SUMMARY
Objectives
Randomized controlled trials of dornase alfa have shown forced expiratory volume in 1 second (FEV1) to improve in patients with cystic fibrosis (CF) but have not assessed change in the rate of lung function decline. We assessed the relationship of dornase alfa use and FEV1 decline using the Epidemiologic Study of CF (ESCF).
Methodology
Patients aged 8–38 years who had been enrolled in ESCF for 2 years when initially treated with dornase alfa were selected if they remained on treatment during the following 2 years. A comparator group included patients aged 8–38 who were not yet reported to have received dornase alfa. For each patient we estimated the annual rate of decline in FEV1 % predicted before and after the index using a mixed-effects model adjusted for age, gender, pulmonary exacerbations, respiratory therapies, and nutritional supplements.
Results
The dornase alfa group (n = 2,230) had a lower FEV1 % predicted at index and a more rapid decline during the pre-index period. The mean rate of FEV1 decline improved for the dornase alfa group; the improvement was similar in adults and children 8–17 years old but was not statistically significant in adults. The comparator group (n = 5,970) showed no change among adults and an increased rate of decline among children 8–17 years old.
Conclusions
The use of dornase alfa for a 2-year period is associated with a reduction in the rate of FEV1 decline. These results also demonstrate the value of using an observational study to assess the association of instituting new therapies in the clinical setting with changes in the rate of FEV1 decline in patients with CF.
Keywords: dornase alfa, cystic fibrosis, epidemiology, pulmonary function
INTRODUCTION
Cystic fibrosis (CF) lung disease is characterized by airway obstruction, chronic infection, and neutrophil-dominated inflammation leading to progressive and irreversible lung destruction.1 Neutrophil-derived DNA contributes to the increased viscosity of airway secretions. Dornase alfa (rhDNase) hydrolyzes DNA, reducing the viscosity of CF sputum.2 Clinical trials have shown that dornase alfa improves forced expiratory volume in 1 second (FEV1) in patients 5 years of age and older.3–5
Although improvement in lung function is important to the health of individuals with CF, long- term survival requires slowing the rate of decline of lung function. Demonstrating changes in rate of lung function decline requires long-term studies with many patients, which are impractical for clinical trials.6 Observational studies such as the Epidemiologic Study of Cystic Fibrosis (ESCF) have the potential to address rate of decline in lung function more effectively because of large numbers of subjects monitored for longer periods of time.7, 8 Therefore, we used ESCF data to evaluate the effectiveness of dornase alfa in clinical practice and hypothesized that the rate of decline in pulmonary function would be reduced in association with long-term use of this therapy.
METHODS
The ESCF is a large, multicenter, longitudinal, prospective observational study of the clinical course of patients with CF in the United States and Canada from 1994 through 2005.8 Informed consent was obtained based on decisions by a central or a local human subjects’ review board.
Patient Population
We compared patients treated with dornase alfa (the dornase alfa group) to patients not treated with dornase alfa (the comparator group). The dornase alfa group included patients enrolled in ESCF for at least 2 years before starting dornase alfa who were aged 8–38 years when initially started on dornase alfa and were reported to be taking dornase alfa for at least 80% of the clinic visits over the following 2 years. A pulmonary function test (PFT) within 30 days of starting dornase alfa (defined as the index PFT) was required to separate a 2-year pre-index period from a 2-year post-index period, but the index PFT was not included in either. The comparator group included patients aged 8–38 years who were not yet reported to have received dornase alfa. The index PFT for comparators was defined as the PFT closest (within 30 days) to the first clinic encounter within 1 year following the eighth or subsequent even-numbered birthday. For both groups, the pre-index and post-index periods were each required to have at least 1 clinic encounter and at least 3 FEV1 values spanning at least 6 months to estimate the slope of FEV1. We elected to use 2-year periods because shorter time periods would have given less precise estimates of slope6 and longer time periods would have reduced the number of patients available for analysis. Comparator patients could contribute more than one set of pre-index and post-index periods and could also subsequently be included in the dornase alfa group.
Statistical Methods
We estimated the annual rate of decline in FEV1 % predicted before and after the index PFT for each patient using a mixed-effects model with random slopes and intercepts for each period. Values for FEV1 % predicted were calculated from Wang9 for males through age 17 years and for females through age 15 years and from the equations of Hankinson10 for patients over these ages.
The model used for this analysis was similar to that used by Ren11 to evaluate the effect of inhaled corticosteroids on change in slope; however, the current model also allowed for change in intercept. Separate estimates were obtained by age group (8–17 and 18–38) for each of 4 categories of FEV1 percent predicted from the index PFT (<40, 40 to <70, 70 to <100, ≥100). The model adjusted for age in years, gender, and time-dependent use of routine therapies and pulmonary exacerbations treated with intravenous (IV) antibiotics. Routine therapies were based on data recorded at the closest visit before or within 14 days after each PFT. Repeated use of patients was accounted for in the modeled covariance structure.
