Abstract
An aortic aneurysm (AA) is a silent but life-threatening disease that involves rupture. It occurs mainly in aging and severe atherosclerotic damage of the aortic wall. Even though surgical intervention is effective to prevent rupture, surgery for the thoracic and thoraco-abdominal aorta is an invasive procedure with high mortality and morbidity. Therefore, an alternative strategy for treatment of AA is required. Recently, the molecular pathology of AA has been clarified. AA is caused by an imbalance between the synthesis and degradation of extracellular matrices in the aortic wall. Chronic inflammation enhances the degradation of matrices directly and indirectly, making control of the chronic inflammation crucial for aneurysmal development. Meanwhile, mesenchymal stem cells (MSCs) are known to be obtained from an adult population and to differentiate into various types of cells. In addition, MSCs have not only the potential anti-inflammatory and immunosuppressive properties but also can be recruited into damaged tissue. MSCs have been widely used as a source for cell therapy to treat various diseases involving graft-versus-host disease, stroke, myocardial infarction, and chronic inflammatory disease such as Crohn’s disease clinically. Therefore, administration of MSCs might be available to treat AA using anti-inflammatory and immnosuppressive properties. This review provides a summary of several studies on “Cell Therapy for Aortic Aneurysm” including our recent data, and we also discuss the possibility of this kind of treatment.
Keywords: Aortic aneurysm, Mesenchymal stem cells, Cell therapy, Elastin, Chronic inflammation, Extracellular matrices, Macrophages, Matrix metalloproteinases
Core tip: Aortic aneurysm (AA) is caused by an imbalance between synthesis and degradation of extracellular matrices (ECMs) such as collagen and elastin in the aortic wall. The chronic inflammation enhances the degradation of ECMs directly and indirectly. We hypothesized that administration of mesenchymal stem cells (MSCs) might be able to treat AA given the anti-inflammatory and immune-suppressive potential of MSC. In this article, we review papers that attempt to treat AA using MSCs with our recent results, as well as review the molecular pathogenesis of AA and characteristics of MSC.
INTRODUCTION
Trend of aortic aneurysm
An aortic aneurysm (AA) occurs mainly in aging and chronic inflammation associated with atherosclerosis. It is a common and silent disease but also a life-threatening one involving rupture. AA has an incidence of 6%-9% in men over the age of 65 in abdominal aorta[1,2]. AA larger than 55 mm in diameter in the abdominal aorta and 60 mm in diameter in the thoracic aorta increase the risk of rupture. Therefore, patients of the kind require surgical intervention such as prosthetic graft replacement to prevent rupture[3]. However, surgery for thoracic and thoraco-abdominal aorta is a highly invasive procedure with high mortality and morbidity rate. On the other hand, abdominal or thoracic endovascular aneurysm repair (EVAR, TEVAR), which are catheter-based interventions, called internal aortic stent grafting, might be used for conventional surgically inapplicable patients with a high risk for surgical repair. However, EVAR and TEVAR have drawbacks such as limitations of anatomic and clinical criteria, complications of endoleaks, and graft migrations[4]. Thus, an alternative less invasive strategy is required for treatment of AA.
Development of medical treatment for aortic aneurysm
Recently, the molecular pathology of AA has been clarified, and control of chronic inflammation is crucial for AA progression. AA is caused by an imbalance between synthesis and degradation of the extracellular matrices (ECMs) such as collagen and elastin in the aortic wall. Chronic inflammation enhances the degradation of ECMs directly and indirectly. Therefore, control of inflammation may be an alternative strategy for treatment of AA. A number of experimental investigations and clinical studies have attempted to treat AA using various drugs and factors to control the inflammation; for example, angiotensin converting enzyme inhibitor and statin associated with reduced abdominal AA (AAA) rupture in a case-control study[5,6], doxycycline decrease in aneurysmal expansion rate in an experimental model[7] and in a randomized double-blinded clinical trial[8], nonsteroidal anti-inflammatory drugs decrease AAA expansion rate in a case control study[9], and c-jun N-terminal kinase inhibitor regresses AAA in a CaCl2-treated mice model[10]. However, these pharmacotherapies have still not been established for clinical application because of their array of side effects caused by systemic administration of these agents. Another disadvantage of using these agents is that special equipment might be required to deliver them locally for treatment of AA.
Mesenchymal stem cell therapy
Meanwhile, the recent progress in stem cell research in regenerative medicine is remarkable. Stem cell is one of the most important cell sources for treatment of damaged organs using regenerative technology. Mesenchymal stem cells (MSCs) can be obtained from adult tissue such as bone marrow[11,12], adipose tissue[13,14] and others. MSCs can be differentiated into various types of cells such as osteoblast, adipocyte and chondrocyte. In addition, MSCs have anti-inflammatory and immunosuppressive properties as well that can be recruited into damaged tissue[15,16]. By utilizing their unique potential, MSCs have been widely used as a cell source for cell therapy to treat various diseases involving graft-versus-host disease, stroke, myocardial infarction (MI), and chronic inflammatory disease such as Crohn’s disease clinically[17-21].
In this article, we review papers that attempt to treat AA using MSCs with our recent results, and we also discussed the update status of the molecular pathogenesis of AA and characteristics of MSC.
