Abstract
Promising new drugs are being evaluated for treatment of multiple myeloma (MM), but their impact should be measured against the expected outcome in patients failing current therapies. However, the natural history of relapsed disease in the current era remains unclear. We studied 286 patients with relapsed MM, who were refractory to bortezomib and were relapsed, refractory, or ineligible, to an IMiD (Immunomodulatory Drug), with measurable disease and ECOG PS of 0, 1 or 2. The date patients satisfied the entry criteria was defined as time zero (T0). The median age at diagnosis was 58 years and time from diagnosis to T0 was 3.3 years. Following T0, 213 (74%) patients had a treatment recorded with one or more regimens (median=1; range 0-8). The first regimen contained bortezomib in 55 (26%) patients and an IMiD in 70 (33%). A minor response or better was seen to at least one therapy after T0 in 94 patients (51%) including >=partial response in 69 (38%). The median overall survival and event free survival from T0 were 9 and 5 months respectively. This study confirms the poor outcome once patients become refractory to current treatments. The results provide context for interpreting ongoing trials of new drugs.
Keywords: multiple Myeloma, relapse, natural history, survival
INTRODUCTION
Survival of patients with multiple myeloma (MM) has improved during the past decade with the introduction of Immunomodulatory Drugs (IMiDs; thalidomide and lenalidomide), and the proteasome inhibitor bortezomib.(1-10) However, MM remains incurable and new therapies are required for continued disease control. In fact, several new drugs are currently undergoing evaluation, and many appear promising based on initial results.(5, 11) One of the difficulties in interpreting the early results of these newer therapies from the small single arm studies has been the lack of information about natural history of MM in the relapsed patient population. While this type of information is available for patients receiving the older therapies, such data is lacking for patients relapsing after the new therapies. However, this information can be beneficial for development of new therapies as early and accurate identification of the most promising treatments can allow prioritization of current clinical trials. Hence, the International Myeloma Working Group (IMWG) undertook this current study with the aim of determining the outcome of patients who have become refractory to bortezomib and at least one of the IMiDs. We also wanted to assess the types of therapy administered in this patient group and the response rates and duration of response to these treatments, to establish a context for assessing the results of ongoing trials with new drugs in myeloma.
PATIENTS AND METHODS
Patients were identified by review of medical records at multiple centers from across United States, Europe, and Asia. Patients had to be refractory to bortezomib (administered either alone or in combination with other agents), defined as no response (less than partial response) while receiving therapy with a prior bortezomib-containing regimens or progression on or within 60 days of a bortezomib-containing regimen. In addition, patients should have relapsed and/or were refractory, intolerant, or ineligible (in the opinion of the treating physician) to receive treatment with an immunomodulatory drug (IMiD; thalidomide OR lenalidomide). The date they met this criteria was defined as time zero (T0). Given the goal of using this data as a benchmark for assessing future clinical trial results, we only included patients who would typically be considered for participation in a clinical trial. Hence, patients had to have ECOG performance status of 0, 1, or 2 as well as measurable disease at T0 (defined conventionally as a serum M protein ≥1.0 g/dL or 24 hour urine M-protein excretion ≥200 mg or bone marrow plasma cells ≥30%). Patients with prior allogeneic stem cell transplantation were excluded from the study.
Clinical and laboratory data pertaining to the time of diagnosis and from the time of individual relapses were obtained from clinical records. The dates of initiation and discontinuation of each treatment regimen as well as the reason for discontinuation were identified, with specific attention to confirm use and discontinuation of IMiDs and bortezomib due to emergence of resistance or toxicity. Detailed data collection sheets were developed, that were used at all the study sites for uniformity of data collection. The data were sent to a centralized area (Cancer Research And Biostatistics, Seattle, WA) for analysis in a de-identified fashion. Institutional Review Boards from each site approved the study and the use of patient medical records and was conducted in accordance with the principles of the Declaration of Helsinki.
The response categories were defined according to the EBMT or IMWG criteria, and the response rate was defined as the proportion of patients achieving at least a partial response, from among those patients with valid response data. Patients who did not receive a myeloma regimen following time zero were not included in the response rate analysis. The response rate and best response were calculated for each regimen used after T0. Duration of response was defined as the length of time between the date a patient first achieved a partial response or greater response level, following time zero and the earlier of the dates at which criteria for progression (defined by EBMT or IMWG criteria) were met or the date of death. Patients who did not have a documented progression after achieving at least a partial response and who were still alive at last contact were censored for duration of response at the date of last contact. Patients who did not achieve a partial response or better following T0, and patients for whom the date of such response was missing, were excluded from the duration of response analysis. Duration of response was estimated using the Kaplan-Meier method with the median duration of response summarized.
Overall survival (OS) was defined as the length of time between T0 and the date of death. Patients without a recorded death date were censored for OS at their last contact date. Progression-free survival (PFS) was defined as the length of time between T0 until the earlier of the date at which criteria for progression were met or the date of death. Patients who did not have a documented progression after T0 and who did not have a recorded death date were censored for PFS at their last contact date. OS and PFS were estimated using the Kaplan-Meier method with the median survival durations summarized. A Cox regression analysis was performed to determine which prognostic factors at T0 and/or at baseline were correlated with improved OS or PFS from T0. Prognostic factors were dichotomized, where appropriate, using standard myeloma cutoffs. Prognostic factors with univariate p-values < 0.100 were considered for inclusion in the multivariate model. The multivariate model used a stepwise selection with an entry level of p<0.10; with backwards elimination set at p<0.05.
Time to Next Treatment (TNT) was defined as the length of time between the start of the first regimen following T0 and the start of the second regimen following T0. Patients who started both a first and second regimen following T0, who do not have recorded start dates for these regimens, were excluded from this analysis. Patients who did not start a second regimen following T0 were censored for time to next treatment at the date of last contact. TNT was estimated using cumulative incidence methodology, with the median TNT summarized. Death preceding the start of a second regimen following T0 was treated as a competing risk. Additional TNT estimates were generated for subsequent regimens where a sufficient number of patients have recorded start dates for the required treatment regimens. All analyses were performed using SAS version 9.1.3.
RESULTS
Complete data were available on 286 patients (from among 300 patients enrolled) and were included in the current analysis. These included patients from 14 sites (107 pts from 3 US sites; 115 from 5 European sites; and 64 from 1 Asian site. The median (range) age for the patient group was 58 years (30, 85) at diagnosis and 62 (35, 87) at time zero, and 176 (62%) were male. The median estimated follow up for the entire cohort from diagnosis was 5.8 years (95% CI; 5.1, 6.3) and the median time from diagnosis to T0 was 3.3 years (range, 0.2-18.7). The baseline characteristics from diagnosis and from T0 are as shown in Table 1. In terms of prior therapy, by definition all patients had previous therapy with bortezomib and were considered refractory to bortezomib. With respect to prior IMiD exposure, 205 and 79 patients respectively met the entry criteria based on their previous treatment with thalidomide or lenalidomide. The eligibility reasons for the thalidomide patients were: 81 relapse, 23 refractory, 69 intolerant, 11 both relapse and refractory, 5 both refractory and intolerant, 5 both relapse and intolerant and 1 person was missing this information. The eligibility reasons for the Lenalidomide patients were: 37 relapse, 20 refractory, 9 intolerant, 8 both refractory and relapse, 1 both refractory and intolerant and 4 relapse and intolerant. The drug that patients were relapsing on or refractory to immediately prior to (or closest to) T0 was bortezomib in 73% and an IMiD in 27%.
Table 1.
