I/R. Arterial blood samples were drawn from individual rats, the plasma PaO2 concentrations were measured (*P<0.05 vs. others, n=6 in each group, ANOVA, mean ± SEM). C and D. Effect of rhMG53 precondition on serum IL-1β and IL-6 concentrations in I/R injured SD rats. Serum levels of IL-1β (C) and IL-6 (D) were measured by ELISA (*P<0.05 vs. others, n=5, ANOVA, mean ± SEM).
In Supplementary Fig. 3, we show that the IV injected rhMG53 appeared in the bronchial alveolar lavage fluid (BALF) following lung injury, and rhMG53 protein could associate with both lung alveolar cells and endothelial cells to protect against stress-induced injury to the lung. It is known that edema in ALI is a consequence of increased permeability of the alveolar-capillary barrier, the protective effect of rhMG53 against damage to both alveolar epithelial and endothelial cells could contribute to the improved pulmonary function in animals treated with rhMG53. To support this conclusion, we show in Supplementary Fig. 4 that the levels of IL-1β and IL-6 in the BALF were significantly reduced with rhMG53 administration, and I/R-induced elevation of HSP70 was also ameliorated by rhMG53 administration.
The above studies show the prophylactic effect of rhMG53 in prevention of I/R-induced lung injury. To test whether rhMG53 has therapeutic value for treatment of ALI, we intravenously injected rhMG53 to rats either immediately prior to reperfusion or at 0.5 h after reperfusion. The data shown in Supplementary Fig. 5 showed that application of rhMG53 at either of these times could ameliorate I/R induced injury to the lung tissue, since both wet/dry weight ratio and PaO2 measurements were restored close to the sham operated animal group. In addition, serum IL-1β and IL-6 concentrations were lower in rhMG53 treatment group.
We performed histopathological analyses to evaluate the changes in lung structure following I/R injury with or without rhMG53 treatment. These analyses were quantified in a blinded fashion using standard methods established by Takil et al25, as outlined in Supplementary Table 2. As shown in Supplementary Fig. 6A, lung sections in the sham group displayed normal alveolar architecture, whereas the lung in the I/R group showed obvious edema, hemorrhage, extensive alveolar wall disruption and a great number of infiltrating inflammatory cells in the interstitium and alveoli. These symptoms were alleviated with rhMG53 administration. Data from multiple experiments are summarized in Supplementary Fig. 6B, which illustrates that pulmonary protective effects of rhMG53 were observed under all three paradigms of rhMG53 application, e.g. precondition, treatment and 0.5 h treatment.
The pulmonary protective effect of rhMG53 was further examined in the mg53−/− mice following I/R lung injury. These results are summarized in Supplementary Fig. 7. Consistent with the data obtained with the rat model, we found that rhMG53 administration into the mg53−/− mice after I/R induced lung injury could improve the survival rate, wet/dry ratio and blood gas exchange function.
Intra tracheal delivery of rhMG53 protects against PPE-induced lung injury
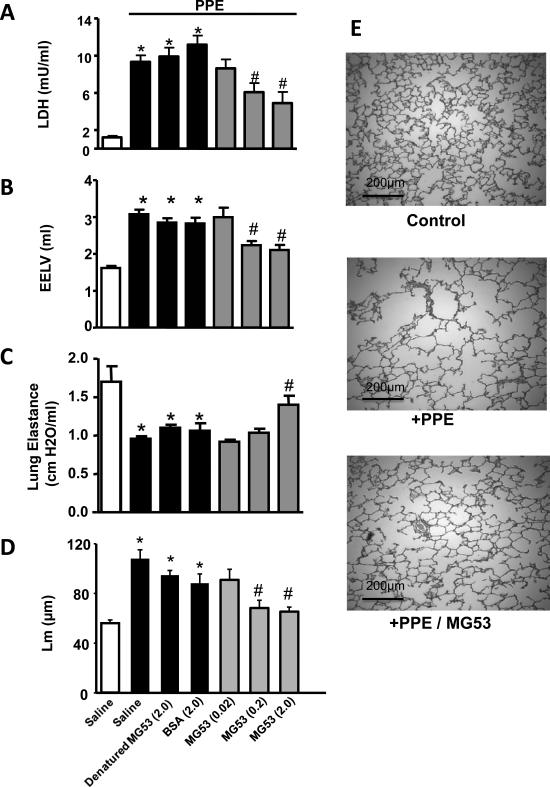
We next used an enzyme-induced emphysema rat model to examine whether intra tracheal delivery of rhMG53 can produce beneficial effects on the structure and function of the lung following acute injury. In this model, rats were treated with the porcine pancreas elastase (PPE, 500 U/kg) by intratracheal spray to produce damage to the airway epithelial lining26. As shown in Fig. 6A, 30 min after PPE application, LDH levels in BALF was significantly increased, indicating that the plasma membrane of the airway epithelium was damaged by PPE. Intratracheal application of rhMG53 at 5 minutes before PPE decreased this LDH release into BALF in a concentration dependent manner. Denatured rhMG53 (boiled for 20 min) or BSA was ineffective at alleviating PPE-induced LDH release, suggesting rhMG53 can prevent PPE-induced acute cell damage to the airway epithelium.
