Abstract
Intestinal drug efflux proteins play a major role in the pharmacokinetics of many drugs. We assessed diurnal rhythmicity in the expression of ten major drug transporters.
We acquired male Sprague-Dawley rats and harvested jejunal mucosa at 3-hourly intervals across a 24-hour period. qPCR was performed for ten transporters: MDR1-3, MRP1, MRP3, MCT1, BRCP, PepT1, OCTN2, OATP-B. Rhythmicity was assessed with the cosinor procedure.
Diurnal rhythmicity was observed for MDR1, MCT1, MRP2, PepT1, and BCRP (1.6–5.4 fold-changes). Mesors occurred during fasting hours.
We conclude many drug transporters display profound diurnal rhythms in transcription, which may underlie diurnal rhythms in drug pharmacokinetics.
Keywords: Diurnal rhythm, intestinal, drug efflux transporter
Recent advances in molecular biology have identified large families of membrane transport proteins capable of carrying drugs and other non-nutrient compounds across the otherwise impermeable cell membrane. In particular, the ATP-binding cassette (ABC) superfamily of transporters is of recognized importance in the membrane transport of many drugs, ranging from digoxin to chemotherapy agents(1, 2). Most of these transporters were initially identified as proteins conveying chemoresistance to cancer cell-lines, due to their ability to extrude cytotoxic agents. However, their wider physiological function is beginning to be appreciated(3).
Many members of the ABC superfamily have now been documented in intestinal mucosal enterocytes, predominantly on the brush-border membrane(4, 5). Their role in drug pharmacokinetics remains unclear, but there is increasing evidence that they both mediate absorption of enterally-delivered drugs and contribute to intestinal clearance of systemic drugs(2).
Multiple classes of drugs show evidence of diurnal variations in pharmacokinetics(6). Whilst this is mediated in part by circadian rhythmicity in hepatic metabolism(7), diurnal changes in intestinal absorptive and efflux capacity may be contributory factors. We have previously shown robust diurnal rhythms in intestinal glucose absorption, matching transporter expression to nutrient delivery patterns(8, 9). Similar rhythmicity is displayed by the peptide transporter 1 PepT1(10). Rats, which are nocturnal feeders, display peaks in transcription for glucose transporters during the light (fasting) phase in anticipation of nocturnal feeding. These are similar in magnitude (but offset by twelve hours) to those seen in primates feeding preferentially during daylight hours(11).
We hypothesized that intestinal drug transporters may vary with a diurnal periodicity, and that this might contribute to circadian rhythmicity in drug metabolism. We therefore assessed the transcriptional signal of ten drug transporters known to be expressed in intestine, using a diurnal rat model.
All animal studies were in accordance with protocols prospectively approved by the Harvard Medical Area Standing Committee on Animals. Male Sprague-Dawley rats (200–211g) were acquired from Harlan (Indianapolis, IN) and acclimatized for seven days under a fixed 12:12 hour light: dark cycle, with lights-on at 7am (Zeitgeber Time ZT0). Animals were allowed ad libitum access to food and water.
On the study day, jejunal mucosa was harvested at ZT0 and subsequently at three-hourly intervals (n=6 per time point) over a 24-hour period. Animals were anesthetized, a midline laparotomy performed, and bowel harvested from 15cm distal to the Ligament of Trietz. This was flushed with ice-cold phosphate buffered saline and opened along the antimesenteric border on a glass plate over ice. Mucosa was retrieved from two 6cm lengths of bowel by scraping with glass microscope slides.
Total RNA was extracted using a commercial isolation kit (mirVana, Ambion, Austin, TX) according to the manufacturer’s protocol. RNA was quantified using optical density ratios on a microplate reader (Spectramax M5, Molecular Devices, Sunnyvale, CA) and simultaneously reverse transcribed using a Superscript III kit (Invitrogen) and oligo(dT) priming.
