Abstract
Herpetic gingivostomatitis represents the most commonly observed clinical manifestation of primary herpes simplex virus (HSV) infection. HSV-1 has been associated with oro-labial disease, with most infections occurring during childhood, and HSV-2 with genital disease. However, it is possible for HSV-2 to cause oro-labial herpes and HSV-1 to cause genital herpes. An unusual case of acute herpetic gingivostomatitis (AHGS) that presented as extremely painful multiple ulcerations of the gingiva and hard palate in a 32-year-old male patient is presented. The association of HSV-2 in the etiology of oral lesions is highlighted. The clinical presentation, course, differential diagnosis and management of AHGS are discussed. How to cite the article: George AK, Anil S. Acute herpetic gingivostomatitis associated with herpes simplex virus 2: Report of a case. J Int Oral Health 2014;6(3):99-102.
Key words: : Acyclovir, gingivostomatitis, herpes simplex virus, HSV-2, oral lesions
Introduction
Acute herpetic gingivostomatitis (AHGS) is a primary infection caused by herpes simplex virus-1 (HSV-1 in >90% of the cases) or HSV-2. Primary human HSV-1 infection usually occurs in childhood and mostly presents as herpetic gingivostomatitis. It is usually subclinical in early childhood and only a small percentage of patients develop an acute primary infection. This usually occurs in older children and consists of fever, malaise, headache, cervical lymphadenopathy and a vesiculo-ulcerative eruption on the peri-oral skin, vermilion or any intra-oral mucosal surface. 1 It has been estimated that up to 90% of the population worldwide is seropositive by the age of 40 years, and around 40% of individuals harboring the virus are affected by recurrent infections. 2 A similar clinical manifestation may be caused by HSV-2, the serotype most often associated with genital herpes. Although HSV-2 is primarily responsible for most genital and cutaneous lower body herpetic lesions, it can also be the cause of primary herpetic gingivostomatitis. The orogenital contact may allow either serotype to cause oral or genital lesions. The two forms of HSV have a similar structure but differ in antigenicity, and HSV-2 is reputed to be of greater virulence. 3
Acute herpetic gingivostomatitis is characterized by a sudden onset, and the severity of symptoms is related to the virulence of the HSV and the host’s immune response. Symptoms such as cervical lymphadenopathy, malaise and low grade fever, can occur in the absence of any discrete clinical lesions. The general course of the infection is 10-14 days, which is usually preceded by an incubation period of up to 26 days. 4 , 5