Figure 6.
Intra-tracheal instillation of rhMG53 protects PPE-induced injury to rat lung. A. PPE was applied through intra tracheal (i.t.) spray. LDH in BALF was measured 30 min after PPE application to evaluate cell damage. Different concentrations of MBP-MG53 or control proteins (denatured MBP-MG53 or BSA) were applied through intra tracheal spray 5 min before PPE. 0.2 mg/kg and 2 mg/kg MBP-MG53 significantly reduced LDH in BALF. The measurements of chronic responses were conducted 4 weeks after PPE application with repetitive application of MBP-MG53 (i.t., 5 days per week for 4 weeks). EELV (B), lung elastance (C), and alveolar structure (quantified by Lm) of the lung (D) were compared to control treatment groups. n=7-10 per group. E. Representative histology images of lung parenchyma in PPE-induced injury to rat lung with or without administration of MG53 (scale bars are equal to 200 μm). *: P<0.05 vs. saline control group (open bar). #: P<0.05 vs. denatured MBP-MG53 group (Mann-Whitney U analysis).
The elastase-induced rat model of emphysema allowed us to further probe if the protective effect of rhMG53 on the acute tissue injury can be translated into long-term improvement in the structure and function of the lung. Chronic treatment with rhMG53 in rats subjected to PPE-induced lung injury was conducted with daily application of rhMG53 for 4 weeks. By 4 weeks after PPE application, this model develops several emphysema related changes41. Accordingly, end expiratory lung volume (EELV) increased from 1.62 ± 0.06 ml to 3.07 ± 0.13 ml and lung elastance decreased from 1.70 ± 0.21 to 0.96 ± 0.03 cm H2O/ml in PPE treated rats. rhMG53 treatment significantly attenuated PPE-induced functional changes in a dose dependent manner (Fig. 6B and 6C). When animals were treated with 2 mg/ml rhMG53, EELV was attenuated to 2.10 ± 0.14ml and the lung elastance was maintained at 1.40 ± 0.12 cm H2O/ml (Fig. 6B and 6C). These rats displayed significant enlargement of the alveolar space, which was partially attenuated by application of rhMG53 (Fig. 6E). The linear mean (Lm) intercept distance in the control group was 56.0 ± 2.7 μm, which increased to 107.8 ± 7.3 μm in the PPE group (p < 0.05 compared with the control group, Mann-Whitney U analysis). Mean Lm in the PPE plus MG53 group (65.3 ± 3.6 μm) was significantly smaller than in the PPE group (P < 0.05, Mann-Whitney U analysis) (Fig. 6D). These data suggest that intra tracheal delivery of rhMG53 could have beneficial effect on structure and functional changes in the lung associated with emphysema.
Intra tracheal delivery of rhMG53 protects LPS-induced injury to the lung
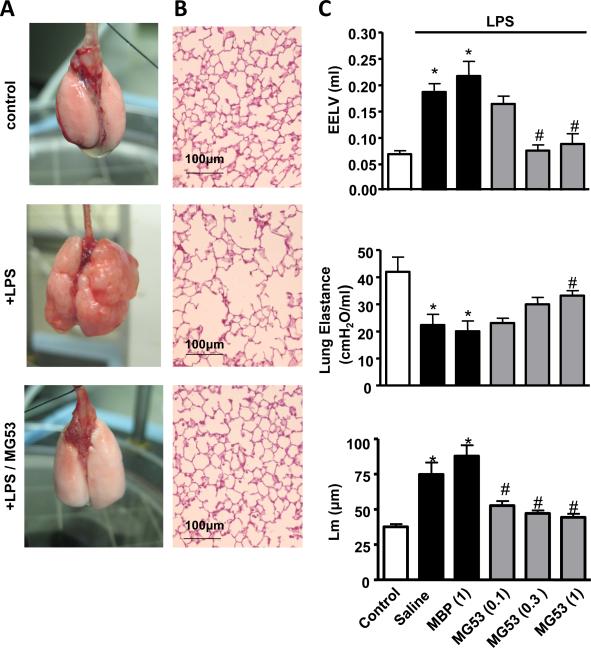
The effect of rhMG53 in prevention of chronic injury to the lung was further evaluated using a model of emphysema involving intra tracheal application of lipopolysaccharide (LPS; 0.2 mg/ml) in C57BL/6J mice27. Mice were treated with LPS 3 times a week for 4 weeks. All animals were housed for another week before the measurements of emphysema related changes. As shown in Fig. 7A, the whole mount specimen of lungs from mice treated with LPS demonstrated bullae on the lung surface, a typical pathological change in emphysema. Remarkably, injecting mice with rhMG53 prevented the appearance of LPS-induced bullae on the lung surface. Histological examination showed expected levels of airspace enlargement and disrupted alveolar septa in LPS treated mice. These changes were greatly reduced by rhMG53 treatment (Fig. 7B).
Figure 7.