The following transporters were studied (NCBI gene bank name is shown in Table 1): multidrug-resistance like protein 1, 2 and 3 (MDR1-3); multidrug resistance protein 1 and 3 (MRP1, 3); monocarboxylate transporter (MCT1); breast cancer resistance protein (BRCP); peptide transporter 1 (PepT1); organic cation transporter 2 (OCTN2); and organic anion transporter B (OATP-B). Primers were designed using rat sequences obtained from the NCBI gene bank database, and the BLAST tool used to ensure specificity. Primer sequences are presented in Table 1.
Table 1. List of primers used for qPCR.
Primer sequences used for quantitative PCR. The corresponding NCBI gene name is given alongside the protein name.
| Transporter | Gene Name | Sense | Primer Sequence |
|---|---|---|---|
| MDR1 | ABCB1 | Sense | 5′- GACGACCTTTTCACTCTCCG |
| Antisense | 5′- TCGTCAGACAGCCTCACATCTT | ||
| MDR3 | ABCB4 | Sense | 5′- ACAGAGGATTGCTATCGCCCG |
| Antisense | 5′- GACGTGGCTTCATCCAGCAG | ||
| MRP1 | ABCC1 | Sense | 5′- TTCATATCTGCTTCGTCACCG |
| Antisense | 5′- GGTAAACAGCACCCACCACAGC | ||
| MRP2 | ABCC2 | Sense | 5′- TCAGTACCAGATCCAGCTCCG |
| Antisense | 5′- TGGCCCAGACATGGTGAGAT | ||
| MRP3 | ABCC3 | Sense | 5′- CAGCCTAAACATTCAAATCCCG |
| Antisense | 5′- ACTTCCCACAGCCCACAGGT | ||
| BCRP | ABCG2 | Sense | 5′- AGGTTGGAACTCAGTTTACCCG |
| Antisense | 5′- GATGGAAGGGTCAGTGATGAGC | ||
| PepT1 | SLC15A1 | Sense | 5′- TGCACGTAGCACTGTCCATGA |
| Antisense | 5′- CAGGGCTTGATTCCTCCTGTAC | ||
| MCT1 | SLC16A1 | Sense | 5′- GCTGGCTGTCATGTATGCCG |
| Antisense | 5′- ACGGCTGCCATATTTATTCACCA | ||
| OCTN2 | SLC22A5 | Sense | 5′- AGGGCAGCAGAGATAGAATACCG |
| Antisense | 5′- CTATGTGTTGGCCTGGCTGTTG | ||
| OATP-B | SLCO2B1 | Sense | 5′- GGTTTCTCTTCTGCTGCCCG |
| Antisense | 5′- GACAGACCTGGTCCTGCTTTC |
For qPCR analysis, all samples were run in duplicate on a single 384-well plate for each transporter, using a SYBR green master mix (Applied Biosystems, Foster City, CA). Actin was measured as a loading control. qPCR was performed on an Applied Biosystems ABI 7900HT thermal cycler (2 minutes at 50°C, 10 minutes at 95°C, then 40 cycles of 15 seconds at 95°C followed by 1 minute at 60°C). A dissociation protocol was subsequently performed and melting curves analyzed to ensure a single amplicon.
Transporter expression (relative to other drug transporters) was calculated from mean cycle threshold (Ct) data. Then, for each sample the transporter mRNA signal was expressed relative to that of Actin. Post-hoc analysis of variance (ANOVA) was employed to assess significant changes across the diurnal cycle.