Baseline characteristics at diagnosis and at Time zero (T0)
| Factor | n/N (%) | |
|---|---|---|
| Male | 176/286 (62%) | |
| Age >= 65 years at diagnosis | 69/284 (24%) | |
| Serum heavy chain at diagnosis | None | 27/250 (11%) |
| IgG | 155/250 (62%) | |
| IgA | 60/250 (24%) | |
| Durie Salmon stage at diagnosis | Stage 1 | 14/216 (6%) |
| Stage 2a | 47/216 (22%) | |
| Stage 3a | 152/216 (70%) | |
| International Staging System (ISS) at diagnosis |
Stage 1 | 63/208 (30%) |
| Stage 2 | 87/208 (42%) | |
| Stage 3 | 58/208 (28%) | |
| Diagnosis Creatinine > ULN | 84/212 (40%) | |
| No bone lesions at diagnosis | 63/256 (25%) | |
| >= 4 bone lesions at diagnosis | 102/256 (40%) | |
| Diagnosis FISH | All abnormalities | 63/95 (66%) |
| del 17p, t(4;14), t(14;16) | 21/95 (22%) | |
| 13q- | 41/95 (43%) | |
| t(11;14) | 9/95 (9%) | |
| Diag. cytogenetic abnormalities | 50/132 (38%) | |
| Time zero (T0) | ||
| Age >= 65 yr at time zero | 115/284 (40%) | |
| International Staging System (ISS) at T0 | Stage 1 | 31/172 (18%) |
| Stage 2 | 82/172 (48%) | |
| Stage 3 | 59/172 (34%) | |
| FISH at T0 | All abnormalities | 30/38 (79%) |
| del 17p, t(4;14), t(14;16) | 9/38 (26%) | |
| 13q- | 13/38 (34%) | |
| t(11;14) | 3/38 (8%) | |
| T0 cytogenetic abnormalities | 23/47 (49%) | |
| At least 1 transplant prior to T0 | 178/286 (62%) | |
| >= 2 transplants prior to T0 | 42/286 (15%) | |
n- Number with Factor, N- Number with Valid Data for Factor
Initial therapy following time zero
We first examined the types of therapy that were employed immediately following T0. Only 213 patients (74%) had a treatment identified in the medical records following T0 and the median time to first treatment following T0 was 0.5 months. The drugs utilized (alone or in combinations) for the initial treatment of the relapsed refractory disease are detailed in Table 2. Interestingly, in this group of patients who met the criteria for having bortezomib refractory disease, 55 patients (25%) received a bortezomib containing treatment regimen immediately following T0. Bortezomib alone or with dexamethasone was the most common bortezomib based regimen used (41%) followed by the combination of bortezomib, lenalidomide or thalidomide, and dexamethasone (17%). Thalidomide or lenalidomide was included in the initial treatment in 70 patients (32%). As would be expected, corticosteroids were part of the treatment in 157 (74%) patients, including 17 (8%) patients receiving steroids as single agents. Alkylating agents (melphalan and cyclophosphamide) was the most common class of drugs employed at this stage of the disease with 97 (46%) patients receiving a regimen that contained one of these drugs. Interestingly, 22 (11%) and 25 (12%) of patients received cisplatin and etoposide respectively, likely a reflection of use of regimens such as DT-PACE.
Table 2.
Response rate by regimen number, following time zero (T0)
|
Drugs included in the
regimen |
Regimen number following time zero (T0) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Number of patients | 213 | 90 | 49 | 27 | 18 |
| BCNU (Carmustine) | 4 (2) | 1 (1) | 2 (4) | 1 (4) | 0 (0) |
| Bortezomib | 55 (26) | 22 (24) | 19 (39) | 7 (26) | 8 (44) |
| Cisplatin | 22 (10) | 6 (7) | 3 (6) | 0 (0) | 2 (11) |
| Cyclophosphamide | 66 (31) | 22 (24) | 10 (20) | 6 (22) | 3 (17) |
|
Corticosteroids (part of
combination) |
140 (66) | 47 (52) | 26 (53) | 20 (74) | 9 (50) |
| Corticosteroids alone | 17 (8) | 6 (7) | 2 (4) | 1 (4) | 0 (0) |
| Doxorubicin | 43 (20) | 11 (12) | 6 (12) | 1 (4) | 3 (17) |
| Etoposide | 25 (12) | 4 (4) | 3 (6) | 0 (0) | 2 (11) |
| Lenalidomide | 41 (19) | 13 (14) | 8 (16) | 6 (22) | 3 (17) |
| Melphalan | 31 (15) | 15 (17) | 9 (18) | 7 (26) | 0 (0) |
| Thalidomide | 29 (14) | 15 (17) | 7 (14) | 3 (11) | 2(11) |
| Vincristine | 18 (8) | 3 (3) | 2 (4) | 2 (7) | 1 (6) |
| Best response (>=PR) % | 51/213 (24%) |
17/90 (19%) |
12/49 (24%) |
6/27 (22%) |
1/18 (6%) |
| Best response (>=MR) % | 73.213 (34%) |
25/90 (28%) |
14/49 (29%) |
8/27 (30%) |
3/18 (17%) |
|
Best Response with a regimen
containing bortezomib, lenalidomide or thalidomide %(number of patients) |
25/106 (24%) |
6/42 (14%) |
7/27 (26%) |
1/14 (7%) |
0/10 (0%) |
|
Best Response with a regimen
without bortezomib, lenalidomide or thalidomide %(number of patients) |
26/107 (24%) |
11/48 (23%) |
5/22 (23%) |
5/13 (38%) |
1/8 (13%) |
|
Median duration of treatment
(mos.) |
1.9 | 1.3 | 1.4 | 1.7 | 1.9 |
PR: partial response; MR: Minor response
Nearly a quarter of patients achieved a partial response or better to the first regimen used after T0 (50/213, 24%) including a very good partial response (VGPR) or better in 7% of the patients. Another 22 (7%) patients had a minor response and 36 (10%) had stable disease as their best response to the treatment. Nearly half of the patients (104; 49%) had progressive disease to the first line of therapy following T0 or a response was not assessable. The response rates and categories of responses observed are as detailed in Table 3. We also analyzed responses by regimen based on whether patients received a regimen containing the newer drugs (bortezomib, lenalidomide or thalidomide) or not. The response rate to the first treatment regimen was 24% among the 106 patients treated with a regimen containing a bortezomib, lenalidomide or thalidomide compared to 25% among the 107 patients receiving a regimen not containing one of these three drugs (Table 2). The breakdown of the response rates and the response categories for the newer drug containing regimen and those without these three drugs are provided in supplementary tables 1 and 2. The primary reasons for discontinuing the regimens are detailed in supplementary table 3. The most common reason for discontinuation of a treatment regimen was lack of response or disease progression followed by adverse event or completion of planned course of treatment. A clear reason for discontinuation could not be ascertained for about 17% of the regimens.
Table 3.
Best response to regimen, by regimen number, for the initial regimens following time zero
| Regimen | 1st | 2nd | 3rd | 4th | 5th |
|---|---|---|---|---|---|
| Factor | |||||
|
Number of
patients |
213 | 90 | 49 | 27 | 18 |
|
Complete
response |
4/213 (2%) | 1/90 (1%) | 0/49 (0%) | 1/27 (4%) | 0/18 (0%) |
|
Very good
partial response |
10/213 (5%) |
2/90 (2%) | 1/49 (2%) | 2/27 (7%) | 0/18 (0%) |
|
Partial
response |
36/213 (17%) |
14/90 (16%) |
11/49 (22%) |
3/27 (11%) | 1/18 (6%) |
| Minor response | 22/213 (10%) |
8/90 (9%) | 3/49 (6%) | 2/27 (7%) | 2/18 (11%) |
| Stable disease | 36/213 (17%) |
16/90 (18%) |
8/49 (16%) | 6/27 (22%) | 4/18 (22%) |
| Progression | 48/213 (23%) |
25/90 (28%) |
15/49 (31%) |
5/27 (19%) | 3/18 (17%) |
|
No or Unknown
response |
56/213 (26%) |
24/90 (27%) |
11/49 (22%) |
8/27 (30%) | 8/18 (44%) |
n/N (%): n- Numlber with Factor, N- Number with Valid Data for Factor
Subsequent therapies
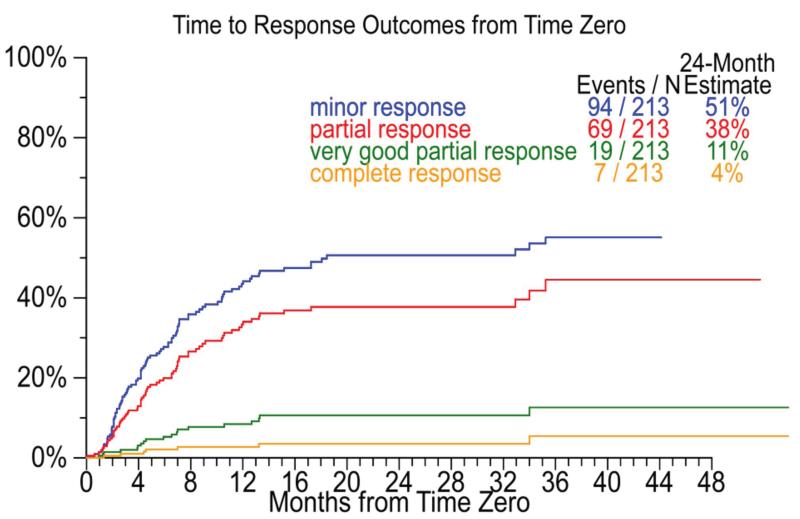
The subsequent drugs used for treatment within the different lines of therapy are detailed in Table 2, along with the best responses by regimen number (for the first five regimens) in Table 3. The median time to next treatment following the first regimen after T0 was 0.5 months. Interestingly, bortezomib and the IMiDs continued to be used in the subsequent regimens in a significant proportion of patients. Overall, 75 (35%), 51 (24%) and 63 (30%) patients received bortezomib, thalidomide or lenalidomide at some point after T0. The breakdown of the response categories for the newer drug containing regimen and those without these three drugs are provided in supplementary tables 1 and 2. Overall, 94 (44%) of patients had a minimal response or better including a partial response or better in 69 (32%) patients at some point during the post T0 period. The median times to achieving any degree of response are shown in Figure 1. The primary reasons for discontinuing the regimens are detailed in supplementary table 3 (supplementary data).
