Abstract
Background: American Heart Association/American Stroke Association guidelines recommend initiating treatment with IV tissue plasminogen activator (tPA) in acute ischemic stroke patients without suspected coagulopathy prior to availability of clotting results; however, little or no data support this practice. We sought to identify how often blood clotting abnormalities were responsible for withholding IV tPA at our institution.
Methods: We conducted a retrospective review of our prospectively acquired Get With the Guidelines Stroke database from January 2003 to April 2008. All patients underwent clinical evaluation by a neurologist, diagnostic neuroimaging, and laboratory testing on admission. We classified patients with absolute contraindications to IV tPA as ineligible, and those with warnings/relative contraindications or potentially treatable factors as potentially eligible.
Results: Of 2,335 considered for analysis, 470 (20.1%) patients presented to our emergency department (ED) within 3 hours. Among these, 147 (31.3%) received IV tPA in our ED, 102 (21.7%) had an absolute contraindication, and 221 (47%) had a reason to consider withholding tPA. Only 30/470 (6.4%) of potential thrombolysis patients were discovered to have international normalized ratio ≥1.7 or platelets ≤100,000/μL, and of these, 28 were suspected a priori due to known coagulopathy from medication or illness. Only 2/470 (0.4%) patients had an unsuspected coagulopathy that ultimately prevented thrombolysis.
Conclusions: Based on the experience of a large thrombolysis referral center, stroke patients without suspected clotting abnormality can safely begin thrombolytic therapy before clotting results are available. These data support the current practice guidelines, and may reassure clinicians that the benefits of early administration greatly outweigh the risks due to an unsuspected bleeding diathesis.
Intravenous thrombolysis using recombinant tissue plasminogen activator (IV tPA) is the single most effective evidence-based therapy available for acute ischemic stroke.1–6 Despite efforts to expand the treatment window,7–9 experts agree that the faster IV tPA is administered following symptom onset, the greater the benefits of the treatment.10–12 Multiple reports indicate that anywhere from one third to a half of all stroke patients presenting within the 3-hour window are potentially eligible for acute thrombolysis12–14; however, the majority of those go untreated.15–20
The time delays associated with performing diagnostic investigations, including laboratory testing for suspected coagulopathy in patients with acute ischemic stroke, is cited frequently as a reason for failure to administer IV tPA within the 3-hour time window.21–24 To avoid delay, the latest guidelines from the American Heart Association/American Stroke Association recommend starting IV tPA in patients with acute ischemic stroke without suspected coagulopathy while clotting results are pending.1 However, there are no data cited to support this practice and to our knowledge, little or no reported data support this practice.24,25 We sought to identify how often the detection of blood clotting abnormalities was the critical reason responsible for withholding IV tPA at our institution.
METHODS
Patient characteristics.
We conducted a retrospective review of 2,872 prospectively enrolled patients at our institution as part of the Get With the Guidelines Stroke (GWTG-S) (January 1, 2003, to April 1, 2008). Patient characteristics, clinical presentation, contraindications, or warnings documented as the reason for withholding IV tPA were abstracted, as per GWTG-S. All patients underwent clinical evaluation by a neurologist, diagnostic neuroimaging, and laboratory testing on admission to the Emergency Department (ED). Time window of presentation was used as a principal inclusion criterion for this study. Among those who arrived at our hospital within 3 hours of symptom onset but were not treated with IV tPA, patients with absolute contraindications as listed in the Food and Drug Administration drug label or consensus guidelines were classified as ineligible for IV tPA. These reasons included prior stroke or serious head trauma within past 3 months (defined as “recent”); known history of intracranial hemorrhage (ICH) or aneurysm; symptom suggestive of subarachnoid hemorrhage (SAH); history of gastrointestinal or urinary hemorrhage in previous 21 days or evidence of active bleeding on examination (defined as “internal bleeding”); seizure at symptom onset with residual postictal neurologic impairment; major surgery in the previous 14 days or acute trauma on examination (defined as “recent surgery/trauma”); and coagulopathy (including international normalized ratio [INR] ≥1.7, abnormal activated partial thromboplastin time [aPTT], or platelet count ≤100,000/μL).