A case of AHGS associated with HSV-2 is presented. The unusual occurrence of HSV-2 in the oral cavity is highlighted.
Case Report
A 32-year-old male reported to the Pushpagiri College of Dental Sciences, Kerala, India complaining of severe pain of the gums. The excruciating and incapacitating nature of pain since 1 month had made this patient to abstain from work. The patient experienced severe pain radiating to his maxillary and temporal regions.
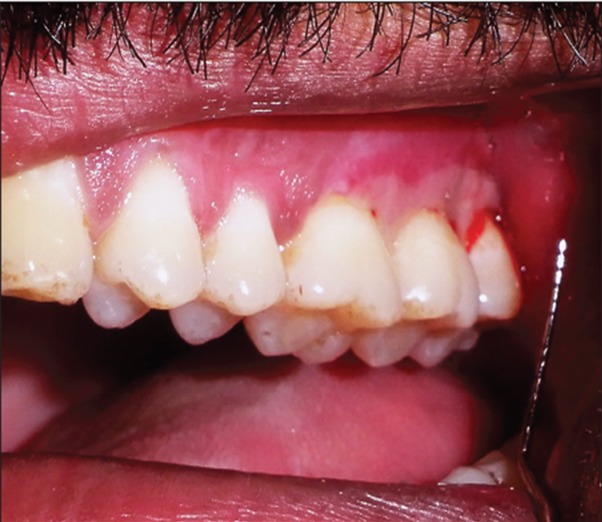
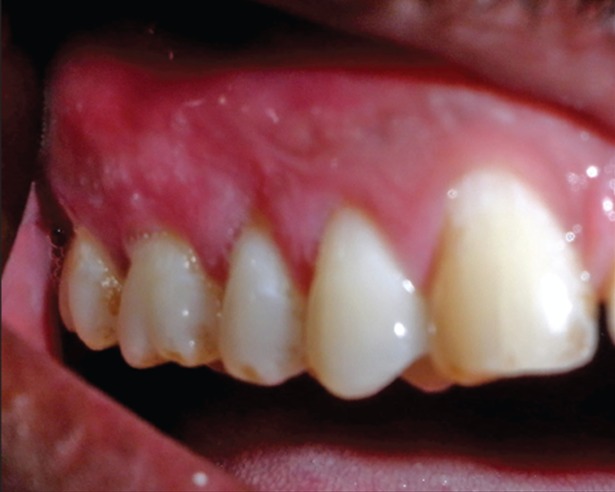
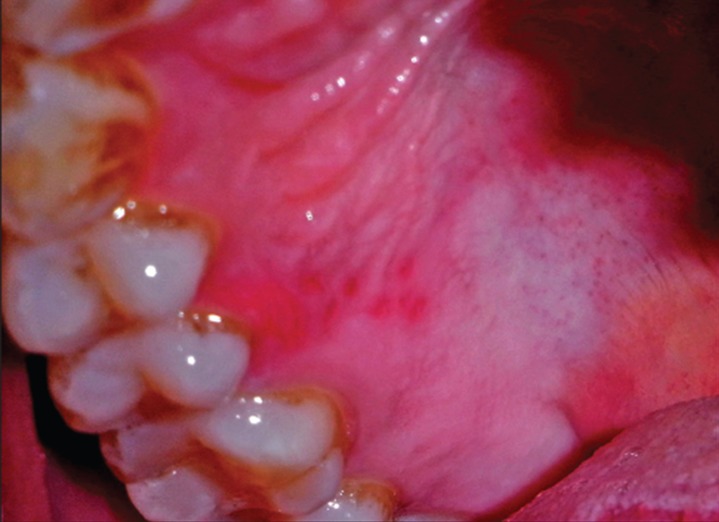
On clinical examination an extremely tender ulcerated area was seen on the marginal and attached gingiva of the maxillary left molars. The lesion extended in a band like manner 3-4 mm wide, extending posteriorly from the mid buccal aspect of 26-28. The area was covered by a yellowish white slough, and there was an erythematous halo surrounding the lesion ( Figure 1 ). There was bleeding on probing without any periodontal pockets. Radiographs revealed marginal alveolar bone loss. Palatal aspect of maxillary right premolars had irregular ulcers measuring less than a centimeter in diameter resembling a bunch of grapes ( Figure 2 ). Patient complained of pain in the maxillary right buccal and palatal areas also ( Figure 3 ). Based on the history and clinical presentation a provisional diagnosis of herpes viral infection was made.
Figure 1: Ulcerated area (yellowish white slough) on left maxillary buccal gingiva.
Figure 2: Lesions on the right buccal gingiva.
Figure 3: Irregular ulcers on the palate.
A hematologic examination was carried out, and investigations for hepatitis B and HIV were done. Swabs were collected from the site and bacterial and viral culture tests were carried out. An incisional biopsy of the lesion was also done from the interdental papilla distal to 27. All these investigations did not yield any significant finding or result. Patient was then sent for serum IgG and IgM antibody testing for HSV-1 and 2. The observed serum value for HSV-2 IgM antibody by enzyme-linked immunosorbent assay (ELISA) was 1.7 and serum value for HSV-2 IgG antibody was 1.15. Results indicate that the patient was reactive to HSV-2 (A reference range > 1.1 is reactive). The observed serum value for HSV-1 IgM antibody by ELISA was 0.14 and serum value for HSV-1 IgG antibody was 0.17 indicating that the patient was not reactive to HSV-1. The diagnosis was confirmed as herpetic gingivostomatitis associated with HSV-2.