MOLECULAR PATHOGENESIS OF AORTIC ANEURYSMS
The molecular pathology of AA is a failure in the balance between synthesis and degradation of ECMs in the aortic wall. These phenomena are induced by chronic inflammation associated with atherosclerosis. Aortic ECMs are mainly composed of elastin and collagen and play an important role in the aortic strength and flexibility to withstand arterial blood pressure. Especially, elastin is a major fibrillar component in the arterial wall, and destruction of elastin fiber directly leads to expansion of AA[22]. Elastin polypeptide is known to be synthesized by vascular smooth muscle cells (VSMCs)[23], and its gene expression is modulated by transforming growth factor (TGF)-β1 and insulin-like growth factor (IGF)-1[24,25]. On the other hand, degradation of ECMs is caused by mainly secretion and activation of matrix metalloproteinases (MMPs), leading to the weakening of the aortic wall. In particular, MMP-2 and MMP-9 are known as a powerful proteinase that degrades elastin fiber, and they are secreted from macrophages which have infiltrated the inflammatory site[26,27]. Macrophage plays a major role of inflammatory cells in the development and progression of AA, and also secretes various cytokines, chemokines and proteinases. Many studies have been reported that interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α and monocyte chemotactic protein (MCP)-1 were up-regulated in the AA wall of human or experimental animal aortic aneurysm[28-30]. These cytokines and chemokines induce recruitment of monocytes[31], apoptosis of VSMCs[32] and regulation of MMP secretion[33]. On the other hand, failure of ECM synthesis is reportedly due to a disability of ECM synthesis and decrease of cell number by apoptosis of VSMCs in the AA wall[34]. Therefore, the inhibition of excessive inflammation and the recovery of ECM synthesis are key factors for treatment of AA.
DETERMINATION OF MSCS
Surface maker of the MSCs
MSC is one of the adult somatic stem cells which can be isolated from adult organs including bone marrow and adipose tissue[13,35]. Early in culture, the spindle-shaped plastic-adherent cells do not appear uniformly by contamination of hematopoietic cells, but this heterogeneity gradually decreases influenced by culture conditions and consecutive passages[36,37]. The International Society of Cell Therapy criteria propose (ISCT) that human MSCs should be positive for the expression of CD73, CD90 and CD105 (≥ 95% positive), and lack expression of CD34, CD45, CD11b or CD14, CD19 or CD79α, and HLA-DR (≤ 2% positive). Also, MSC should differentiate into osteogenic, adipogenic and chondrogenic lineage (Table 1)[38]. However, CD73 and CD105 are also expressed on fibroblast and endothelial lineage cells and CD90 is also expressed on haematopoietic stem cells[39,40]. To improve purity of the human MSC population, several studies have been performed using a combination such as Stro-1, CD271, CD146 and PDGFR-α, not only CD73, CD90 or CD105[41-43].
Table 1.
Mesenchymal stem cells phenotypic characteristics
| Positive marker | Negative marker | Pluripotency | Ref. | ||
| ISCT criteria | Human MSC | CD73, CD90, CD105 | CD34, CD45, CD11b or CD14, CD19 or CD79α, HLA-DR | Osteogenic Chondrogenic Adipogenic | [38] |
| In AA experimental studies | Mouse BM-MSC | CD44, CD106, Sca-1 | CD11b, CD31, CD34, CD45, CD86, CD117 | Osteogenic Chondrogenic Adipogenic | [51,53] |
| Human placental-MSC | CD29, CD44, CD73, CD90, CD105 | CD14, CD19, CD34, CD45, HLA-DR | Data not shown | [54] | |
| Rat BM-MSC | CD44, CD73, CD90, CD105 | CD11b, CD45 | Data not shown | [56] | |
| Pig ASC | CD73, CD90, CD105 | CD14, CD11b | Osteogenic Chondrogenic Adipogenic | [57] | |
| Pig BM-MSC | CD13, CD29 | CD31, CD34, CD45 | Data not shown | [58] |
ISCT: International society of cell therapy; MSC: Mesenchymal stem cell; AA: Aortic aneurysm; BM-MSC: Bone marrow-derived MSC; HLA-DR: Human leukocyte antigen-DR; ASC: Adipose tissue-derived MSC.
Migration mechanism of MSCs
Through a CXCR4 signaling pathway of damaged tissue stimuli migration and activation of MSC via stromal cell-derived factor-1, MSCs are known to accumulate in damaged tissue sites[44]. In addition, it also has been reported that the migration of MSCs is accelerated through up-regulation of pro-MMP-2 and membrane-type 1-MMP complex by stimulation of the inflammatory cytokines IL-1β[45,46].
Immunosuppression and anti-inflammation properties of MSCs
MSCs have the capability of immunosuppression and anti-inflammation properties. Several investigations were reported regarding the mechanisms of immunosuppression and anti-inflammation of MSCs. MSCs do not express the costimulatory molecules CD80, CD86 and CD40, which have been identified to play a role in the initiation of immune responses by T and B lymphocytes[47,48]. Also, MSCs can inhibit activation of T-cells immune response and proliferation by expression of indoleamine 2,3-dioxygenase, which degrades tryptophan and suppresses T-cell proliferation. Moreover, MSCs reduce the secretion of interferon (IFN)-γ, which regulates several aspects of the immune response, from T-helper 1 (Th1) cells, and conversely increase secretion of IL-4, which plays a central role in the inhibitory regulation of immune response, from Th2 cells. In addition, MSCs inhibit proliferation of natural killer cells through soluble factor prostaglandin E2 (PGE2), which inhibits actions on T cells depending on their maturation and activation state, and TGF-β which were secreted from MSC, and reduce the proinflammatory potential of dendritic cell-1 (DC1) by inhibition of their secretion TNF-α, IFN-γ and IL-12 and conversely increase IL-10 secretion from DC-2[49,50].
