Abstract
Guidelines have been established for the management of acute ischemic stroke; however, specific recommendations for endovascular revascularization therapy are lacking. Burgeoning investigation of endovascular revascularization therapies for acute ischemic stroke, rapid device development, and a diverse training background of the providers performing the procedures underscore the need for practice recommendations. This review provides a concise summary of the Society of Vascular and Interventional Neurology endovascular acute ischemic stroke roundtable meeting. This document was developed to review current clinical efficacy of pharmacologic and mechanical revascularization therapy, selection criteria, periprocedure management, and endovascular time metrics and to highlight current practice patterns. It therefore provides an outline for the future development of multisociety guidelines and recommendations to improve patient selection, procedural management, and organizational strategies for revascularization therapies in acute ischemic stroke.
In an effort to improve outcome in patients with acute ischemic stroke (AIS), recent initiatives have outlined the best medical management and developed protocols to facilitate timely identification and administration of the US Food and Drug Administration (FDA)–approved IV recombinant tissue plasminogen activator (rtPA) to eligible patients.1,2
Restoration of blood flow after AIS is associated with improved outcome and reduced mortality.3,4 A meta-analysis including over 2,000 patients in 53 studies confirmed a strong correlation between recanalization and good functional outcome at 3 months, in comparison with nonrecanalization (odds ratio [OR] 4.43; 95% confidence interval [CI] 3.32–5.91).4 Intra-arterial (IA) thrombolysis has not received FDA approval, but randomized trials and several case series have led to endorsements by multiple associations for select patients.5–9 Endovascular revascularization therapy (ERT) currently has a Class Ib recommendation for IA thrombolysis for select patients and a Level IIb recommendation for mechanical thrombus extraction in the American Heart Association (AHA) guidelines.1–9 Two device families have FDA approval for ERT: the Merci Retriever (Concentric Medical, Inc., Mountain View, CA) and the Penumbra Aspiration System (Penumbra Inc., Alameda, CA); and multiple new devices are rapidly approaching FDA approval and market availability.10,11 Established guidelines and recommendations are available for the early treatment of adults with AIS1 and for the development of comprehensive stroke centers7 and training standards for endovascular ischemic stroke treatment.9 However, guidelines for ERT for AIS are lacking. Ongoing clinical trials and the brisk pace of emerging technologies have fostered enthusiasm for endovascular therapy for AIS, resulting in the need for development of practice recommendations.
This outline was developed by a panel of physicians with a range of expertise in neurointerventional procedures, vascular neurology, neurocritical care, neurosurgery, and neuroradiology. In many instances, definitive clinical trial–based data are lacking, and practices are discussed on the basis of pathophysiologic rationale and expert opinion, not on the basis of randomized clinical trials.
SAFETY AND EFFICACY OF ENDOVASCULAR REVASCULARIZATION THERAPY FOR ACUTE ISCHEMIC STROKE
Endovascular treatment options for intracerebral revascularization have evolved considerably over the past decade. Several trials evaluating the various therapies are summarized in table 1. The Prolyse in Acute Cerebral Thromboembolism (PROACT) and PROACT II studies evaluated the use of IA thrombolysis with prourokinase in middle cerebral artery (MCA) occlusions.5,6 The initial phase 2 trial demonstrated higher recanalization rates with prourokinase.5 The phase 3 trial, PROACT II, demonstrated the effectiveness of IA thrombolysis with prourokinase in patients with an MCA occlusion treated within 6 hours from symptom onset.6 A minimum requirement NIH Stroke Scale (NIHSS) score of 4, except for isolated aphasia or hemianopia, was required for enrollment. Patients treated with prourokinase had a higher rate of recanalization (66% vs 18%; p < 0.001) and were more likely to have a good outcome (modified Rankin Scale [mRS] score of 0–2 at 90 days, 40% vs 25%; p = 0.04), despite a higher rate of symptomatic intracranial hemorrhage (sICH) (10% vs 2%; p = 0.06). The MCA-Embolism Local fibrinolytic intervention Trial (MELT) was a similarly designed trial comparing urokinase to placebo in patients with MCA occlusions, which was terminated early because of the approval of the IV administration of rtPA in Japan.12 Although the MELT findings are underpowered, the results are consistent with those of the PROACT trials, suggesting higher recanalization rates (74%) with IA thrombolysis.12 A meta-analysis of these 3 trials and 2 additional smaller trials combined 395 randomized patients and showed that IA thrombolysis increased the odds of both nondisabled outcome (mRS score 0–1; OR 2.5; 95% CI 1.33–3.14; p < 0.001) and nondependent outcome (mRS score 0–2; OR 14; 95% CI 1.31–3.51; p < 0.003).13 A case-control analysis from Japan's Multicenter Stroke Investigators' Collaboration (J-MUSIC) compared 91 patients with an acute cardioembolic stroke treated with IA urokinase within 4.5 hours of symptom onset to a matched control group that did not receive IA therapy. The analysis showed that a favorable outcome (mRS score of 0–2) was more frequently observed in the urokinase group (50.5% vs 34.1%; p = 0.0124), and there was no difference in mortality rate.14 Although confirmatory trials required for FDA approval of IA therapy have not been performed, these randomized trials and numerous case series support the use of IA thrombolysis in select patients who are ineligible for IV thrombolysis.
Table 1.
Data from selected trials of endovascular revascularization therapy for acute ischemic stroke
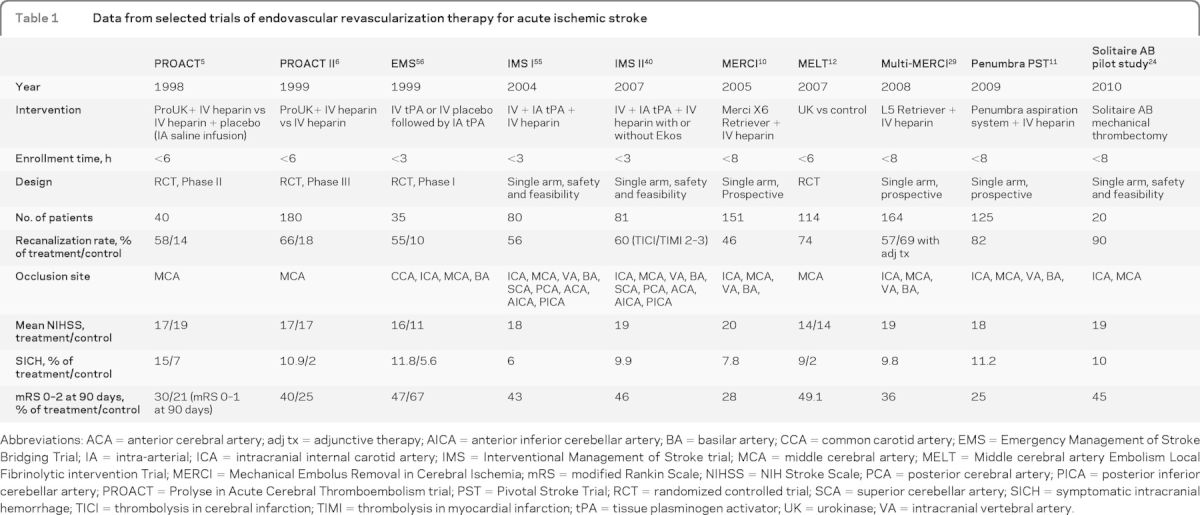
Abbreviations: ACA = anterior cerebral artery; adj tx = adjunctive therapy; AICA = anterior inferior cerebellar artery; BA = basilar artery; CCA = common carotid artery; EMS = Emergency Management of Stroke Bridging Trial; IA = intra-arterial; ICA = intracranial internal carotid artery; IMS = Interventional Management of Stroke trial; MCA = middle cerebral artery; MELT = Middle cerebral artery Embolism Local Fibrinolytic intervention Trial; MERCI = Mechanical Embolus Removal in Cerebral Ischemia; mRS = modified Rankin Scale; NIHSS = NIH Stroke Scale; PCA = posterior cerebral artery; PICA = posterior inferior cerebellar artery; PROACT = Prolyse in Acute Cerebral Thromboembolism trial; PST = Pivotal Stroke Trial; RCT = randomized controlled trial; SCA = superior cerebellar artery; SICH = symptomatic intracranial hemorrhage; TICI = thrombolysis in cerebral infarction; TIMI = thrombolysis in myocardial infarction; tPA = tissue plasminogen activator; UK = urokinase; VA = intracranial vertebral artery.