Intra-tracheal application of rhMG53 protects LPS-induced lung remodeling in mice. Animals were first treated with LPS (i.t., 3 times per week, for 5 weeks) followed by MBP-MG53 or MBP-MBP in control group (i.t., 5 days/week for 5 weeks). Lungs were collected 5 weeks after initial LPS application for evaluation. A. MBP-MG53 prevents emphysema in LPS-induced chronic lung remodeling. B. Histology H/E staining of parenchyma in LPS-induced emphysematous lung (Scale bars are equal to 100 μm). C. Statistical results for EELV, lung elastance and Lm in LPS-induced emphysematous lung treated with different doses of rMG53 (mg/kg, 0.1, 0.3, 1.0) and 1 mg/kg control protein, n=7-10, *P<0.05 compared to no LPS control group (open bar) and #P<0.05 compared with saline-treated, LPS-challenged group. n=7-10 (Mann-Whitney U analysis).
LPS treatment increased the mean linear intercept (Lm) and EELV from 37.5 ± 2.0 μm and 69 ± 6 μl to 74.7 ± 8.5 μm and 187 ± 16 μl, respectively (Fig. 7C). LPS also decreased lung elastance from 42.0 ± 5.4 cmH2O/ml to 22.4 ± 3.9 cmH2O/ml. Treating these animals with rhMG53 (0.1-1.0 mg/ml, i.t. 5 weeks) significantly improved the pathological changes. In the MG53 treatment group (1.0 mg/ml), the value of Lm, EELV, and lung elastance are 44.4 ± 2.5 μm, 88 ± 19μl and 33.2 ± 1.8 cmH2O/ml, respectively. These results provide direct evidence that rhMG53 restored emphysema-related morphological and functional changes in the LPS-induced emphysema model in mice.
DISCUSSION
The data presented in this study is consistent with the hypothesis that MG53-mediated cell membrane repair contributes to the normal physiology function of the lung, and that defects in membrane repair due to the absence of MG53 can lead to increased susceptibility of the lung to stress-induced injury. We also present evidence that application of the exogenous rhMG53 protein have beneficial effects in treatment or prevention of lung injury in various animal model systems.
Since our previously published studies with MG53 function in cell membrane repair were mostly conducted in skeletal and cardiac muscles5-7, 9, 10, 28, the observation of MG53 expression in the lung tissue is novel. Using RACE analysis, we found that the mRNA coding sequence for mg53 in the lung is identical to that in muscle cells, suggesting that the mg53 gene expresses one product in both tissues without alternative splicing. Interestingly, there is a slight mobility change of MG53 in the lung tissue compared with that in the muscle tissue. Further efforts will be required to test if this apparent shift in mobility was related to the differential posttranslational mechanisms that may exist in these two tissues. The cell membrane in lung epithelial cells and striated muscle cells are both exposed to mechanical stresses during the course of normal physiology. These cell types constantly undergo stretch or deformation induced injury in the course of their function in their resident tissue. Even though the level of MG53 protein in the lung tissue is substantially less than that in the muscle cells, a clear lung phenotype was observed with the mg53−/− mice under stress conditions as these animals are vulnerable to I/R and over-ventilation induced injury to the lung. Based on immunoblotting and histological studies, we show that MG53 is present in both type I and type II alveolar cells, but absent from endothelial cells.
The lung epithelium represents an important barrier that protects the internal organs from exposure to external environment. While normal tidal breathing produces little mechanical strain across alveolar cells, over-ventilation may cause stress to the lung and lead to injury to alveolar cells29. In pathophysiological settings such as mechanical ventilation, I/R injury, sepsis, trauma and shock, these insults can lead to damage to the organ. If the injured lung epithelial cells cannot repair, an excessive pro-inflammatory response may occur in the lung and trigger a cascade of detrimental events leading to extensive damage of the lung and eventual failure of pulmonary function. Targeting repair of cell membrane injury has recently emerged as an attractive avenue for prevention and/or treatment of ALI. Several studies have demonstrated protective effect for P188, a polymer with membrane sealing capacity, against injury to spinal cord30, 31 and heart32, 33. A previous study by Plataki et al showed that P188 has protective effect in cultured lung epithelial cells from stretch-induced injury24. However, this protective effect of P188 can only be seen in isolated perfused lungs but not in living rats subjected to over ventilation for reasons that are currently unknown24. Compared with P188, rhMG53 has several advantages for treatment of cell membrane injuries. MG53 is an intrinsic tissue-repair gene present in the human body, and the primary amino acid sequence of MG53 is highly conserved among various animal species. Native MG53 protein is constantly present in blood circulation due to tissue injury, thus reducing the potential immunogenic responses associated with systemic delivery19. Our published data showed that rhMG53 is effective at facilitating cell membrane repair at lower concentrations than P188. Thus, targeting MG53-mediated membrane repair in lung epithelial cells could potentially be an effective and integrative component of ALI therapies.