Figure 1.
Figure shows the time to response at any time after time zero (T0) for the different categories of responses among 213 patients who received at least one treatment after T0.
We also examined the frequency of use of high dose therapy and stem cell transplantation in this population. There were 44 patients who received a transplant after time zero, the median time to transplant was 96 days (approximately 3 months) with the first transplant received after 5 days and the last one received after 936 days (approximately 2 years and 5 months). Half of the patients who received a transplant after T0 received it between 37 days and 203 days. Among the 44 patients receiving a transplant after T0, this was the first transplant in 16 patients (i.e., no transplants done before T0).
Survival outcomes
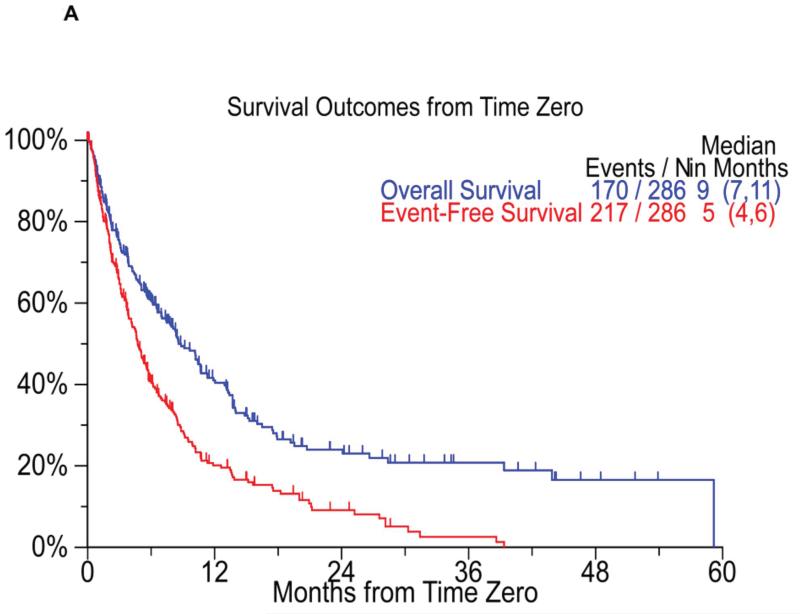
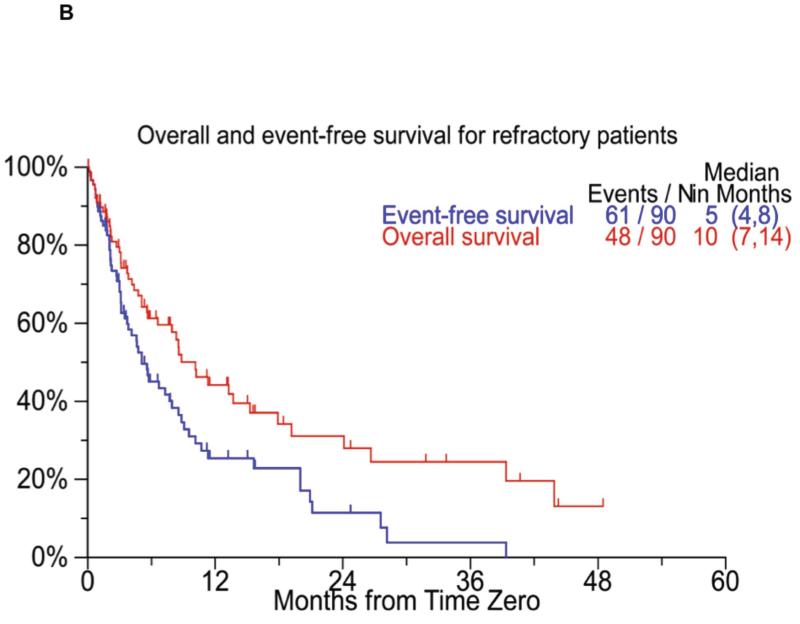
The median event free survival (EFS) for the entire cohort was 5 months (95% CI; 4, 6) from T0 and the median overall survival (OS) was 9 months (95% CI; 7, 11) from T0 (Figure 2A). The overall survival from diagnosis for the entire cohort was 56 months (95% CI; 44, 72). When considering only the patients considered refractory at T0 (n=90) the median EFS and OS from T0 was 5 months (95% CI; 4,8) and 10 months (95% CI; 7,14) respectively (Figure 2B). We also examined the overall survival from T0 based on whether the patients first met criteria for bortezomib refractoriness or the IMiD criteria for inclusion in the study. The median OS from T0 was 9 months (95% CI; 7,11) for patients meeting the bortezomib criteria first, compared to 9 months (95% CI; 7,13) for patients meeting criteria for IMiDs first (P=0.44). We also separately examined the outcome from the date they became refractory to bortezomib. The median overall survival from the time they were considered refractory to bortezomib (as defined for the purposes of the study) was 11 months (95% CI; 10,14). Similarly, the median overall survival from the date patients were considered to be relapsed/ refractory/ ineligible to an IMiD was 22 months (95% CI; 15, 26) for lenalidomide patients and 16 months (95% CI; 14, 22) for thalidomide patients. The median OS from the time they were refractory to anyone of the novel agent was 10 months (95% CI: 7, 14).
Figure 2.
Panel A shows the Kaplan Meier curves for event free survival (red curve, median 5 months) and overall survival (blue curve, median 9 months) from T0 all patients (n=286) enrolled on the study. Panel B shows the Kaplan Meier curves for event free survival (blue curve, median 5 months) and overall survival (red curve, median 10 months) from T0 for refractory patients (n=90).
The per regimen outcome of patients on this study is detailed in Table 4, which provides patient disposition data in terms of treatment status and survival at various time points from T0. The number of patients in each successive treatment regimen who died during that regimen, received another treatment, or are still receiving that regimen are shown in the Table. The median event-free survival (in months) for each regimen is shown in Table 4.
Table 4.