TREATMENT OF AORTIC ANEURYSMS USING MSCs
Recently, several studies using MSCs as a cell source for treatment of AA have been reported including our own studies. Published experimental studies were summarized in Table 2.
Table 2.
Animal studies for treatment of aortic aneurysmusing mesenchymal stem cells
| Experimental AA model | Cell source | Number of cells | Injection time | Delivery | Efficiency | Ref. |
| ATII-infusion mouse model | BM-MSC | Cell-sheet | Same time as ATII-infusion | Implantation of MSC-sheet around infrarenal aorta | 4 wk after implantation, inhibition of AA development and growth, and elastin degradation downregulation of IL-1β, IL-6, MCP-1 and TNF-α protein expression, and MMP-2 and -9 enzymatic activity Up-regulation of IGF-1 and TIMP-1 protein expression Positive for MSC specific surface marker | [51] |
| ATII-infusion mouse model | BM-MSC | 1 × 106/every week, 4 times | Same time as ATII-infusion | iv-administration | 4 wk after injection, inhibition of AA development and growth, elastin degradation, Mφ infiltration downregulation of IL-1β, IL-6 and MCP-1 protein expression, and MMP-2 and -9 enzymatic activity Up-regulation of IGF-1 and TIMP-1 protein expression Detection of MSC in the aortic wall | [53] |
| Elastase-perfusion mouse model | Placental-MSC | 1 × 106 | 1 d after elastase-perfusion | iv injection | 2 wk after injection, inhibition of AA expansion, inflammatory cell infiltration, and elastin degradation, downregulation of IL-17, IL-23, INF-γ, TNF-α, RANTES and MCP-1 protein expression Increase of α-SMA expression | [54] |
| Xenograft rat model | BM-MSC | 1 × 106 | Same time as surgical intervention | Catheter | 1 wk after surgical intervention inhibition of inflammatory cells infiltration and MMP-9 gene expression, and increase of TIMP-1 gene expression, after 4 wk, inhibition of AA expansion, increase of α-SMA expression, elastin and collagen content | [56] |
| Dacron-patch pig model | ASC | 1 × 106 | Same time as surgical intervention | Catheter | Attenuation of inflammation reaction, detection of ASC 3 wk after surgical intervention | [57] |
| Balloon injury with type 1 collagen and elastase-perfusion porcine model | BM-MSC | 1 × 106 | Same time as balloon-injury | Direct injection into aortic wall | 72 h after injection, Increase of VEGF-A mRNA expression level 1 wk after injection, detection of GFP-labeled MSC at aortic wall and vWF positive cells formed tubuloluminal structures within outer layer of media and throughout the adventitia | [54] |
AA: Aortic aneurysm; ATII: Angiotensin II; MSC: Mesenchymal stem cell; BM-MSC: Bone marrow-derived MSC; iv: Intravenous; VEGF: Vascular endothelial growth factor; GFP: Green fluorescent protein; TIMP-1: Tissue inhibitor of metalloproteinase 1; MMP: Matrix metalloproteinases; MCP-1: Monocyte chemotactic protein 1; IL: Interleukin; TNF: Tumor necrosis factor; IGF: Insulin-like growth factor; IFN: Interferon; ASC: Adipose tissue-derived MSC; vWF: von Willebrand factor.
Implantation of bone marrow-derived MSC cell-sheet for aortic aneurysm
We earlier reported that AA formation and growth were attenuated by intraperitoneal implantation of bone marrow-derived MSC (BM-MSC) cell-sheet using an angiotensin II (ATII)-infused apolipoprotein E-deficient (apoE-/-) mouse model[51]. The BM-MSC cell-sheet was prepared using an Upcell® which is a thermoresponsive polymer-grafted dish surface, and the BM-MSC cell-sheet was implanted into the nearby abdominal aortic adventitia at the time of implantation of Alzet osmotic mini-pump to infuse the ATII (Figure 1). Four weeks after implantation of BM-MSC cell-sheet, the aortic diameter of the BM-MSC cell-sheet implanted group was significantly lower than that of the apoE-/- + ATII group at the infrarenal aorta (Figure 2A). The enzymatic activities of MMP-2 and MMP-9 were suppressed in the BM-MSC cell-sheet implanted mice group. The downregulation of MMP enzymatic activity may be influenced via the paracrine effect of soluble factors secreted from BM-MSC because we showed that gene expression of MMPs in macrophages was decreased by indirect co-culture with BM-MSCs in vitro in this paper. In addition, the protein expression of tissue inhibitor of metalloproteinase (TIMP)-1 was increased in the BM-MSC cell-sheet implanted group. The BM-MSC cell-sheet implanted group also showed decreased inflammatory cytokines including IL-6, MCP-1 and TNF-α. These results suggested that BM-MSC cell-sheet might suppress the excess inflammatory reaction which caused ATII-induced AA. On the other hand, degradation of elastin was inhibited by implantation of the BM-MSC cell-sheet compared with control. This result could be supported by the increase of the gene expression of elastin in VSMCs co-cultured with BM-MSCs in vitro. Moreover, the protein expression of IGF-1 and TIMP-1 in AA tissue with BM-MSC cell-sheet implantation was deemed to be in a paracrine manner, because the IGF-1 and TIMP-1 are identified to be present in the condition medium of MSCs[46,52]. Our study showed a new approach by treating AA through implantation of BM-MSC cell-sheet. However, such implantation using laparotomy is a relatively invasive procedure, even less invasive than prosthetic graft replacement for AA.