Patients ineligible for IV thrombolysis were classified as having either “suspected” or “unsuspected” coagulopathy. Patients were classified as “suspected coagulopathy” if they were taking oral anticoagulants, had received heparin in the previous 48 hours, or had a known medical condition predisposing them to a bleeding diathesis (e.g., thrombocytopenia, end-stage liver disease, hematologic malignancy), whereas patients without the above conditions but who were discovered to have abnormal laboratory clotting values during the ED evaluation for acute stroke symptoms were classified as “unsuspected” coagulopathy.
The remaining patients who were without an absolute contraindication were classified as “potentially eligible.” These were those with warnings or relative contraindications (e.g., stroke severity too severe, stroke severity too mild, rapidly improving symptoms, advanced age, life expectancy less than 1 year), or with potentially treatable conditions (poorly controlled hypertension defined as sustained systolic blood pressure >185 mm Hg and/or diastolic blood pressure >110 mm Hg despite best medical treatment) or potentially modifiable factors (inability to determine patient eligibility, family or patient refusal of treatment, delay in arrival precluding timely treatment, in-hospital delay, unrecorded contraindications).
While we had no prespecified clinical criteria with respect to minimum NIH Stroke Scale (NIHSS) threshold for treatment eligibility, our approach to the acute stroke patient has been to treat those patients who have “a significant neurologic deficit expected to result in long-term disability.” In practice, we treated patients with NIHSS ≥4, and among those with NIHSS <4, we treated those with isolated, severe aphasia, cannot ambulate independently, or in whom we did not “anticipate ability to discharge to home” due to the stroke deficits.
Standard protocol approvals, registrations, and patient consents.
All acute stroke patients at our institution are entered in the GWTG-Stroke quality improvement database during their admission. Because de-identified data are collected for quality improvement purposes, only data available in the medical record are abstracted and our institutional review board has waived the need for informed consent and approved the analysis of our GWTG-Stroke database.