Mechanical devices for ERT have evolved as a means of achieving faster rates of recanalization in medium- to large-vessel occlusions. The Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi-MERCI were prospective, single-arm, multicenter trials designed to test the efficacy and safety of a corkscrew thrombectomy device in the treatment of medium- to large-vessel occlusions (anterior and posterior circulation) within 8 hours of symptom onset.10 A combined analysis of the 2 studies demonstrated a successful recanalization rate (defined as Thrombolysis in Myocardial Infarction 2 or 3 score) of 64.6%, with good clinical outcome (mRS score of 0–2) in 32.4%, despite an sICH rate of 7.8% in the first study and 9.8% in the second.15 The Penumbra Pivotal Stroke Trial provided registry data on a novel aspiration-thrombectomy device, the Penumbra system, used within 8 hours for large-artery cerebrovascular occlusion.11 A quarter of the patients achieved an mRS score of less than or equal to 2 at 90 days. Different techniques for measuring recanalization preclude a direct comparison between the rates achieved with MERCI and Penumbra, but both exceed the natural history rate.16
Randomized trials are ongoing, such as the Local Versus Systemic Thrombolysis for Acute Ischemic Stroke (SYNTHESIS EXP) and the Multicenter Randomized Clinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands (MR CLEAN), comparing endovascular recanalization vs standard medical treatment alone (including IV rtPA or supportive care alone).17,18 The NIH-funded Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) trial is comparing the effectiveness of endovascular therapy within 8 hours of symptom onset and standard medical care to standard medical treatment alone.19 Several trials are testing bridging therapies combining early administration of IV rtPA with the endovascular approach, including the Interventional Management of Stroke III (IMS III) and Assess the Penumbra System in the Treatment of Acute Stroke (THERAPY) trials.20 In the United States, the ongoing IMS, a randomized, multicenter trial, will enroll 900 subjects with AIS within 3 hours of symptom onset to compare combined IV and IA rtPA to IV rtPA alone.21
Alternative revascularization methods continue to evolve and have included acute intracranial stent implantation22 and temporary endovascular bypass and thrombectomy with a retrievable stent.23,24 Initial open series reports with stent retrievers suggest potentially higher recanalization rates and shorter procedure times.
PATIENT SELECTION FOR ENDOVASCULAR REVASCULARIZATION THERAPY IN ACUTE ISCHEMIC STROKE
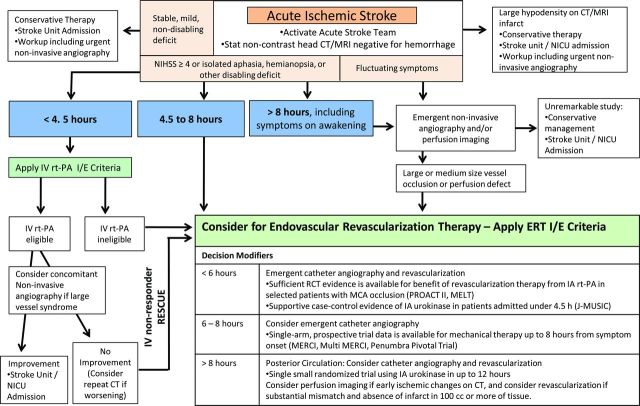
Designing a decision algorithm for patient selection for ERT in AIS is hindered by variable enrollment criteria in the trials cited previously. The presented outline for the development of a decision algorithm is based on findings from available randomized controlled trials and extrapolated from criteria from recent and ongoing clinical trials (figure). This is an example of one possible algorithm, and further investigation is necessary prior to clinical use.
Figure. Possible decision algorithm for revascularization therapies in acute ischemic stroke.
Outside of clinical trials, IV therapy remains first-line treatment for eligible patients presenting with clinical symptoms of AIS. Through a systematic review of the literature, the American Stroke Association (ASA) guidelines outline the best medical management as well as protocols to facilitate timely administration of IV rtPA to patients eligible for thrombolysis.1 For patients with moderate to severe deficits and minimal or no early ischemic changes on brain imaging, therapy triage is largely governed by time from symptom onset. There is strong evidence from multiple clinical trials to support the use of IV rtPA within 3 hours.2,25,26 Current FDA approval exists for patients presenting up to 3 hours from symptom onset, and a science advisory from the ASA/AHA has recommended expanding the time window to 4.5 hours in a subgroup of patients, on the basis of results of the European Cooperative Acute Stroke Study III (ECASS III).25,27
Patients presenting after 4.5 hours are not eligible for systemic thrombolysis; however, data exist, as cited previously, for the consideration of IA fibrinolytic administration up to 6 hours from symptom onset in patients with a large- to medium-vessel occlusion.6,12,28 For patients in whom endovascular therapy can be initiated within 8 hours from symptom onset, 2 mechanical revascularization device families have demonstrated safe and feasible rates of recanalization in single-arm, prospective trials.10,11,29 The optimal device for mechanical revascularization has not been identified, and the rapid growth of device technology will likely continue to challenge rigorous clinical evaluation.
Vertebrobasilar artery occlusion (VBO) has an invariably poor outcome if recanalization is not achieved early. The recent literature shows that mortality with acute VBO treated with nonthrombolytic drugs is 80% to 90%, although lower rates of 42% to 60% can be achieved with IA therapy.28,30,31 Success of recanalization and neurologic status before treatment are independent predictors of a favorable outcome after IA therapy.30,31 Multiple studies failed to establish a time window that would definitively exclude patients from IA therapy.30 One study found a significantly better clinical outcome in patients with acute VBO treated within 6 hours after symptom onset than in patients treated after 6 hours (favorable outcome of 36% vs 7%; mortality of 52% vs 70%; p = 0.005).28 Other studies demonstrated trends toward better outcome, with shorter duration of symptoms, and no significant association between time to treatment and clinical outcome.30,31 When patients are in a coma or have had prolonged symptoms, additional imaging such as MRI with diffusion and perfusion or CT perfusion might help in identifying those who are likely to benefit from intervention. However, the current application of CT perfusion results to the posterior circulation may be limited.
The trials that shape the current decision patterns have been largely based on time from symptom onset. Data are lacking on the efficacy of ERT beyond 12 hours from symptom onset in patients with posterior circulation occlusion and beyond 8 hours in anterior circulation occlusion.10,11,29,31,32 Given the poor natural history of VBO, revascularization has been considered beyond 12 hours from symptom onset. Enthusiasm continues for a perfusion imaging–based decision algorithm, although rigorous data to support this approach are lacking.33 Further study of perfusion imaging may assist with selection of patients who would benefit from revascularization beyond 8 hours.34
At the very least, noncontrast head CT or diffusion- and susceptibility-weighted MRI are required to exclude hemorrhage and identify early ischemic changes that could pose increased hemorrhagic risk following revascularization. Larger regions of well-defined hypoattenuation (CT) or hyperintensity (MRI) indicating infarcted tissue may carry a considerably higher risk of hemorrhage following revascularization. Careful consideration may be needed for patients with CT hypodensity or MRI hyperintensity in greater than 1/3 of the MCA territory or with prominent sulcal effacement.35 Alternative standardized scoring systems may include the Alberta Stroke Program Early CT Score (ASPECTS).36
Future studies may show that for patients who receive IV rtPA and have a clinical presentation suggestive of a large-vessel occlusion, early consideration of ERT may be important. The limited efficacy of IV rtPA in large vessel occlusions is demonstrated by recanalization rates as low as 30% in the proximal MCA and 6% in the terminal internal carotid artery (ICA).37 Urgent noninvasive vascular imaging can identify patients with a large-vessel occlusion. The interval from a decision to pursue IA intervention to reaching the clot can be long, with time required to obtain consent, transport and prepare the patient, and negotiate tortuous anatomy. Accordingly, an efficient strategy may be to activate the neurointerventional team when a large-vessel occlusion is suspected, without delay in IV rtPA initiation. If dramatic clinical improvement occurs, patients can be rerouted to repeat noninvasive vessel assessment. One retrospective study has shown that in those patients with a contraindication to IV rtPA or whose IV therapy fails, the use of ERT within the first 3 hours after stroke symptom onset has a low sICH rate, of 5.3%.38
Patients with fluctuating deficits or continued mild deficits (NIHSS score ≤4) following rapid improvement from presentation carry a risk of harboring a large-vessel occlusion with tenuous collateral supply. Failure of collateral supply could lead to acute deterioration; therefore, emergent noninvasive angiography to identify vessel occlusions amenable to ERT may be considered. To date, no randomized clinical trial has compared the natural history of medical treatment alone to early recanalization with ERT in this subset of patients.