Patient experience by regimen (from initiation of each regimen)
| Regimen number since time zero | |||||
|---|---|---|---|---|---|
| First (n=213) |
Second (n=91) |
Third (n=49) |
Fourth (n=27) |
Fifth (n=18) |
|
| 1 mos. | 14/7/127/65 | 3/7/47/33 | 3/3/27/16 | 0/0/17/10 | 1/0/9/8 |
| 2 mos. | 30/19/93/71 | 9/15/30/36 | 6/11/17/15 | 1/3/9/14 | 2/3/5/8 |
| 3 mos. | 40/36/64/73 | 13/22/24/31 | 9/14/1214 | 2/4/9/12 | 2/5/4/7 |
| 4 mos. | 44/46/52/71 | 15/26/17/32 | 9/16/9/15 | 2/9/7/9 | 2/5/4/7 |
| 5 mos. | 48/59/36/70 | 17/31/12/30 | 9/16/8/16 | 2/12/5/8 | 2/5/3/8 |
| 6 mos. | 52/67/29/65 | 19/35/6/30 | 12/18/6/13 | 3/13/5/6 | 4/5/1/8 |
| 9 mos. | 60/74/13/66 | 20/42/4/24 | 13/21/4/11 | 4/18/0/5 | 4/7/0/7 |
| 12 mos. | 64/80/6/63 | 21/45/2/22 | 13/23/2/11 | 4/18/0/5 | 4/7/0/7 |
| 15 mos. | 68/84/5/56 | 22/46/2/20 | 13/24/1/11 | 4/18/0/5 | 4/7/0/7 |
| 18 mos. | 68/84/5/56 | 22/47/2/19 | 13/27/0/9 | 4/18/0/5 | 4/7/0/7 |
| 21 mos. | 68/86/4/55 | 22/48/1/19 | 13/27/0/9 | 4/18/0/5 | 4/7/0/7 |
| 24 mos. | 68/87/3/55 | 22/48/0/20 | 13/27/0/9 | 4/18/0/5 | 4/7/0/7 |
|
Median
EFS |
3.2 mos. | 2.6 mos. | 2.2 mos. | 4.6 mos. | 3.6 mos. |
XX/XX/XX =cumulative number of patients receiving regimen who died during treatment/cumulative number of patients started a new regimen/number of patients who were still on regimen/cumulative number of patients still alive who went off regimen and did not start another regimen
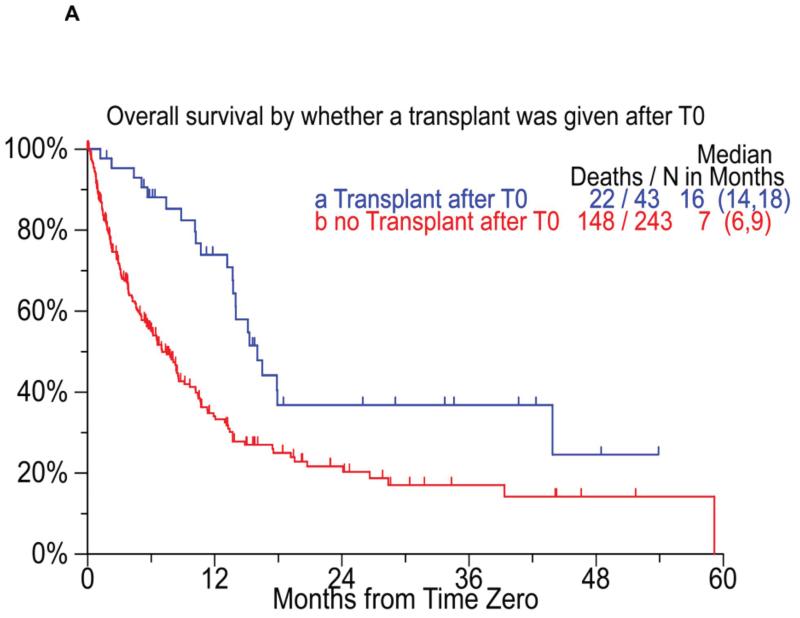
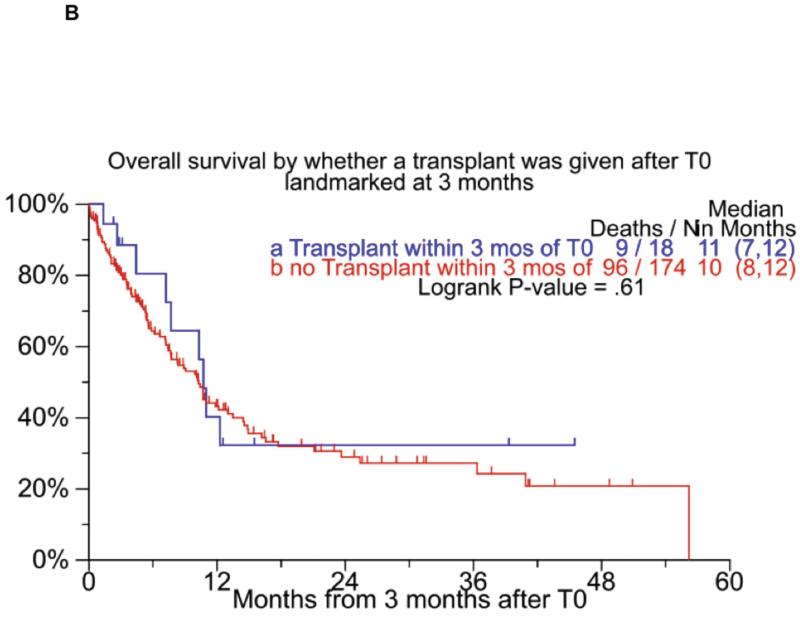
We also examined outcome on the basis of whether a transplant was performed following T0. The median overall survival following T0 among the 43 patients who had at least one transplant after T0 was 15 months (95% CI; 14, 18) compared to 7 months (95% CI; 6, 9) for patients without a transplant post T0 (Figure 3A). As the transplanted patients had a guaranteed survival time till they got to transplant, we also did a landmark analysis comparing patients who had a transplant within 3 months of T0 with patients who survived at least 3 months, but did not have a transplant during that time period, and found that the OS from T0 was comparable between the groups (Figure 3B). A similar analysis was performed using different time points after T0 for landmark (6, 9 and 12 months) and as with the previous analysis, no differences were seen in the OS from T0 based on whether a transplant was performed or not. Since transplant is often applied in a delayed fashion with comparable results as an early transplant, we separately examined the outcome of 16 patients who had received their first transplant after T0 as these patients likely represent those who opted for a delayed transplant. The median EFS and OS for these 16 patients were 13 months (95% CI; 10, 21) and 18 months (95% CI; 13, 44) respectively.
Figure 3.
Panel A shows the overall survival among patients who did or did not receive an autologous stem cell transplant at any time after T0. Panel B shows a similar comparison, but is landmarked at 3 months by considering only transplants done within 3 months from T0.
Prognostic Factors
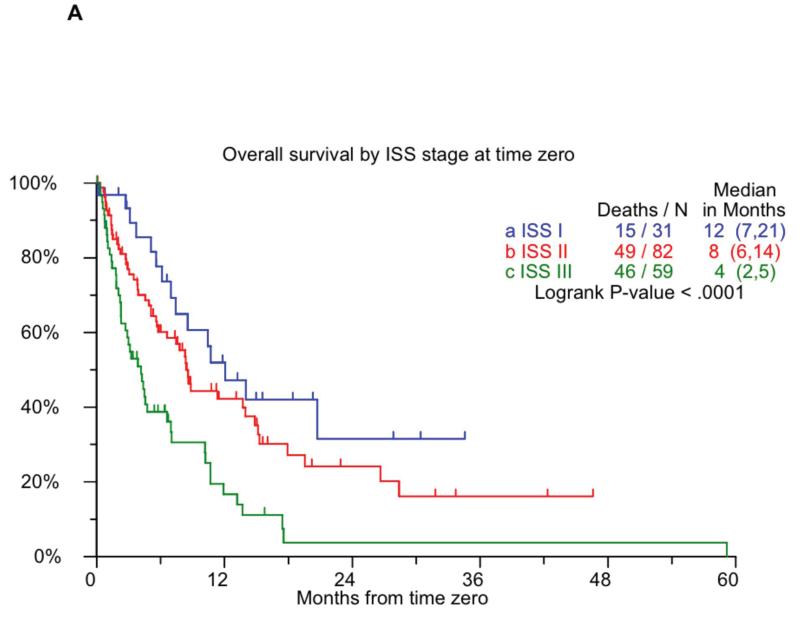
We performed additional analyses to identify prognostic factors predicting event free survival and overall survival following T0. Factors impacting the OS and EFS from T0 identified in a univariate analysis are shown in Table 5. In a multivariate model employing step wise selection that included most of these variables only B2M > 5.5 mg/L at T0 (HR: 3.58; P=0.047) and an albumin < 3.5 mg/dL at T0 (HR: 5.62; P=0.009) were independently significant for overall survival. Given that B2M and serum albumin, the two components of ISS, was prognostic for survival in this patients group, we examined the outcome based on ISS stage at T0. As shown in Figure 4A, the ISS stage was prognostic for overall survival following T0, with median survivals of 12, 8, and 4 months for ISS stages 1, 2, and 3 respectively. However, the ISS stage did not predict event free survival in this group.