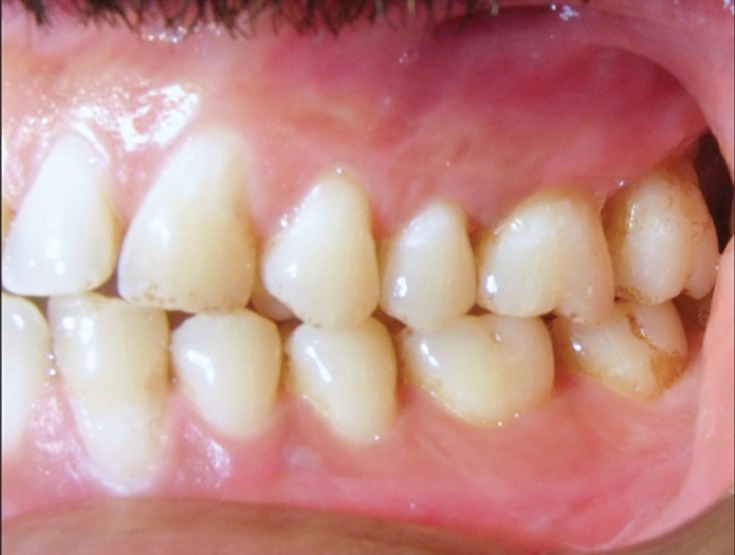
Dermatologic consultation ruled out the presence of any genital or extraoral lesions. Systemic antiviral therapy was initiated with acyclovir 400 mg thrice daily for 3 weeks. Supportive therapy such as beta dine mouth wash and oral analgesics were given. The lesions resolved after 4 weeks of antiviral therapy (Figures 4 - 6 ).
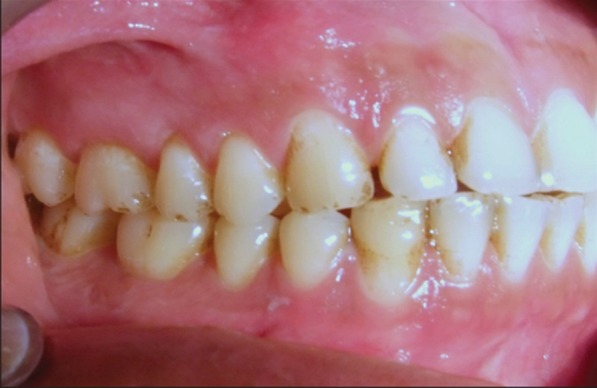
Figure 4: Clinical appearance after anti-viral therapy-complete resolution of ulcerations on left buccal gingiva.
Figure 6: Clinical appearance after anti-viral therapy-complete resolution of ulcerations on palatal aspect.
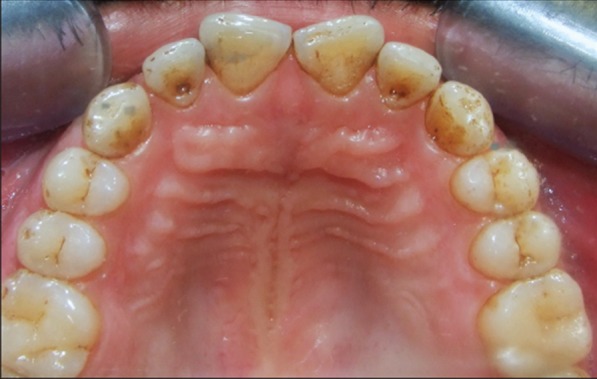
Figure 5: Clinical appearance after anti-viral therapy-complete resolution of ulcerations on right buccal gingiva.