For patients in whom ERT is considered, inclusion and exclusion criteria will be needed. Based on the existing clinical trials and guidelines, a framework for the future development of criteria can be outlined (table 2).
Table 2.
Possible selection criteria for acute ischemic stroke endovascular revascularization therapy
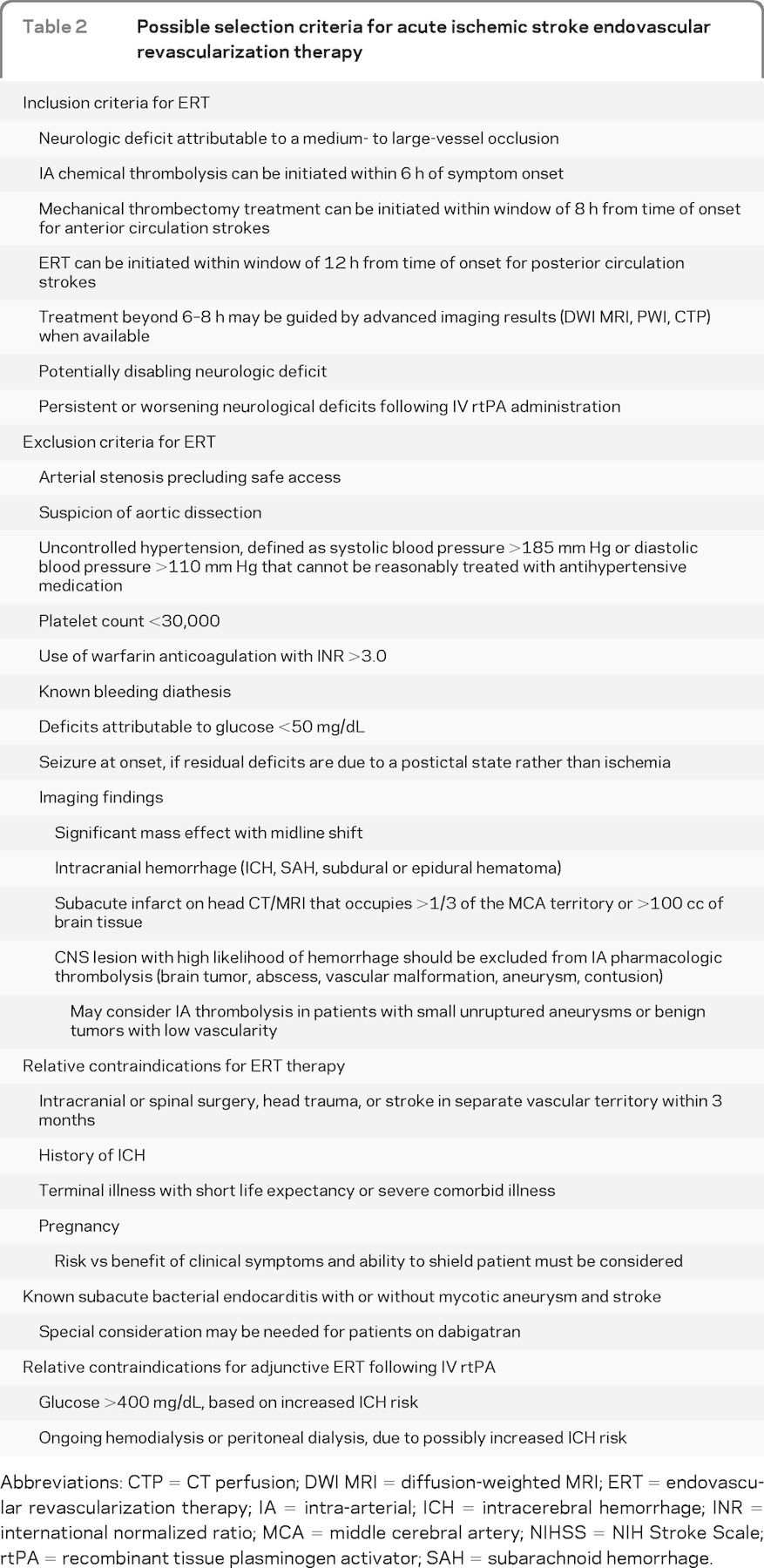
Abbreviations: CTP = CT perfusion; DWI MRI = diffusion-weighted MRI; ERT = endovascular revascularization therapy; IA = intra-arterial; ICH = intracerebral hemorrhage; INR = international normalized ratio; MCA = middle cerebral artery; NIHSS = NIH Stroke Scale; rtPA = recombinant tissue plasminogen activator; SAH = subarachnoid hemorrhage.
SELECTION OF ENDOVASCULAR REVASCULARIZATION THERAPY TECHNIQUE
The heterogeneity of AIS characteristics, including thrombus composition, occlusion location, thrombus volume burden, and collateral perfusion, may demand tailored interventions. For example, greater efficacy and safety may be demonstrated in distal vessel revascularization by use of IA fibrinolytic therapy, vs a mechanical device that may be more difficult to deliver. Alternatively, in large proximal vessel occlusions, greater benefit may be achieved with mechanical thrombectomy. Furthermore, carotid occlusion at the origin of the ICA may be better treated with balloon angioplasty and stent implantation.
Pharmacologic thrombolysis.
Local IA thrombolysis efficacy was demonstrated in PROACT II.6 This led to an AHA Class I, level of evidence B recommendation that IA thrombolysis is an option for the treatment of selected patients who have AIS under 6 hours duration due to occlusions of the MCA and who are not otherwise candidates for IV rtPA.1 Although variability in study designs prohibits direct comparison of the data, theoretically there may be a higher risk of intracerebral hemorrhage (ICH) with chemical IA thrombolysis than with mechanical revascularization. However, increased ICH was not substantiated in a multicenter study.39
Microcatheter position during thrombolytic infusion may also theoretically affect recanalization rates. The microcatheter position varies among the studies; in some instances it is placed distal to the thrombus, within the thrombus, or proximal to the thrombus. Some operators will use multiple locations to infuse rtPA throughout the thrombus. The maximum safe dose for IA rtPA is not known; however, if we extrapolate from large clinical trial experience, then a maximum dose of 22 mg, as in the IMS trials, may be a reasonable initial limit.21,40
Bridging therapies.
Bridging therapy trials evaluating the combined approach have shown better recanalization rates for medium- to large-vessel occlusions. However, they have shown only trends toward better outcomes in comparison with the IV rtPA–treated subjects in the National Institute of Neurological Disorders and Stroke (NINDS) rtPA Stroke Study or a database registry.40,41 Potential benefit of bridging therapy increases when the target population is limited to IV rtPA nonresponders (40% IV-IA patients reached functional independence at 3 months, vs 14.9% of recipients of only IV rtPA, among the nonresponders [p = 0.012]). This benefit came at the cost of a higher morbidity associated with the bridging therapy (OR 2.14; 95% CI 0.58–7.83 for sICH).42 The early Emergency Management of Stroke Bridging Trial/IMS trials used a protocol of 0.6 mg/kg IV rtPA with up to an additional maximum of 22 mg IA rtPA, which in most patients allowed for the total dose to remain below the NINDS maximum amount of 90 mg (table 3). However, newer bridging studies and the amended IMS III are using full-dose IV rtPA in the combined IV-IA treatment arm.20,21
Table 3.
