Abstract
Background: Study of the clinical application of bioactive glass in treating periodontal defects has been gaining momentum. Studies in the past have hypothesized that bioactive glass resulted in an improvement of bony lesion when compared with open flap debridement. Considering that there were very few studies in the Indian dental literature involving the analysis of PerioGlas®- A particulate Bioglass in intrabony defects, the present clinical trial aimed to clinically and radiographically evaluate the efficacy of PerioGlas® and compare it to open debridement as control in the treatment of human periodontal osseous (three and two wall) defects in South Indian population. Materials and Methods: Ten patients with chronic periodontitis within the age group of 30-45 years having at least two pockets with depth of ≥6 mm exhibiting vertical osseous defects were selected for the study. A total of 20 defect sites were randomly assigned to one of the two treatment modalities such that 10 sites (experimental) received PerioGlas® material after open flap debridement and 10 sites with open flap debridement (controls). Plaque index and gingival index (GI) were recorded at baseline, 6 weeks, 3 months, 6 months and 9 months, whereas probing pocket depth (PPD), clinical attachment level and gingival recession (GR) were recorded at baseline, 6 and 9 months postoperatively. Linear radiographic measurements were carried out at baseline, 6 and 9 months to evaluate the defect fill, defect resolution and change in the alveolar crest height (ACH). Results: Both experimental and control site showed a significant reduction in plaque and GI, and a slight increase in GR. The mean reduction in PPD for experimental and control site was 4.4 ± 0.34 mm and 3.2 ± 0.1 mm, respectively. Gain in clinical attachment at experimental and control site was 4.4 ± 0.21 and 3.4 ± 0.11, respectively which on comparison was statistically non-significant for both sites. The radiographic mean defect fill for experimental site was 1.73 mm. The mean defect resolution was 46.5% and 15.3% for the experimental group and control group, respectively, with a slight increase in the ACH at the experimental site. Conclusion: Comparison of experimental and control sites revealed a statistically significant improvement in both clinical and radiographic parameters, but experimental sites showed better results when compared with control. How to cite the article: Chacko NL, Abraham S, Rao HN, Sridhar N, Moon N, Barde DH. A clinical and radiographic evaluation of periodontal regenerative potential of PerioGlas®: A synthetic, resorbable material in treating periodontal infrabony defects. J Int Oral Health 2014;6(3):20-6.
Key words: : Debridement, graft, periodontal regeneration, perioglas, surgical flaps
Introduction
Periodontal disease leaves a historical record of the damage to periodontium in the form of periodontal attachment and bone loss. 1 In teeth in which continued function requires additional periodontal support, optimal treatment involves not only controlling periodontal infection, but also regeneration of the lost periodontium. 2
Among the plethora of regenerative modalities, bone grafts and their synthetic substitutes have been used in an attempt to gain their therapeutic endpoint; the use of which dates back to Hegedes in 1923. 3 Since then a number of techniques and materials have been used for regeneration.