For further circadian statistical analysis, animals were analysed as if harvested with a single animal at 3-hourly intervals over a six-day period (cross-sectional analysis). This allowed assessment of the data using the cosinor procedure (Cosinor Periodicity, downloaded from www.circadian.org), assuming a period of 24±0.1 hour(12). Diurnal rhythmicity was considered significant with a P value <0.05. Mesor, amplitude and acrophase (peak change) were abstracted from the program output, and fold-change calculated from:
Our results showed that all transporters were expressed in jejunum. However, there was a wide range in expression levels, approximately 1000-fold, between different transporters. The order of expression was as follows:
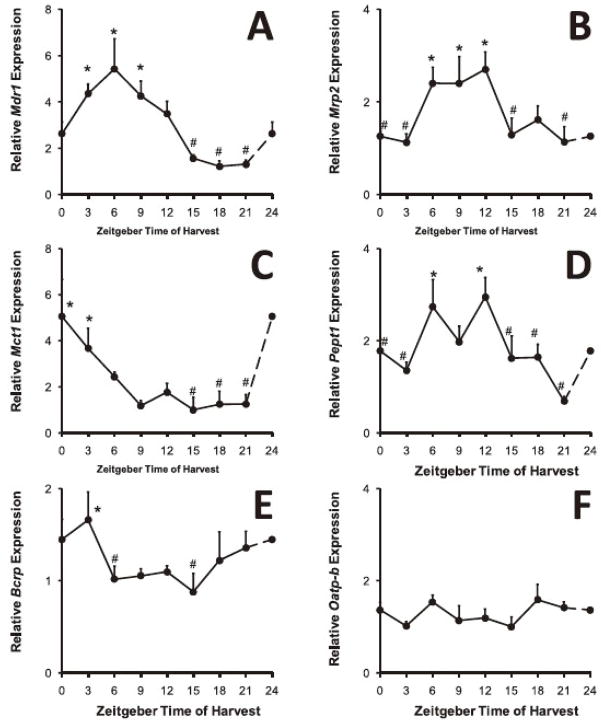
Using ANOVA, significant differences in expression (P<0.05) were identified in six of the ten transporters across the time periods studied (BCRP, MCT1, MDR1, MRP2, PepT1 and OCTN2, P<0.02). The peak levels of expression for these were all between ZT0 and ZT12, the daylight fasting period. Fold changes between maximum and minimum levels ranged from 1.9 to 5.1-fold. OATP-B, MRP1, MRP3 and MDR3 showed no significant diurnal variation. A representative graph, showing mRNA signal for MDR1, is displayed in Figure 1.
Fig. 1.
Time-dependent expression of intestinal drug transporters. Plots of relative mRNA signal against time for Mdr1 multidrug resistance like protein 1 (A), Mrp2 multidrug resistance protein 2 (B), Mct1 monocarboxylate transporter 1 (C), Pept1 peptide transporter 1 (D), and Bcrp breast cancer resistance protein (E). All show both significance on ANOVA between time points (* compared to # P<0.05 and an overall significant rhythm on cosinor analysis. In contrast, panel F shows expression of Oatp-b organic anion transporter B, which shows no significant variation on either ANOVA or cosinor analysis. The first time point (Zeitgeber time ZT0) has been repeated at ZT24 to complete the cycle.
With cosinor analysis, significant rhythms were observed in all transporters previously identified by ANOVA, with the exception of OCTN2. The acrophase (peak time) ranged from ZT1 to ZT10, again daylight fasting hours. The data are summarized in Table 2. In particular, we noted that those transporters displaying a rhythm showed a significantly higher expression (as determined by mean Ct value on qPCR) than those without a rhythm (P=0.047, Mann-Whitney non-parametric test).
Table 2. Summary data for jejunal drug transporters.
Table showing summary data for all the transporters studied. Also included in the table is the gene name as referenced in the NCBI database, and example substrates. The peak expression time (acrophase) is given according to Zeitgeber Time (ZT), where ZT0 is lights-on at 7am. Fold-changes are estimated from the cosinor amplitude, and differ from those observed on ANOVA. For a detailed review of transporter substrates, please see Reference (1).