The relationship between stage of lung disease and the modeled slopes and intercepts was further evaluated using the FEV1 % predicted at the index PFT categorized into age-specific FEV1 deciles obtained by using all PFTs recorded in ESCF (N = 535,344), regardless of whether the PFT was taken at a time of clinical stability. The mixed model was then re-estimated, replacing the age group and index PFT category variables with the FEV1 decile. Additional details on the statistical methods and the rationale for the adjustment for FEV1 decile are presented in the online supplement.
Statistical analyses were performed using SAS 9.1 (SAS Institute, Inc., Cary, NC). P values < 0.05 were considered significant. No adjustments were made for multiple comparisons.
RESULTS
Patient Characteristics
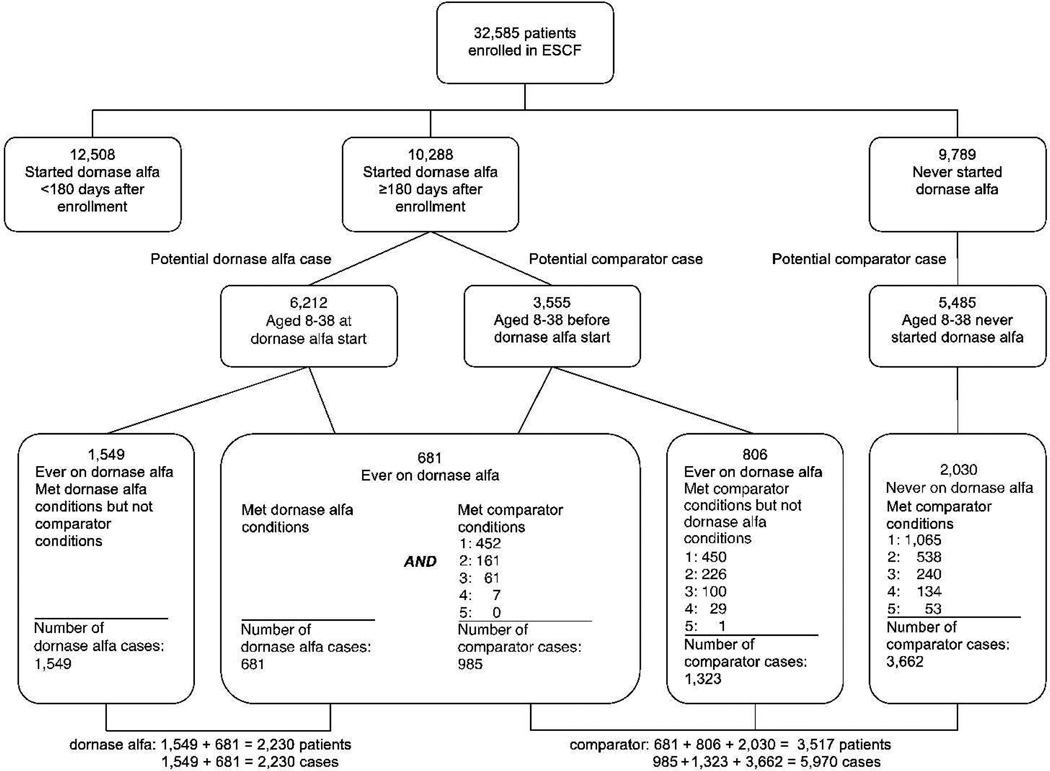
Of the 32,585 patients enrolled in the ESCF between 1994 and 2001, 22,796 received dornase alfa at least one time. A total of 10,288 patients started dornase alfa at least 180 days after enrollment, and 6,212 of those were aged 8 to 38 (Figure 1). Of those started on dornase alfa, 3,132 had sufficient data (at least 1 encounter and 3 PFTs spanning at least 6 months in both the 2-year pre-index and the 2-year post-index period), and 2,230 of those (71%) were recorded as receiving dornase alfa for at least 80% of visits. Comparator cases were drawn from patients who never received dornase alfa (3,662 cases from 2,030 unique patients), patients who later received dornase alfa but never met the criteria to be included in the dornase alfa group (1,323 cases from 806 unique patients), and patients subsequently included in the dornase alfa group (985 cases from 681 unique patients).
Fig. 1.
Identification of patients and cases for the dornase alfa and comparator groups.
Demographic and clinical characteristics of the 2,230 unique patients in the dornase alfa group and the 5,970 cases (3,517 unique patients) in the comparator group are presented in Table 1. Dornase alfa patients were more often female, had lower index FEV1, had lower weight for age, were more often positive for Pseudomonas aeruginosa on a respiratory tract culture, and had more clinical signs and symptoms. For the routine therapies most often recorded (other than dornase alfa), the frequency of use before and after the index PFT for the dornase alfa and comparator groups is shown in Table 2. Overall, therapies were more frequent in the dornase alfa group before index. Therapies increased after index except for oral antibiotics, oral bronchodilators, and mast cell stabilizers. This increase in therapies was greater in the dornase alfa group for inhaled antibiotics, IV antibiotics for exacerbations, inhaled corticosteroids, and oral nutritional supplements. Oral antibiotic use was unchanged in the dornase alfa group, but decreased in the comparator group.
TABLE 1.
