Abstract
Background: Since ancient times, use of graft materials to promote healing of defects of bone is wellknown. Traditionally, missing bone is replaced with material from either patient or donor. Multiple sources of bone grafts have been used to graft bone defects to stimulate bone healing. Hydroxyapatite is naturally occurring mineral component of bone, which is osteoconductive. This versatile biomaterial is derived from many sources. The aim of this study is to evaluate the efficacy of eggshell derived hydroxyapatite (EHA) in the bone regeneration of human maxillary cystic bone defects secondary to cystic removal/apicoectomy and compare the material properties of EHA in vitro. Materials and Methods: A total of eight maxillary bone defects were grafted after cystic enucleation and/or apicoectomy in the year 2008 and completed the study at 1 year. The patients were followed-up 2 weeks after surgery for signs and symptoms of infection or any other complications that may have been related to surgical procedure. Follow-up radiographs were obtained immediately after surgery followed by 1, 2, and 3 months to assess the efficacy of EHA in bone healing. Physicochemical characterization of the EHA was carried out in comparison with synthetic hydroxyapatite (SHA), also compared the biocompatibility of EHA using in vitro cytotoxicity test. Results: By the end of the 8th week, the defects grafted with EHA showed complete bone formation. However, bone formation in non-grafted sites was insignificant. The values of density measurements were equal or more than that of surrounding normal bone. These results indicate that the osseous regeneration of the bone defect filled with EHA is significant. EHA showed the superior material properties in comparison with SHA. Conclusion: EHA is a versatile novel bone graft substitute that yielded promising results. Because of its biocompatibility, lack of disease transfer risks, ease of use and unlimited availability, EHA remains a viable choice as regenerative material. EHA is very cost-effective, efficient bone graft substitute, which can be prepared in a very economical way. It is a worthwhile bone substitute because it is safe and easily available material. How to cite the article: Kattimani VS, Chakravarthi PS, Kanumuru NR, Subbarao VV, Sidharthan A, Kumar TS, Prasad LK. Eggshell derived hydroxyapatite as bone graft substitute in the healing of maxillary cystic bone defects: A preliminary report. J Int Oral Health 2014;6(3):15-9.
Key words: : Bone regeneration, bone substitute, eggshell derived hydroxyapatite, hen's eggshell, hydroxyapatite, osteoconductivity, synthetic hydroxyapatite
Introduction
Since ancient times use of graft material to promote healing of large defects of bone is well-known. 1 - 3 Traditionally, missing bone is replaced with allograft. 1 - 3 Because of morbidity of donor site, second surgery to harvest graft, quantity required, and so many other factors made the clinicians and scientists to search for alternative bone graft substitutes. 4 -9 Recently, use of processed or synthetic bone graft substitute has gained popularity over traditional methods. 3 , 7 - 9
Hydroxyapatite (HA) is a naturally occurring mineral component of bone. HA is osteoconductive. 3 , 7 , 8 Few studies shown that nanocrystalline HA is osteoinductive in nature 10 , 11 and stimulates cells for periodontal tissue regeneration. 12 This versatile biomaterial derived from many sources, e.g., bone, corals, synthetic, etc. HA is used to graft bone defects to stimulate bone healing. 2 , 3 , 7 - 9 The aim of our study is to evaluate the efficacy of indigenously prepared eggshell derived hydroxyapatite (EHA) in bone healing and compare the material properties with synthetic hydroxyapatite (SHA).