Statistical methods.
Continuous variables were analyzed using t test or Wilcoxon rank sum test, as appropriate. Fisher exact test was used to analyze proportions. Significance level for all analyses was set at p < 0.05.
RESULTS
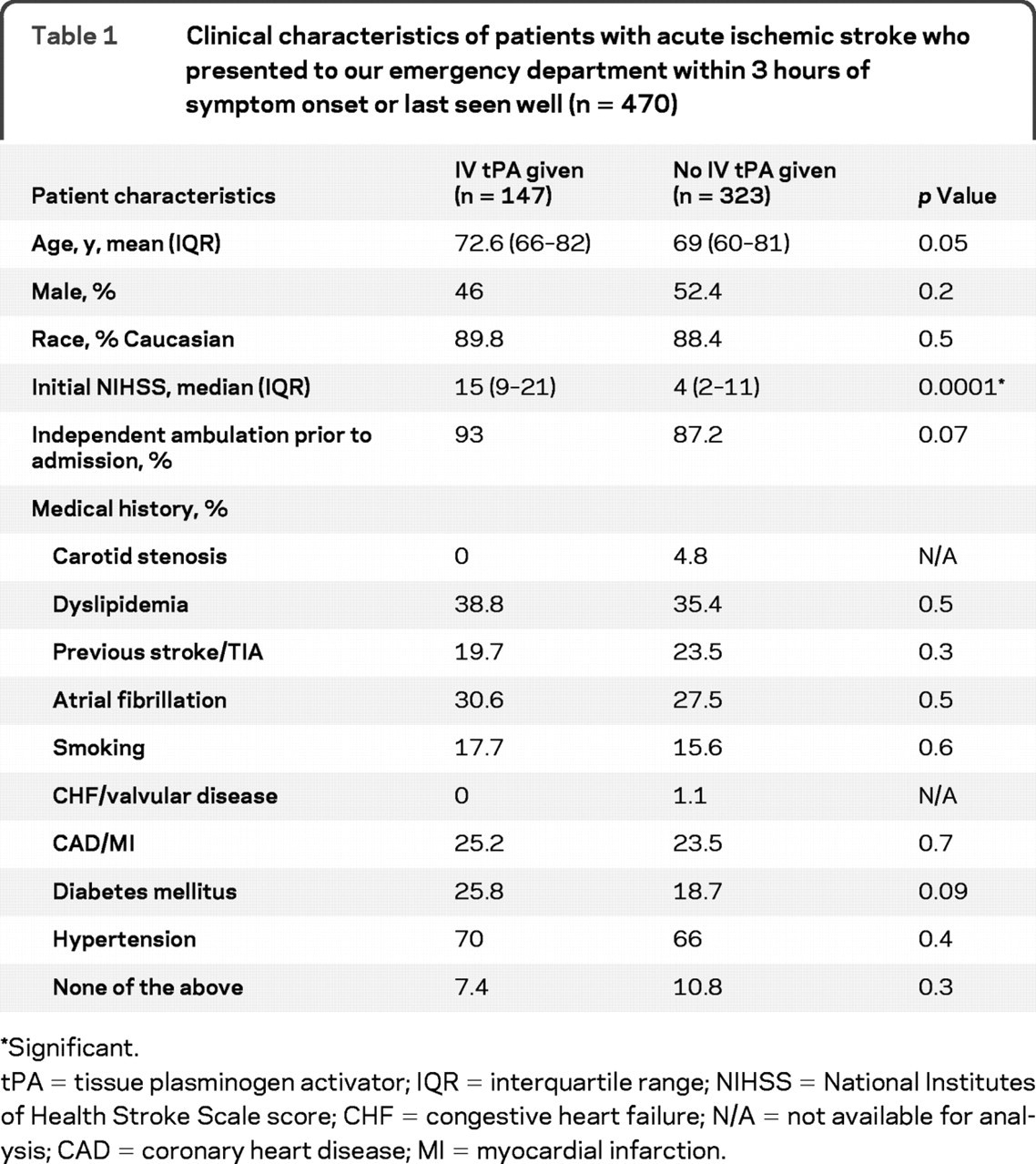
Of 2,872 patients recorded as part of our prospective acute stroke registry, we excluded from analysis patients with TIA (n = 317), those who arrived to our center within 3 hours of symptom onset but were treated with IV tPA at an outside hospital (n = 30) or as inpatients (n = 23), and those without explicit time last seen well (n = 167). Of 1,865 patients who presented to our hospital beyond 3 hours after symptom onset, 148 (7.9%) had received IV tPA at the referring hospital. Of the 2,335 patients we considered for final analysis, 470 (20.1%) patients presented to our ED within 3 hours from the symptom onset and formed the cohort for analysis in this report. Baseline clinical characteristics of these 470 patients are reported in table 1. The cohort was predominantly Caucasian (94%), with African American subjects representing 4.5% and Asian subjects 1.5% of all subjects. The patients who received IV tPA had higher NIHSS score (p < 0.0001) on admission but otherwise did not differ significantly from the group that did not receive tPA.
Table 1Clinical characteristics of patients with acute ischemic stroke who presented to our emergency department within 3 hours of symptom onset or last seen well (n = 470)
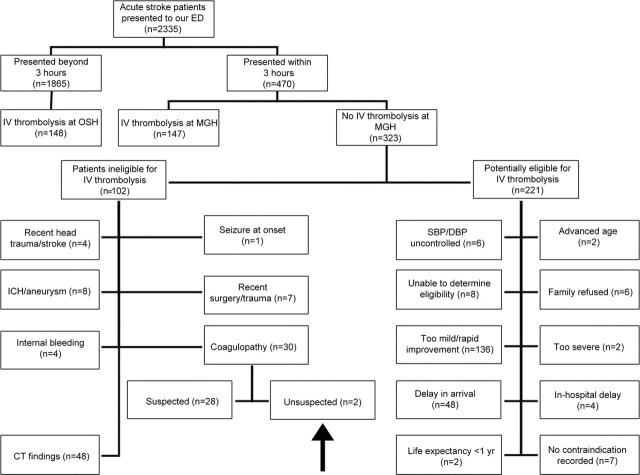
Among the patients who presented to our ED within 3 hours from symptom onset, 147 (31.3%) received IV t-PA in our ED, 102 (21.7%) had an absolute contraindication, and 221 (47%) had a reason to consider withholding tPA (figure).
Figure Reasons for exclusion from IV thrombolytic therapy in patients with acute ischemic stroke
Arrow points to the box that identifies patients with unsuspected coagulopathy. DBP = diastolic blood pressure; ED = emergency department; ICH = intracerebral hemorrhage; OSH = outside hospital; SBP = systolic blood pressure.