Newer ceramic alloplast like bioactive glass has shown the ability to help bone regeneration and clinical insertion gain, with better results than other materials available. 4 - 8 Bioactive glasses are a group of surface reactive glass-ceramics composed entirely of elements naturally occurring in the body (silica, calcium, phosphorous, oxygen, and sodium). It is the formation of a biologically active hydrated calcium phosphate layer at the surface of the bioactive glass which plays a key role in the formation of the bone/graft bond. 4 Hench et al. at the University of Florida first developed these materials for limb prosthesis in the late 1960’s. 9 The first bioactive glass compositions created that bonded to both living bone and tissue was Bioglass® that proved to have multiple useful applications and thus was cleared by Food and Drug Administration for clinical use in 1984. In the mid-90’s particulate Bioglass® was introduced to dentists and oral surgeons as PerioGlas®. 10
This material has demonstrated osteoconductive and osteopromotive abilities in the biocompatible interface for osseous migration, and a bioactive surface colonized by osteogenic cells free in the surgical wound. 11 , 12 Their ability to bond to soft and osseous tissues seems to make a difference when compared to other alloplastic materials available. 13 - 15 It was recently discovered that the bioactive glasses slowly dissolve and the dissolution products, the soluble silicon and soluble calcium, actually activates six families of genes in old bone cells that then form new bone cells. 16 , 17 These cells not only increase in number, but also generate collagen and other extracellular matrix proteins that mineralized form new bone. 18 , 19 Histologic studies have shown that the use of bioactive glass induces a significant increase in newly formed cementum and attachment and that apically directed growth of the junctional epithelium can be prevented. 20 , 21 Potential antibacterial effect for this material is also reported, which is due to the surface reactions undergone by Bioglass. 22 , 23
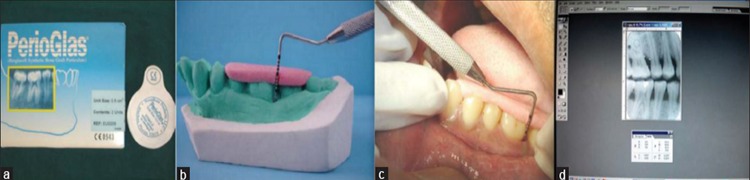
The bioactive glass used in the present study is a granulated form of Bioglass 45S5 - PerioGlas® (US Biomaterials Corporation, Florida, USA) ( Figure 1a ). The granules have a grain size of 90-710 μm. Studies in the past have hypothesized that using bioactive glass for the treatment of intraosseous defects resulted in an improvement of the bony lesion when compared with the open flap debridement procedure among western populations. 4 , 24 , 25
Figure 1: (a) Perioglas bone graft. (b) Acrylic stent on cast. (c) Pocket probing with UNC-15 probe. (d) Radiographic measurements using Adope photoshop.
Considering that there are very few studies in the Indian dental literature involving the analysis of PerioGlas® in intrabony defects among Indian population, the present investigation was designed to clinically and radiographically evaluate particulate bioactive ceramic - PerioGlas® and compare it to open debridement controls in the treatment of human infrabony vertical osseous defects (three and two walled) in South Indian population.
Materials and Methods
The patients for this study were selected from the outpatient Department of Periodontics, R.V. Dental College and Hospital, Bangalore, Karnataka. Patients with chronic adult periodontitis, exhibiting at least two or more infrabony pockets of ≥6 mm, with radiographic evidence of vertical bone loss, in the age group between 30 and 45 years of either sex, having good systemic health, with no contraindication for periodontal surgery and who had not received any type of periodontal therapy for the past 6 months were considered for the study. The study excluded patients who were smokers, pregnant and lactating women, and patients showed unacceptable oral hygiene during pre-surgical (Phase I) period and those who had taken antibiotics 1 month prior to the study. The nature of the study was explained to patients and consent form was obtained from them.
A total of 20 sites from 10 patients were selected for the study, after completion of pre-surgical phase of treatment. The selected sites were randomly divided into control and experimental site by using split mouth design. The control sites were treated with flap surgery alone, whereas the experimental sites received flap surgery with PerioGlas® grafting. The clinical parameters assessed were plaque index (PI) (Silness and Loe, 1964) gingival index (GI) (Loe and Silness, 1963), probing pocket depth (PPD), clinical attachment level (CAL) and gingival recession (GR). All clinical parameters were recorded preoperatively at baseline. PI and GI were recorded at 6 weeks, 3 months, 6 months and 9 months, whereas the PPD, CAL and GR were recorded at 6 and 9 months postoperatively. Radiographic evaluation was made on standardized bitewing radiographs at baseline, 6 and 9 months postoperatively.
For the clinical measurements, alginate impressions were taken and study models were prepared and acrylic stents were fabricated on the study model of each patient to fit over the selected teeth. Vertical grooves were made on the stent with burs for proper guidance and orientation of periodontal probe.4 All the customized acrylic stents were stored on the prepared study casts to minimize distortion ( Figure 1b ). The measurements were performed by UNC-15 periodontal probe ( Figure 1c ). The radiographic assessment involved no. 2 adult size E-speed film was placed in a bitewing film holder with a bitewing metal guiding arm attached to a cone-positioning device (Xcp-Ds, Gendex, Dentsply), which would hold the tube head during exposure. All the radiographs were evaluated for radiographic fill of the osseous defects with a computer assisted method for making linear radiographic measurements. All the radiographs were scanned and digitized using HP -
transparency scanner. The digitized images were displayed on the monitor at ×5 magnification and the linear measurements were made on the digitized images using Adobe Photoshop 5.5 computer software from Adobe Systems Inc. ( Figure 1d ).