Materials and Methods
Sample preparation and characterization
Hen's eggshells washed thoroughly and heated in box furnace at 900oC for 2 hrs to decompose organic matter and convert it to calcium hydroxide after exposure to the atmosphere. The product was finely ground in an agate pestle and mortar. Calcium hydroxide weighed and mixed with distilled water to form 0.3 M suspension and reacted with 0.5 M di ammonium hydrogen phosphate solution corresponding to the stoichiometric ratio of Ca/p = 1.67. The mixed reactants were irradiated in a domestic microwave oven (BPL India, 245 GHz, 800 W). The product was then washed repeatedly with distilled water to remove unwanted ions and dried overnight in an oven at 100oC to produce EHA. 13 The procedure was repeated to check the reproducibility. The SHA was prepared in an identical manner by heat processing using synthetic calcium hydroxide (analytical grade, Merck, Germany). A small amount of both the samples were heated at 900oC for 2 hrs. Followed by cooling to improve the crystallinity and to check the purity.1 3
Cell culture and cell viability assay
The in vitro cytotoxicity test was performed as per direct contact method 13 (CISO 10993-5, 1999) using osteoblast cells maintained in minimum essential media supplemented with fetal bovine serum. These cells were seeded onto sintered HA pallets of 4 mm diameter and 2 mm thickness for 24 hr, which were viewed under an optical microscope. High density polyethylene and copper were used as negative and positive control samples, respectively. 13
Clinical and radiological evaluation
The total of eight patients treated in the year 2008 for periapical lesions such as residual and radicular cysts were included in this study. All the patients were grafted with EHA after cystic enucleation and/or apicoectomy. Clinically, the wound healed uneventfully in all cases. Suture removal was done on the 7 th day after surgery. Patients were excluded if the graft was lost or infected or were lost during the follow-up examination. The patients were followed-up at 1st and 2nd week after surgery for signs and symptoms of infection or any other complications that may have been related to the surgical procedures. At 1st, 2nd, and 3rd month and later 6th month radiographs were obtained to assess the amount of osseous fill. Mucosal color, post-operative pain and swelling was noted during clinical evaluation. Visual analog scale was used for clinical pain measurements.
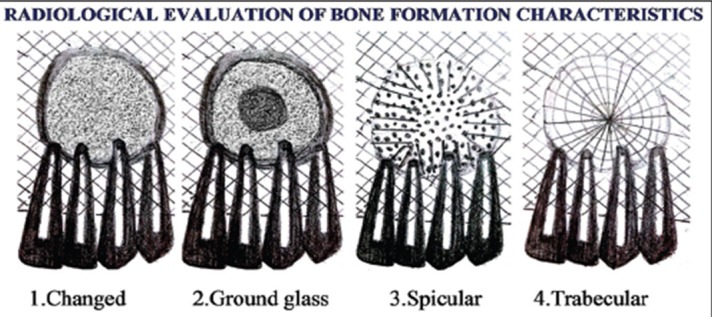
Observer strategy was modified (Figures 1 and 2 ) to assess the efficacy of EHA in the healing of bone after grafting of cystic defects. 14 - 18 The radiographic measures were collected at the following times: (1) Presurgically, (2) immediately after surgery, (3) 1st month after surgery, (4) 2nd month after surgery, (5) 3rd month after surgery, and (6) 6th month after surgery. Radiographs were evaluated and documented for (1) change in the surgical site outline, (2) change in the internal portion of surgical site, and (3) the density of bone formation. All the radiographs were examined blindly by two examiners. In case of any gross inconsistency with observations, the third examiner observed the radiographs to prevent bias and the results were tabulated. Density was noted in comparison with surrounding normal bone, as the surrounding bone density is considered as reference since beginning of the study and correlated until last follow-up. The comparison of the images were performed with variable intensity light. The radiographic changes in surgical site outline, internal portion of surgical site after surgery correlated with density using Mann–Whitney U-test and Wilcoxon matched paired test.
Figure 1: Schematic diagram showing radiological evaluation of surgical site outline.

Figure 2: Schematic diagram showing radiological evaluation of bone formation characteristics.