Within the group with absolute contraindications to IV tPA, the most common reason for exclusion was abnormal brain imaging on unenhanced head CT findings (n = 48/102, 47.1%), followed by coagulopathy (n = 30/102, 29.4%), history of ICH and/or evidence of aneurysm (n = 8/102, 7.8%), recent trauma or major surgery (n = 7/102, 6.8%), recent stroke or head trauma (n = 4/102, 3.9%), evidence or history of internal bleeding (n = 4/102, 3.9%), or seizure at onset (n = 1/102, 0.9%).
Among the patients who were potentially eligible for IV thrombolysis but in whom a reason to withhold IV tPA was discovered, the majority presented with symptoms deemed to be too mild or who were rapidly improving (n = 136/221, 61.5%). The remaining patients were excluded due to delay in arrival (n = 48/221, 21.7%), being unable to determine eligibility (n = 8/221, 3.6%), having difficulty in controlling hypertension (n = 6/221, 3.6%), family refusing the treatment (n = 6/221, 2.7%), in-hospital delay (n = 4/221, 1.8%), deemed to have symptoms that were too severe (n = 2/221, 0.9%), advanced age (n = 2/221, 0.9%), or life expectancy less than 1 year (n = 2/221, 0.9%). There were no documented reasons for nontreatment recorded in 7/221 patients (3.2%) in our database who were potentially eligible for IV tPA treatment but did not receive it.
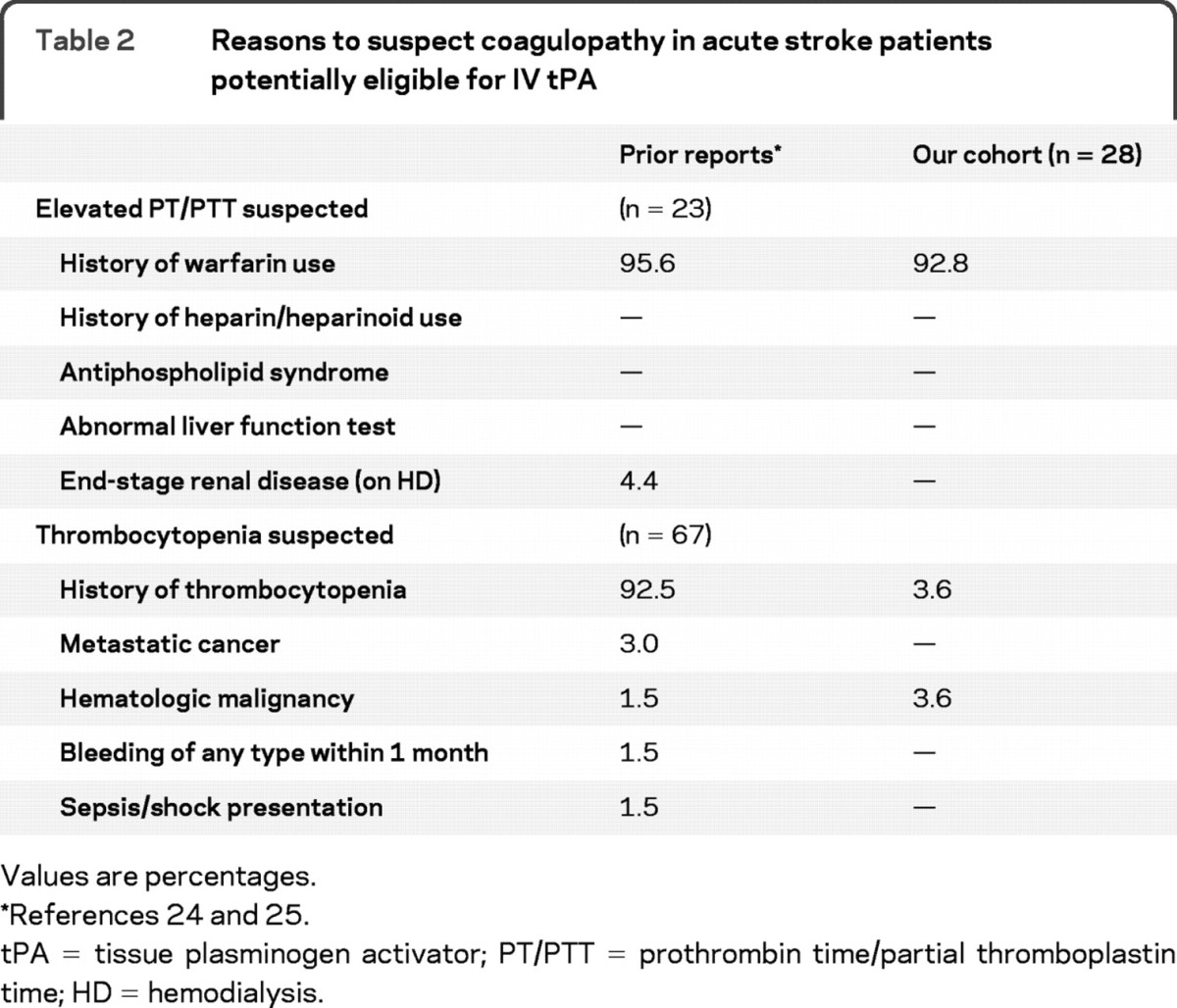
A total of 30/470 (6.4%) of potential thrombolysis patients were discovered to have INR ≥1.7 or platelets ≤100,000/μL. Of these 30 patients, 28 (93.3%) were suspected a priori due to known coagulopathy from medication or illness. Only 2/470 (0.4%, 95% CI −0.16, 1.02) patients had an unsuspected coagulopathy that ultimately prevented thrombolysis. One patient had an elevated PT/INR in the setting of longstanding alcohol abuse but no evidence of abnormal liver function (as measured by liver function tests). A second patient was discovered to have previously unrecognized thrombocytopenia (platelet count ≤100,000/μL), possibly due to undiagnosed myelodysplastic disorder. There were no patients in our cohort with abnormal markers of coagulation due to antiphospholipid syndrome, abnormal liver function tests, end-stage renal disease and/or on hemodialysis, metastatic cancer, recent bleeding of any type, or shock/sepsis (table 2).
Table 2 Reasons to suspect coagulopathy in acute stroke patients potentially eligible for IV tPA
DISCUSSION