Figure 1.
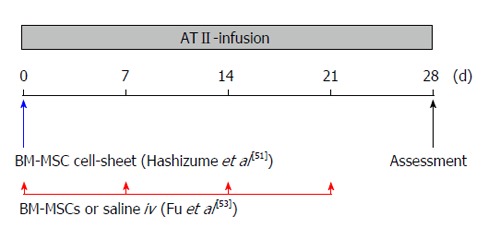
Diagram of bone marrow-derived mesenchymal stem cell cell-sheet implantation or bone marrow-derived mesenchymal stem cell intravenous-administration protocol. At the time of Alzet osmotic minipump implantation, the BM-MSC cell-sheet was implanted into the nearby abdominal aortic adventitia[51], and 1 × 106 BM-MSCs (in 0.2 mL saline) or 0.2 mL saline were injected intravenously via the tail vein every week[53]. Mice were sacrificed and assessed on day 28. ATII: Angiotensin II; iv: Intravenous; BM-MSC: Bone marrow-derived mesenchymal stem cell.
Figure 2.

Bone marrow-derived mesenchymal stem cell cell-sheet implantation or bone marrow-derived mesenchymal stem cell IV-administration attenuates aortic aneurysm progression and expansion. Aortic diameter was measured at the infrarenal aorta in the bone marrow-derived mesenchymal stem cell (BM-MSC) cell-sheet (A) or the BM-MSC IV-administration. Data are assessed by one-way ANOVA with Bonferroni correction. aP < 0.05 vs apoE-/- group, cP < 0.05 vs apoE-/- + ATII group. Data are from Hashizume et al[51] and Fu et al[53]. ATII: Angiotensin II; iv: Intravenous.
Intravenous administration for aortic aneurysm
To treat AA by a less-invasive BM-MSC delivery, we demonstrated multiple intravenous (iv) administration of BM-MSC for an ATII-infusion AA mouse model[53]. At the time of Alzet osmotic minipump implantation, 1 × 106 BM-MSCs (in 0.2 mL saline) or 0.2 mL saline were injected intravenously via the tail vein every week (Figure 1). After the treatment (4 wk later), the BM-MSC (iv)-administration group reduced the incidence of AA compared with that of the saline group, and attenuated the progression and expansion at the infrarenal levels of the aorta (Figure 2B). The BM-MSC IV-administration group also suppressed MMP-2 and MMP-9 enzymatic activity and protein expression of inflammatory cytokines including IL-1β, IL-6 and MCP-1 in the aortic tissue. In addition, the infiltration of macrophages was suppressed by BM-MSC IV-administration. Moreover, the BM-MSC IV-administration group showed inhibition of elastin degradation, which might have been affected by the up-regulation of IGF-1 and TIMP-2 protein expression. This study showed that the multiple IV-administration of BM-MSC inhibits AA development and progression as a less-invasive procedure. Our studies suggest that the attenuation of AA development and growth is associated with improvement of the imbalance between degradation and synthesis of ECMs due to the anti-inflammation, immunosuppression and tissue repair potential of BM-MSC (Figure 3).
Figure 3.
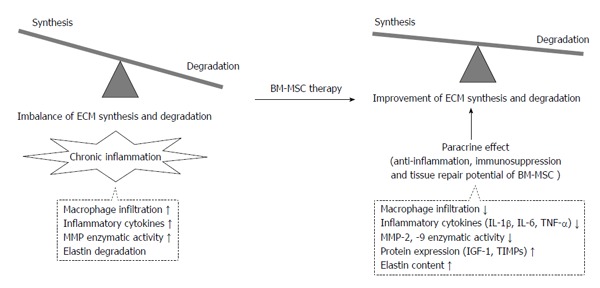
Attenuation of aortic aneurysm development and growth is associated with improvement of the imbalance between degradation and synthesis of extracellular matrices by bone marrow-derived mesenchymal stem cell therapy. ECM: Extracellular matrices; BM-MSC: Bone marrow-derived mesenchymal stem cell; TIMP: Tissue inhibitor of metalloproteinase; MMP: Matrix metalloproteinase; IL: Interleukin; IGF: Insulin-like growth factor.