Results
All the cases healed well with no significant adverse clinical observations. The bone density had reached that of surrounding normal bone or more in all cases by the end of 8 weeks indicating the bone regeneration. Significant density changes were observed between 1st, 2nd, and 3rd month as summarized in Table 1 and Figure 3 . Density remained steady afterwards indicating complete bone healing. Control group showed very less bone regeneration and density measurements were insignificant
Table 1: Comparison at 1st week, 1st month, 2rd month, and 3rd month with mean bone density by paired t-test in EHA.
| Treatment durations | Mean | Standard deviation | Mean difference | SD difference | Paired t value | P value |
| 1st week | 107.3333 | 5.6782 | −21.5833 | 7.4524 | −10.0326 | 0.0000* |
| 1st month | 128.9167 | 5.9001 | ||||
| 1st week | 107.3333 | 5.6782 | −39.9167 | 13.3652 | −10.3459 | 0.0000* |
| 2nd month | 147.2500 | 10.6269 | ||||
| 1st week | 107.3333 | 5.6782 | −55.2500 | 22.3653 | −8.5575 | 0.0000* |
| 3rd month | 162.5833 | 20.2460 | ||||
| 1st month | 128.9167 | 5.9001 | −18.3333 | 11.8807 | −5.3455 | 0.0002* |
| 2nd month | 147.2500 | 10.6269 | ||||
| 1st month | 128.9167 | 5.9001 | −33.6667 | 20.7247 | −5.6273 | 0.0002* |
| 3rd month | 162.5833 | 20.2460 | ||||
| 2nd month | 147.2500 | 10.6269 | −15.3333 | 11.2439 | −4.7240 | 0.0006* |
| 3rd month | 162.5833 | 20.2460 |
*Significant at 5% level (P<0.05). EHA: Eggshell derived hydroxyapatite
Figure 3: Graph showing measurements of mean bone density at 1st week, 1st month, 2nd month, and 3rd month.

for correlation. The margin blending with material margin was progressive indicating the bone regeneration from the periphery to the center. These changes observed at 1 st, 2nd, and 3rd month were significant as summarized in Table 2 and were well-correlated with density changes ( Figure 4 ). The internal portion of surgical site was trabecular or specular in all the cases at the end of 2 nd month after surgery showing successful healing and osteoid regeneration as summarized in Table 3 . The in vitro cell viability test showed the material is biocompatible. The material characterization revealed the EHA is superior compared to SHA as summarized in Table 4 .
Table 2: Comparison at 1st week, 1st month, 2nd month, and 3rd month with radiological evaluation of surgical site outline by Wilcoxon matched pairs test by ranks in EHA.
| Treatment durations | t value | Z value | P level |
| 1st week-1st month | 0.0000 | 3.0594 | 0.0022* |
| 1st week-2nd month | 0.0000 | 3.0594 | 0.0022* |
| 1st week-3rd month | 0.0000 | 3.0594 | 0.0022* |
| 1st month-2nd month | 0.0000 | 2.9341 | 0.0033* |
| 1st month-3rd month | 0.0000 | 2.9341 | 0.0033* |
| 2nd month-3rd month | 0.0000 | 1.8257 | 0.0679 |
*Significant at 5% level (P<0.05). EHA: Eggshell derived hydroxyapatite
Figure 4: Intraoral periapiacal radiographs showing radiological evaluation of surgical site outline and bone formation characteristics.

Table 3: Comparison at 1st week, 1st month, 2nd month, and 3rd months with radiological evaluation of bone formation by Wilcoxon matched pairs test by ranks in EHA.
| Treatment durations | t value | Z value | P level |
| 1st week-1st month | 0.0000 | 3.0594 | 0.0022* |
| 1st week-2nd month | 0.0000 | 3.0594 | 0.0022* |
| 1st week-3rd month | 0.0000 | 3.0594 | 0.0022* |
| 1st month-2nd month | 0.0000 | 3.0594 | 0.0022* |
| 1st month-3rd month | 0.0000 | 3.0594 | 0.0022* |
| 2nd month-3rd month | 0.0000 | - | - |
*Significant at 5% level (P<0.05). EHA: Eggshell derived hydroxyapatite
Table 4: Comparison of physicochemical properties of EHA and SHA.