| Transporter | Substrate Examples | Mean Ct on qPCR | Relative Expression | Peak | Fold Change | P Value |
|---|---|---|---|---|---|---|
| MDR1 | Small hydrophobic drugs, digoxin | 28.4 | ++++ | ZT7 | 5.4 | 0.000003 |
| MCT1 | Monocarboxylates, aspirin | 31.7 | ++ | ZT2 | 5.4 | 0.002 |
| MRP2 | Ceftriaxone, doxorubicin | 28.5 | ++++ | ZT10 | 2.5 | 0.001 |
| PepT1 | Small peptides, β-lactams | 28.3 | ++++ | ZT9 | 2.2 | 0.0048 |
| BCRP | Purine analogs, methotrexate | 26.5 | +++++ | ZT1 | 1.6 | 0.039 |
| OCTN2 | Verapamil | 28.6 | ++++ | None | N/A | 0.20 |
| MRP3 | Glucuronides, Vincristine | 31.5 | ++ | None | N/A | 0.30 |
| OATP-B | Organic anions, Pravastatin | 29.9 | +++ | None | N/A | 0.55 |
| MDR3 | Phospholipids, vinblastin | 34.4 | + | None | N/A | 0.84 |
| MRP1 | Hydrophobic peptides, vincristine | 34.2 | + | None | N/A | 0.93 |
We have confirmed detectable transcriptional signal of ten drug transport channels within the jejunum, with a broadly similar order of expression as previously described in human jejunum(4). We have also demonstrated that drug transporters from several different classes vary with a profound diurnal rhythmicity, at least at a transcriptional level. This is of importance for a number of reasons. Firstly, diurnal variations in drug metabolism and pharmacokinetics are well described. Diurnal rhythms in drug transporters expression may lead to variations in oral bioavailability of certain drugs, such as digoxin (transported by MDR1). Furthermore, variations in transporter expression may impact on metabolism of parenteral drugs through drug efflux mechanisms. For example, intestinal MDR1 clears approximately 11% of total intravenously delivered digoxin within 3 hours by secretion into the intestinal lumen(13). Synchronizing parenteral drug delivery in antiphase to intestinal transporter expression may reduce drug metabolism, promoting more steady-state drug kinetics.
Secondly, this establishes diurnal rhythms as a model for examining the regulation of several of the important drug transporters. For example, we have previously used diurnal rhythmicity as a model for examining the neuronal regulation of intestinal nutrient transporters(14). Similarly, it underlines the need to consider diurnal rhythmicity when investigating drug transporters in vivo, and therefore perform harvests across both peak and nadir expression times.
Lastly, most of the intestinal transporters we describe as possessing inherent diurnal rhythmicity at a transcriptional level (eg. MDR1, MRP2 and BCRP) were initially described as chemoresistance proteins over-expressed in cancer cell lines. It may be interesting to investigate whether these transporters display a diurnal rhythmicity in visceral cancers in vivo, as this would have clear therapeutic implications.
In this study, we have only studied transcriptional signal rather than protein or functional expression. However, there is good supporting evidence to suggest that protein expression will follow diurnal changes in transcription. Notably, diurnal rhythmicity of the peptide transporter PepT1 has been well characterized at transcriptional level as well as protein level. Indeed, the acrophase for PepT1 has shown remarkable consistency between studies(10). This is followed by peak protein expression and function around six to nine hours later. This pattern is repeated with intestinal glucose transporters, with similar peak times for transcription, protein and function(8).
Whilst there is no work to date to our knowledge documenting diurnal variation in any intestinal transporters in humans, there is abundant documentation of circadian rhythmicity in the gastrointestinal tract and virtually every other organ system in man. Furthermore, glucose transporters in a primate model vary with an identical pattern to rats but offset by 12 hours- as expected given Rhesus monkeys are diurnal feeders(11). We would therefore project that in humans the transcription for those intestinal drug transporters that vary diurnally would also be offset by twelve hours, peaking overnight during fasting.
In summary, we have demonstrated that a number of intestinal drug transporters vary on a transcriptional level with a profound diurnal rhythm. These transporters, namely MDR1, MRP2, BCRP, MCT1 and PepT1, probably have a significant impact both on the oral bioavailability of drugs, but also on the pharmacokinetics of parenteral drugs. Further work to characterize the protein and functional expression of these transporters is now required.