The therapeutic effect of rhMG53 on ALI was assessed in two different rodent models of emphysema involving tracheal application of LPS or elastase. Chronic administration of LPS in the airway likely induces airway inflammation and emphysematous related changes through endogenous inflammatory mediators which induce oxidant stress, protease/antiprotease imbalance, and apoptosis27, 34. This model resembles some of the changes seen in human emphysema35, although the mechanisms at work in the model are not clearly defined. The elastase induced emphysema results from destruction of lung parenchyma tissues. Such models have been widely used to evaluate pharmacological effect on emphysema targeting lung regeneration36,37. In both experimental models of emphysema, we showed that rhMG53 could significantly attenuate the emphysema-related changes including air space enlargement, loss of lung elastance, and elevation of EELV when compared to that in vehicle control-treated animals. As lung elastic recoil property is the driving force for expiration, loss of lung elastic recoil function in the emphysematous lungs leads to expiratory air flow limitation and chronic lung hyperinflation, that is known to play a significant role in the development of dyspnea and exercise limitation that are major clinical manifestations of chronic obstructive pulmonary disease (COPD)38.
Our studies with the rodent animal models show that intravenous as well as intra tracheal delivery of rhMG53 are both effective in protecting injury to the lung epithelial cells and restoring the pulmonary function following ALI. While intra tracheal delivery of rhMG53 can be conveniently achieved through nebulization, further formulation efforts will be needed to efficiently target the lower airway epithelial cells to increase the potential efficacy for rhMG53 in treatment or prevention of ALI. The systemic delivery of rhMG53 via intravenous route is potentially an attractive avenue for treatment of ALI, especially for patients with more severe pulmonary dysfunction. We have previously shown that intravenous delivery of rhMG53 does not produce overt toxicity in rodent models19. Here we find that intravenous application of rhMG53 can have protective effects against I/R mediated acute injury to the lungs.
Overall, we provide evidence that injury to lung resident cells is involved in the pathogenesis of acute and chronic lung diseases, and targeting lung cell repair represents a novel disease modifying treatment for ALI. Future studies should focus on understanding the mechanism for MG53-mediated repair, e.g. identification of co-factors that participate in the nucleation process of membrane repair in pulmonary cells. Moreover, elucidating the mechanism of action for the exogenous rhMG53 protein in protection of acute lung injury should improve the potential of rhMG53 as a therapeutic agent for treatment of ALI.
METHODS
Immunoblotting and RACE analysis
Isolated lung tissues were washed twice with ice-cold PBS and lysed in lysis buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 1 mM PMSF, and 10 mg/ml each leupeptin and aprotinin). After centrifugation at 12,000 g for 15 min, the supernatants were collected and their protein concentrations measured. The lung tissue homogenates (50 μg of protein) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (PVDF) (Bio-Rad). For quantification purpose, different amounts of skeletal muscle tissue lysates were loaded into the same gel. The blots were washed with Tris-buffered saline Tween-20 (TBST), blocked with 5% milk in TBST buffer for 1 hr, and incubated with custom-made rabbit monoclonal anti-MG53 and the appropriate secondary antibody coupled to horseradish peroxidase (HRP) (Sigma, 1:5000 dilution). Bands were visualized with an ECL+ kit (Pierce). The amount of protein transferred onto the PVDF membranes was verified by Ponceau S staining.
RACE was conducted using a mouse lung Marathon-Ready cDNA library purchased from Clontech (Catalog No. 639411). The primer sequences used for 5’-RACE and 3’-RACE were listed in Supplemental Table 1. RACE amplifications were performed in 20 μl reaction volume using 2 μl mouse lung cDNA, 0.3 μM primers, 0.4 mM dNTPs and 0.4 unit of KOD Fx (TOYOBO, Japan). Conditions were as follows: 94°C for 2 min; 5 cycles of 98°C for 10 s and 74°C for 2 min; 5 cycles of 98°C for 10 s and 72°C for 2 min; and 30 cycles of 98°C for 10 s and 70°C for 2 min and 72°C for 5 min. PCR products were subcloned into pBS-SK vector (Stratagene) and sequenced.
Isolation and administration of recombinant human MG53 protein
Purification of the recombinant human MG53 (rhMG53) protein has been described previously19. The present study employed two different forms of MG53 protein, MBP-MG53 and untagged rhMG53. MBPMBP control proteins contained two copies of the MBP (maltose-binding protein) tag attached in tandem and were isolated by identical purification methods. Untagged rhMG53 was produced by cleavage of MBP from MBP-MG53 using thrombin and separation of these two using gel filtration high pressure liquid chromatography. Untagged rhMG53 was lyophilized and stored at 4 °C as dry powder in a desiccator. For intravenous injection of rhMG53, the protein was diluted in 0.9% sterile saline, filtered through a 0.2 μm filter and injected via the tail vein. For intra-tracheal application of rhMG53, animals were placed under anesthesia (2% to 5% isoflurane, inhaled in a chamber) and the protein was applied into the trachea, using a laryngoscope and a micro sprayer (Penn-Century Inc. Philadelphia, PA).