Intra-arterial thrombolytic dosing and methods from selected trials
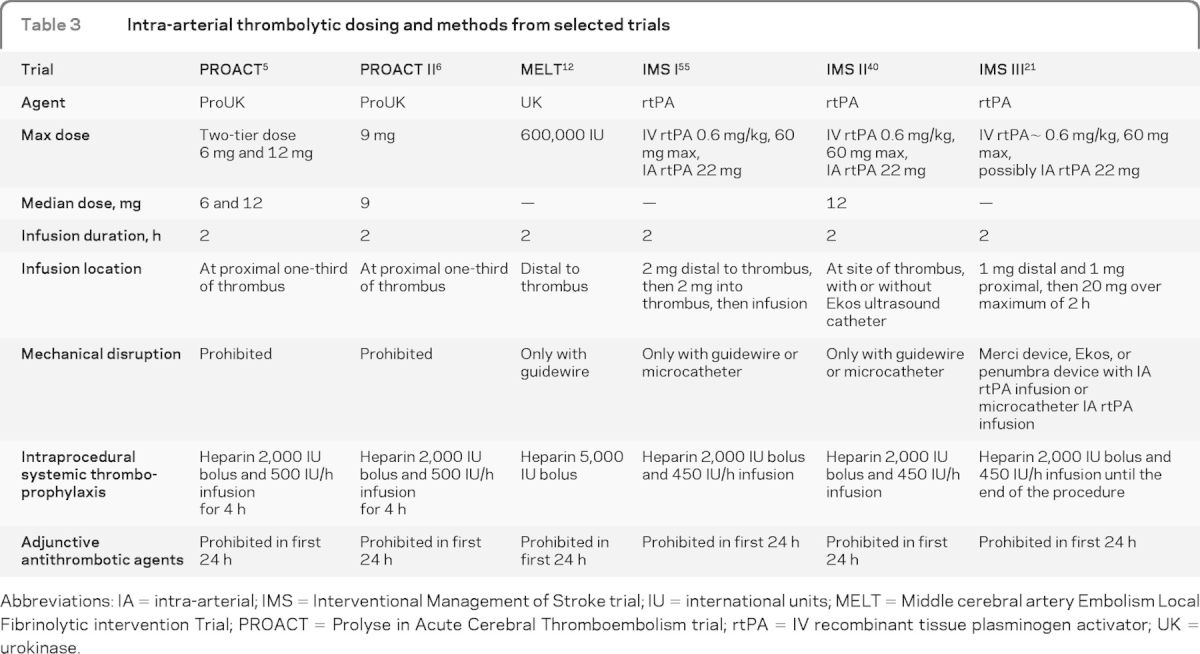
Abbreviations: IA = intra-arterial; IMS = Interventional Management of Stroke trial; IU = international units; MELT = Middle cerebral artery Embolism Local Fibrinolytic intervention Trial; PROACT = Prolyse in Acute Cerebral Thromboembolism trial; rtPA = IV recombinant tissue plasminogen activator; UK = urokinase.
Mechanical revascularization.
Mechanical techniques for ERT, including thrombectomy, clot retrieval, and thromboaspiration, have shown comparable or slightly higher recanalization rates than IA thrombolysis alone (table 1). Newer devices, such as the Solitaire FR retrievable stent (eV3, Irvine, CA), have shown even higher recanalization rates (84%–90%).23,24 In appropriately selected patients, mechanical revascularization may theoretically have a lower risk of hemorrhagic complications, given the absence or reduced need for a thrombolytic agent. In patients who are considered for ERT after full-dose IV rtPA, a mechanical approach might be favorable. Limitations of mechanical revascularization include device failure, deliverability to distal locations, and embolization.
Multimodal revascularization.
Given the heterogeneity of vessel occlusion etiology in AIS, a combination of multiple techniques may afford the highest success for revascularization. Small series suggest that multimodal therapies including IA thrombolysis and stent implantation lead to higher recanalization rates.39 Future studies may find mechanical thrombectomy to be more successful in proximal large-vessel occlusions, whereas local IA thrombolysis would be preferred in distal small-vessel occlusions. Also, stent implantation may be most effective for in situ intracranial atherosclerosis with supervening thrombosis, but retrieval and aspiration techniques may be more effective for thromboemboli occlusive in relatively normal recipient arteries.4
Posterior circulation modality selection.
The best treatment modality for patients with VBO remains poorly defined. A large prospective, observational registry and a separate systematic analysis of published case series analyzed a total of 1,012 patients; both studies did not support unequivocal superiority of IA therapy vs IV thrombolysis.32,43 However, the heterogeneity of the data between the patients within the groups analyzed limits interpretation of the clinical conclusions. Early recanalization is an important prognostic factor for good clinical outcome; as such, higher and safer rates of recanalization are being achieved with newer therapeutic strategies utilizing mechanical embolectomy devices, retrievable stents, angioplasty with or without stenting, use of glycoprotein IIb/IIIa inhibitors, and combinations thereof.23,28,30,44,45
TARGET TIME INTERVALS, AND TRIAGE AND TRANSFER STRATEGIES
Time to revascularization is an independent predictor of good outcome in patients with AIS.46,47 Randomized trials of IV rtPA have demonstrated the greatest benefit in subjects treated within 90 minutes of symptom onset.2,25 Recanalization rates with ERT are higher than with IV rtPA alone, although the delay to treatment may attenuate the benefit. This illustrates the importance of establishing benchmark door-to-revascularization times. The Brain Attack Coalition has recommended that IV rtPA be administered within 60 minutes from arrival to the emergency department (ED) for eligible patients.48 The established time intervals target a multidisciplinary goal. Each component of the process—ED physicians, ancillary staff, laboratory and radiology services, neurology team, and radiology staff—is essential for the time goal. As such, ERT time intervals should integrate into the existing model, beginning with patient arrival to the ED. Separate time intervals can be established for patients transferred from another institution and patients who receive adjunctive ERT following IV rtPA therapy. Because vascular anatomy can add unpredictable delays in procedural times, the endpoint should reflect the last modifiable variable. Therefore, ERT time intervals should reflect door-to-puncture (more predictable), puncture-to-clot, and clot-to-close goals. A clot-to-close time of 120 minutes, as described in IMS III, may be warranted to establish procedural termination times. More variables, including anatomy, evidence of persistent penumbra, and ERT method, may be used in the future to modify time benchmarks.
In the limited case series discussing time intervals to ERT, there is variability in which interval is utilized (table 4).5,6,11,12,15,41,43,49–56 Randomized trials show feasibility in achieving time intervals of approximately 4 to 5 hours from stroke onset to IA rtPA administration.6,55 In PROACT II, median time from stroke onset to randomization and IA rtPA administration was 282 minutes, whereas the IMS study demonstrated an interval of 231 minutes from stroke onset to IA rtPA administration.6,55 Time from CT scan to microcatheter placement in the cerebrovasculature had a mean time of 174 ± 60 minutes in 91 patients undergoing ERT for AIS, demonstrating wide variability and a need for time standards.53 Transferred patients whose laboratory tests and CT scan have already been completed may still have a door-to-puncture time of up to 60 minutes.57 Further study is needed to identify barriers to rapid access to endovascular therapy.
Table 4.
Treatment times in selected studies, in minutes
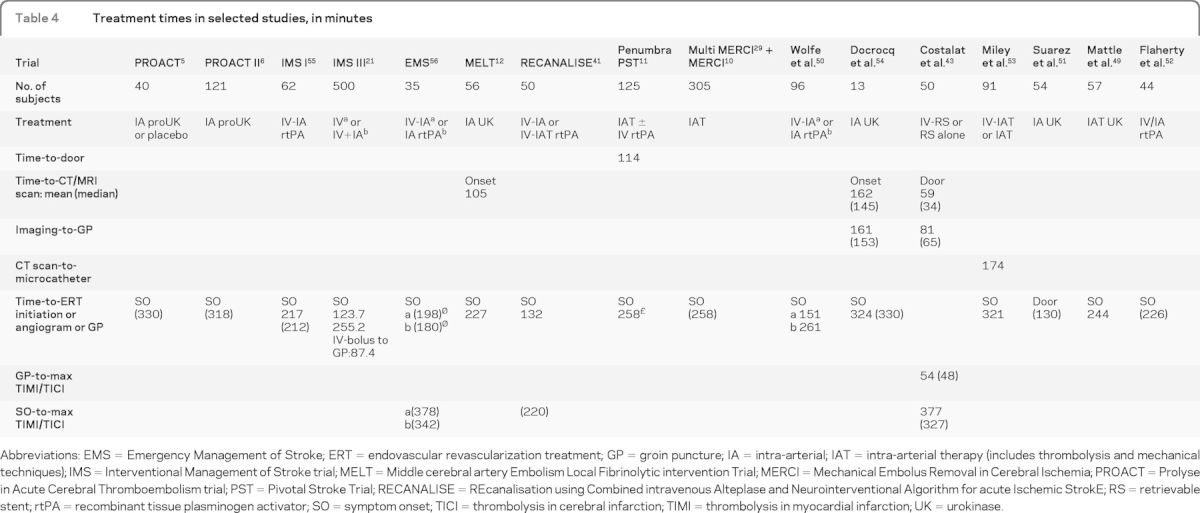
Abbreviations: EMS = Emergency Management of Stroke; ERT = endovascular revascularization treatment; GP = groin puncture; IA = intra-arterial; IAT = intra-arterial therapy (includes thrombolysis and mechanical techniques); IMS = Interventional Management of Stroke trial; MELT = Middle cerebral artery Embolism Local Fibrinolytic intervention Trial; MERCI = Mechanical Embolus Removal in Cerebral Ischemia; PROACT = Prolyse in Acute Cerebral Thromboembolism trial; PST = Pivotal Stroke Trial; RECANALISE = REcanalisation using Combined intravenous Alteplase and Neurointerventional Algorithm for acute Ischemic StrokE; RS = retrievable stent; rtPA = recombinant tissue plasminogen activator; SO = symptom onset; TICI = thrombolysis in cerebral infarction; TIMI = thrombolysis in myocardial infarction; UK = urokinase.