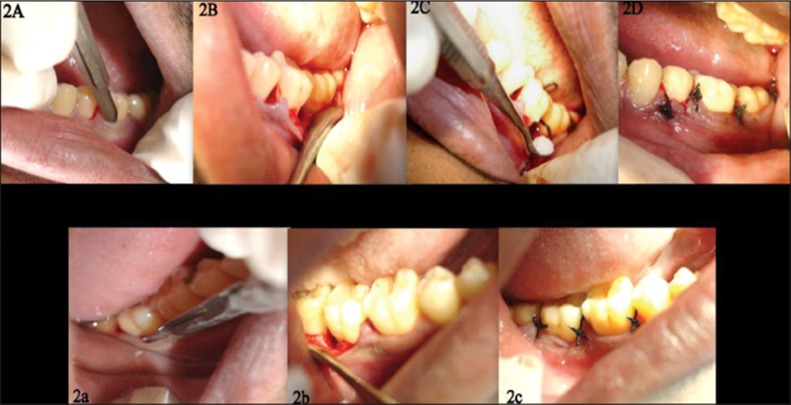
The surgical protocol involved comfortable seating of the patient followed by a preprocedural rinse of 10 ml of 0.2% chlorhexidine digluconate solution. The extra oral surfaces of the patient were swabbed with 5% povidone iodine solution and the operative site was anaesthetized with 2% xylocaine hydrochloride with adrenaline (1:80000), using block and infiltration techniques. The crevicular incisions were given on the facial and lingual/palatal sides using the Bard Parker handle no. 3 with blade no. 2. Full thickness mucoperiosteal flaps were reflected by using the periosteal elevator, taking care that, the interdental papillary tissue was retained as much as possible. After reflection of the flap and exposure of the osseous defect, thorough surgical debridement of both soft and hard tissues was done using curettes. The surgical site was thoroughly irrigated with normal saline. At the experimental site, the defect was filled with PerioGlas® ( Figures 2A-D ). The required quantity of PerioGlas® was transferred to a dappen dish, mixed with saline or autologous blood and was delivered into osseous defect incrementally with the help of cumine scaler. The material was placed from the base of the defect coronally to the approximate level of the crest or the remaining osseous walls. Care was taken that the defect was gently placed and not packed in the defect. The operative site was closed with 4-0 black silk sutures and protected with a non eugenol dressing. The control sites were left unfilled after surgical debridement and thorough root planning ( Figures 2a-c ). Thorough irrigation of surgical wound was done with normal saline. The mucoperiosteal flaps were repositioned and secured in place using black braided (4-0) interrupted silk sutures to obtain primary closure of the interdental space and protected with a non eugenol dressing. All patients were prescribed systemic amoxicillin 250 mg for 5 days, and a combination of ibuprofen (400 mg) and paracetamol (500 mg) thrice daily for 3 days and appropriate postoperative instructions were given to patients. Recall appointments were made for clinical and radiographic evaluation at 6 weeks, 3 months, 6 months and 9 months. At each visit, oral hygiene were reinforced and scaling was done if necessary.
Figure 2: (A-D) Clinical pictures of the experimental site. (a-c) Clinical pictures of control cite.
All the clinical and radiographic parameters were subjected to statistical analysis. For intragroup variations, paired t-test was performed. In case of failure of normality test, Wilcoxon signed rank test was used. For inter-group variations t-test was performed. In case of failure of normality test, two sample rank test (Mann–Whitney U-test) a non-parametric test was utilized.