| Sample | Crystalline size | Surface area (m2/g) | Density(g/cm2) | Cytotoxicity |
| EHA | 21 | 106 | 3.12 | Noncytotoxic |
| SHA | 19 | 104 | 3.06 | Noncytotoxic |
EHA: Eggshell derived hydroxyapatite, SHA: Synthetic hydroxyapatite
Discussion
Currently in the United States alone, number of bone graft procedures done per year exceeds 500,000 and approximately 2.2 million worldwide. 19 The estimated cost of these procedures approaches $2.5 billion per year. Harvesting the autograft requires an additional surgery that can result in its own complications such as inflammation, infection and chronic pain that occasionally outlasts the pain of the original surgical procedure. 4 - 6 Quantities of bone tissue that can be harvested is also limited, thus creating a supply problem. 2 - 6 , 19 , 20 Risk of human immunodeficiency virus transmission with allograft was reported to be one case in 1.6 million population. 20 , 21 Cases of hepatitis transmission and development of septic arthritis from the donor tissue have been reported with allograft. 22 - 24 The complement-dependent cytotoxicity reported25 other cases of allograft-related infection or illness and death of patient due to Clostridium sordellii . 25
The limitations 4 - 6 of autografts, and allografts have necessitated the pursuit of alternatives. 2 , 3 , 7 - 9 Two basic criteria for successful grafting (i.e., osteoconduction and osteoinduction) were used by investigators and several alternatives were also developed; some of which are available for clinical use and others are still in the developmental stage. 2 , 3 , 7 - 9 Many of these alternatives use a variety of materials, including natural and synthetic polymers, ceramics and composites, whereas others have incorporated factor and cell-based strategies that are used either alone or in combination. 2 - 3 , 7 - 9 , 26 -2 9 This article introduces the EHA as a novel bone graft material.
The formulations of eggshell are being used as mineral and trace element supplying agent. 29 , 30 The various formulations comprising eggshell powder have been examined in rats. 29 - 32 In recent times, this eggshell derived material has been introduced as bone graft substitute. 29 There are few studies with surface modified eggshell as osteoconductive bone filling material for bone regeneration with variable benefit. 31 , 32 After histomorphometrical evaluation at 4 and 8 weeks interval, it was confirmed that the eggshell-derived powders have excellent new bone formation ability. 31 - 33 This has led to the curiosity to prepare the EHA from eggshell waste in a very economical way. 34 Even the material properties are superior to the commercially available graft materials. 13 The material is chemically pure form of nanocrystalline HA with eggshell origin alike any other SHA. 13 , 34 The different forms of HA and origin are in use as bone graft substitute since long time. 7 - 10
In our study 10 patients enrolled, over the course of 1 year, two patients were lost follow-up. However, all 10 patients were followed-up through first 2 months. All attempts to contact the two patients failed in locating them. If the patient could not be followed-up for the remaining period, his or her data were not included for the purpose of reporting levels of osseous fill and bone healing. At the initial post-operative visits at 1 and 2 weeks, there are no signs of infection. At the 1-week follow-up visit, all patients reported less pain associated with grafted site than with the non-grafted site.
Radiographic changes in surgical site outline and bone formation characteristics were significant between 1 st week and 1 st month (P < 0.05) showing specular or ground glass appearance with merging of material and bone margin. 18 The radiographic bone healing observed in all the patients confirms findings reported by other authors 29 - 32 that EHA is biocompatible and well-tolerated by oral tissues in humans. Baliga et al. 33 have shown the biocompatibility of surface modified eggshell material in cystic cavities of jaw bones with centripetal ossification, which occurred within 6 weeks. The enhancement of bone regeneration could be explained by the ability of the HA to facilitate bone adsorption and calcium release, which stimulates osteoblast differentiation and bone formation. 35 Compared to SHA, the EHA seems to have better morphology, stoichiometry, sinterability, stability at high temperatures and an osteoblast adhesion. 13 , 34
Eggshell HA seems to be promising graft material with excellent properties for grafting. EHA is hydrophilic, absorbing surrounding fluids and blood, making it easy to handle and place it in the surgical site. This study did not include collection of tissue for histologic examination because of ethical considerations. The histologic examination of EHA has been studied in animal models 32 and surface modified eggshell in humans showing early bone regeneration. 33
The EHA showed biocompatibility and good in vitro material properties. It is available in unlimited quantity. It can be easily sterilized by autoclaving without altering its biological properties. 30 EHA can be used as graft material for grafting of the bone defects secondary to periodontal diseases, trauma, tooth extractions, developmental imperfections, intra bony defects, sinus lift procedures, and so on.