Table 5.
Univariate analysis of prognostic factors for OS and EFS from T0
| OS from Time Zero | EFS from Time Zero | ||||
|---|---|---|---|---|---|
| Variable | n/N (%) | HR (95% CI) |
P- value |
HR (95% CI) |
P- value |
| At Diagnosis | |||||
| Serum heavy chain: None | 27/250 (11%) | 1.73 (1.03,2.89) |
0.038 | 1.51 (0.94,2.42) |
0.085 |
| Serum heavy chain: G | 155/250 (62%) | 0.62 (0.45,0.86) |
0.005 | 0.68 (0.51,0.91) |
0.010 |
| Serum heavy chain: A | 60/250 (24%) | 1.40 (0.98,2.01) |
0.064 | 1.27 (0.92,1.76) |
0.148 |
| B2M >= 3.5 mg/L | 123/226 (54%) | 1.59 (1.12,2.26) |
0.009 | 1.58 (1.15,2.16) |
0.004 |
| Platelet < 150,000/uL | 50/229 (22%) | 1.57 (1.06,2.32) |
0.024 | 1.20 (0.83,1.72) |
0.325 |
| FISH t(4;14) | 9/95 (9%) | 2.14 (0.90,5.10) |
0.086 | 2.15 (0.97,4.74) |
0.058 |
| Hypodiploidy | 14/132 (11%) | 1.86 (1.01,3.41) |
0.045 | 1.53 (0.85,2.77) |
0.158 |
| At Time zero | |||||
| Age >= 65 yr | 115/284 (40%) | 1.34 (0.98,1.82) |
0.063 | 1.11 (0.84,1.46) |
0.471 |
| Serum heavy chain: None | 23/176 (13%) | 1.86 (1.10,3.14) |
0.021 | 1.50 (0.91,2.46) |
0.114 |
| Serum heavy chain: G | 108/176 (61%) | 0.49 (0.33,0.74) |
<.001 | 0.58 (0.41,0.83) |
0.002 |
| Serum heavy chain: A | 41/176 (23%) | 1.69 (1.09,2.61) |
0.020 | 1.54 (1.04,2.27) |
0.029 |
| Albumin < 3.5 g/dL | 152/279 (54%) | 1.73 (1.26,2.37) |
<.001 | 1.47 (1.12,1.93) |
0.006 |
| B2M >= 3.5 mg/L | 108/173 (62%) | 2.36 (1.55,3.60) |
<.001 | 1.71 (1.20,2.44) |
0.003 |
| B2M > 5.5 mg/L | 59/173 (34%) | 2.20 (1.50,3.25) |
<.001 | 1.55 (1.09,2.21) |
0.015 |
| ISS Stage 3 | 59/172 (34%) | 2.24 (1.52,3.31) |
<.001 | 1.57 (1.10,2.24) |
0.013 |
| Creatinine > ULN | 64/185 (35%) | 2.19 (1.48,3.25) |
<.001 | 1.50 (1.06,2.11) |
0.022 |
| FISH t(14;16) | 3/38 (8%) | 5.04 (0.97,26.16) |
0.054 | 2.43 (0.54,10.98) |
0.250 |
| Time zero cytogenetic abnormalities |
23/47 (49%) | 3.71 (1.43,9.66) |
0.007 | 1.82 (0.93,3.55) |
0.080 |
| Time zero hypodiploidy | 12/47 (26%) | 3.57 (1.52,8.38) |
0.003 | 3.77 (1.72,8.27) |
<.001 |
| At least 1 transplant prior to time zero |
178/286 (62%) | 1.17 (0.85,1.61) |
0.331 | 1.29 (0.98,1.71) |
0.072 |
HR- Hazard Ratio, 95% CI- 95% Confidence Interval, P-value from Wald Chi-Square Test in Cox Regression
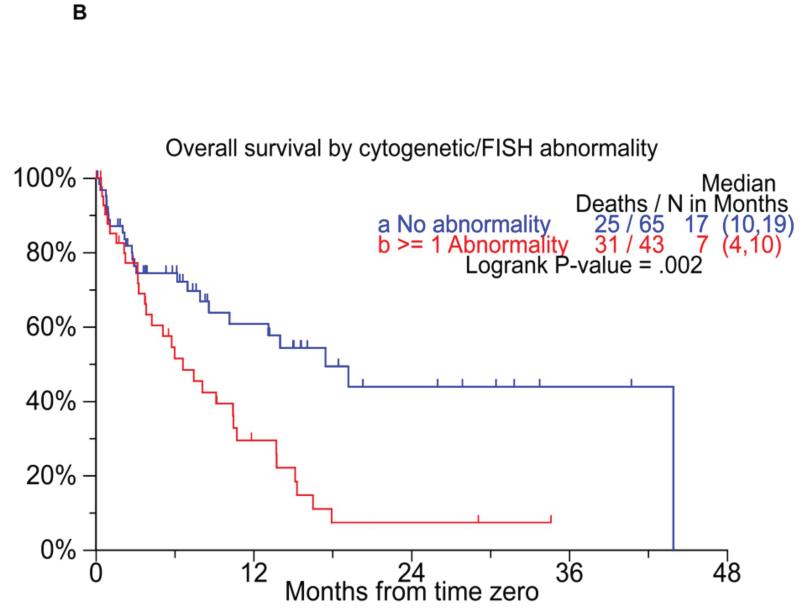
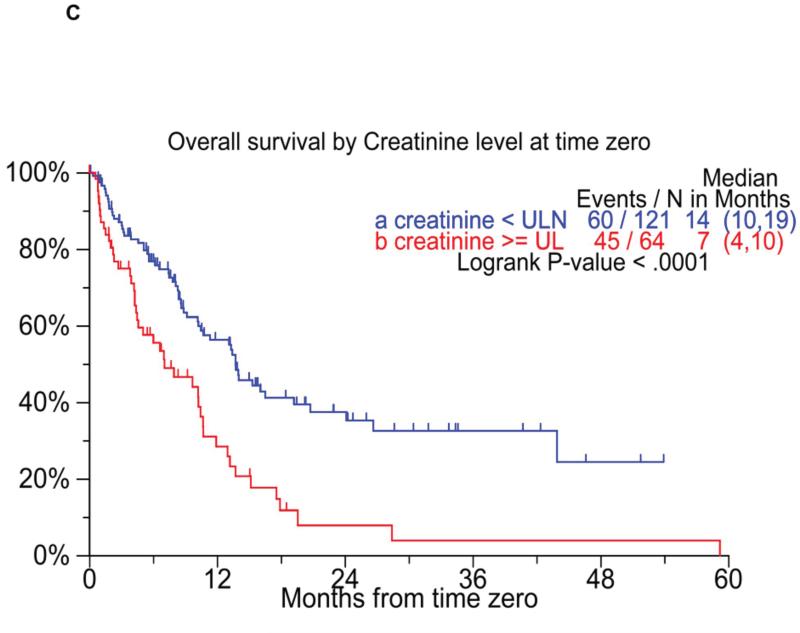
Figure 4.
Panel A shows the overall survival by ISS stage at T0. Panel B compares the overall survival following T0 among patients with with either t(4;14) or hypodiploidy, compared to the remaining patients. Panel C shows the overall survival among patients with elevated creatinine at T0, compared to the remaining patients.