Currently, the American College of Cardiology and the AHA recommend that door-to-balloon time in ST segment elevation myocardial infarction should be within 90 minutes. A similar future proposal could be made for ERT in AIS, with a goal door-to-puncture time of 90 minutes (table 5). This would include activation of the stroke team, technologists, and nurses. Adjunctive time benchmarks can be developed, including puncture-to-clot and clot-to-close goals. This target is more difficult to achieve for cerebral than cardiac revascularization, as stroke patients require more time-consuming neurologic evaluation and brain imaging before proceeding to the angiography laboratory.
Table 5.
Benchmark times for revascularization therapies and possible intervals for ERT
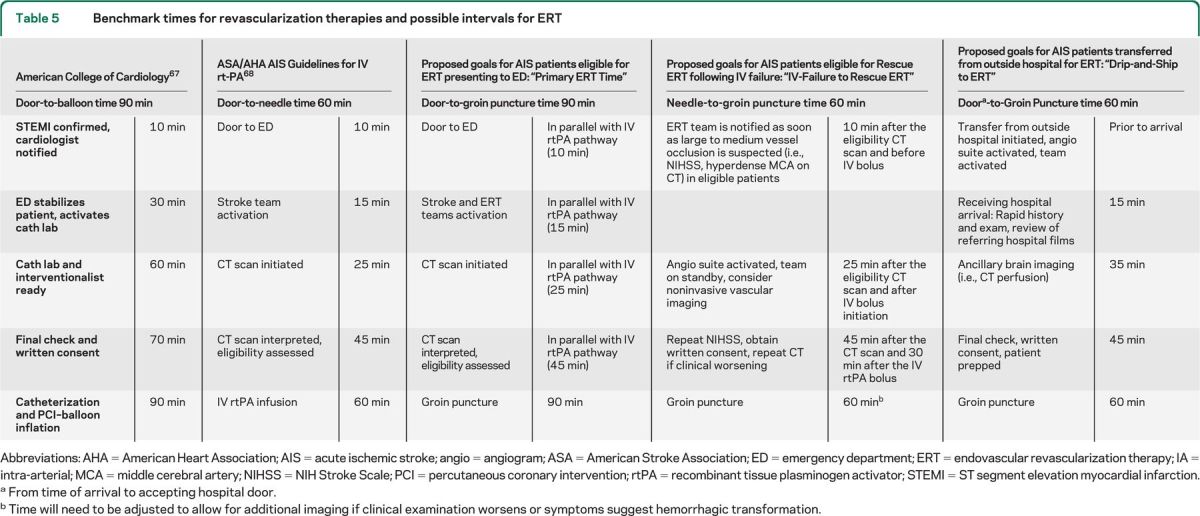
Abbreviations: AHA = American Heart Association; AIS = acute ischemic stroke; angio = angiogram; ASA = American Stroke Association; ED = emergency department; ERT = endovascular revascularization therapy; IA = intra-arterial; MCA = middle cerebral artery; NIHSS = NIH Stroke Scale; PCI = percutaneous coronary intervention; rtPA = recombinant tissue plasminogen activator; STEMI = ST segment elevation myocardial infarction.
From time of arrival to accepting hospital door.
Time will need to be adjusted to allow for additional imaging if clinical examination worsens or symptoms suggest hemorrhagic transformation.
Achieving a 90-minute door-to-puncture time would likely require the neurointerventionalist to play an integral part in the stroke team, because the decisions on treatment strategy may evolve as the patient proceeds through the AIS protocol and IV rtPA evaluation pathway. The ERT protocol should integrate into the IV rtPA pathway. For interhospital transfers, the completed imaging and laboratory studies as well as additional lead time may reduce the target time interval to 60 minutes for door-to-puncture. However, significant delay in hospital transfer may warrant repeat neuroimaging when the patient arrives at the recipient institution. Benchmark times will need to be established for IV nonresponders, with special consideration for additional delay when clinical deterioration following IV rtPA requires a repeat brain imaging prior to ERT.
Triage and transfer strategies.
The considerable decline in efficacy of revascularization therapies at around 6 to 8 hours from symptom onset demands well-organized triage and transfer strategies.46 Emergency department reorganization has been an area of focus to improve early identification of stroke patients. Tracking door-to-thrombolysis times, positioning a CT/MRI scanner within the department, and having emergency medical services (EMS) send a prehospital notification are steps that have improved thrombolysis access.58 However, given the limited availability of Comprehensive Stroke Center infrastructure, few centers in a given geographic region will have capabilities for providing comprehensive stroke care and 24/7 ERT. This will lead to a high proportion of patients eligible for ERT arriving by interhospital transfer. Transfer delay has been shown to be a major factor limiting the use of ERT in stroke patients, accounting for an estimated odds of treatment decrease by 2.5% for every minute of transfer time.59 To avoid transfer delay, regional protocols for triage of AIS patients by EMS personnel to designated stroke centers has become a focus of prehospital stroke triage policy.60
Alternative strategies include initiation of IV thrombolytic in a referring hospital prior to transfer “drip-and-ship,” followed by further management at the accepting hospital, which may include ERT. A described model of “drip, ship, and retrieve” used full-dose IV rtPA (0.9 mg/kg) followed by ERT with mechanical thrombectomy and thrombo-aspiration, suggesting feasibility in basilar artery occlusion.61 Different models may evolve where the patient receives ERT in an outside hospital and is transferred for further care at a comprehensive stroke center where neurosurgery, neurocritical care, and vascular neurology expertise are available, known as “retrieve-and-ship.” Pay-for-performance measures similar to those for acute myocardial infarction could help facilitate transfer of appropriate patients from primary to comprehensive stroke centers (the hub-and-spoke model).
GENERAL PREPROCEDURAL AND INTRAPROCEDURAL MANAGEMENT
Anesthesia and monitoring.
The type of anesthesia for ERT has been a topic of controversy, with recent reports suggesting worse outcome with use of general endotracheal anesthesia, possibly due to treatment delays and complications from intubation.62,63 Alternatively, conscious sedation may pose a different set of risks related to patient cooperation, especially in those with severe aphasia or neglect, which may negatively influence time to revascularization and procedural success. Furthermore, ancillary monitoring requiring invasive arterial access for blood pressure monitoring and central IV access may also be of limited value and add delay to initiation of therapy. Further study is needed to evaluate sedation methods for ERT. Sedation methods may currently vary among centers.
Thromboprophylaxis with systemic anticoagulation.
Arterial catheterization carries a risk of thromboembolism, often requiring systemic anticoagulation. Randomized clinical trials of ERT report variable protocols for thromboprophylaxis, including bolus IV heparin infusion of 2,000 to 5,000 units at procedure onset, followed by continuous infusions of approximately 500 units of IV heparin per hour for the procedure duration.6,21 Alternatively, activated clotting time (ACT) values can be obtained with heparin boluses to maintain an ACT at a therapeutic goal. Limited data exist on the safety of heparin anticoagulation during ERT procedures. A subgroup analysis of the MERCI trial showed no association with hemorrhage or 90-day mortality and heparin use.64 A reasonable ACT range may be 250 to 300 seconds during ERT.
Renal prophylaxis.
Patients with AIS may also have chronic renal impairment, which may worsen with contrast administered during angiography. Interventions designed to prevent contrast-induced nephropathy have not been rigorously studied. Reasonable prophylaxis strategies include hydration with isotonic saline. Recent data have not provided strong support for the administration of N-acetylcysteine.65 The use of sodium bicarbonate infusion may be reasonable for patients with renal insufficiency, but it can be limited by the large volume and time to acquire the solution from the pharmacy. Periprocedural renal prophylaxis for ERT in select AIS patients is an important area in need of further investigation.
POSTPROCEDURAL MANAGEMENT
Imaging.
Patients may benefit from postprocedural imaging, including noncontrast head CT or susceptibility-weighted MRI within 16 to 32 hours from ERT. Given the associated risk of hemorrhagic complications with revascularization therapy, urgent head CT or MRI may be needed for clinical deterioration in the postprocedure period. Intraprocedure imaging is also possible in many angiography suites and can offer rapid diagnostic information.
Neuromonitoring.
Intensive care unit monitoring with staff trained in neurologic patient care may be important for postprocedure neuromonitoring, including frequent neurologic examination assessments by nursing staff experienced and trained in neurovascular diseases. Intensive monitoring would also include surveillance for groin-access complications and the appropriate management. Stroke severity and outcome scales may be important in performance monitoring.