Discussion
Two of the known herpesviridae, HSV-1 and HSV-2 are responsible for primary and recurrent mucocutaneous herpetic infections. The HSV is a double-stranded DNA virus of which the HSV-1 type is responsible for oral, facial and ocular infections including primary herpetic gingivostomatitis. HSV-2 accounts for most genital and cutaneous lower body herpetic lesions. Orogenital contact may allow either serotype to cause oral or genital lesions. Even though herpetic gingivostomatitis is primarily an HSV-1 infection isolated cases of HSV-2 association have been reported in older patients, probably transmitted sexually and causing genital infection. Oral infection with HSV-2 is also an unusual complication of long-term immunosuppression, such as those for cicatricial pemphigoid, 6 or HIV disease. 7 , 8 Oral HSV-2 infection has also been reported in HIV-negative and HSV-2-seropositive gay men. 9
Herpetic infection, both acute and recurrent, is a self-limiting disease with a healing period of 1 to 2 weeks. Complications are rare and include keratoconjunctivitis, esophagitis, pneumonitis, meningitis and encephalitis. 10 The most common mode of transmission of HSV is the saliva of the carriers. Infection on the hands of health care personnel from patients shedding HSV may result in herpetic whitlow. Transmission of HSV-2 is usually by sexual contact. Both types 1 and 2 may be transmitted to various sites by oral-genital, oral-anal or anal-genital contact. 10 , 11 Primary infection occurs in childhood from infected saliva or herpetic lesions. Reactivation can occur at any time and may be triggered by immunosuppression, stress, trauma, ultraviolet irradiation, or fever. Recurrences are generally less severe than the primary infection and severity and frequency tend to diminish with time. 12
The diagnosis of AHG is usually made by clinical presentation and history. In the present case, the typical grape-like cluster appearance of ulcers on the palate, surrounded by erythema and extreme tenderness was conclusive for the diagnosis of a herpes simplex infection. The diagnosis can be confirmed via laboratory tests: Serological assays (anti-HSV IgM and IgG), the Tzanck test and immunofluorescence, but the culture of viral isolates is still considered to be the gold standard. 13 , 14 HSV antibody testing can detect both viral types - HSV-1 and HSV-2. Primary HSV infection is associated with an increase in IgM titer followed several weeks later by permanent IgG titers. Individuals with recent infections are more likely to test positive for herpes IgG and IgM or herpes IgM alone. The present case tested positive for HSV-2 IgM and HSV-2 IgG. IgM is usually negative in HSV reactivation. 15
The differential diagnosis of primary herpetic gingivostomatitis includes acute necrotizing ulcerative gingiv itis, herpangina, aphthous stomatitis, candidiasis of the mouth, Steven-Johnson syndrome and hand, foot and mouth disease. 3 , 16 The presence of multiple ulcerations alerted us to a number of other possibilities like varicella zoster infection, erythema multiforme, allergic stomatitis, recurrent aphthous infection, behcet’s disease, recurrent herpes simplex, pemphigus, bullous pemphigoid and erosive and bullous lichen planus. Varicella zoster Infection was ruled out due to the bilateral distribution of the lesions. The absence of characteristic skin lesions helped to rule out erythema multiforme. Since lesions were on the keratinized gingiva and the palate, recurrent aphthous infection was ruled out. The biopsy report of the lesions ruled out pemphigus, bullous pemphigoid and lichen planus.
Treatment of the acute herpetic infection includes symptomatic measures; if the disease is diagnosed early, systemic antiviral therapy is advised in order to accelerate clinical resolution. Recurrent herpetic lesions are frequently managed with topical application of antiviral agents. In cases of frequent recurrences or association with viral-induced erythema multiforme, long-term preventive systemic antiviral therapy may be warranted. Systemic administration of acyclovir accelerates the resolution of viral shedding and healing time, and reduces pain. Acyclovir is generally well-tolerated. Common adverse effects include nausea, vomiting, and headaches. Palliative and supportive management of orolabial herpetic infections variably consists of controlling fever and pain, preventing dehydration, and shortening the duration of lesions. Antiviral chemotherapy is available for the treatment of patients at increased risk of complications. Topical anesthetics, analgesics, and antipyretics, rinsing with lidocaine viscous (2%), before each meal, effectively reduces pain during eating.
Conclusion
A case of acute herpetic gingivostomatitis associated with of herpes simplex virus 2 is presented. The clinical presentation, differential diagnosis and management of acute herpetic gingivostomatitis is discussed.
Footnotes
Source of Support: Nil
Conflict of Interest: None
Contributor Information
Annie Kitty George, Department of Periodontics, Pushpagiri College of Dental Sciences, Tiruvalla, Kerala, India.
Sukumaran Anil, Department of Periodontics and Community Dentistry, College of Dentistry, King Saud University, Riyadh, Kingdom of Saudi Arabia.