Cell culture and in vitro membrane injury assays
The rat lung epithelial cells (RLE), human primary pulmonary artery endothelial cells (PPAEC) and umbilical vein endothelial cells (HUVEC) were all obtained from American Type Culture Collection (ATCC, Manassas, VA) and the standard culture conditions were followed accordingly. Human lung microvascular endothelial cells (MVEC-L) were purchased from Lonza (Alpharetta, GA).
For in vitro membrane injury assays, RLE cells were cultured in F-12 culture medium supplemented with 10% fetal bovine serum (FBS) and 1% Penicillin/Streptomycin. Transfection of GFP-MG53 into RLE cells was performed using the Lipofectamine LTX reagent (Life Technologies) per manufacturer's instructions. RLE cells expressing GFP-MG53 was subjected to microelectrode penetration induced acute injury to the plasma membrane as previously described5. Micro-glass beads induced injury to RLE cells in suspension was conducted according to our published protocol8, 11, 19. 9 × 104 cells were suspended in 150 μl of PBS in the presence or absence of acid washed beads, and/or different concentrations of rhMG53 in a 96-well plate. The plate was shaken on a rotary orbital shaker (Belloco Biotechnology) at 180 rpm for 6 min at room temperature. After shaking, the cells were immediately centrifuged at 1,000 × g for 5 min and 50 μL of supernatant was transferred into a new 96-well plate. LDH activity in this supernatant was determined using a LDH Cytotoxicity Detection kit (Takara Bio Inc.).
Animal care
Animal handling and surgical procedure was performed in accordance with local regulations and all protocols were approved by all participating Institutional Animal Care and Use Committee (IACUC), including The Ohio State University, Daping Hospital-The Third Military Medical University and Schering-Plough Research Institute-Merck Research Labs) and were compliant with the guidelines of the American Association for the Accreditation of Laboratory Animal Care. MG53 knockout mice (mg53−/−) and their wild-type control mice were breed and maintained as previously described5. All other animals were obtained from commercial sources indicated for each procedure.
Over-ventilation-induced lung injury and BALF collection
The study protocol was approved by the IACUC of the Ohio State University. Wild type and mg53−/−mice5 were anesthetized with isoflurane and then over-ventilated at 30 ml/kg (Harvard Apparatus) for 30 min. For animals in the imaging study group, propidium iodide (PI, 1 μg/ml) (Invitrogen, Eugene, OR) was administered into the right ventricle immediately after cessation of the mechanical ventilation, and ex bloc lung was occluded at peak inspiration for confocal imaging of the subpleural lung area. Positive endexpiratory pressure was not used for these studies. Cell membrane injury was expressed as a ratio of the number of PI-positive cells per total number of alveoli in the field from eight random subpleural images. In separate experiments, rhMG53 (2 mg/kg) were administered via intravenous injection into mg53−/−mice immediately before over-ventilation induced acute lung injury described above. The bronchial alveolar lavage fluid (BALF) was collected and immunoblot used for detection of rhMG53 in the BALF.
Assessment of rhMG53 effects on Ischemia/Reperfusion (I/R)-induced lung injury in rats
Sprague-Dawley (SD) rats of both sexes, weighing 210 to 250g, were obtained from the Experimental Animals Centre of Daping Hospital at Chongqing, China. The experimental procedure for I/R-induced ALI was conducted according to published protocols with minor modifications39-41. Rats were randomized into sham-operated, and lung I/R groups with or without rhMG53 treatment. Rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg) and placed on a heating pad to maintain their body temperature. The right femoral arteries and vein of rats were then catheterized with polyethylene tubing (outer diameter = 0.965 mm; inner diameter = 0.58 mm) for arterial blood sampling and rhMG53 (or saline as control) injection. After intubation via tracheotomy, rats were placed on a volume ventilator (tidal volume = 6 ml/kg, respiratory rate = 60 breaths per min, inspiratory/expiratory ratio = 1: 2) with room air. After thoracotomy, the left hilum of I/R group was clamped for 1h, and released for reperfusion for 1h. The left lungs were observed atelectasis and inflation to make sure adequate hilum clamping and reperfusion. The sham group underwent thoracotomy and was placed on ventilator without left hilum clamp. Rats gone through I/R procedures were further divided into the following subgroups: untreated “I/R” group; rhMG53 “precondition” group- rhMG53 was administered in between thoracotomy and ischemia; rhMG53 “treatment” group- rhMG53 was administered immediately after 1h ischemia but before reperfusion; and rhMG53 “0.5h treatment” group-rhMG53 was administered 0.5 h after reperfusion. After reperfusion, blood samples were obtained immediately for arterial blood gas analysis (Gem primer 3000, Instrumentation Laboratory, US) and serum collection for ELISA measurement of IL1β and IL6 (Boster, Wu Han, China).
I/R-induced lung injury in mice followed the same protocol as that used for rats, with the following modifications. mg53−/− and wild type mice (3-4 months) were connected to a Harvard ventilator (Hugo Sachs Elektronik, German) with an inspiratory pressure of 7 ml/kg after anesthetization with sodium pentobarbital and intubation via tracheotomy (normal low-tidal ventilation). The respiratory rate was set at 100 breaths per minute. After thoracotomy, the left hilum of I/R group was clamped for 1hour, and released, reperfusion proceeded for 1hour.