Interpretation of Results
Clinical parameters
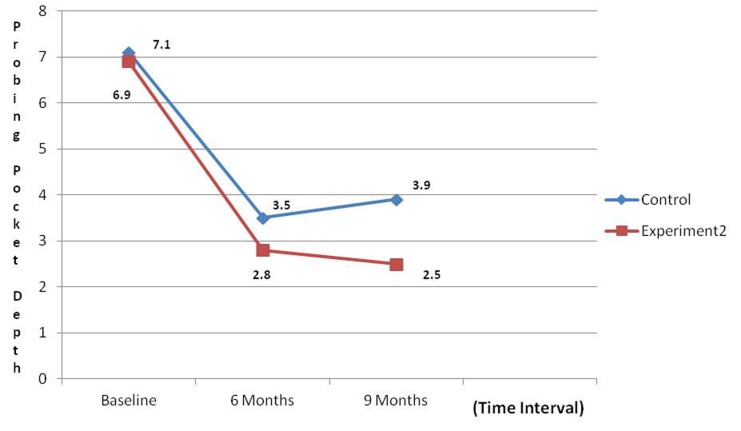
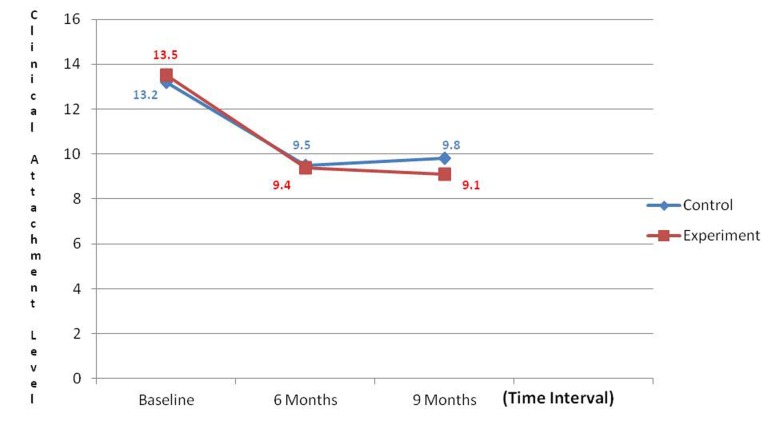
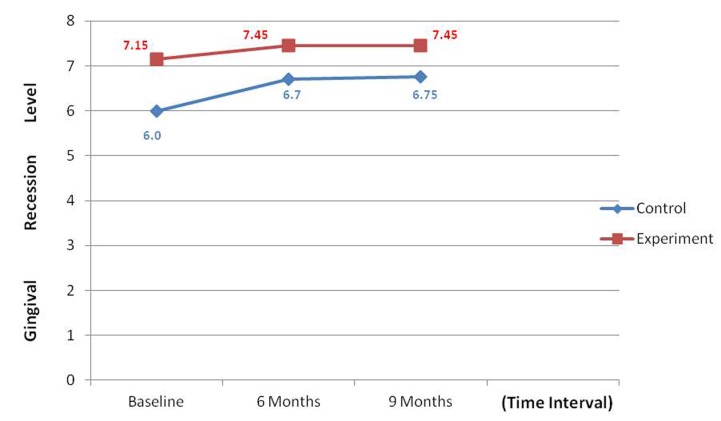
An overall reduction in the PI and GI scores were noticed during the various intervals; however, a non-significant difference was noticed when control and experimental groups were compared. Comparative analysis of PPD at control and experimental site revealed that there was a significant reduction in PPD at the experimental site when compared to control site (P < 0.001) ( Graph 1 ). Comparative analysis of gain in CALs at the control site and experimental site revealed a non-significant difference when control and experimental groups were compared ( Graph 2 ). GR was noticed in both groups and this was found to be a non-significant difference when control and experimental groups were compared ( Graph 3 ).
Graph 1: Comparison of mean reduction in probing pocket depth.
Graph 2: Comparison of mean gain in clinical attachment level.
Graph 3: Comparison of mean change in gingival recession.