We found that patients without suspected coagulopathy represent a minor fraction of all patients who present within a time window for thrombolytic treatment for acute ischemic stroke. These data provide evidence to support the current American Heart Association/American Stroke Association guidelines1 to initiate IV tPA treatment without a delay and before clotting values are available. Under these circumstances, the benefits of prompt administration of IV tPA in patients without suspected coagulopathy may greatly outweigh the risk of potential bleeding complications.2,10–12 Furthermore, advancement of other strategies such as the use of point of care fingerstick devices for measurement of blood chemistries and coagulation values may be warranted in reducing time to IV tPA administration.
Other authors proposed a similar approach in an attempt to avoid unnecessary in-hospital delays in initiation of acute thrombolysis for ischemic stroke in patients who are otherwise eligible for treatment. Several models were compared24 including the one using only the historic information suggestive of abnormal clotting values in order to predict elevated PT/PTT. These were history of warfarin or heparin/heparinoid use, history of antiphospholipid syndrome, and/or abnormal liver function test. This model alone predicted with 100% sensitivity which acute stroke patients in the emergency department had elevated PT/PTT. While the authors did not advocate for elimination of coagulation testing in the process of establishing eligibility for IV tPA treatment, they suggested that in absence of historical information suggesting a clotting abnormality, the likelihood of coagulopathy is negligible, and that delay in administration of thrombolytic due to the laboratory testing could be eliminated.
Similarly, the utility of delaying thrombolytic therapy to check platelet counts has been evaluated.25 The authors have argued that while the risk of harm with treatment without platelet information available may be as high as 3 per 1,000 patients, the potential benefit of early administration of the thrombolytic in ischemic stroke is far greater. Laboratory testing, including determination of the platelet count, is frequently cited as a cause of treatment delay because it requires multiple steps—from venipuncture to sample analysis and communication of results—each of which is fraught with potential for introducing unnecessary delays in the process. Given that no reliable data on absolute risk of administering IV tPA to patients with thrombocytopenia exist,25 the authors suggest that the benefits of earlier thrombolysis outweigh the risks of bleeding complications when treating a patient with unsuspected thrombocytopenia. They also enforce the necessity of rapid laboratory testing that would, otherwise, allow thrombolytics to be discontinued promptly in those patients who subsequently were discovered to be thrombocytopenic.