We also specifically examined the prognostic value of cytogenetic features such as hypodiploidy, t(4,14), or del 17p on metaphase cytogenetics or FISH. High risk patients were identified by the presence of any of these three abnormalities identified at either diagnosis or at T0. Those with none of the abnormalities on cytogenetics/ FISH at either of the time points were considered as the standard risk. Patients with any of the high-risk abnormalities had both an inferior EFS as well as OS (Figure 4B) from T0. We also examined the prognostic value of serum creatinine; an elevated creatinine at T0 predicted for poorer EFS and OS from T0 (Figure 4C). Given that nearly 20% of the patients survive beyond 2 years, we specifically compared the baseline characteristics of those who survived beyond 2 years to those who died within 3 months of reaching T0. The results of the comparison, which is detailed in Supplementary table 4, demonstrated significant differences between the two groups in terms of lower B2M and less patients with ISS stage 3 both at diagnosis and at T0, normal creatinine at T0, and at least a partial response or better prior to T0 among the group with longer survival.
Given the differences in terms of speed of drug approval process and availability in different countries, we also separately examined the outcomes among patients seen at the centers in United States. Among the 107 patients from US sites, the median time from diagnosis to T0 was 4 years and these patients had a median of 3 therapies by T0. Of these patients, 99 (93%) had at least one therapy documented post T0 and the median (95% CI) EFS and OS from T0 was 5 (4,6) and 13 (10,16) respectively.
DISCUSSION
New developments in therapy over the past decade have changed the treatment paradigm for myeloma and resulted in significant improvement in survival.(9, 10, 12) However, myeloma remains incurable and new treatments are currently being studied. The results of the new drugs, especially those from the single arm trials, should be interpreted in the context of the expected outcomes in this group of patients. However, the rapid pace of development in the area of myeloma therapy has precluded a good understanding of the outcome among patients who have exhausted the currently available therapies. The natural history of relapsed myeloma has been studied previously, but before the new drugs became available. Specifically, one study included 578 patients with newly diagnosed MM who were followed up and monitored throughout their clinical course at a single institution between 1985 and 1998.(13) The overall survival (OS) for the 578 patients at 1, 2, and 5 years was 72%, 55%, and 22%, respectively; the median OS from initial therapy was 28.4 months. The median OS of 355 patients who had relapsed at the time of the analysis was 17.1 months from initiation of the second therapy, and 84% died within 5 years. This study revealed decreasing response duration with increasing number of salvage regimens, likely reflecting acquired drug resistance and an increasing proliferative rate of the myeloma cells. The median survival of patients who had 3 previous therapies in the initial trials of bortezomib for similar patients was 12 months compared to the 5 months seen in this study demonstrating clinically relevant activity for the drug.(14) Similarly, the overall survival of heavily pre-treated patients in the initial study of thalidomide demonstrated a 58% overall survival at 12 months, again demonstrating improvement over historical data.(15) However, with the improved survival due to the widespread use of IMiDs and bortezomib this data is not reflective of the current practice.
It is important to understand the clinical course of patients, who have become refractory to one or more of these agents and hence our study was focused on patients considered refractory to bortezomib and at least one of the IMiDs. However, these drug scan be used in combination with a variety of agents, giving rise to multitude of regimens and detailed information regarding the specific combinations these drugs were part of is not available. In the current study, we specifically enrolled patients who would be considered eligible for a clinical trial, by restricting to patients with good performance status and those with measurable disease at the time point where they would be considered refractory to bortezomib and to one of the IMiDs. The definitions for refractory disease were based on the recommendations of the ASH/FDA Panel on clinical endpoints in myeloma.(16) Patients eligible for clinical trials generally have better survival outcomes irrespective of diseases being studied(17, 18) and does limit the generalizability of the results to myeloma patient population as a whole; but at the same time allows better comparison with the current clinical trials. We also required only failure of either one of the IMiDs to be eligible for the study, taking into account the varied availability/ accessibility of the two drugs in different parts of the world. By incorporating patients from several large centers from different geographical regions, similar to what is often seen in the large multicenter trials, we hoped to overcome the effect of heterogeneity of clinical practice. By using a uniform approach, we have therefore sought to minimize the heterogeneity in reporting that can happen in a multicenter study such as this.(19, 20)
One of the most striking aspects of our finding has been the response rates seen in this patient population with the first regimen employed after they become refractory to the new drugs. The overall response seen in a third of the patients can be due to several factors. With the advent of the new drugs, older drugs such as alkylators are increasingly being relegated to later stages of disease. It has been shown in the setting of transplant, that patients relapsing after IMiD therapy can obtain comparable response duration with delayed transplant as with early transplant suggesting preservation of sensitivity of tumor cells to alkylators.(21) In fact, alkylators were the most common drugs employed for treatment of patients once they stopped responding the newer drugs in the current study. In addition, transplant is increasingly being used later in the disease course as well as second transplants as salvage therapy. In fact in the current study nearly 20% of patients received a transplant after T0, a third of which were first time transplants. Finally, many of the new drugs can be used again in patients who initially responded but had stopped responding to it, with variable degree of responses.(22) Bortezomib has activity with retreatment (22-25) and lenalidomide has significant activity in thalidomide refractory population.(6) As in this study, many of the current clinical trials include a similar mix of patients and the response rates seen in these phase 2 trials and phase 3 trials utilizing standard of care for control arm should be considered in the context of these findings. In contrast to previous studies, we do not see a progressive decline in response rates and duration of response.(13) This may be a reflection of increasing treatment choices that are available compared to a decade ago when alkylating agents and steroids formed the basis of myeloma treatment.(26) Also, some degree of selection bias leading to patients with better performance status as well as patients with more indolent disease being considered for multiple therapies cannot be excluded.
Despite the initial responses of over 30% in this group of relapsed and refractory patients, the median EFS of 5 months and OS of 9 months highlight the limited durability of these responses and the poor overall outcome among patients who are no longer responding to the existing newer therapies. This is consistent with recent reports showing poor outcome of patients refractory to IMiDs even in the context of SCT.(27) Another important finding from the study was the continued value of conventional prognostic factors in this patient group. Interestingly, the ISS staging parameters such as B2M and albumin at T0 best predicted survival outcome in this group of patients and should be taken into account when comparing results between different trials and could be incorporated as stratification factors in clinical trials of new drugs.(28) Unfortunately, limited data was available with respect to cytogenetic and FISH features in the current study. However, examination of the available data suggests retained prognostic value for these characteristics. Patients with high risk genetic abnormalities such as t(4;14), t(14;16) and hypodiploidy had shorter duration of responses and poorer overall survival compared to the other patients.(29-31) Similar to previous studies in the context of newly diagnosed disease, the presence of renal insufficiency predicted to poorer survival. This might to some extent reflect the lack of enrollment in clinical trials of patients with compromised renal function. Clearly the results presented here have some drawbacks, particularly the inability to study patients who are refractory to individual IMiDs, the prognostic value of all genetic risk factors in the context of specific therapies and the variations across different geographical areas based on clinical practices and drug availability. An ongoing study is recruiting additional patients to extend the current analyses.
In conclusion, the current study provides valuable insights into the natural history of myeloma after it become non-responsive to the current therapies. Clearly there are some disadvantages with the current study in terms of only including ‘trial eligible’ patients and lack of uniform availability of modern prognostic factors such as cytogenetic and FISH abnormalities. However, the results provide an important reference point for comparison of the results of the ongoing phase 2 and possibly phase 3 trials of new drugs in myeloma.
Supplementary Material
DISCLOSURES Authors Contributions: All authors (except JC, JH, JF and AH) provided patient data and were involved in manuscript preparation. JC, JH, JF and AH were involved in the statistical analysis.