Conclusion
EHA is a versatile novel bone graft substitute that yielded promising results. Because of its biocompatibility, lack of disease transfer risks, ease of use and unlimited availability, EHA remains a viable choice as regenerative material. EHA is very cost effective, efficient bone graft substitute which can be prepared in a very economical way. It is a worthwhile bone substitute because it is safe and easily available material.
Many products are being marketed today as bone grafts. Several of these products capitalize on the necessities of an ideal substitute. As more materials are adapted and discovered, pre-existing products are finding new applications and effectiveness in combination with newly emerging technology. In addition, further research is going on to use it in combination with collagen for bone repair. It would be valuable to study a larger sample size and with variable age group to test hypothesis including hisomorphometry to confirm its nature of bone regeneration. The future of EHA graft material continues to be an expanding topic.
Footnotes
Source of Support: Nil
Conflict of Interest: None
Contributor Information
Vivekanand S Kattimani, Department of Oral and Maxillofacial Surgery, Sibar Institute of Dental Sciences, Guntur, Andhra Pradesh, India.
P Srinivas Chakravarthi, Department of Oral and Maxillofacial Surgery, Sibar Institute of Dental Sciences, Guntur, Andhra Pradesh, India.
Narasimha Reddy Kanumuru, Department of Conservative and Endodontics, Sibar Institute of Dental Sciences, Guntur, Andhra Pradesh, India.
Vummidisetti V Subbarao, Department of Conservative Dentistry and Endodontics, Lenora Institute of Dental Sciences, Rajamundry, Andhra Pradesh, India.
A Sidharthan, Department of Mechanical Engineering, Anna University, Chennai, Tamil Nadu, India.
T S Sampath Kumar, Medical Materials Laboratory, Department of Metallurgical and Materials Engineering, Indian Institute of Technology Madras, Chennai, Tamil Nadu, India.
L Krishna Prasad, Department of Oral and Maxillofacial Surgery, Sibar Institute of Dental Sciences, Guntur, Andhra Pradesh, India.
References
- 1.H Kromer. Bone homografts in minor oral surgery. Proc R Soc Med. 1962;5:607–614. doi: 10.1177/003591576205500727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CJ Damien, JR Parsons. Bone graft and bone graft substitutes: A review of current technology and applications. J Appl Biomater. 1991;2:187–208. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 3.MB Habal, AH Reddi. Different forms of bone grafts. In: Habal MB, Reddi AH (Editor). Bone Grafts and Bone Substitutes. Philadelphia: Saunders. 1992:6–8. [Google Scholar]
- 4.JC Banwart, MA Asher, RS Hassanein. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 5.J Clavero, S Lundgren. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: Comparison of donor site morbidity and complications. Clin Implant Dent Relat Res. 2003;5:154–160. doi: 10.1111/j.1708-8208.2003.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 6.RE Marx, MJ Morales. Morbidity from bone harvest in major jaw reconstruction: A randomized trial comparing the lateral anterior and posterior approaches to the ilium. J Oral Maxillofac Surg. 1988;46:196–203. doi: 10.1016/0278-2391(88)90083-3. [DOI] [PubMed] [Google Scholar]