Blood pressure management.
Patients in whom revascularization was successful may be at risk of reperfusion hemorrhage, thereby warranting aggressive blood pressure control. Common practice has followed a protocol similar to that for post IV rtPA administration with vigilant blood pressure monitoring for at least the first 24 hours. Blood pressure is measured every 15 minutes for 2 hours, then every 30 minutes for 6 hours, and every hour for 18 hours. Goal blood pressure is targeted to remain below 180/105 mm Hg. Bolus dosing of labetolol or continuous infusion of nicardipine has been used to achieve target blood pressure. Adjustments in blood pressure parameters may be necessary to achieve clinical stability.
Antithrombotic regimen.
Postprocedure antithrombotic regimen will likely follow a similar pathway to that in general AIS management. Antithrombotics are usually avoided in the first 24 hours following IV and IA administration of a thrombolytic agent. Certain procedures may present exceptions, such as patients receiving stent implantation, in which the respective preferred regimen will need to be implemented. This may include loading doses of 325 to 650 mg of aspirin (orally or rectally) and 300 to 600 mg of clopidogrel with subsequent dual antiplatelet therapy with daily aspirin (325 mg) and clopidogrel (75 mg) for 4 to 12 weeks, followed by indefinite single-antiplatelet therapy with aspirin 325 mg daily or tailored to the underlying etiology. A potential hazard of dual antiplatelet therapy for acute stent implantation in a patient with a recent large stroke includes hemorrhage.
Glycemia management.
Hyperglycemia may be associated with an increased risk of hemorrhagic transformation of the cerebral infarction.66 An appropriate glycemic-control regimen will likely be modeled after existing management strategies developed for AIS.
Statin therapy.
Comprehensive management strategies for patients with AIS who undergo ERT will likely also adopt statin therapy regimens modeled after those developed for AIS patients in general.
CLINICAL OUTCOME MEASUREMENTS
Monitoring clinical outcomes following ERT is important for quality metrics. Thresholds and benchmarks for acceptable stroke severity–weighted sICH and mortality rates need to be established. The proportion of patients completing 90-day clinical follow-up (from those who are eligible) needs to be established. Similarly, consensus on rates of 90-day good functional mRS outcome (score of 0–2) following ERT needs to be established.
DISCUSSION
This outline can be used as a framework for the development of future practice recommendations and as an interim tool that the practicing neurovascular specialist can use to assess the rapidly evolving management strategies. This evolving field is marked by ongoing intense investigation of various therapies for acute revascularization, which will demand frequent reevaluation and modification of these strategies.
GLOSSARY
- ACT
activated clotting time
- AHA
American Heart Association
- AIS
acute ischemic stroke
- ASA
American Stroke Association
- ASPECTS
Alberta Stroke Program Early CT score
- CI
confidence interval
- ECASS
European Cooperative Acute Stroke Study III
- ED
emergency department
- EMS
emergency medical services
- ERT
endovascular revascularization therapy
- FDA
US Food and Drug Administration
- IA
intra-arterial
- ICH
intracranial hemorrhage
- IMS
Interventional Management of Stroke
- J-MUSIC
Japan Multicenter Stroke Investigators'Collaboration
- MCA
middle cerebral artery
- MELT
MCA-Embolism Local fibrinolytic intervention Trial
- MERCI
Mechanical Embolus Removal in Cerebral Ischemia
- MR CLEAN
Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands
- MR RESCUE
MR and Recanalization of Stroke Clots Using Embolectomy
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- NINDS
National Institute of Neurological Disorders and Stroke
- OR
odds ratio
- PROACT
Prolyse in Acute Cerebral Thromboembolism
- rtPA
recombinant tissue plasminogen activator
- sICH
symptomatic ICH
- SYNTHESIS EXP
Intra-Arterial Versus Systemic Thrombolysis for Acute Ischemic Stroke
- THERAPY
Assess the Penumbra System in the Treatment of Acute Stroke
- VBO
vertebrobasilar occlusion
AUTHOR CONTRIBUTIONS
All authors participated in the design and revision of the manuscript. Dr. Lazzaro: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. Dr. Novakovic: drafting/revising the manuscript, acquisition of data, statistical analysis, study supervision. Dr. Alexandrov: drafting/revising the manuscript, discussion of review content. Dr. Darkhabani: drafting/revising the manuscript, acquisition of data. Dr. Edgell: drafting/revising the manuscript. Dr. English: drafting/revising the manuscript, analysis or interpretation of data. Dr. Frei: drafting/revising the manuscript. Dr. Jamieson: drafting/revising the manuscript. Dr. Janardhan: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, development of stroke algorithms and figures for acute ischemic stroke endovascular therapy. Dr. N. Janjua: drafting/revising the manuscript. Dr. R.M. Janjua: drafting/revising the manuscript. Dr. Katzan: drafting/revising the manuscript. Dr. Khatri: study concept or design. Dr. Kirmani: study concept or design, study supervision. Dr. Liebeskind: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Linfante: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision. Dr. Nguyen: drafting/revising the manuscript. Dr. Saver: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. Dr. Shutter: drafting/revising the manuscript. Dr. Xavier: drafting/revising the manuscript. Dr. Yavagal: drafting/revising the manuscript. Dr. Zaidat: drafting/revising the manuscript, study concept or design, contribution of vital reagents/tools/patients, acquisition of data, statistical analysis, study supervision.
DISCLOSURE
Dr. Lazzaro reports no disclosures. Dr. Novakovic performs endovascular treatments for acute ischemic strokes (has not used a retrievable stent). Dr. Alexandrov serves as an Associate Editor for Frontiers in Interventional Neurology; has a patent for Therapeutic Methods and Apparatus for Use of Sonication to Enhance Perfusion of Tissue; has received publishing royalties for Cerebrovascular Ultrasound in Stroke Prevention and Treatment (first and second editions); has served as a consultant for Cerevast Therapeutics; spends 60% effort on clinical stroke service at Comprehensive Stroke Center, UAB Hospital, monitoring endovascular procedures and evaluating success of recanalization with imaging; has received research support from Cerevast Therapeutics, Inc.; has received research support from NINDS; has received compensation from Cerevast Therapeutics, Inc.; and has received license fee payments from Therapeutic Methods and Apparatus for Use of Sonication to Enhance Perfusion of Tissue. Dr. Darkhabani reports no disclosures. Dr. Edgell serves as an Associate Editor, Frontiers in Interventional Neurology. Dr. English has served on the scientific advisory board of Concentric Medical Inc., Clinical Events Committee; serves on the editorial boards of Neurohospitalist and The Stroke Interventionalist; and serves as Medical Scientific Advisor for Silk Road Medical. Dr. Frei has served as a consultant to Penumbra, Inc. Dr. Jamieson has served as a consultant to Bayer and Boerhinger-Ingelheim; served on the speakers bureau for Boehringer-Ingelheim and Merck; served on the scientific advisory board for Bayer and on the Adjudication Committee for ARRIVE trial; and serves as an Assistant Editor for Neurology Alert. Dr. Janardhan reports no disclosures. Dr. N. Janjua serves on the scientific advisory board for Lundbeck/DSMB and Neurointerventions; receives research support from NIH/NINDS; and holds stock or stock options or board of directors compensation for Neurointerventions. Dr. R.M. Janjua reports no disclosures. Dr. Katzan has served as consultant to Pzifer and Genentech; served as a speaker for and received compensation from Cardionet; serves on the Real World Advisory Board for Pfizer; has received research funding from Novartis, Inc., Hoffman-La Roche Ltd, and Takeda Pharmaceuticals; and has received research funding from Ohio Department of Health. Dr. Khatri is on the Executive Committee of the IMS III Trial, is Neurology PI of the Penumbra THERAPY Trial, and has received research support from Genentech, Inc., for survey implementation; served on the editorial boards of Frontiers in Endovascular and Interventional Neurology; has received research support from the NIH/NINDS; and has served as an expert witness