Sharma et al[54] reported the role of IL-17 in the elastase-perfused mouse AAA model and the effectiveness of iv injection of human placental-derived MSC for experimental AAA. T-cell-produced IL-17, which is known as a regulator of inflammation and VSMC apoptosis, induced the expression of various cytokines, chemokines, and MMPs[55]. On day 1 after elastase-perfused wild-type (WT) mice, 1 × 106 placental-derived MSCs were injected intravenously via the tail vein. After 2 wk, the aortic diameter was attenuated in the placental-derived MSC-treated mice group compared with untreated elastase-perfused WT mice group. In histological analysis, infiltration of inflammatory cells including CD3+ T cells, macrophages and neutrophils was attenuated and elastic fiber disruption decreased in placental-derived MSC-treated mice group. In addition, the placental-derived MSC-treated mice group suppressed the protein production of IL-17, IL-23, IFN-γ, TNF-α, RANTES and MCP-1 in aortic tissue. The same investigators suggested that placental-derived MSC treatment attenuated AAA formation and inflammatory cytokine production including IL-17 via paracrine effect of soluble factors secreted from MSCs such as TGF-β, hepatocyte growth factor, or PGE2. This suggestion was supported by co-culture of placental-derived MSCs and mononuclear cells (MNCs) in an in vitro experiment. The placental-derived MSCs co-cultured with MNCs suppressed the proliferation of activated MNCs and attenuated IL-17 production from MNCs.
Catheter-based MSC therapy for aortic aneurysm
Schneider et al[56] also reported that an already-formed tentative AA was stabilized by BM-MSCs using a xenograft rat AAA model. To obtain xenograft, guinea pig infrarenal aortas were decellularized using 1% sodium dodecyl sulfate. Then, the male Fischer 344 rat aorta was replaced by a decellularized xenograft. Fourteen days after xenograft implantation, 1 × 106 BM-MSCs were injected into the lumen of clamped xenograft aorta through a PE10 catheter, and allowed to attach for 8 min. The results showed down-regulation of MMP-9 mRNA, up-regulation of TIMP-1 mRNA and decrease of macrophages at the xenograft site at 1 wk, and a decrease in aortic diameter at the xenograft site 4 wk after BM-MSC injection. These results suggest that BM-MSCs inhibit xenograft aneurysmal wall injury and heal through paracrine mechanisms and induction of collagen production rather than direct differentiation. This endovascular seeding of BM-MSCs may support the development of catheter-based intervention for AA treatment in the future.
The possibility of catheter-based delivery of MSCs has also been reported by Riera Del Moral et al[57] who demonstrated coadjuvant treatment with MSCs in EVAR based on clinical current treatment. They injected 1 × 107 adipose tissue-derived MSCs (ASCs) (in 1 mL fibrin sealant) inside the aneurysmal sac through a second 5F introductor using a Dacron-patched AAA pig model. This study investigated whether the MSCs induced local immunosuppression, prevention of excessive fibrosis, prevention of apoptosis and induction of intrinsic progenitor cell. The results showed that the ASC-treated group was a lower infiltration of inflammatory cells compared with the non-treated group, and green fluorescent protein (GFP)-linked ASCs were detected 3 wk after. They suggested that ASC endovascular administration into aneurysmal sac assuming common clinical treatment might stabilize AAA.
Direct Injection of MSCs to the aneurysmal aortic wall
Turnbull et al[58] reported the success of implantation of autologous BM-MSC by direct injection into the aortic wall using a porcine AAA model and the potential of cell-based therapies. The aneurysm was created by injection of type 1 collagenase and elastase solution into the aortic lumen, following dilation of the infrarenal aorta using a 12 mm noncompliant angioplasty balloon. After that, 1 × 107 BM-MSCs were directly injected into the aortic wall immediately after the injury. The GFP-labeled BM-MSCs were identified in the aortic wall 1 wk after injection. And, von willebrand factor positive cells formed tubuloluminal structures were detected within the outer layer of the media and throughout the adventitia. In addition, the mRNA level of vascular endothelial growth factor (VEGF)-A was increased at 72 h in BM-MSC-injected aortic tissue compared with non-treated control aorta. Thus, they suggested BM-MSC-enhanced wound healing and angiogenic response through paracrine factor, such as VEGF.
In these studies, MSC phenotypic characteristics have been identified by surface marker and pluripotency. Although these different positive and negative immunophenotypes are concerned with differences in animal species, they resemble human MSC immunophenotypic characteristics.
FUTURE PERSPECTIVE OF MSC THERAPY FOR AA
Unsolved issues
The efficacy of MSC for treatment of AA has been suggested in current experimental studies showing the advantages of inhibition of excess inflammation, decrease of inflammatory cells, suppression of elastic fiber disruption and increase of elastin content by MSC administration. However, some unresolved issues remain in the treatment with MSCs. First, it remains unclear whether the cell numbers, frequency and administration timing of MSCs are required for AA treatment. Thus, these optimizations warrant further investigation. Second, the delivery methods of MSC in these studies are respectively different. Investigators have performed administration using several methods involving implantation of cell-sheet, IV-administration, direct injection into aortic wall, and catheter delivery (Table 3). Among them, although IV-administration is the least invasive and simple procedure, the targeting ability is lower and injected MSCs are trapped in other tissues such as lung, spleen, liver and kidney. In contrast, the implantation of cell-sheet and the direct injection into aortic wall make it possible to target AA. However, these are relatively invasive procedures. On the other hand, endovascular delivery using a catheter is less invasive and has a high targeting ability. Third, the long-term follow-up administration of MSCs has not been reported yet. The injected MSCs may differentiate into adipocyte, osteocyte or other differentiated cells at the aortic wall site. These cells might promote harmful effects to the aorta such as deposition of lipid or calcification. Fourth, the isolation and expansion of MSCs might become difficult with aging. Therefore, we must investigate the therapeutic effect of AA using allogeneic MSCs, not only autologous MSCs. Finally, further investigation using a large animal will be ultimately required to confirm the repeatability.