Demographic and Clinical Characteristics at Index PFT
| Dornase alfa patients | Comparator cases | |||||
|---|---|---|---|---|---|---|
| Characteristic | Combined | 8–17 | 18–38 | Combined | 8–17 | 18–38 |
| N | 2230 | 1712 | 518 | 5970 | 4421 | 1549 |
| Age, mean (SD) | 14.5 (6.5) | 11.6 (2.8) | 24.2 (5.7) | 14.5 (6.5) | 11.4 (2.8) | 23.5 (5.7) |
| Female, % | 48.7 | 48.3 | 49.8 | 42.8 | 42.8 | 42.9 |
| ΔF508 homozygous,a % (n) | 52.2 (n = 1580) | 54.9 (n = 1244) | 42.3 (n = 336) | 51.1 (n = 4231) | 51.6 (n = 3163) | 49.4 (n = 1068) |
| FEV1 (% pred.), mean (SD) | 76.0 (22.9) | 80.5 (21.0) | 61.0 (22.7) | 86.6 (22.3) | 92.4 (18.5) | 70.2 (24.0) |
| FEV1 disease stage groups, % | ||||||
| <40% predicted | 8.7 | 4.4 | 23.0 | 4.3 | 1.1 | 13.3 |
| 40 to <70% predicted | 28.3 | 24.5 | 40.7 | 15.9 | 10.0 | 32.8 |
| 70 to <100% predicted | 48.5 | 53.3 | 32.6 | 50.9 | 52.9 | 45.1 |
| ≥100% predicted | 14.5 | 17.8 | 3.7 | 28.9 | 36.0 | 8.8 |
| Weight for age,a mean (SD) | 30.6 (26.5) | 30.2 (26.1) | 31.8 (28.0) | 37.7 (28.5) | 37.8 (28.3) | 37.5 (29.3) |
| Pseudomonasa positive, % (n) | 52.3 (n = 945) | 45.6 (n = 745) | 77.0 (n = 200) | 43.4 (n = 3020) | 35.6 (n = 2219) | 64.8 (n = 801) |
| Pulmonary signs and symptoms | ||||||
| positive, % | ||||||
| Cough | 90.0 | 88.2 | 96.5 | 82.1 | 79.3 | 90.4 |
| Sputum production | 72.3 | 67.5 | 89.2 | 58.1 | 50.6 | 80.3 |
| Digital clubbing | 58.9 | 54.8 | 73.5 | 49.6 | 45.1 | 62.9 |
| Crackles | 21.8 | 18.2 | 34.7 | 12.3 | 8.1 | 24.7 |
| Wheeze | 6.5 | 6.0 | 8.6 | 5.9 | 4.6 | 9.8 |
Includes only patients with non-missing data at index PFT. Approximately 500 patients are missing weight-for-age and pulmonary signs and symptoms data. (n) indicates the denominator for that particular percentage.
TABLE 2.
Average Proportion of Visits at Which Therapies Were Recorded
| Treatment group |
Pre-index visit, mean |
Post-index visit, mean |
Diff pre–post, mean |
P value* | P value† | |
|---|---|---|---|---|---|---|
| Oral antibiotics | Dornase alfa | 0.39 | 0.40 | 0.00 | 0.56 | 0.004 |
| Comparator | 0.38 | 0.36 | −0.02 | <0.001 | ||
| Inhaled antibiotics | Dornase alfa | 0.16 | 0.29 | 0.13 | <0.001 | <0.001 |
| Comparator | 0.10 | 0.17 | 0.06 | <0.001 | ||
| IV antibiotics for exacerbations | Dornase alfa | 0.10 | 0.13 | 0.02 | <0.001 | <0.001 |
| Comparator | 0.07 | 0.08 | 0.01 | <0.001 | ||
| Oral bronchodilators | Dornase alfa | 0.05 | 0.05 | −0.00 | 0.39 | 0.16 |
| Comparator | 0.05 | 0.04 | −0.01 | <0.001 | ||
| Inhaled bronchodilators | Dornase alfa | 0.82 | 0.87 | 0.05 | <0.001 | 0.047 |
| Comparator | 0.72 | 0.75 | 0.03 | <0.001 | ||
| Oral corticosteroids | Dornase alfa | 0.10 | 0.12 | 0.02 | 0.006 | 0.074 |
| Comparator | 0.08 | 0.08 | 0.01 | 0.13 | ||
| Inhaled corticosteroids | Dornase alfa | 0.30 | 0.40 | 0.10 | <0.001 | 0.004 |
| Comparator | 0.24 | 0.32 | 0.07 | <0.001 | ||
| Mast cell stabilizers | Dornase alfa | 0.19 | 0.15 | −0.04 | <0.001 | 0.38 |
| Comparator | 0.18 | 0.15 | −0.03 | <0.001 | ||
| Oral supplements | Dornase alfa | 0.22 | 0.26 | 0.03 | <0.001 | 0.019 |
| Comparator | 0.19 | 0.20 | 0.01 | 0.004 | ||
P value for difference within treatment group.
P value comparing the difference between treatment groups.