Despite the overall recent improvements in management of acute ischemic stroke, the rates of acute thrombolysis remain unacceptably low.12 Among the estimated 30%–40% of patients who might be receiving IV tPA upon arrival to the treatment center,16 only a significantly smaller proportion of acute ischemic stroke patients actually get the treatment. The reasons for exclusions among the patients who arrive within 3 hours of symptom onset are frequently avoidable13,16,20,26 and include in-hospital delays. In our study, 31.3% of patients who presented within 3 hours from last seen well received IV thrombolysis. The patients who were perceived to be potentially eligible for IV tPA (221/323) were those with warnings, relative contraindications, or with potentially treatable or modifiable factors (68%), whereas those determined as ineligible constituted 32%.
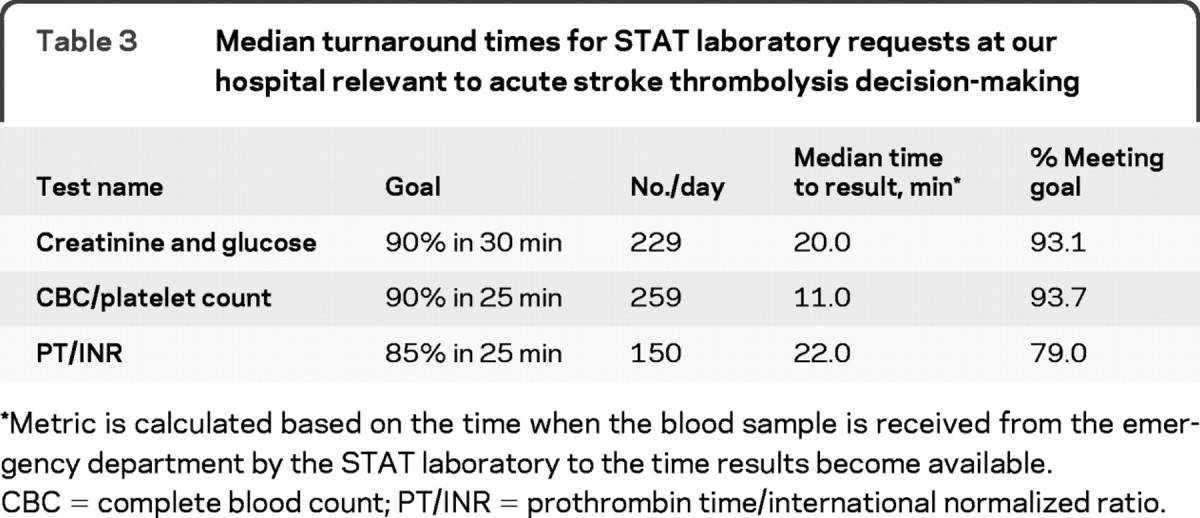
Our study has several limitations. It is a retrospective review of prospectively collected data; thus, we were limited in our ability to review information that was not documented at the time of decision-making. In addition, it is possible that patients with other absolute contraindications did not have additional reasons for nontreatment specified. However, these limitations likely have little impact on our primary finding. Furthermore, these data are based on the experience of a single large, academic referral stroke center with established practice parameters and a laboratory that consistently returns coagulation profile results in a timely manner. At our hospital, median turnaround times for STAT laboratory requests relevant to acute stroke thrombolysis decision-making are 11 to 22 minutes (platelet count vs PT/PTT/INR) (table 3); however, these goal times are achieved, on average, 79% (77%–84%) of the time and could still contribute to delaying IV tPA treatment. We do not have the data to report on whether these laboratory delays ever resulted in treatment delays at our institution, but we do know that no patients were unable to receive drug due to the these delays since this reason for nontreatment is an available option to select in our database.