for stroke cases over the last 2 years. Dr. Kirmani served on the advisory board for Otsuka Pharmaceuticals; served as an Associate Editor for Frontiers in Clinical Trials in Neurology; received publishing royalties from the Taylor and Francis Group for The Stroke Center Handbook; receives research support from Penumbra, Inc., and Genentech, Inc.; received research support from NIH/NINDS; and has served as expert witness for stroke cases. Dr. Liebeskind served as a consultant for Concentric Medical and CoAxia and receives research support from NIH. Dr. Linfante served as a consultant for Codman Neurovascular and Stryker; holds stock options in Surpass Limited; serves on the Scientific Advisory Board for Codman Neurovascular; serves on the editorial boards for Stroke and Journal of Neurointerventional Surgery; and serves on the speakers bureau for Codman. Dr. Nguyen serves as Associate Editor of Frontiers in Vascular and Interventional Neurology and Editor of SVIN newsletter The Core; performs intra-arterial stroke procedures; and serves as a consultant for Penumbra. Dr. Saver serves on the editorial boards of Stroke, Reviews in Neurologic Disease, Journal of Neuroimaging, and Journal of Stroke and Cerebrovascular Diseases; is an employee of the University of California (UC), which holds a patent on retriever devices for stroke; serves on scientific advisory boards, for which the UC Regents receive payments, for CoAxia, Inc., Concentric Medical, Talecris Biotherapeutics, Ferrer, AGA Medical Corporation, BrainsGate, PhotoThera, Ev3, and Sygnis Bioscience GmbH & Co. KG; is an unpaid site investigator in multicenter clinical trials sponsored by AGA Medical Corporation, Lundbeck, Inc., and Ev3, for which the UC Regents received payments based on clinical trial contracts for the number of subjects enrolled; is an unpaid site investigator in the NIH IRIS, CLEAR, IMS 3, SAMMPRIS, and VERITAS multicenter clinical trials, for which the UC Regents receive payments based on clinical trial contracts for the number of subjects enrolled; receives research support from the NIH and NINDS; receives research support from the AHA; and performs acute stroke care (35%). Dr. Shutter serves on the scientific advisory board for Neuren Pharmaceuticals; receives funding for travel or speaker honoraria from Codman, J&J, and NIH; serves as a consultant for Cincinnati Bengals; receives research support from Department of Defense and NIH (NINDS); receives research support from the Mayfield Education and Research Fund; and holds stock options in UCB Pharma. Dr. Xavier receives research support from Concentric Medical and from Medical University of South Carolina/NIH study. Dr. Yavagal received an honorarium from Penumbra Inc. for consultation and speaking; serves as an Associate Editor for Frontiers in Endovascular Neurology; and serves as a consultant to Penumbra Inc., Codman Neurovascular, Micrus, Inc., Genentech, and Boston Scientific. Dr. Zaidat serves on the scientific advisory board for Talecris; served on the adjudication committee for Stryker; received speaker honoraria from Stryker; served on the editorial board of Frontiers in Neurology (Endovascular & Interventional Neurology Section); serves as Editor of The Journal of Neurointerventional Surgery, and serves as Associate Editor and is a member of the Editorial Board of Journal of Stroke & Cerebrovascular Diseases; served as a consultant for Stryker Neurovascular, Codman Neurovascular, and Microvention, Inc; and has received research support from Society of Vascular & Interventional Neurology (SVIN) for this educational activity. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Stroke 2007;38:1655–1711 [DOI] [PubMed] [Google Scholar]
- 2.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group.Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 3.Zaidat OO, Suarez JI, Sunshine JL, et al. Thrombolytic therapy of acute ischemic stroke: correlation of angiographic recanalization with clinical outcome. AJNR Am J Neuroradiol 2005;26:880–884 [PMC free article] [PubMed] [Google Scholar]
- 4.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967–973 [DOI] [PubMed] [Google Scholar]
- 5.del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke: PROACT Investigators: Prolyse in Acute Cerebral Thromboembolism. Stroke 1998;29:4–11 [DOI] [PubMed] [Google Scholar]
- 6.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial: Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003–2011 [DOI] [PubMed] [Google Scholar]
- 7.Alberts MJ, Latchaw RE, Selman WR, et al. Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition. Stroke 2005;36:1597–1616 [DOI] [PubMed] [Google Scholar]
- 8.Leifer D, Bravata DM, Connors JJ, 3rd, et al. Metrics for measuring quality of care in comprehensive stroke centers: detailed follow-up to Brain Attack Coalition comprehensive stroke center recommendations: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:849–877 [DOI] [PubMed] [Google Scholar]
- 9.Meyers PM, Schumacher HC, Higashida RT, et al. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association Council on Cardiovascular Radiology and Intervention, Stroke Council, Council on Cardiovascular Surgery and Anesthesia, Interdisciplinary Council on Peripheral Vascular Disease, and Interdisciplinary Council on Quality of Care and Outcomes Research. Circulation 2009;119:2235–2249 [DOI] [PubMed] [Google Scholar]
- 10.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–1438 [DOI] [PubMed] [Google Scholar]
- 11.The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 2009;40:2761–2768 [DOI] [PubMed] [Google Scholar]
- 12.Ogawa A, Mori E, Minematsu K, et al. Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: the Middle Cerebral Artery Embolism Local Fibrinolytic Intervention Trial (MELT) Japan. Stroke 2007;38:2633–2639 [DOI] [PubMed] [Google Scholar]
- 13.Lee M, Hong KS, Saver JL. Efficacy of intra-arterial fibrinolysis for acute ischemic stroke: meta-analysis of randomized controlled trials. Stroke 2010;41:932–937 [DOI] [PubMed] [Google Scholar]
- 14.Inoue T, Kimura K, Minematsu K, Yamaguchi T. A case-control analysis of intra-arterial urokinase thrombolysis in acute cardioembolic stroke. Cerebrovasc Dis 2005;19:225–228 [DOI] [PubMed] [Google Scholar]
- 15.Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke 2009;40:3777–3783 [DOI] [PubMed] [Google Scholar]
- 16.Saver JL, Liebeskind DS, Nogueira RG, Jahan R. Need to clarify Thrombolysis In Myocardial Ischemia (TIMI) scale scoring method in the Penumbra Pivotal Stroke Trial. Stroke 2010;41:e115–e116 [DOI] [PubMed] [Google Scholar]
- 17.Intra-arterial Versus Systemic Thrombolysis for Acute Ischemic Stroke: SYNTHESIS EXP. Available at: strokecenter.org Accessed November 26, 2011
- 18.MR CLEAN: a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands. 2011. Available at: www.trialregister.nl/trialreg/admin/rctview.asp?TC=1804 Accessed November 26, 2011 [DOI] [PMC free article] [PubMed]
- 19.Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy: MR RESCUE. Available at: clinicaltrials.gov/ct2/show/NCT00389467 Accessed November 26, 2011 [DOI] [PMC free article] [PubMed]
- 20.Assess the Penumbra System in the Treatment of Acute Stroke (THERAPY). Available at: clinicaltrials.gov/ct2/show/NCT01429350 Accessed November 26, 2011
- 21.The Interventional Management of Stroke (IMS) trials. Available at: http://www.strokecenter.org/trials/trialDetail.aspx?tid=747 Accessed August 26, 2011
- 22.Zaidat OO, Wolfe T, Hussain SI, et al. Interventional acute ischemic stroke therapy with intracranial self-expanding stent. Stroke 2008;39:2392–2395 [DOI] [PubMed] [Google Scholar]
- 23.Roth C, Papanagiotou P, Behnke S, et al. Stent-assisted mechanical recanalization for treatment of acute intracerebral artery occlusions. Stroke 2010;41:2559–2567 [DOI] [PubMed] [Google Scholar]
- 24.Castano C, Dorado L, Guerrero C, et al. Mechanical thrombectomy with the Solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke 2010;41:1836–1840 [DOI] [PubMed] [Google Scholar]
- 25.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329 [DOI] [PubMed] [Google Scholar]
- 26.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (alteplase) for ischemic stroke 3 to 5 hours after symptom onset: the ATLANTIS study: a randomized controlled trial: Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999;282:2019–2026 [DOI] [PubMed] [Google Scholar]
- 27.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke 2009;40:2945–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckert B, Kucinski T, Pfeiffer G, Groden C, Zeumer H. Endovascular therapy of acute vertebrobasilar occlusion: early treatment onset as the most important factor. Cerebrovasc Dis 2002;14:42–50 [DOI] [PubMed] [Google Scholar]