A total of 16,556 pre-index visits and 20,783 post-index visits exist for dornase alfa patients (n = 2230).
A total of 38,189 pre-index visits and 41,324 post-index visits exist for comparator patients (n = 5970).
Estimated Changes in FEV1 % Predicted by Age Group
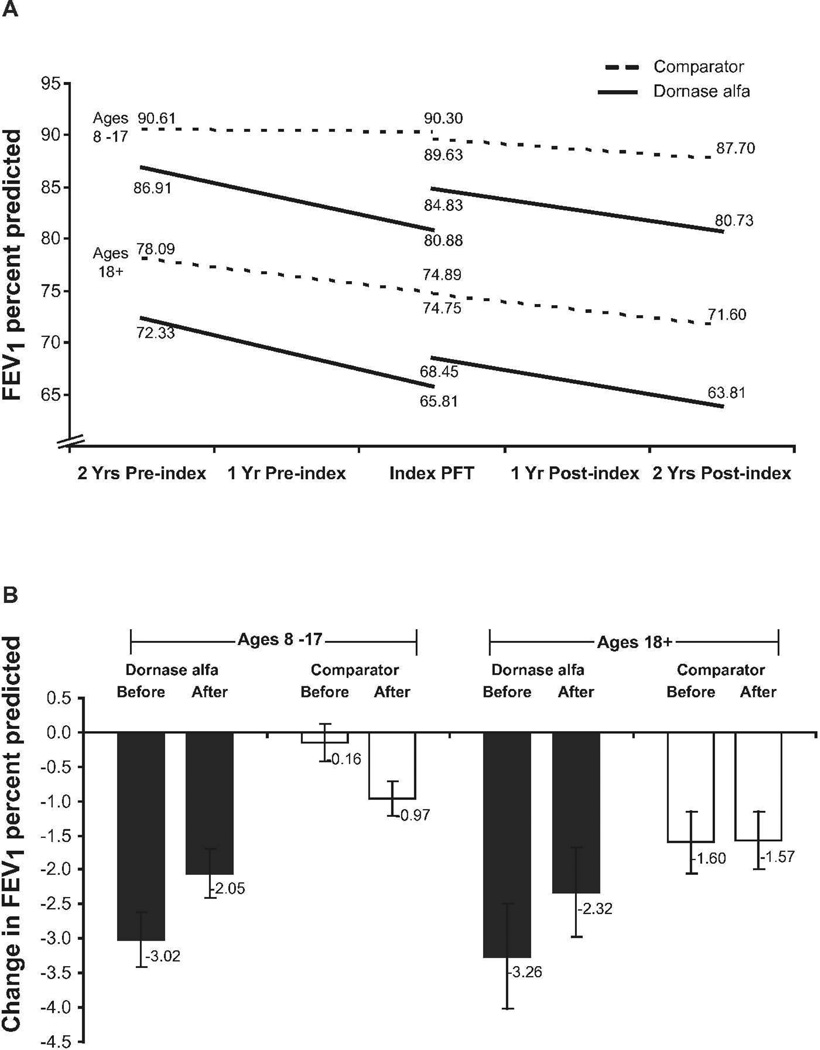
The dornase alfa patients showed a significant improvement in FEV1 (change in intercept) soon after starting dornase alfa (3.95% predicted in 8- to 17-year-olds and 2.64% predicted in ≥18-year-olds, both P < 0.001) (Figure 2A). In contrast, children (8–17 years old) in the comparator group showed a decrease in FEV1 after the index PFT (−0.67 percentage points, P < 0.001). Adults (≥18 years old) in the comparator group showed a non-significant decrease in FEV1 after the index PFT (−0.14 percentage points, P = 0.66).
Fig. 2.
FEV1 % predicted before and after index PFT in children and adults for the dornase alfa and comparator groups. Estimated linear trends (2A), and annual rates of decline (2B). Error bars indicate 95% CI for the estimated slope.
The estimated changes in FEV1 % predicted per year during the 2-year periods before and after the index PFT for the dornase alfa and comparator patients are shown in Figure 2B. Before the index PFT, dornase alfa patients had a more rapid annual decline in FEV1 % predicted than comparator patients (P < 0.001 for both 8–17 and ≥18 age groups). After the index PFT, the mean annual rate of decline for the dornase alfa group improved by similar amounts in children (32.1%, P < 0.001) and adults (28.8%), but was not statistically significant in adults (P = 0.068). In contrast, the age 8–17 comparator group had a worsened mean annual rate of decline (P < 0.001), while those age ≥18 had no change (P = 0.92)). Despite the improvement in rate of decline post-index, children in the dornase alfa group still had a greater rate of decline than those in the comparator group (P < 0.001).