Table 3.
Methodology of delivery system
| Delivery system | Administration site | Localization, timing |
Delivery system |
Ref. | |
| Merits | Demerits | ||||
| Cell-sheet | Adventitia of abdominal aorta | Adventitia, 4 wk after implantation | High targeting ability | Invasive procedure by laparotomy | [51] |
| iv | Tail vein | Media and/or adventitia, at 4 wk | Least invasive | Low targeting ability and trapping in other tissue | [53] |
| iv | Tail vein | Data not shown | Least invasive | Low targeting ability and trapping in other tissue | [54] |
| Catheter | Clamped endovascular | Intima 1 wk after injection | Less invasive and high targeting ability | Requirement of a surgical procedure or advanced catheter intervention | [56] |
| Catheter | Clamped endovascular | Media 3 wk after injection | Less invasive and high targeting ability | Requirement of a surgical procedure or advanced catheter intervention | [57] |
| Direct injection | Injured aortic wall | Aortic wall, 1 wk after injection | High targeting ability | Risk of rupture | [58] |
iv: Intravenous.
Future perspective
Meanwhile, it is important to elucidate the mechanisms by which MSCs induced negative effects for progression of AA. One of the mechanisms was suggested to be the paracrine effect of MSCs. Recently, trophic factors of MSC-conditioned medium (MSC-CM) were profiled by proteomic analysis using mass spectrometry, protein microarrays and bioinformatics; as a result, many candidates such as TGF-β, IGF-1, epidermal growth factor, fibroblast growth factor, interleukins, MMPs, or TIMPs were identified[59]. TGF-β is an important signal that induces smooth muscle cell (SMC) differentiation and increases serum response factor (SRF) expression through an increase in transcription of the SRF gene[60]. Moreover, SRF controls vasoconstriction via SMC phenotypic modulation[61]. This fact might be supported by cellular activities in the treatment of AA using MSC therapy. Timmers et al[62,63] demonstrated that iv injection of human MSC-CM for treatment of MI in porcine model, resulted in reduced myocardial apoptosis, oxidative stress, myocardial infract size, preserved systolic and diastolic function through reduction of TGF-β signaling including phospho-Smad2 and apoptosis including active caspase 3 following MSC-CM treatment. These studies also revealed that the fraction of the MSC-CM containing products > 1000 kDa improved cardiac function rather than the fraction of products < 1000 kDa. This result indicates that a large complex protein such as a combination of angiogenic factors rather than a single protein may be the responsible paracrine factor. Regarding MSC for AA, it is not clear which factors can induce a better effect. Although iv injection of MSC-CM provides easy delivery compared to direct MSC injection, the effects would be of short-term duration by degradation of molecules immediately.
Study of MSC therapy for AA has only just begun, and MSCs are indeed a promising tool for AA treatment. Some studies have suggested that inflammation and ECM degradation at the AA wall site were inhibited by various anti-inflammatory cytokines, inhibitor of proteases and stimulator of ECMs synthesis which were induced by various growth factors secreted from MSCs. In addition, MSC therapy has been demonstrated to have an efficacy not only for prevention of AA development and progression but also regression of already-formed AA. These healing mechanisms remain unknown, and so further research will be warranted in the future.
CONCLUSION
Treatment of AA using MSCs has been demonstrated to be effective, and promises to be a new non-surgical therapeutic strategy. These effects might be promoted in a paracrine manner from MSCs as one possible mechanism.
Footnotes
P- Reviewers: Coleti D, Evans T S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
References
- 1.Lederle FA, Johnson GR, Wilson SE, Ballard DJ, Jordan WD, Blebea J, Littooy FN, Freischlag JA, Bandyk D, Rapp JH, et al. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA. 2002;287:2968–2972. doi: 10.1001/jama.287.22.2968. [DOI] [PubMed] [Google Scholar]
- 2.Alcorn HG, Wolfson SK, Sutton-Tyrrell K, Kuller LH, O’Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1996;16:963–970. doi: 10.1161/01.atv.16.8.963. [DOI] [PubMed] [Google Scholar]
- 3.Hollier LH, Taylor LM, Ochsner J. Recommended indications for operative treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the Society for Vascular Surgery and the North American Chapter of the International Society for Cardiovascular Surgery. J Vasc Surg. 1992;15:1046–1056. [PubMed] [Google Scholar]
- 4.SVN Task Force for Clinical Practice Guideline. 2009 clinical practice guideline for patients undergoing endovascular repair of abdominal aortic aneurysms (AAA) J Vasc Nurs. 2009;27:48–63. doi: 10.1016/j.jvn.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Hackam DG, Thiruchelvam D, Redelmeier DA. Angiotensin-converting enzyme inhibitors and aortic rupture: a population-based case-control study. Lancet. 2006;368:659–665. doi: 10.1016/S0140-6736(06)69250-7. [DOI] [PubMed] [Google Scholar]