Histological analysis and wet/dry ratio determination
Left lung from SD rats and mice were washed with ice-cold oxygenated saline to remove blood and then fixed in 4% neutral-buffered paraformaldehyde for 1-2 days at 4 °C before processing for paraffin embedding. Blocks were sectioned (4 μm), mounted on slides then deparaffinized and rehydrated by successive incubations in xylene, 100% ethanol, 95% ethanol, 75% ethanol, and phosphate buffer saline (PBS). Sections were stained with hematoxylin and eosin (H&E) using standard procedures and scored according to a four-point scale25 by an experienced histologist from the Department of Pathology at Daping Hospital under blinded conditions. The histological analyses were performed in 10 randomly selected microscope fields (200×) from each section.
Fresh lungs from separate groups of mice or rats were weighed and then desiccated until a stable dry weight was achieved. To measure wet lung, the left lungs of rats and mice were weighted immediately after dissection. To measure dry weight, the tissues were desiccated then placed in an oven at 80 °C for 24h and reweighted as a dry weight for calculation of the wet/dry weight ratio.
Anoxia and reoxygenation induced injury in A549 cells
A549 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). To induce anoxia, cells were placed in an anoxic chamber with 5% CO2 and 95% N2 at 37 °C for 2 h followed by reoxygenation for 2 h. Exogenous rhMG53 was conjugated with Rhodamine by a dye labeling kit (GBiosciences, St Louis, US.), and applied to cells immediately after anoxia. After the reoxygenation period, the cells were used for Annexin V staining or immunofluorescence staining. Cells were fixed with 4% paraformaldehyde (30 min), and custom rabbit anti-MG53 antibody was applied (1:200 dilution) over night at 4 °C, which was followed by FITC–conjugated goat anti-rabbit IgG antibody (Jackson ImmunoResearch Laboratory, West Grove, Pa). Immunofluorescence confocal images were acquired (Olympus AX70 laser confocal microscopy) at excitation wavelengths of 350 nm and 507 nm; emission was detected at 450 and 529 nm. Cells that were treated with only FITC–conjugated goat anti-rabbit IgG antibody revealed no immunofluorescence, and omission of the anti-MG53 antibody showed no green fluorescence after merging the images.
Intra-tracheal PPE- or LPS-induced emphysema models in rodent
Emphysema animal models were induced as described previously27, 36. Emphysema was induced in Wistar rats by one time intratracheal (i.t.) application of porcine pancreas elastase (PPE, 500U/kg, Elastin Products Co., Owensville, MO), using a laryngoscope and a micro sprayer (Penn-Century Inc. Philadelphia, PA), under the anesthesia by 2% to 5% isoflurane (inhaled in a chamber). MG53 or control proteins were applied intratrachealy using the same method, 5 days per week for 4 weeks, starting on the day of PPE application. The emphysema related changes were evaluated 4 weeks after the PPE application36. Emphysema was also induced in mice by lipopolysaccharide (LPS, Sigma-Aldrich, Saint Louis, Missouri) as described27. Briefly, animals under anesthesia (2% to 5% isoflurane, inhaled in a chamber) was applied with LPS (i.t. application in mice: 0.2 mg/ml, 50 μl/animal) into the trachea, using a laryngoscope and a micro sprayer (Penn-Century Inc. Philadelphia, PA), 3 times a week for 5 weeks. MBP-MG53, or MBP-MBP in the control group, was applied into the trachea once daily, 5 days a week for 5 weeks, starting on the first day of the LPS application. All the measurements were conducted 5 weeks after initial LPS application.
End expiratory lung volume (EELV) was measured ex vivo according to Borzone et al42. Briefly, rats or mice were euthanized and trachea was occluded at the end of expiration with the chest wall intact. The lungs with the occluded trachea were then removed and the whole lung volume was estimated by saline solution volume displaced by the isolated lungs. The lung tissue volume was estimated by lung tissue weight divided by tissue density. EELV was calculated as the difference between the whole lung volume and the lung tissue volume. Here we used the water density (1 g/ml) to estimate lung tissues density as described previously38-40. The true tissue density may be greater than water density (for example, fat/water free liver density is 1.4 g/ml39-40). In addition, the saline on the surface of lung tissues cannot be completely excluded from the lung weight measurements. These factors may over estimate lung tissue volume and thus under estimate EELV. However, these systemic error applies to all the groups equally and do not affect the comparisons among groups. Static deflationary volume-pressure relationship was determined in isolated lungs to assess the lung elastic properties according to Borzone et al42. Briefly, immediately after isolation, the lungs were connected to a syringe filled with air and a differential pressure transducer through trachea.