Estimated Changes in FEV1 % Predicted by Disease Stage
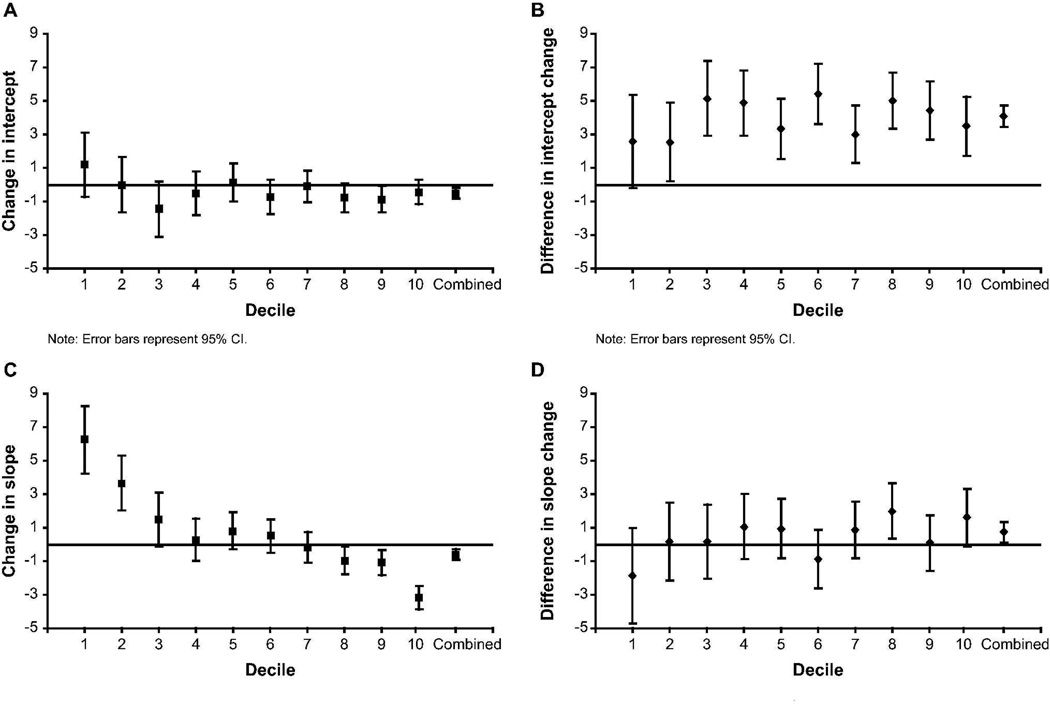
There are substantial differences in the stage of lung disease between the dornase alfa and comparator patients. As shown in the online supplement, differences in disease stage can lead to differences in expected change in the annual rate of decline, with the healthiest patients expected to have an accelerating rate of decline. To provide a more refined evaluation of the effect of disease stage, cases were categorized by using the age-specific deciles of FEV1 % predicted described in the online supplement. The dornase alfa group median decile was 6, and the comparator group was 8. Intercepts and rate of decline were estimated before and after index by decile. The pre-index rate of decline for the dornase alfa group was greater than the comparator in all but the lowest decile, where the groups were similar. The post-index rate of decline for the dornase alfa group was greater in all but the highest decile, where the groups were similar.
Figure 3A shows the change in intercept for the comparator group. The comparator group showed a negative change in intercept in 7 of the 10 FEV1 deciles and an overall small negative change (−0.50, P = 0.003). Figure 3B shows the between-group difference in change in intercept (dornase alfa–comparator) favored dornase alfa in every decile. The change in rate of decline for the comparator group was negative overall (−0.60, P < 0.001) and inversely related to decile (Figure 3C). The positive change in the low deciles represents a flattening of the trajectory of the FEV1 decline. The negative change in the highest deciles represents an acceleration in the FEV1 decline. The between-group difference in the change in rate of FEV1 decline favored the dornase alfa group in 8 of the 10 deciles (Figure 3D) and was statistically significant overall (0.73, P = 0.020). Details are presented in Table 3.
Fig. 3.
Estimated change from before to after index PFT in intercept and slope by age-specific deciles of FEV1 % predicted and overall. Error bars indicate 95% CI. (3A) Change in intercept for comparator patients. (3B) Difference in change in intercept between dornase alfa and comparator patients. (3C) Change in slope (% predicted per year) for comparator patients. (3D) Difference in change in slope (% predicted per year) between dornase alfa and comparator patients.
TABLE 3.