Table 3 Median turnaround times for STAT laboratory requests at our hospital relevant to acute stroke thrombolysis decision-making
While the efforts to decrease arrival time to the hospital for patients with acute ischemic stroke are still a priority, the determination of tPA eligibility must be expedited. This is critical because every additional minute delay in initiation of IV tPA results in the “death of 1.9 million neurons, 14 billion synapses, and 12 km (7.5 miles) of myelinated fibers.”27 Therefore, reducing delays in administration of IV tPA in patients with unsuspected coagulopathy may be a powerful step in improving the overall benefit of acute thrombolysis. Both national guidelines and the rt-PA Food and Drug Administration approved label or “package insert” indicate that “treatment can be initiated prior to the availability of coagulation study results.”28 Our data now provide evidence for this practice recommendation. While the risk of harm due to initiating IV tPA treatment prior to information on potential coagulopathy may be as high as 4–10 per 1,000 patients (given point estimate 0.4%, 95% CI −0.16, 1.02), the benefit of early administration of IV tPA may be up to 333 per 1,000 patients.29 These data should reassure clinicians that the benefits of early administration greatly outweigh the risk of harm due to an unsuspected bleeding diathesis.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Natalia Rost.
DISCLOSURE
Dr. Rost receives research support from the Bugher Foundation/American Stroke Association (training fellowship grant). Dr. Masrur and Dr. Pervez report no disclosures. Dr. Viswanathan receives research support from the NIH [NIA 2P50AG005134-268382 (Co-I)]. Dr. Schwamm serves as vice chair of the American Heart Association GWTG Steering Committee; serves on scientific advisory boards for CoAxia, Inc., Phreesia, and Lundbeck Inc.; serves on the editorial board of Neurocritical Care; may accrue revenue on US Patent 6,542,769, Issued: 1 Apr 2003: Imaging system for obtaining quantitative perfusion indices; his wife receives royalties from publishing Obstetric Anesthesia (Cambridge Pocket Clinicians, 2007); has received honoraria for lectures or educational activities not funded by industry; serves/has served as a consultant to CryoCath® Technologies Inc., Research Triangle Inc., Massachusetts Department of Public Health, and the Canadian Stroke Network; receives research support from Forest Laboratories, Inc., the NIH [NINDS P50 NS051343-01 (Co-I), NINDS U01 NS052220 IMS-3 (Site PI), NCRR 1 UL 1 RR025758-01 (CRC Staff)], and the Department of Health and Human Services [CDC 5 U13 DP001176-02 (PI)]; and has provided expert medical opinions in malpractice lawsuits [RE: stroke treatment and prevention].
Glossary
- aPTT
activated partial thromboplastin time
- ED
emergency department
- GWTG-S
Get With the Guidelines Stroke
- ICH
intracranial hemorrhage
- INR
international normalized ratio
- NIHSS
NIH Stroke Scale
- SAH
subarachnoid hemorrhage
- tPA
tissue plasminogen activator.
Footnotes
e-Pub ahead of print on November 25, 2009, at www.neurology.org.
Disclosure: Author disclosures are provided at the end of the article.
Received May 5, 2009. Accepted in final form September 9, 2009.
REFERENCES
- 1.Adams HP, Jr., del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007;38:1655–1711. [DOI] [PubMed] [Google Scholar]
- 2.The National Institute of Neurological Disorders and Stroke rt PASSG. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1588. [DOI] [PubMed] [Google Scholar]
- 3.Kwiatkowski TG, Libman RB, Frankel M, et al. The National Institute of Neurological Disorders, Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group: effects of tissue plasminogen activator for acute ischemic stroke at one year. N Engl J Med 1999;340:1781–1787. [DOI] [PubMed] [Google Scholar]
- 4.Wahlgren N, Ahmed N, Eriksson N, et al, for the S-MI. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke–Monitoring Study (SITS-MOST). Stroke 2008;39:3316–3322. [DOI] [PubMed] [Google Scholar]