Transpulmonary pressure was measured as the intratracheal pressure. The lungs were slowly inflated with air to 20 cmH2O twice. Step lung deflation maneuvers were performed. The intratracheal pressure and the lung volume at each step were recorded after waiting for at least 10 seconds. The deflationary static volume-pressure curve of the lung was established, and the lung elastance at low lung volumes (transpulmonary pressure of 0 to 5 cmH2O) was calculated as (P5cmH2O-P0cmH2O)/(V5cmH2O-V0cmH2O), where P is the transpulmonary pressure and V is the lung volume. The isolated lungs were fixed with 10% formalin through trachea at a constant pressure of 20 cmH2O. The lung tissue was processed, sliced and stained with hematoxylin and eosin. An image from the same area in the right middle lobe of the lung was chosen for measurements of mean linear intercept (Lm). Three lines of equal distance on each image were analyzed and Lm was calculated as the length of each line divided by the number of alveolar septum on the line. Lm was expressed as mean value analyzed from the 3 lines.
Statistical Analysis
The data are expressed as mean ± SEM. Comparison within groups was made by student t test when comparing two experimental groups and by ANOVA for repeated measures. Comparison among groups was made by ANOVA with Holm-Sidak test. The product limit (Kaplan-Meier) estimate of the cumulative survival was assessed with the log-rank test to evaluate significance differences. A value of P<0.05 was considered significant. A subset between group comparisons employed a Mann-Whitney U statistical analysis. The statistical significance level was set at P<0.05 or P<0.01 as specified in figure legends.
Supplementary Material
Acknowledgement
This work was supported by grants from the National Institutes of Health (NIH) to JM, HT, and NW; grants from the National Science Foundation of China (30925018 and 31130029) and National Basic Research Program of China (973 Program, 2013CB531104, and 2012CB517801) to CZ; and a Small Business Innovation Research grant from NIH awarded to TRIM-edicine, Inc.
Footnotes
Author contributions:
YJ and JM developed the concept for the studies. YJ, GL, RLM, and JH performed studies with the PPE-and LPS-induced lung injury model. KC, ZW, YY, YL and CZ conducted I/R induced lung injury study. PL, MN, RY, BW, PD, HL, HZ, XZ, HT, and NW performed biochemical and in vitro studies of endogenous MG53 in the lung and exogenous rhMG53 characterization. JM and CZ oversaw the entire project. YJ, KC, NW, CZ and JM wrote the manuscript and all authors contributed to revision of the manuscript.
Competing financial interests
JM and NW have an equity interest in TRIM-edicine, which develops rhMG53 for treatment of human diseases. Patents on the use of MG53 are held by Rutgers University – Robert Wood Johnson Medical School.
References
- 1.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- 2.Andrews NW. Membrane repair and immunological danger. EMBO Rep. 2005;6:826–830. doi: 10.1038/sj.embor.7400505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han R, Campbell KP. Dysferlin and muscle membrane repair. Curr Opin Cell Biol. 2007;19:409–416. doi: 10.1016/j.ceb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeckler RA, Hubmayr RD. Cell wounding and repair in ventilator injured lungs. Respir Physiol Neurobiol. 2008;163:44–53. doi: 10.1016/j.resp.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai C, et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11:56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai C, et al. MG53 regulates membrane budding and exocytosis in muscle cells. J Biol Chem. 2009;284:3314–3322. doi: 10.1074/jbc.M808866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai C, et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin P, et al. Nonmuscle myosin IIA facilitates vesicle trafficking for MG53-mediated cell membrane repair. FASEB J. 2012;26:1875–1883. doi: 10.1096/fj.11-188599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao CM, et al. MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation. 2010;121:2565–2574. doi: 10.1161/CIRCULATIONAHA.110.954628. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, et al. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res. 2010;107:76–83. doi: 10.1161/CIRCRESAHA.109.215822. [DOI] [PubMed] [Google Scholar]
- 11.Zhu H, et al. Polymerase transcriptase release factor (PTRF) anchors MG53 protein to cell injury site for initiation of membrane repair. J Biol Chem. 2011;286:12820–12824. doi: 10.1074/jbc.C111.221440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweeney RM, Griffiths M, McAuley D. Treatment of acute lung injury: current and emerging pharmacological therapies. Semin Respir Crit Care Med. 2013;34:487–498. doi: 10.1055/s-0033-1351119. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosio G, Tritto I. Reperfusion injury: experimental evidence and clinical implications. Am Heart J. 1999;138:S69–75. doi: 10.1016/s0002-8703(99)70323-6. [DOI] [PubMed] [Google Scholar]
- 15.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 16.Ng CS, Wan S, Arifi AA, Yim AP. Inflammatory response to pulmonary ischemiareperfusion injury. Surg Today. 2006;36:205–214. doi: 10.1007/s00595-005-3124-2. [DOI] [PubMed] [Google Scholar]