| Treatment group | Decile | N | Pre-index slope (SE) | Post-index slope (SE) | Slope difference (SE) | P difference | Post-index increase (SE) | P increase | Pre-index start | Pre-index stop | Post-index start | Post-index stop |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dornase alfa | 1 | 109 | −5.43 (0.80) | −1.04 (0.66) | 4.39 (1.04) | <0.001 | 3.75 (1.02) | <.001 | 57.78 | 46.93 | 50.68 | 48.60 |
| 2 | 169 | −4.13 (0.68) | −0.30 (0.53) | 3.84 (0.87) | <0.001 | 2.54 (0.84) | 0.002 | 64.59 | 56.32 | 58.86 | 58.27 | |
| 3 | 216 | −4.03 (0.58) | −2.39 (0.49) | 1.64 (0.76) | 0.031 | 3.68 (0.75) | <.001 | 69.52 | 61.47 | 65.15 | 60.36 | |
| 4 | 235 | −3.40 (0.57) | −2.06 (0.47) | 1.34 (0.74) | 0.071 | 4.34 (0.73) | <.001 | 75.07 | 68.27 | 72.62 | 68.51 | |
| 5 | 268 | −3.39 (0.53) | −1.62 (0.45) | 1.76 (0.70) | 0.012 | 3.46 (0.70) | <.001 | 79.06 | 72.29 | 75.75 | 72.51 | |
| 6 | 242 | −2.88 (0.54) | −3.24 (0.48) | −0.35 (0.73) | 0.63 | 4.68 (0.75) | <.001 | 83.46 | 77.69 | 82.37 | 75.90 | |
| 7 | 257 | −2.63 (0.54) | −1.93 (0.47) | 0.69 (0.72) | 0.33 | 2.90 (0.73) | <.001 | 88.14 | 82.88 | 85.79 | 81.92 | |
| 8 | 262 | −3.46 (0.54) | −2.44 (0.48) | 1.02 (0.73) | 0.16 | 4.20 (0.74) | <.001 | 92.32 | 85.40 | 89.61 | 84.73 | |
| 9 | 247 | −1.59 (0.56) | −2.58 (0.50) | −1.00 (0.75) | 0.19 | 3.57 (0.78) | <.001 | 94.78 | 91.60 | 95.17 | 90.00 | |
| 10 | 225 | −1.07 (0.60) | −2.64 (0.53) | −1.57 (0.81) | 0.052 | 3.05 (0.82) | <.001 | 103.91 | 101.77 | 104.82 | 99.54 | |
| Comparator | 1 | 134 | −5.25 (0.74) | 0.99 (0.69) | 6.24 (1.02) | <0.001 | 1.19 (0.99) | 0.23 | 59.30 | 48.81 | 50.00 | 51.99 |
| 2 | 206 | −2.97 (0.62) | 0.70 (0.54) | 3.66 (0.83) | <0.001 | −0.00 (0.84) | 1.00 | 65.52 | 59.59 | 59.59 | 60.98 | |
| 3 | 210 | −1.15 (0.61) | 0.33 (0.54) | 1.48 (0.83) | 0.073 | −1.46 (0.85) | 0.085 | 69.63 | 67.33 | 65.87 | 66.53 | |
| 4 | 344 | −1.15 (0.49) | −0.87 (0.42) | 0.28 (0.65) | 0.67 | −0.53 (0.66) | 0.43 | 73.81 | 71.51 | 70.98 | 69.24 | |
| 5 | 480 | −1.63 (0.41) | −0.81 (0.37) | 0.81 (0.56) | 0.15 | 0.13 (0.58) | 0.81 | 78.91 | 75.66 | 75.79 | 74.17 | |
| 6 | 594 | −0.93 (0.38) | -0.43 (0.34) | 0.50 (0.51) | 0.33 | −0.74 (0.53) | 0.16 | 81.45 | 79.59 | 78.85 | 77.99 | |
| 7 | 740 | −0.74 (0.34) | −0.89 (0.31) | −0.15 (0.46) | 0.74 | −0.10 (0.48) | 0.84 | 84.79 | 83.31 | 83.21 | 81.42 | |
| 8 | 879 | −0.26 (0.31) | −1.23 (0.28) | −0.97 (0.42) | 0.021 | −0.79 (0.44) | 0.075 | 89.14 | 88.62 | 87.83 | 85.36 | |
| 9 | 1090 | −0.26 (0.28) | −1.36 (0.26) | −1.10 (0.38) | 0.004 | −0.87 (0.41) | 0.033 | 94.44 | 93.92 | 93.05 | 90.33 | |
| 10 | 1293 | 0.72 (0.26) | −2.45 (0.24) | −3.17 (0.35) | <0.001 | −0.45 (0.37) | 0.23 | 101.33 | 102.77 | 102.32 | 97.42 | |
Covariates include treatment group and decile interacted with time, age, female, oral antibiotics (including azithromycin), inhaled antibiotics, oral bronchodilator, inhaled bronchodilator, oral corticosteroid, inhaled corticosteroid, mast cell stabilizer, oral supplements, enteral supplements, parenteral supplements, and pulmonary exacerbations treated with IV antibiotics.
FEV1% predicted is calculated based on Wang and Hankinson algorithms.
Sensitivity Analyses
Although there were over 32,000 patients enrolled in ESCF, only 2,230 were included in the dornase alfa group. In order to establish the FEV1 slope before initiation of dornase alfa, we required a lead-in period without dornase alfa. Because ESCF started about the time of the introduction of dornase alfa, many patients started on dornase alfa before or shortly after enrollment and therefore did not have the required lead-in period (12,508 out of 22,796). An additional 3,810 patients were ineligible because they started dornase alfa before their 8th birthday, 266 patients because they started after their 38th birthday, and 937 because they did not have an index pulmonary function test. Of the remaining 5,275 patients, 2,230 (42%) were evaluable. They differed from the 3,045 nonevaluable patients by being an average of almost 2.5 years younger (14.5 vs. 16.9 years) and had higher FEV1 % predicted (76.0 vs. 73.9). When the groups were compared by age groups (8–12, 13–17, 18–24, 25–38), the evaluable patients were similar to the nonevaluable patients on most characteristics, but in the youngest age group the evaluable patients had lower FEV1 % predicted (82.7 vs. 85.3) and lower weight for age percentile (29.5 vs. 33.2).