- 6.Schouten O, van Laanen JH, Boersma E, Vidakovic R, Feringa HH, Dunkelgrün M, Bax JJ, Koning J, van Urk H, Poldermans D. Statins are associated with a reduced infrarenal abdominal aortic aneurysm growth. Eur J Vasc Endovasc Surg. 2006;32:21–26. doi: 10.1016/j.ejvs.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Yamawaki-Ogata A, Hashizume R, Satake M, Kaneko H, Mizutani S, Moritan T, Ueda Y, Narita Y. A doxycycline loaded, controlled-release, biodegradable fiber for the treatment of aortic aneurysms. Biomaterials. 2010;31:9554–9564. doi: 10.1016/j.biomaterials.2010.08.069. [DOI] [PubMed] [Google Scholar]
- 8.Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Kent KC, Upchurch GR, Chaikof EL, Mills JL, Fleckten B, et al. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J Vasc Surg. 2002;36:1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- 9.Walton LJ, Franklin IJ, Bayston T, Brown LC, Greenhalgh RM, Taylor GW, Powell JT. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation. 1999;100:48–54. doi: 10.1161/01.cir.100.1.48. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura K, Aoki H, Ikeda Y, Fujii K, Akiyama N, Furutani A, Hoshii Y, Tanaka N, Ricci R, Ishihara T, et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med. 2005;11:1330–1338. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 12.Jackson L, Jones DR, Scotting P, Sottile V. Adult mesenchymal stem cells: differentiation potential and therapeutic applications. J Postgrad Med. 2007;53:121–127. doi: 10.4103/0022-3859.32215. [DOI] [PubMed] [Google Scholar]
- 13.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Villiers JA, Houreld N, Abrahamse H. Adipose derived stem cells and smooth muscle cells: implications for regenerative medicine. Stem Cell Rev. 2009;5:256–265. doi: 10.1007/s12015-009-9084-y. [DOI] [PubMed] [Google Scholar]
- 15.Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 17.Panfilov IA, de Jong R, Takashima S, Duckers HJ. Clinical study using adipose-derived mesenchymal-like stem cells in acute myocardial infarction and heart failure. Methods Mol Biol. 2013;1036:207–212. doi: 10.1007/978-1-62703-511-8_16. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigo SF, van Ramshorst J, Hoogslag GE, Boden H, Velders MA, Cannegieter SC, Roelofs H, Al Younis I, Dibbets-Schneider P, Fibbe WE, et al. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J Cardiovasc Transl Res. 2013;6:816–825. doi: 10.1007/s12265-013-9507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 20.Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, Herrmann RP. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Thompson RW, Geraghty PJ, Lee JK. Abdominal aortic aneurysms: basic mechanisms and clinical implications. Curr Probl Surg. 2002;39:110–230. doi: 10.1067/msg.2002.121421. [DOI] [PubMed] [Google Scholar]
- 23.Uitto J, Christiano AM, Kähäri VM, Bashir MM, Rosenbloom J. Molecular biology and pathology of human elastin. Biochem Soc Trans. 1991;19:824–829. doi: 10.1042/bst0190824. [DOI] [PubMed] [Google Scholar]
- 24.Liu JM, Davidson JM. The elastogenic effect of recombinant transforming growth factor-beta on porcine aortic smooth muscle cells. Biochem Biophys Res Commun. 1988;154:895–901. doi: 10.1016/0006-291x(88)90224-0. [DOI] [PubMed] [Google Scholar]
- 25.Foster J, Rich CB, Florini JR. Insulin-like growth factor I, somatomedin C, induces the synthesis of tropoelastin in aortic tissue. Coll Relat Res. 1987;7:161–169. doi: 10.1016/s0174-173x(87)80007-9. [DOI] [PubMed] [Google Scholar]
- 26.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palombo D, Maione M, Cifiello BI, Udini M, Maggio D, Lupo M. Matrix metalloproteinases. Their role in degenerative chronic diseases of abdominal aorta. J Cardiovasc Surg (Torino) 1999;40:257–260. [PubMed] [Google Scholar]
- 28.Middleton RK, Lloyd GM, Bown MJ, Cooper NJ, London NJ, Sayers RD. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: a protein array study. J Vasc Surg. 2007;45:574–580. doi: 10.1016/j.jvs.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjälä H, Airaksinen J, Leinonen M, Saikku P, Juvonen T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–2847. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 30.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 32.von Wnuck Lipinski K, Keul P, Lucke S, Heusch G, Wohlschlaeger J, Baba HA, Levkau B. Degraded collagen induces calpain-mediated apoptosis and destruction of the X-chromosome-linked inhibitor of apoptosis (xIAP) in human vascular smooth muscle cells. Cardiovasc Res. 2006;69:697–705. doi: 10.1016/j.cardiores.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86:1259–1265. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- 34.Huffman MD, Curci JA, Moore G, Kerns DB, Starcher BC, Thompson RW. Functional importance of connective tissue repair during the development of experimental abdominal aortic aneurysms. Surgery. 2000;128:429–438. doi: 10.1067/msy.2000.107379. [DOI] [PubMed] [Google Scholar]
- 35.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boiret N, Rapatel C, Veyrat-Masson R, Guillouard L, Guérin JJ, Pigeon P, Descamps S, Boisgard S, Berger MG. Characterization of nonexpanded mesenchymal progenitor cells from normal adult human bone marrow. Exp Hematol. 2005;33:219–225. doi: 10.1016/j.exphem.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Eslaminejad MB, Nadri S, Hosseini RH. Expression of Thy 1.2 surface antigen increases significantly during the murine mesenchymal stem cells cultivation period. Dev Growth Differ. 2007;49:351–364. doi: 10.1111/j.1440-169X.2007.00932.x. [DOI] [PubMed] [Google Scholar]