- 17.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 18.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Weisleder N, et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med. 2012;4:139ra185. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisleder N, Takeshima H, Ma J. Immuno-proteomic approach to excitation--contraction coupling in skeletal and cardiac muscle: molecular insights revealed by the mitsugumins. Cell Calcium. 2008;43:1–8. doi: 10.1016/j.ceca.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CS, et al. TRIM72 negatively regulates myogenesis via targeting insulin receptor substrate-1. Cell Death Differ. 2010;17:1254–1265. doi: 10.1038/cdd.2010.1. [DOI] [PubMed] [Google Scholar]
- 22.Kracklauer MP, et al. Discontinuous thoracic venous cardiomyocytes and heart exhibit synchronized developmental switch of troponin isoforms. FEBS J. 2013;280:880–891. doi: 10.1111/febs.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gajic O, et al. Ventilator-induced cell wounding and repair in the intact lung. Am J Respir Crit Care Med. 2003;167:1057–1063. doi: 10.1164/rccm.200208-889OC. [DOI] [PubMed] [Google Scholar]
- 24.Plataki M, Lee YD, Rasmussen DL, Hubmayr RD. Poloxamer 188 facilitates the repair of alveolus resident cells in ventilator-injured lungs. Am J Respir Crit Care Med. 2011;184:939–947. doi: 10.1164/rccm.201104-0647OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takil A, et al. Histopathologic effects of lipid content of enteral solutions after pulmonary aspiration in rats. Nutrition. 2003;19:666–669. doi: 10.1016/s0899-9007(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 26.Kimmel EC, Winsett DW, Diamond L. Augmentation of elastase-induced emphysema by cigarette smoke. Description of a model and a review of possible mechanisms. Am Rev Respir Dis. 1985;132:885–893. doi: 10.1164/arrd.1985.132.4.885. [DOI] [PubMed] [Google Scholar]
- 27.Brass DM, et al. Chronic LPS inhalation causes emphysema-like changes in mouse lung that are associated with apoptosis. Am J Respir Cell Mol Biol. 2008;39:584–590. doi: 10.1165/rcmb.2007-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yi JS, et al. MG53-induced IRS-1 ubiquitination negatively regulates skeletal myogenesis and insulin signalling. Nat Commun. 2013;4:2354. doi: 10.1038/ncomms3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir Physiol. 2000;119:1–17. doi: 10.1016/s0034-5687(99)00092-4. [DOI] [PubMed] [Google Scholar]
- 30.Misra A, et al. Preventing neuronal damage and inflammation in vivo during cortical microelectrode implantation through the use of Poloxamer P-188. J Neural Eng. 2013;10:016011. doi: 10.1088/1741-2560/10/1/016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo CL, et al. Poloxamer 188 attenuates in vitro traumatic brain injury-induced mitochondrial and lysosomal membrane permeabilization damage in cultured primary neurons. J Neurotrauma. 2013;30:597–607. doi: 10.1089/neu.2012.2425. [DOI] [PubMed] [Google Scholar]
- 32.Juneman EB, et al. The effects of poloxamer-188 on left ventricular function in chronic heart failure after myocardial infarction. J Cardiovasc Pharmacol. 2012;60:293–298. doi: 10.1097/FJC.0b013e31825f6f88. [DOI] [PubMed] [Google Scholar]
- 33.Spurney CF, et al. Membrane sealant Poloxamer P188 protects against isoproterenol induced cardiomyopathy in dystrophin deficient mice. BMC Cardiovasc Disord. 2011;11:20. doi: 10.1186/1471-2261-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vernooy JH, Dentener MA, van Suylen RJ, Buurman WA, Wouters EF. Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am J Respir Cell Mol Biol. 2002;26:152–159. doi: 10.1165/ajrcmb.26.1.4652. [DOI] [PubMed] [Google Scholar]
- 35.Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol. 2008;294:L612–631. doi: 10.1152/ajplung.00390.2007. [DOI] [PubMed] [Google Scholar]
- 36.Massaro GD, Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med. 1997;3:675–677. doi: 10.1038/nm0697-675. [DOI] [PubMed] [Google Scholar]
- 37.Belloni PN, Garvin L, Mao CP, Bailey-Healy I, Leaffer D. Effects of all-trans-retinoic acid in promoting alveolar repair. Chest. 2000;117:235S–241S. doi: 10.1378/chest.117.5_suppl_1.235s. [DOI] [PubMed] [Google Scholar]
- 38.O'Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:180–184. doi: 10.1513/pats.200508-093DO. [DOI] [PubMed] [Google Scholar]
- 39.Bozok S, et al. Protective effects of hyperbaric oxygen and iloprost on ischemia/reperfusion-induced lung injury in a rabbit model. Eur J Med Res. 2012;17:14. doi: 10.1186/2047-783X-17-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karapanos NT, et al. Does lung ischemia and reperfusion have an impact on coronary flow? A quantitative coronary blood-flow analysis with inflammatory cytokine profile. Eur J Cardiothorac Surg. 2012;41:154–161. doi: 10.1016/j.ejcts.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nowak K, Weih S, Post S, Gebhard MM, Hohenberger P. Bradykinin in ischemiareperfusion injury of the rat lung. J Physiol Pharmacol 58 Suppl. 2007;5:513–522. [PubMed] [Google Scholar]
- 42.Borzone G, et al. Rat and hamster species differences in susceptibility to elastase-induced pulmonary emphysema relate to differences in elastase inhibitory capacity. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1342–1349. doi: 10.1152/ajpregu.00343.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.