To reduce potential bias, we chose to include in the comparator group patients who subsequently were started on dornase alfa. If these patients were omitted from the comparator group, then two otherwise identical patients would be treated differently based on whether or not they take dornase alfa in the future, possibly the distant future. Therefore, we included patients in the comparator group up to the start of dornase alfa. As a sensitivity analysis, we repeated the decile-adjusted analysis after omitting from the comparator group those patients who later started dornase alfa, which reduced the number of comparator cases from 5,970 to 3,662. The estimated effects were similar but with larger standard errors reflecting the nearly 39% overall reduction in sample size (data not shown).
DISCUSSION
This study indicates that dornase alfa is associated with a reduction in the annual rate of decline in FEV1 % predicted in patients with CF across a range of disease stages, even after adjusting for other therapies. We confirmed previous findings that initiation of treatment is associated with improvement in FEV1, regardless of age or disease stage.3–5, 7 Although improvement in FEV1 is important to the health of patients with CF, long-term survival is associated with slower rates of decline in lung function, particularly FEV1.12, 13 Thus therapeutic interventions resulting in a slower rate of FEV1 decline are more likely to have a greater impact on the course of CF lung disease than those showing only a step change in lung function. The only CF therapies previously shown to have a beneficial effect on rate of FEV1 decline are high-dose ibuprofen (in both a clinical trial and an observational study)14, 15 and inhaled corticosteroids (in an observational study).11 Although a previous clinical trial evaluated dornase alfa over a 2-year period, the sample size was not sufficient to assess for rate of FEV1 decline.5
Observational studies such as ESCF have the potential to demonstrate how real-world clinical use of CF therapies affects lung function over the long term, but are subject to certain limitations. Most importantly, they are subject to indication bias: patients selected by physicians for any specific treatment are likely to be different from those not selected. In observational studies, indication bias can overwhelm positive therapeutic effects that can be shown in randomized trials.16 As expected, we found in this study that patients receiving dornase alfa had more advanced lung disease than those not receiving dornase alfa. Moreover, patients receiving dornase alfa in this study received more CF-related therapies at the outset. The dornase alfa patients also had a greater increase in their use of these therapies after index compared with patients who were not treated with dornase alfa. Although we used time-varying covariates to adjust for within-patient changes in pulmonary therapies, such adjustments are limited to available data. An increase in nonpulmonary interventions for these patients could potentially explain some of the associated beneficial effect of dornase alfa on the decline in FEV1.17
One way some studies address the problem of indication bias is to include a control group. In this study, the indication bias is likely to be too large to overcome with adjustments for measured covariates. Accordingly, we had no control group; we used patients as their own controls by comparing slopes before and after a point in time. We included a comparator group to help elucidate the usual pattern of change in FEV1 slope about an arbitrary point in time, not to act as matched controls. The online supplement includes additional information on this point. The dornase alfa group and comparator group were different in many ways. Patients started on dornase alfa were likely to have more advanced lung disease. Advanced lung disease has been shown to be associated with a slower rate of FEV1 decline.18 However, the dornase alfa group also had a higher prevalence of other risk factors that are associated with a more rapid decline in FEV1, including having Pseudomonas, more signs and symptoms of lung disease, and poorer nutritional status.18 The net effect of these opposing influences was a more rapid decline among the dornase alfa group. That is, these other risk factors outweighed the effect of the more advanced lung disease. We chose not to attempt to adjust for between-patient risk factors because the focus was on the within-patient change in slope. However, in the model we did control for CF-related respiratory therapies to avoid confounding by the fact that the dornase alfa group received more therapies and to account for any change in therapy that coincided with initiation of dornase alfa.
Although the FEV1 rate of decline in the adults in the comparator group stayed the same, it significantly accelerated in the children. This may be explained in part by age-related differences in disease stage (as measured by FEV1 % predicted). By using deciles, we adjusted for the combined effect of age and disease stage. Patients in the lower deciles had a decelerating decline, whereas those in the higher deciles had an accelerating decline, in both the comparator and dornase alfa groups. The decelerating decline among patients with lower age-adjusted FEV1 % predicted may be partially due to a survivor effect (mortality tends to exclude those with worsening FEV1). Among patients in the lower deciles, the dornase alfa patients had a greater improvement in the rate of decline than the comparator patients. For those patients in the higher deciles, the rate of decline for the dornase alfa patients did not worsen as much as for the comparator patients.
Another potential limitation of observational studies is that although a large number of patients may be enrolled, the number of patients with adequate data for analysis may be limited. In this study we required patients to have at least 6 months of data before and after index, an adequate number of PFTs, and to have received dornase alfa at least 80% of the time after index. This 80% requirement presumably resulted in the dornase alfa patients being more likely to have had a beneficial response to therapy. Ideally, an observational study should be able to demonstrate a dose effect or the effect of discontinuing therapy. Unfortunately, because patients who start dornase alfa tend to receive this therapy continuously for a long time, ESCF does not have sufficient data to evaluate a dose effect based on the percent of time on dornase alfa nor to evaluate the effect of discontinuation. This study also is subject to other limitations inherent in observational studies, such as lack of standardization across sites and lack of monitoring of patient compliance with prescribed treatments. In addition we did not address whether the identified reduction in FEV1 decline lasted more than the 2 years studied.