- 7.LL Hench, J Wilson. An Introduction to Bioceramics. Singapore: World Scientific. 1993 [Google Scholar]
- 8.LL Hench. Bio ceramics: From concept to clinic. J Am Ceram Soc. 1991;74:1487–1510. [Google Scholar]
- 9.AS Greenwald, SD Boden, VM Goldberg, Y Khan, CT Laurencin, RN Rosier, et al. Bone-graft substitutes: Facts, fictions, and applications. J Bone Joint Surg Am. 2001;83:98–103. doi: 10.2106/00004623-200100022-00007. [DOI] [PubMed] [Google Scholar]
- 10.U Ripamonti. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials. 1996;17:31–35. doi: 10.1016/0142-9612(96)80752-6. [DOI] [PubMed] [Google Scholar]
- 11.AK Gosain, L Song, P Riordan, MT Amarante, PG Nagy, CR Wilson, et al. A 1-year study of osteoinduction in hydroxyapatite-derived biomaterials in an adult sheep model: Part I. Plast Reconstr Surg. 2002;109:619–630. doi: 10.1097/00006534-200202000-00032. [DOI] [PubMed] [Google Scholar]
- 12.A Kasaj, B Willershausen, C Reichert, B Rohrig, R Smeets, M Schmidt. Ability of nanocrystalline hydroxyapatite paste to promote human periodontal ligament cell proliferation. J Oral Sci. 2008;50:279–285. doi: 10.2334/josnusd.50.279. [DOI] [PubMed] [Google Scholar]
- 13.D Siva Rama Krishna, A Siddharthan, SK Seshadri, TS Sampath Kumar. A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J Mater Sci Mater Med. 2007;18:1735–1743. doi: 10.1007/s10856-007-3069-7. [DOI] [PubMed] [Google Scholar]
- 14.JO Andreasen, J Rud. Correlation between histology and radiography in the assessment of healing after endodontic surgery. Int J Oral Surg. 1972;1:161–173. doi: 10.1016/s0300-9785(72)80006-1. [DOI] [PubMed] [Google Scholar]
- 15.J Rud, JO Andreasen, JE Jensen. Radiographic criteria for the assessment of healing after endodontic surgery. Int J Oral Surg. 1972;1:195–214. doi: 10.1016/s0300-9785(72)80013-9. [DOI] [PubMed] [Google Scholar]
- 16.J Rud, JO Andreasen, JE Jensen. Radiographic criteria for the assessment of healing after endodontic surgery. Int J Oral Surg. 1972;1:195–214. doi: 10.1016/s0300-9785(72)80013-9. [DOI] [PubMed] [Google Scholar]
- 17.PV Oltramari, L Navarro Rde, JF Henriques, R Taga, TM Cestari, G Janson, et al. Evaluation of bone height and bone density after tooth extraction: An experimental study in minipigs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:9–16. doi: 10.1016/j.tripleo.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 18.VS Kattimani, NV Bajantai, SK Sriram, RR Sriram, VK Rao, PD Desai. Observer strategy and radiographic classification of healing after grafting of cystic defects in maxilla: A radiological appraisal. J Contemp Dent Pract. 2013;14(2):227–232. doi: 10.5005/jp-journals-10024-1304. [DOI] [PubMed] [Google Scholar]
- 19.PV Giannoudis, MK Al-Lami, C Tzioupis, D Zavras, MR Grotz. Tricortical bone graft for primary reconstruction of comminuted distal humerus fractures. J Orthop Trauma. 2005;19(10):741–743. doi: 10.1097/01.bot.0000177121.70367.3f. [DOI] [PubMed] [Google Scholar]
- 20.T Boyce, J Edwards, N Scarborough. Allograft bone. The influence of processing on safety and performance. Orthop Clin North Am. 1999;30(4):571–581. doi: 10.1016/s0030-5898(05)70110-3. [DOI] [PubMed] [Google Scholar]
- 21.RJ Simonds, SD Holmberg, RL Hurwitz, TR Coleman, S Bottenfield, LJ Conley, et al. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med. 1992;326:726–732. doi: 10.1056/NEJM199203123261102. [DOI] [PubMed] [Google Scholar]