References
- 1.MJ McCullough, NW Savage. Oral viral infections and the therapeutic use of antiviral agents in dentistry. Aust Dent J. 2005;50(4 Suppl 2):31–35. doi: 10.1111/j.1834-7819.2005.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 2.JA Embil, RG Stephens, FR Manuel. Prevalence of recurrent herpes labialis and aphthous ulcers among young adults on six continents. Can Med Assoc J. 1975;113(7):627–630. [PMC free article] [PubMed] [Google Scholar]
- 3.PJ Chauvin, AH Ajar. Acute herpetic gingivostomatitis in adults: A review of 13 cases, including diagnosis and management. J Can Dent Assoc. 2002;68(4):247–251. [PubMed] [Google Scholar]
- 4.H Faden. Management of primary herpetic gingivostomatitis in young children. Pediatr Emerg Care. 2006;22(4):268–269. doi: 10.1097/01.pec.0000218982.46225.f5. [DOI] [PubMed] [Google Scholar]
- 5.A Kolokotronis, S Doumas. Herpes simplex virus infection, with particular reference to the progression and complications of primary herpetic gingivostomatitis. Clin Microbiol Infect. 2006;12(3):202–211. doi: 10.1111/j.1469-0691.2005.01336.x. [DOI] [PubMed] [Google Scholar]
- 6.TK Gilmour, PA Meyer, E Rytina, PM Todd. Antiepiligrin (laminin 5) cicatricial pemphigoid complicated and exacerbated by herpes simplex virus type 2 infection. Australas J Dermatol. 2001;42(4):271–274. doi: 10.1046/j.1440-0960.2001.00518.x. [DOI] [PubMed] [Google Scholar]
- 7.GS Liang, GL Daikos, U Serfling, WY Zhu, Pecoraro M, CL Leonardi, et al. An evaluation of oral ulcers in patients with AIDS and AIDS-related complex. J Am Acad Dermatol. 1993;29(4):563–568. doi: 10.1016/0190-9622(93)70222-f. [DOI] [PubMed] [Google Scholar]
- 8.PH Itin, S Lautenschlager. Viral lesions of the mouth in HIV-infected patients. Dermatology. 1997;194(1):1–7. doi: 10.1159/000246047. [DOI] [PubMed] [Google Scholar]
- 9.MR Krone, A Wald, SR Tabet, M Paradise, L Corey, CL Celum. Herpes simplex virus type 2 shedding in human immunodeficiency virus-negative men who have sex with men: Frequency, patterns, and risk factors. Clin Infect Dis. 2000;30(2):261–267. doi: 10.1086/313647. [DOI] [PubMed] [Google Scholar]
- 10.PG Arduino, SR Porter. Oral and perioral herpes simplex virus type 1 (HSV-1) infection: Review of its management. Oral Dis. 2006;12(3):254–270. doi: 10.1111/j.1601-0825.2006.01202.x. [DOI] [PubMed] [Google Scholar]
- 11.MA Huber. Herpes simplex type-1 virus infection. Quintessence Int. 2003;34(6):453–467. [PubMed] [Google Scholar]
- 12.RJ Whitley, B Roizman. Herpes simplex virus infections. Lancet. 2001;357(9267):1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 13.MA Siegel. Diagnosis and management of recurrent herpes simplex infections. J Am Dent Assoc. 2002;133(9):1245–1249. doi: 10.14219/jada.archive.2002.0366. [DOI] [PubMed] [Google Scholar]
- 14.CM Lee, DD Damm, BW Neville, C Allen, Bouquot J Oral and Maxillofacial Pathology, 3rd ed. St Louis: Elsevier-Saunders. 2009;St Louis:Elsevier. [Google Scholar]
- 15.MS Greenberg. Ulcerative vesicular and bullous lesions. In: Greeberg MS, Glick M, (Editors). Burket’s Oral Medicine, Diagnosis and Treatment, 10th ed.: BC Decker Inc.; USA. 2003:68–71. [Google Scholar]
- 16.J Amir, L Harel, Z Smetana, I Varsano. The natural history of primary herpes simplex type 1 gingivostomatitis in children. Pediatr Dermatol. 1999;16(4):259–263. doi: 10.1046/j.1525-1470.1999.00072.x. [DOI] [PubMed] [Google Scholar]