- 5.Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke–Monitoring Study (SITS-MOST): an observational study. The Lancet 2007;369:275–282. [DOI] [PubMed] [Google Scholar]
- 6.Albers GW, Bates VE, Clark WM, Bell R, Verro P, Hamilton SA. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA 2000;283:1145–1150. [DOI] [PubMed] [Google Scholar]
- 7.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. [DOI] [PubMed] [Google Scholar]
- 8.Wahlgren N, Ahmed N, Dávalos A, et al. Thrombolysis with alteplase 3–4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet 2008;372:1303–1309. [DOI] [PubMed] [Google Scholar]
- 9.De Keyser J, Gdovinova Z, Uyttenboogaart M, Vroomen PC, Luijckx GJ. Intravenous alteplase for stroke: beyond the guidelines and in particular clinical situations. Stroke 2007;38:2612–2618. [DOI] [PubMed] [Google Scholar]
- 10.Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: The NINDS rt-PA Stroke Study. Neurology 2000;55:1649–1655. [DOI] [PubMed] [Google Scholar]
- 11.The ATLANTIS, ECASS, and NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768–774. [DOI] [PubMed] [Google Scholar]
- 12.Lyden P. Thrombolytic therapy for acute stroke: not a moment to lose. N Engl J Med 2008;359:1393–1395. [DOI] [PubMed] [Google Scholar]
- 13.Kaste M. Do not wait, act now. Stroke 2007;38:3119–3120. [DOI] [PubMed] [Google Scholar]
- 14.Katzan IL, Furlan AJ, Lloyd LE, et al. Use of tissue-type plasminogen activator for acute ischemic stroke: The Cleveland Area Experience. JAMA 2000;283:1151–1158. [DOI] [PubMed] [Google Scholar]
- 15.The Paul Coverdell Prototype Registries. Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell national acute stroke registry. Stroke 2005;36:1232–1240. [DOI] [PubMed] [Google Scholar]
- 16.Bambauer KZ, Johnston SC, Bambauer DE, Zivin JA. Reasons why few patients with acute stroke receive tissue plasminogen activator. Arch Neurol 2006;63:661–664. [DOI] [PubMed] [Google Scholar]
- 17.Schestatsky P, Picon PD, Kleindorfer DO, Khatri P, Katzan I, Cocho D. Reasons for exclusion from thrombolytic therapy following acute ischemic stroke. Neurology 2005;65:1844. [PubMed] [Google Scholar]
- 18.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology 2001;56:1015–1020. [DOI] [PubMed] [Google Scholar]
- 19.Fink JN, Selim MH, Kumar S, Schlaug G, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology 2001;57:1739–1740. [DOI] [PubMed] [Google Scholar]
- 20.Hills NK, Johnston SC. Why are eligible thrombolysis candidates left untreated? Am J Prev Med 2006;31:S210–S216. [DOI] [PubMed] [Google Scholar]
- 21.Romano JG, Muller N, Merino JG, Forteza AM, Koch S, Rabinstein AA. In-hospital delays to stroke thrombolysis: paradoxical effect of early arrival. Neurol Res 2007;29:664–666. [DOI] [PubMed] [Google Scholar]
- 22.Huang P, Yang Y-H, Lin R-T, et al. Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke. Cerebrovasc Dis 2006;22:423–428. [DOI] [PubMed] [Google Scholar]
- 23.Kwan J, Hand P, Sandercock P. A systematic review of barriers to delivery of thrombolysis for acute stroke. Age Ageing 2004;33:116–121. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman RF, Alt J, Wityk RJ, Llinas RH. Predicting abnormal coagulation in ischemic stroke: reducing delay in rt-PA use. Neurology 2006;67:1665–1667. [DOI] [PubMed] [Google Scholar]
- 25.Cucchiara BL, Jackson B, Weiner M, Messe SR. Usefulness of checking platelet count before thrombolysis in acute ischemic stroke. Stroke 2007;38:1639–1640. [DOI] [PubMed] [Google Scholar]
- 26.Cocho D, Belvis R, Marti-Fabregas J, et al. Reasons for exclusion from thrombolytic therapy following acute ischemic stroke. Neurology 2005;64:719–20. [DOI] [PubMed] [Google Scholar]
- 27.Saver JL. Time is brain–quantified. Stroke 2006;37:263–266. [DOI] [PubMed] [Google Scholar]
- 28.Genentech Inc. Activase Prescribing Information. Genentech Inc.: 2002.
- 29.Saver JL. Number needed to treat estimates incorporating effects over the entire range of clinical outcomes: novel derivation method and application to thrombolytic therapy for acute stroke. Arch Neurol 2004;61:1066–1070. [DOI] [PubMed] [Google Scholar]