Acknowledgments
We thank Jan Rounds for invaluable managerial assistance. This study is supported by National Institute of Health 5 R01 DK047326 (SWA), Harvard Clinical Nutrition Center Grant P30-DK040561 (AT), March of Dimes #1-FY99-221 (DBR), Berkeley Fellowship (ATS) and the Nutricia Research Foundation (AB).
References
- 1.Chan LM, Lowes S, Hirst BH. The ABCs of drug transport in intestine and liver: efflux proteins limiting drug absorption and bioavailability. Eur J Pharm Sci. 2004;21 (1):25–51. doi: 10.1016/j.ejps.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97 (7):3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietrich CG, Geier A, Oude Elferink RP. ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut. 2003;52 (12):1788–1795. doi: 10.1136/gut.52.12.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taipalensuu J, Tornblom H, Lindberg G, Einarsson C, Sjoqvist F, Melhus H, et al. Correlation of gene expression of ten drug efflux proteins of the ATP-binding cassette transporter family in normal human jejunum and in human intestinal epithelial Caco-2 cell monolayers. J Pharmacol Exp Ther. 2001;299 (1):164–170. [PubMed] [Google Scholar]
- 5.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84 (21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemmer B. The clinical relevance of chronopharmacology in therapeutics. Pharmacol Res. 1996;33 (2):107–115. doi: 10.1006/phrs.1996.0016. [DOI] [PubMed] [Google Scholar]
- 7.Lake BG, Tredger JM, Burke MD, Chakraborty J, Bridges JW. The circadian variation of hepatic microsomal drug and steroid metabolism in the golden hamster. Chem Biol Interact. 1976;12 (1):81–90. doi: 10.1016/0009-2797(76)90069-7. [DOI] [PubMed] [Google Scholar]
- 8.Tavakkolizadeh A, Berger UV, Shen KR, Levitsky LL, Zinner MJ, Hediger MA, et al. Diurnal rhythmicity in intestinal SGLT-1 function, V(max), and mRNA expression topography. Am J Physiol Gastrointest Liver Physiol. 2001;280 (2):G209–215. doi: 10.1152/ajpgi.2001.280.2.G209. [DOI] [PubMed] [Google Scholar]
- 9.Balakrishnan A, Stearns AT, Rounds J, Irani J, Giuffrida M, Rhoads DB, et al. Diurnal rhythmicity in glucose uptake is mediated by temporal periodicity in the expression of the sodium-glucose cotransporter SGLT1. Surgery. 2008 doi: 10.1016/j.surg.2008.03.018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan X, Terada T, Irie M, Saito H, Inui K. Diurnal rhythm of H+-peptide cotransporter in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2002;283 (1):G57–64. doi: 10.1152/ajpgi.00545.2001. [DOI] [PubMed] [Google Scholar]
- 11.Rhoads DB, Rosenbaum DH, Unsal H, Isselbacher KJ, Levitsky LL. Circadian periodicity of intestinal Na+/glucose cotransporter 1 mRNA levels is transcriptionally regulated. J Biol Chem. 1998;273 (16):9510–9516. doi: 10.1074/jbc.273.16.9510. [DOI] [PubMed] [Google Scholar]
- 12.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6 (4):305–323. [PubMed] [Google Scholar]
- 13.Drescher S, Glaeser H, Murdter T, Hitzl M, Eichelbaum M, Fromm MF. P-glycoprotein-mediated intestinal and biliary digoxin transport in humans. Clin Pharmacol Ther. 2003;73 (3):223–231. doi: 10.1067/mcp.2003.27. [DOI] [PubMed] [Google Scholar]
- 14.Stearns AT, Balakrishnan A, Rounds J, Rhoads DB, Ashley SW, Tavakkolizadeh A. Capsaicin-sensitive vagal afferents modulate posttranscriptional regulation of the rat Na+/glucose cotransporter SGLT1. Am J Physiol Gastrointest Liver Physiol. 2008;294 (4):G1078–1083. doi: 10.1152/ajpgi.00591.2007. [DOI] [PubMed] [Google Scholar]