CONFLICTS OF INTEREST
| Shaji Kumar | |
| Jae Hoon Lee | No COI |
| Juan J. Lahuerta | No COI. Scientific advisory boards Celgene and Janssen-Cilag |
| Gareth Morgan | No COI |
| Paul G. Richardson | NO COI. Advisory board participant member - Celgene, Millenium, Johnson and Johnson |
| John Crowley | None |
| Jeff Haessler | None |
| John Feather | None |
| Antje Hoering | None |
| Philippe Moreau | No COI |
| Xavier LeLeu | No COI |
| Cyrille Hullin | No COI |
| Saskia K. Klein | No COI |
| Pieter Sonneveld | No COI. |
| David Siegel | No COI. Speakers Bureau for Celgene and Millenium |
| Joan Bladé | No COI. Honoraria for lectures and advisory boards from Celgene, Jansen Cilag. Grant support from Celgene and Jansen- Cilag. |
| Hartmut Goldschmidt | No COI |
| Sundar Jagannath | No COI |
| Jesus San Miguel | No COI, Advisory Board participant Celgene, Millennium J&J |
| Robert Orlowski | No COI. |
| Antonio Palumbo | No COI. Advisory Board participant Celgene, Johnson & Johnson |
| Orhan Sezer | None |
| S. Vincent Rajkumar | No COI |
| Brian G.M. Durie | No COI. Advisory Board participant Celgene, Millennium |
***.
International Myeloma Working Group Niels Abildgaard, Syddansk Universitet, Odense, Denmark
Rafat Abonour, Indiana University School of Medicine, Indianapolis, Indiana, USA
Ray Alexanian, MD Anderson, Houston, Texas, USA
Melissa Alsina, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida, USA
Kenneth C. Anderson, DFCI, Boston, Massachusetts, USA
Michael Attal, Purpan Hospital, Toulouse, France
Hervé Avet-Loiseau, Institute de Biologie, Nantes, France
Ashraf Badros, University of Maryland, Baltimore, Maryland, USA
Dalsu Baris, National Cancer Institute, Bethesda, Maryland, USA
Bart Barlogie, M.I.R.T. UAMS Little Rock, Arkanas, USA
Régis Bataille, Institute de Biologie, Nantes, France
Meral Beksaç, Ankara University, Ankara, Turkey
Andrew Belch, Cross Cancer Institute, Alberta, Canada
Dina Ben-Yehuda, Hadassah University Hospital, Hadassah, Israel
Bill Bensinger, Fred Hutchinson Cancer Center, Seattle, Washington, USA
P. Leif Bergsagel, Mayo Clinic Scottsdale, Scottsdale, Arizona, USA
Jenny Bird, Bristol Haematology and Oncology Center, Bristol, UK
Joan Bladé, Hospital Clinica, Barcelona, Spain
Mario Boccadoro, University of Torino, Torino, Italy
Michele Cavo, Universita di Bologna, Bologna, Italy
Asher Chanan-Khan, Roswell Park Cancer Institute, Buffalo, New York USA
Wen Ming Chen, MM Research Center of Beijing, Beijing, China
Tony Child, Leeds General Hospital, Leeds, United Kingdom
James Chim, Department of Medicine, Queen Mary Hospital, Hong Kong
Wee-Joo Chng, National University Health System, Singapore
Ray Comenzo, Tufts Medical School, Boston, Massachusetts, USA
John Crowley, Cancer Research and Biostatistics, Seattle, Washington, USA
William Dalton, H. Lee Moffitt, Tampa, Florida, USA
Faith Davies, Royal Marsden Hospital, London, England
Cármino de Souza, Univeridade de Campinas, Caminas, Brazil
Michel Delforge, University Hospital Gasthuisberg, Leuven, Belgium
Meletios Dimopoulos, University of Athens School of Medicine, Athens, Greece
Angela Dispenzieri, Mayo Clinic, Rochester, Minnesota, USA
Johannes Drach, University of Vienna, Vienna, Austria
Matthew Drake, Mayo Clinic Rochester, Rochester, Minnesota, USA
Brian G.M. Durie, Cedars-Sinai Samuel Oschin Cancer Center, Los Angeles, California, USA
Hermann Einsele, Universitätsklinik Würzburg, Würzburg, Germany
Theirry Facon, Centre Hospitalier Regional Universitaire de Lille, Lille, France
Dorotea Fantl, Socieded Argentinade Hematolgia, Buenos Aires, Argentina
Jean-Paul Fermand, Hopitaux de Paris, Paris, France
Rafael Fonseca, Mayo Clinic Arizona, Scottsdale, Arizona, USA
Gösta Gahrton, Karolinska Institute for Medicine, Huddinge, Sweden
Ramón García-Sanz, University Hospital of Salamanca, Salamanca, Spain
Christina Gasparetto, Duke University Medical Center, Durham, North Carolina, USA
Morie Gertz, Mayo Clinic, Rochester, Minnesota, USA
John Gibson, Royal Prince Alfred Hospital, Sydney, Australia
Sergio Giralt, MD Anderson Cancer Center, Houston, Texas, USA
Hartmut Goldschmidt, University Hospital Heidelberg, Heidelberg, Germany
Philip Greipp, Mayo Clinic, Rochester, Minnesota, USA
Roman Hajek, Brno University, Brno, Czech Republic
Izhar Hardan, Tel Aviv University, Tel Aviv, Israel
Parameswaran Hari, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
Jean-Luc Harousseau, Institute de Biologie, Nantes, France
Hiroyuki Hata, Kumamoto University Hospital, Kumamoto, Japan
Yutaka Hattori, Keio University School of Medicine, Tokyo, Japan
Tom Heffner, Emory University, Atlanta, Georgia, USA
Joy Ho, Royal Prince Alfred Hospital, Sydney, Australia
Vania Hungria, Clinica San Germano, Sao Paolo, Brazil
Shinsuke Ida, Nagoya City University Medical School, Nagoya, Japan
Peter Jacobs, Constantiaberg Medi-Clinic, Plumstead, South Africa
Sundar Jagannath, Mt. Sinai Cancer Institute, New York, New York, USA
Hans Johnsen, AHSIC Aarhus University, Aalbor, Denmark
Hou Jian, Shanghai Chang Zheng Hospital, Shanghai, China
Douglas Joshua, Royal Prince Alfred Hospital, Sydney, Australia
Artur Jurczyszyn, The Myeloma Treatment Foundation, Poland
Michio Kawano, Yamaguchi University, Ube, Japan
Nicolaus Kröger, University Hospital Hamburg, Hamburg, Germany
Shaji Kumar, Department of Hematology, Mayo Clinic, Minnesota, USA
Robert A. Kyle, Department of Laboratory Med. and Pathology, Mayo Clinic, Minnesota, USA
Martha Lacy, Mayo Clinic Rochester, Rochester, Minnesota, USA
Juan José Lahuerta, Grupo Español di Mieloma, Hospital Universitario 12 de Octubre, Madrid, Spain
Ola Landgren, National Cancer Institute, Bethesda, Maryland, USA
Jacob Laubach, Dana-Farber Cancer Institute, Boston, Massachusetts, USA
Jae Hoon Lee, Gachon University Gil Hospital, Incheon, Korea
Xavier LeLeu, Hospital Huriez, CHRU Lille, France
Suzanne Lentzsch, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
Henk Lokhorst, University Medical CenterUtrecht, Utrecht, The Netherlands
Sagar Lonial, Emory University Medical School, Atlanta, Georgia, USA
Heinz Ludwig, Wilhelminenspital Der Stat Wien, Vienna, Austria
Angelo Maiolino, Rua fonte da Saudade, Rio de Janeiro, Brazil
María Mateos, University of Salamanca, Salamanca, Spain
Jayesh Mehta, Northwestern University, Chicago, Illinois, USA
Ulf-Henrik Mellqvist, Sahlgrenska University Hospital, Gothenburg, Sweden
GiamPaolo Merlini, University of Pavia, Pavia, Italy
Joseph Mikhael, Mayo Clinic Arizona, Scottsdale, Arizona, USA
Angelina Rodríguez Morales, Bonco Metro Politano de Sangre, Caracas, Venezuela
Philippe Moreau, University Hospital, Nantes, France
Gareth Morgan, Royal Marsden Hospital, London, England
Hareth Nari, Karolinska University Hospital, Stockholm, Sweden
Nikhil Munshi, Diane Farber Cancer Institute, Boston, Massachusetts, USA
Ruben Niesvizky, Weill Medical College of Cornell University, New York, New York, USA
Amara Nouel, Hospital Rutz y Paez, Bolivar, Venezuela
Yana Novis, Hospital SírioLibanês, Bela Vista, Brazil
Robert Orlowski, MD Anderson Cancer Center, Houston, Texas, USA
Antonio Palumbo, Cathedra Ematologia, Torino, Italy
Santiago Pavlovsky, Fundaleu, Buenos Aires, Argentina
Linda Pilarski, University of Alberta, Alberta, Canada
Raymond Powles, Leukemia & Myeloma, Wimbledon, England
Noopur Raje, Massachusetts General Hospital, Boston, Massachusetts, USA
S. Vincent Rajkumar, Mayo Clinic, Rochester, Minnesota, USA
Donna Reece, Princess Margaret Hospital, Toronto, Canada
Tony Reiman, Cross Cancer Institute, Alberta, Canada
Paul G. Richardson, Dana Farber Cancer Institute, Boston, Massachusetts, USA