- 29.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008;39:1205–1212 [DOI] [PubMed] [Google Scholar]
- 30.Schulte-Altedorneburg G, Hamann GF, Mull M, et al. Outcome of acute vertebrobasilar occlusions treated with intra-arterial fibrinolysis in 180 patients. AJNR Am J Neuroradiol 2006;27:2042–2047 [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold M, Nedeltchev K, Schroth G, et al. Clinical and radiological predictors of recanalisation and outcome of 40 patients with acute basilar artery occlusion treated with intra-arterial thrombolysis. J Neurol Neurosurg Psychiatry 2004;75:857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schonewille WJ, Wijman CA, Michel P, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol 2009;8:724–730 [DOI] [PubMed] [Google Scholar]
- 33.Mishra NK, Albers GW, Davis SM, et al. Mismatch-based delayed thrombolysis: a meta-analysis. Stroke 2010;41:e25–e33 [DOI] [PubMed] [Google Scholar]
- 34.Jovin TG, Liebeskind DS, Gupta R, et al. Imaging-based endovascular therapy for acute ischemic stroke due to proximal intracranial anterior circulation occlusion treated beyond 8 hours from time last seen well: retrospective multicenter analysis of 237 consecutive patients. Stroke 2011;42:2206–2211 [DOI] [PubMed] [Google Scholar]
- 35.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS) JAMA 1995;274:1017–1125 [PubMed] [Google Scholar]
- 36.Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 2001;22:1534–1542 [PMC free article] [PubMed] [Google Scholar]
- 37.Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke 2007;38:948–954 [DOI] [PubMed] [Google Scholar]
- 38.Mathews MS, Sharma J, Snyder KV, et al. Safety, effectiveness, and practicality of endovascular therapy within the first 3 hours of acute ischemic stroke onset. Neurosurgery 2009;65:860–865, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Gupta R, Tayal AH, Levy EI, et al. Intra-arterial thrombolysis or stent placement during endovascular treatment for acute ischemic stroke leads to the highest recanalization rate: results of a multicenter retrospective study. Neurosurgery 2011;68:1618–1623 [DOI] [PubMed] [Google Scholar]
- 40.The Interventional Management of Stroke (IMS) II study. Stroke 2007;38:2127–2135 [DOI] [PubMed] [Google Scholar]
- 41.Mazighi M, Serfaty JM, Labreuche J, et al. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol 2009;8:802–809 [DOI] [PubMed] [Google Scholar]
- 42.Rubiera M, Ribo M, Pagola J, et al. Bridging intravenous-intra-arterial rescue strategy increases recanalization and the likelihood of a good outcome in nonresponder intravenous tissue plasminogen activator-treated patients: a case-control study. Stroke 2011;42:993–997 [DOI] [PubMed] [Google Scholar]
- 43.Costalat V, Machi P, Lobotesis K, et al. Rescue, combined, and stand-alone thrombectomy in the management of large vessel occlusion stroke using the solitaire device: a prospective 50-patient single-center study: timing, safety, and efficacy. Stroke 2011;42:1929–1935 [DOI] [PubMed] [Google Scholar]
- 44.Eckert B, Koch C, Thomalla G, et al. Aggressive therapy with intravenous abciximab and intra-arterial rtPA and additional PTA/stenting improves clinical outcome in acute vertebrobasilar occlusion: combined local fibrinolysis and intravenous abciximab in acute vertebrobasilar stroke treatment (FAST): results of a multicenter study. Stroke 2005;36:1160–1165 [DOI] [PubMed] [Google Scholar]
- 45.Pfefferkorn T, Mayer TE, Opherk C, et al. Staged escalation therapy in acute basilar artery occlusion: intravenous thrombolysis and on-demand consecutive endovascular mechanical thrombectomy: preliminary experience in 16 patients. Stroke 2008;39:1496–1500 [DOI] [PubMed] [Google Scholar]
- 46.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology 2009;73:1066–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nogueira RG, Smith WS, Sung G, et al. Effect of time to reperfusion on clinical outcome of anterior circulation strokes treated with thrombectomy: pooled analysis of the MERCI and Multi MERCI trials. Stroke Epub 2011 Sept 15 [DOI] [PubMed] [Google Scholar]
- 48.NINDS: Proceedings of a National Symposium on Rapid Identification and Treatment of Acute Stroke. National Institute of Neurological Disorders and Stroke, 2011. Available at: http://www.ninds.nih.gov/news_and_events/proceedings/stroke_proceedings/execsum.htm Accessed September 15, 2011
- 49.Mattle HP, Arnold M, Georgiadis D, et al. Comparison of intraarterial and intravenous thrombolysis for ischemic stroke with hyperdense middle cerebral artery sign. Stroke 2008;39:379–383 [DOI] [PubMed] [Google Scholar]
- 50.Wolfe T, Suarez JI, Tarr RW, et al. Comparison of combined venous and arterial thrombolysis with primary arterial therapy using recombinant tissue plasminogen activator in acute ischemic stroke. J Stroke Cerebrovasc Dis 2008;17:121–128 [DOI] [PubMed] [Google Scholar]
- 51.Suarez JI, Sunshine JL, Tarr R, et al. Predictors of clinical improvement, angiographic recanalization, and intracranial hemorrhage after intra-arterial thrombolysis for acute ischemic stroke. Stroke 1999;30:2094–2100 [DOI] [PubMed] [Google Scholar]
- 52.Flaherty ML, Woo D, Kissela B, et al. Combined IV and intra-arterial thrombolysis for acute ischemic stroke. Neurology 2005;64:386–388 [DOI] [PubMed] [Google Scholar]
- 53.Miley JT, Memon MZ, Hussein HM, et al. A multicenter analysis of “time to microcatheter” for endovascular therapy in acute ischemic stroke. J Neuroimaging 2011;21:159–64 [DOI] [PubMed] [Google Scholar]
- 54.Ducrocq X, Bracard S, Taillandier L, et al. Comparison of intravenous and intra-arterial urokinase thrombolysis for acute ischaemic stroke. J Neuroradiol 2005;32:26–32 [DOI] [PubMed] [Google Scholar]
- 55.Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke 2004;35:904–911 [DOI] [PubMed] [Google Scholar]
- 56.Lewandowski CA, Frankel M, Tomsick TA, et al. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke: Emergency Management of Stroke (EMS) Bridging Trial. Stroke 1999;30:2598–2605 [DOI] [PubMed] [Google Scholar]
- 57.Lazzaro MA LV, Mohammad Y, Chen M, Lopes DK, Prabhakaran S. Weekend effect is not observed in time to angiography for transferred acute ischemic stroke patients. Cerebrovasc Diseases 2010;29(suppl 2):329 [Google Scholar]
- 58.Lindsberg PJ, Happola O, Kallela M, Valanne L, Kuisma M, Kaste M. Door to thrombolysis: ER reorganization and reduced delays to acute stroke treatment. Neurology 2006;67:334–336 [DOI] [PubMed] [Google Scholar]
- 59.Prabhakaran S, Ward E, John S, et al. Transfer delay is a major factor limiting the use of intra-arterial treatment in acute ischemic stroke. Stroke 2011;42:1626–1630 [DOI] [PubMed] [Google Scholar]
- 60.Crocco TJ, Grotta JC, Jauch EC, et al. EMS management of acute stroke–prehospital triage (resource document to NAEMSP position statement). Prehosp Emerg Care 2007;11:313–317 [DOI] [PubMed] [Google Scholar]
- 61.Pfefferkorn T, Holtmannspotter M, Schmidt C, et al. Drip, ship, and retrieve: cooperative recanalization therapy in acute basilar artery occlusion. Stroke 2010;41:722–726 [DOI] [PubMed] [Google Scholar]
- 62.Abou-Chebl A, Lin R, Hussain MS, et al. Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: preliminary results from a retrospective, multicenter study. Stroke 2010;41:1175–1179 [DOI] [PubMed] [Google Scholar]
- 63.Gupta R. Local is better than general anesthesia during endovascular acute stroke interventions. Stroke 2010;41:2718–2719 [DOI] [PubMed] [Google Scholar]
- 64.Nahab F, Walker GA, Dion JE, Smith WS. Safety of periprocedural heparin in acute ischemic stroke endovascular therapy: the Multi MERCI trial. J Stroke Cerebrovasc Dis Epub 2011 June 1 [DOI] [PubMed] [Google Scholar]
- 65.Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrast-Induced Nephropathy Trial (ACT). Circulation 2011;124:1250–1259 [DOI] [PubMed] [Google Scholar]
- 66.Kase CS, Furlan AJ, Wechsler LR, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology 2001;57:1603–1610 [DOI] [PubMed] [Google Scholar]
- 67.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). J Am Coll Cardiol 2004;44:E1–E211 [DOI] [PubMed] [Google Scholar]
- 68.Fonarow GC, Smith EE, Saver JL, et al. Improving door-to-needle times in acute ischemic stroke: the design and rationale for the American Heart Association/American Stroke Association's target: stroke initiative. Stroke 2011;42:2983–2989 [DOI] [PubMed] [Google Scholar]