- 38.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 39.Craig W, Kay R, Cutler RL, Lansdorp PM. Expression of Thy-1 on human hematopoietic progenitor cells. J Exp Med. 1993;177:1331–1342. doi: 10.1084/jem.177.5.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishii M, Koike C, Igarashi A, Yamanaka K, Pan H, Higashi Y, Kawaguchi H, Sugiyama M, Kamata N, Iwata T, et al. Molecular markers distinguish bone marrow mesenchymal stem cells from fibroblasts. Biochem Biophys Res Commun. 2005;332:297–303. doi: 10.1016/j.bbrc.2005.04.118. [DOI] [PubMed] [Google Scholar]
- 41.Gronthos S, Zannettino AC. A method to isolate and purify human bone marrow stromal stem cells. Methods Mol Biol. 2008;449:45–57. doi: 10.1007/978-1-60327-169-1_3. [DOI] [PubMed] [Google Scholar]
- 42.Tormin A, Li O, Brune JC, Walsh S, Schütz B, Ehinger M, Ditzel N, Kassem M, Scheding S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117:5067–5077. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bühring HJ, Treml S, Cerabona F, de Zwart P, Kanz L, Sobiesiak M. Phenotypic characterization of distinct human bone marrow-derived MSC subsets. Ann N Y Acad Sci. 2009;1176:124–134. doi: 10.1111/j.1749-6632.2009.04564.x. [DOI] [PubMed] [Google Scholar]
- 44.Ozawa K, Sato K, Oh I, Ozaki K, Uchibori R, Obara Y, Kikuchi Y, Ito T, Okada T, Urabe M, et al. Cell and gene therapy using mesenchymal stem cells (MSCs) J Autoimmun. 2008;30:121–127. doi: 10.1016/j.jaut.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 45.De Becker A, Van Hummelen P, Bakkus M, Vande Broek I, De Wever J, De Waele M, Van Riet I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440–449. doi: 10.3324/haematol.10475. [DOI] [PubMed] [Google Scholar]
- 46.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 47.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 48.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 49.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 50.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 51.Hashizume R, Yamawaki-Ogata A, Ueda Y, Wagner WR, Narita Y. Mesenchymal stem cells attenuate angiotensin II-induced aortic aneurysm growth in apolipoprotein E-deficient mice. J Vasc Surg. 2011;54:1743–1752. doi: 10.1016/j.jvs.2011.06.109. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 53.Fu XM, Yamawaki-Ogata A, Oshima H, Ueda Y, Usui A, Narita Y. Intravenous administration of mesenchymal stem cells prevents angiotensin II-induced aortic aneurysm formation in apolipoprotein E-deficient mouse. J Transl Med. 2013;11:175. doi: 10.1186/1479-5876-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma AK, Lu G, Jester A, Johnston WF, Zhao Y, Hajzus VA, Saadatzadeh MR, Su G, Bhamidipati CM, Mehta GS, et al. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation. 2012;126:S38–S45. doi: 10.1161/CIRCULATIONAHA.111.083451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 56.Schneider F, Saucy F, de Blic R, Dai J, Mohand F, Rouard H, Ricco JB, Becquemin JP, Gervais M, Allaire E. Bone marrow mesenchymal stem cells stabilize already-formed aortic aneurysms more efficiently than vascular smooth muscle cells in a rat model. Eur J Vasc Endovasc Surg. 2013;45:666–672. doi: 10.1016/j.ejvs.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 57.Riera Del Moral L, Aramburu CL, García JR, de Cubas LR, García-Olmo D, García-Arranz M. Experimental model for coadjuvant treatment with mesenchymal stem cells for aortic aneurysm. Am J Stem Cells. 2012;1:174–181. [PMC free article] [PubMed] [Google Scholar]
- 58.Turnbull IC, Hadri L, Rapti K, Sadek M, Liang L, Shin HJ, Costa KD, Marin ML, Hajjar RJ, Faries PL. Aortic implantation of mesenchymal stem cells after aneurysm injury in a porcine model. J Surg Res. 2011;170:e179–e188. doi: 10.1016/j.jss.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie. 2013;95:2196–2211. doi: 10.1016/j.biochi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 60.Kawai-Kowase K, Sato H, Oyama Y, Kanai H, Sato M, Doi H, Kurabayashi M. Basic fibroblast growth factor antagonizes transforming growth factor-beta1-induced smooth muscle gene expression through extracellular signal-regulated kinase 1/2 signaling pathway activation. Arterioscler Thromb Vasc Biol. 2004;24:1384–1390. doi: 10.1161/01.ATV.0000136548.17816.07. [DOI] [PubMed] [Google Scholar]
- 61.Galmiche G, Labat C, Mericskay M, Aissa KA, Blanc J, Retailleau K, Bourhim M, Coletti D, Loufrani L, Gao-Li J, et al. Inactivation of serum response factor contributes to decrease vascular muscular tone and arterial stiffness in mice. Circ Res. 2013;112:1035–1045. doi: 10.1161/CIRCRESAHA.113.301076. [DOI] [PubMed] [Google Scholar]
- 62.Timmers L, Lim SK, Hoefer IE, Arslan F, Lai RC, van Oorschot AA, Goumans MJ, Strijder C, Sze SK, Choo A, et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011;6:206–214. doi: 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1:129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]