- 22.WW Tomford. Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg Am. 1995;17(11):1742–1754. doi: 10.2106/00004623-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 23.EU Conrad, DR Gretch, KR Obermeyer, MS Moogk, M Sayers, JJ Wilson, et al. Transmission of the hepatitis-C virus by tissue transplantation. J Bone Joint Surg Am. 1995;77(2):214–224. doi: 10.2106/00004623-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Public Health Service Guideline on Infectious Disease Issues in Xenotransplantation. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2001;50(RR-15):1–46. [PubMed] [Google Scholar]
- 25.MA Greenwald, MJ Kuehnert, JA Fishman. Infectious disease transmission during organ and tissue transplantation. Emerg Infect Dis. Available from: http://www.nc.cdc.gov/eid/article/18/8/12-0277_article.htm . 2012;18:1. doi: 10.3201/eid1808.120277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.T Turunen, J Peltola, A Yli-Urpo, RP Happonen. Bioactive glass granules as a bone adjunctive material in maxillary sinus floor augmentation. Clin Oral Implants Res. 2004;15:135–141. doi: 10.1111/j.1600-0501.2004.00989.x. [DOI] [PubMed] [Google Scholar]
- 27.MJ Coughlin, JS Grimes, MP Kennedy. Coralline hydroxyapatite bone graft substitute in hindfoot surgery. Foot Ankle Int. 2006;27:19–22. doi: 10.1177/107110070602700104. [DOI] [PubMed] [Google Scholar]
- 28.N Velich, Z Nemeth, C Toth, G Szabo. Long-term results with different bone substitutes used for sinus floor elevation. J Craniofac Surg. 2004;15:38–41. doi: 10.1097/00001665-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 29.L Dupoirieux, D Pourquier, F Souyris. Powdered eggshell: A pilot study on a new bone substitute for use in maxillofacial surgery. J Craniomaxillofac Surg. 1995;23:187–194. doi: 10.1016/s1010-5182(05)80009-5. [DOI] [PubMed] [Google Scholar]
- 30.L Dupoirieux. Ostrich eggshell as a bone substitute: A preliminary report of its biological behaviour in animals - A possibility in facial reconstructive surgery. Br J Oral Maxillofac Surg. 1999;37:467–471. doi: 10.1054/bjom.1999.0041. [DOI] [PubMed] [Google Scholar]
- 31.JW Park, SR Bae, JY Suh, DH Lee, SH Kim, H Kim, et al. Evaluation of bone healing with eggshell-derived bone graft substitutes in rat calvaria: A pilot study. J Biomed Mater Res A. 2008;87A:203–214. doi: 10.1002/jbm.a.31768. [DOI] [PubMed] [Google Scholar]
- 32.SH Kim, W Kim, JH Cho, NS Oh, MH Lee, SJ Lee. Comparison of bone formation in rabbits using hydroxyapatite and ß-tricalcium phosphate scaffolds fabricated from egg shells. Adv Mater Res. 2008;47-50:999–1002. [Google Scholar]
- 33.M Baliga, P Davies, L Dupoirieux. La poudre de coquille d,oeuf dans le comblement des cavites cystiques des maxillaires. Rev Stomatol Chir Maxilofac. 1998;99:86–88. [PubMed] [Google Scholar]
- 34.Application no. 848/CHE/2006, PG Journal Number: 13/2009, Publication Date 27/03/2009, Patent No. 232313. Available from: http://www.ipindiaservices.gov.in/patentsearch/GrantedSearch/frmGrantedSearch.aspx/ [Google Scholar]
- 35.A El-Ghannam, H Amin, T Nasr, A Shama. Enhancement of bone regeneration and graft material resorption using surface-modified bioactive glass in cortical and human maxillary cystic bone defects. Int J Oral Maxillofac Implants. 2004;19:184–191. [PubMed] [Google Scholar]