In conclusion, this study not only confirms that initiating dornase alfa therapy leads to an acute improvement in FEV1 but also shows that initiation and consistent use of dornase alfa is associated with an improvement in the annual rate of decline in FEV1 over a 2-year period. Although survival was not evaluated in this study, slowing the progression of lung disease, as indicated by a reduced rate of FEV1 decline, should ultimately relate to this important outcome.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the participation of the more than 400 site investigators and coordinators in the ESCF in collecting this comprehensive database.
Disclosure of
This study is sponsored by Genentech, Inc. Michael Konstan, Jeffrey Wagener, and Wayne Morgan have received honoraria from Genentech for serving as members of the Scientific Advisory Group for the Epidemiologic Study of Cystic Fibrosis (ESCF). Michael Konstan, Wayne Morgan, and Jeffrey Wagener have served as consultants to Genentech. No compensation was provided to these authors in exchange for production of this manuscript. David Pasta and Stefanie Millar are employees of ICON Clinical Research, which was paid by Genentech for providing analytical services for this study.
All sources of support for the Epidemiologic Study of Cystic Fibrosis in the form of grants, case report forms, and data analysis were provided by Genentech, Inc., South San Francisco, California. The authors were responsible for the study design, interpretation of data, and writing of the manuscript. The decision to submit the manuscript was made by the authors and was approved by Genentech, Inc.
Footnotes
Conflict of Interest
Joan Jacobs and Ashley Yegin are currently and Jeffrey Wagener was previously an employee of Genentech.
REFERENCES
- 1.Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol. 1997;24:137–142. doi: 10.1002/(sici)1099-0496(199708)24:2<137::aid-ppul13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci U S A. 1990;87:9188–9192. doi: 10.1073/pnas.87.23.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 4.McCoy K, Hamilton S, Johnson C. Effects of 12-week administration of dornase alfa in patients with advanced cystic fibrosis lung disease. Pulmozyme Study Group. Chest. 1996;110:889–895. doi: 10.1378/chest.110.4.889. [DOI] [PubMed] [Google Scholar]
- 5.Quan JM, Tiddens HA, Sy JP, McKenzie SG, Montgomery MD, Robinson PJ, Wohl ME, Konstan MW. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr. 2001;139:813–820. doi: 10.1067/mpd.2001.118570. [DOI] [PubMed] [Google Scholar]
- 6.Davis PB, Byard PJ, Konstan MW. Identifying treatments that halt progression of pulmonary disease in cystic fibrosis. Pediatr Res. 1997;41:161–165. doi: 10.1203/00006450-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Johnson CA, Butler SM, Konstan MW, Breen TJ, Morgan WJ. Estimating effectiveness in an observational study: a case study of dornase alfa in cystic fibrosis. The Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. J Pediatr. 1999;134:734–739. doi: 10.1016/s0022-3476(99)70290-8. [DOI] [PubMed] [Google Scholar]
- 8.Morgan WJ, Butler SM, Johnson CA, Colin AA, Fitzsimmons SC, Geller DE, Konstan MW, Light MJ, Rabin HR, Regelmann WE, Schidlow DV, Stokes DC, Wohl ME, Kaplowitz H, Wyatt MM, Stryker S. Epidemiologic Study of Cystic Fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S. and Canada. Pediatr Pulmonol. 1999;28:231–241. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 10.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 11.Ren CL, Pasta DJ, Rasouliyan L, Wagener JS, Konstan MS, Morgan WJ. Relationship between inhaled corticosteroid therapy and rate of lung function decline in children with cystic fibrosis. J Pediatr. 2008;153:746–751. doi: 10.1016/j.jpeds.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131:809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 13.Schluchter MD, Konstan MW, Davis PB. Jointly modelling the relationship between survival and pulmonary function in cystic fibrosis patients. Stat Med. 2002;21:1271–1287. doi: 10.1002/sim.1104. [DOI] [PubMed] [Google Scholar]
- 14.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332:848–854. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 15.Konstan MW, Schluchter MD, Wei X, Davis PB. Clinical use of ibuprofen is associated with slower rate of FEV1 decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2007;176:1084–1089. doi: 10.1164/rccm.200702-181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothman KJ, Wentworth CE., III Mortality of cystic fibrosis patients treated with tobramycin solution for inhalation. Epidemiology. 2003;14:55–59. doi: 10.1097/00001648-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Johnson C, Butler SM, Konstan MW, Morgan W, Wohl ME. Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest. 2003;123:20–27. doi: 10.1378/chest.123.1.20. [DOI] [PubMed] [Google Scholar]
- 18.Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, Stokes DC, Wohl ME, Wagener JS, Regelmann WE, Johnson CA. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151:134–139. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.