David Roodman, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania USA
Laura Rosiñol, Hospital Clinic, Barcelona, Spain
Jesús San Miguel, University of Salamanca, Salamanca, Spain
Orhan Sezer, Universität Hamburg, Hamburg, Germany
Jatin J. Shah, MD Anderson Cancer Institute, Houston, Texas, USA
John Shaughnessy, M.I.R.T. UAMS, Little Rock, Arkansas, USA
Kazuyuki Shimizu, Nagoya City Midori General Hospital, Nagoya, Japan
Chaim Shustik, McGill University, Montreal, Canada
David Siegel, Hackensack, Cancer Center, Hackensack, New Jersey, USA
Seema Singhal, Northwestern University, Chicago, Illinois, USA
Pieter Sonneveld, Erasmus MC, Rotterdam, The Netherlands
Andrew Spencer, The Alfred Hospital, Melbourne, Australia
Edward Stadtmauer, University of Pennsylvania, Philadelphia, Pennsylvania, USA
Keith Stewart, Mayo Clinic Arizona, Scottsdale, Arizona, USA
Evangelos Terpos, University of Athens School of Medicine, Athens, Greece
Patrizia Tosi, Italian Cooperative Group, Istituto di Ematologia Seragnoli, Bologna, Italy
Guido Tricot, Huntsman Cancer Institute, Salt Lake City, Utah, USA
Ingemar Turesson, SKANE University Hospital, Malmo, Sweden
Ben Van Camp, Vrije Universiteit Brussels, Brussels, Belgium
Brian Van Ness, University of Minnesota, Minneapolis, Minnesota, USA
Ivan Van Riet, Brussels Vrija University, Brussels, Belgium
Isabelle Vande Broek, Vrije Universiteit Brussels, Brussels, Belgium
Karin Vanderkerken, Vrije University Brussels VUB, Brussels, Belgium
Robert Vescio, Cedars-Sinai Cancer Center, Los Angeles, California, USA
David Vesole, Hackensack Cancer Center, Hackensack, New Jersey, USA
Anders Waage, University Hospital, Trondheim, Norway NSMG
Michael Wang, MD Anderson, Houston, Texas, USA
Donna Weber, MD Anderson, Houston, Texas, USA
Jan Westin, Sahlgrenska University Hospital, Gothenburg, Sweden
Keith Wheatley, University of Birmingham, Birmingham, United Kingdom
Jeffrey Zonder, Karmanos Cancer Institute, Detroit, Michigan, USA
REFERENCES
- 1.Rajkumar SV, Blood E, Vesole DH, Fonseca R, Greipp PR. Phase III Clinical Trial of Thalidomide Plus Dexamethasone Compared With Dexamethasone Alone in Newly Diagnosed Multiple Myeloma: A Clinical Trial Coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Hayman S, Gertz MA, Dispenzieri A, Lacy MQ, Greipp PR, et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol. 2002;20:4319–4323. doi: 10.1200/JCO.2002.02.116. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar SV, Hayman SR, Lacy MQ, Dispenzieri A, Geyer SM, Kabat B, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. New England Journal of Medicine. 2005;352(24):2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 5.Lacy MQ, Hayman SR, Gertz MA, Dispenzieri A, Buadi F, Kumar S, et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009 Oct 20;27(30):5008–5014. doi: 10.1200/JCO.2009.23.6802. [DOI] [PubMed] [Google Scholar]
- 6.Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007 Nov 22;357(21):2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007 Nov 22;357(21):2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010 Jan;11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008 Mar 1;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008 Mar 1;111(5):2521–2526. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- 11.Badros A, Burger AM, Philip S, Niesvizky R, Kolla SS, Goloubeva O, et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009 Aug 15;15(16):5250–5257. doi: 10.1158/1078-0432.CCR-08-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar SK, Mikhael JR, Buadi FK, Dingli D, Dispenzieri A, Fonseca R, et al. Management of newly diagnosed symptomatic multiple myeloma: updated mayo stratification of myeloma and risk-adapted therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009 Dec;84(12):1095–1110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar SK, Therneau TM, Gertz MA, Lacy MQ, Dispenzieri A, Rajkumar SV, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004 Jul;79(7):867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 14.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003 Jun 26;348(26):2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 15.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999 Nov 18 341;(21):1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 16.Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P. Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008 Feb;22(2):231–239. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]
- 17.Davis S, Wright PW, Schulman SF, Hill LD, Pinkham RD, Johnson LP, et al. Participants in prospective, randomized clinical trials for resected non-small cell lung cancer have improved survival compared with nonparticipants in such trials. Cancer. 1985 Oct 1;56(7):1710–1718. doi: 10.1002/1097-0142(19851001)56:7<1710::aid-cncr2820560741>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Karjalainen S, Palva I. Do treatment protocols improve end results? A study of survival of patients with multiple myeloma in Finland. BMJ. 1989 Oct 28;299(6707):1069–1072. doi: 10.1136/bmj.299.6707.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998 Sep;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 20.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006 Sep;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Lacy MQ, Dispenzieri A, Buadi F, Hayman SR, Dingli D, et al. Novel Agents for Initial Therapy of Multiple Myeloma: Comparable Results with Continued Initial Therapy and Delayed Transplantation at Relapse Versus Early Transplantation. ASH Annual Meeting Abstracts 2009.Nov 20, 2009. p. 956. [Google Scholar]
- 22.Sood R, Carloss H, Kerr R, Lopez J, Lee M, Druck M, et al. Retreatment with bortezomib alone or in combination for patients with multiple myeloma following an initial response to bortezomib. Am J Hematol. 2009 Oct;84(10):657–660. doi: 10.1002/ajh.21517. [DOI] [PubMed] [Google Scholar]
- 23.Conner TM, Doan QD, Walters IB, LeBlanc AL, Beveridge RA. An observational, retrospective analysis of retreatment with bortezomib for multiple myeloma. Clin Lymphoma Myeloma. 2008 Jun;8(3):140–145. doi: 10.3816/CLM.2008.n.016. [DOI] [PubMed] [Google Scholar]
- 24.Warzocha K, Kraj M, Poglod R, Kwasniak B. Bortezomib in multiple myeloma: treatment and retreatment. A single center experience. Acta Pol Pharm. 2008 Nov-Dec;65(6):753–756. [PubMed] [Google Scholar]
- 25.Wolf J, Richardson PG, Schuster M, LeBlanc A, Walters IB, Battleman DS. Utility of bortezomib retreatment in relapsed or refractory multiple myeloma patients: a multicenter case series. Clin Adv Hematol Oncol. 2008 Oct;6(10):755–760. [PubMed] [Google Scholar]
- 26.Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. Myeloma Trialists’ Collaborative Group. J Clin Oncol. 1998 Dec;16(12):3832–3842. doi: 10.1200/JCO.1998.16.12.3832. [DOI] [PubMed] [Google Scholar]
- 27.Gertz MA, Kumar S, Lacy MQ, Dispenzieri A, Dingli D, Hayman SR, et al. Stem cell transplant in multiple myeloma: impact of response failure with thalidomide or lenalidomide induction. Blood. 2010 Jan 20; doi: 10.1182/blood-2009-07-235531. [DOI] [PubMed] [Google Scholar]
- 28.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005 May 20;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 29.Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009 Dec;23(12):2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA, et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood. 2003 Jun 1;101(11):4569–4575. doi: 10.1182/blood-2002-10-3017. [DOI] [PubMed] [Google Scholar]
- 31.Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007 Apr 15;109(8):3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.