Radiographic parameters
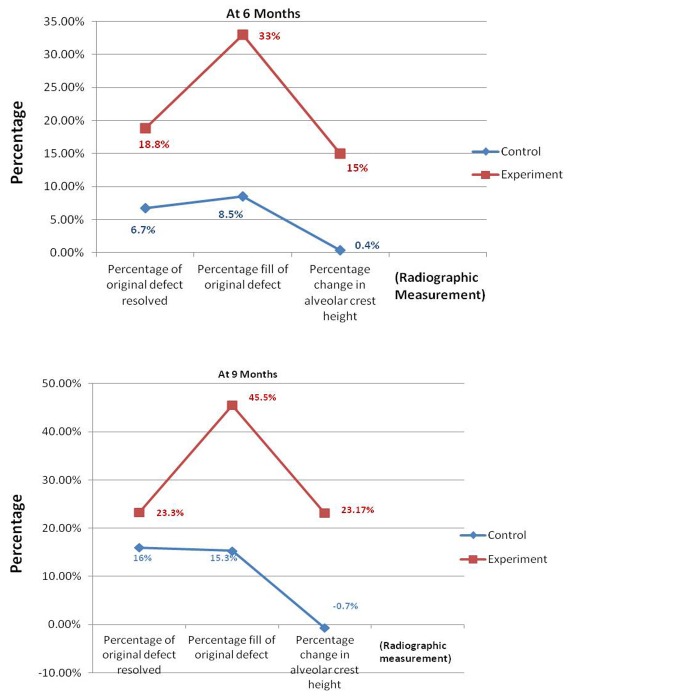
Percentage of original defect resolved (ODR) - The mean percentage of ODR when compared between control and experimental sites, the latter showed a higher percentage of defect resolution than control site with t value of −2.978 at 6 months which was statistically significant (P = 0.008) and t value of −1.389 at 9 months which was statistically non-significant (P = 0.182) ( Graph 4 ).
Graph 4: Comparisons of mean change in percentage of original defect resolution, percentage fill of original defect and percentage change in alveolar crest height between control and experimental sites.
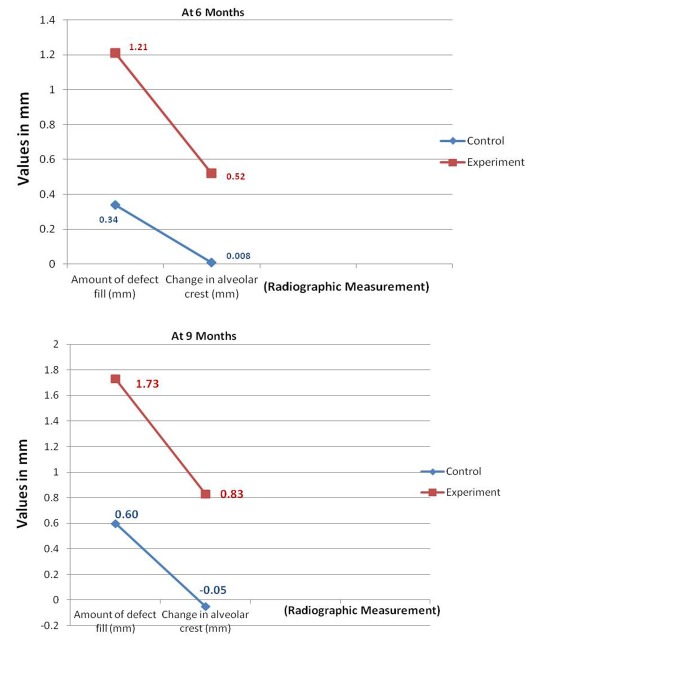
Amount of defect fill (DF) - The mean amount of DF on comparative analysis revealed a better result for experimental site with t value of 94.000 which was statistically significant (P = 0.001) at 6 months and t value of −3.693 at 9 months which was also statistically significant (P = 0.002) ( Graph 5 ).
Graph 5: Comparisons mean change in the amount of defect fill and change in alveolar crest between control and experimental sites.
Percentage fill of original defect (OD) - The mean percentage fill of OD when compared between control and experimental sites revealed experimental site to be better than control, with ‘t’ value of −4.623 at 6 months indicating a statistically significant result (P < 0.001) and −4.102 indicating a significant result (P < 0.001) at 9 months ( Graph 4 ).
Change in alveolar crest (AC) - The AC height when compared between control and experimental sites, the later showed t value of −3.125 at 6 months (P = 0.006) and −3.658 at 9 months
which was significant (P = 0.002) indicating gain in AC height in experimental site ( Graph 5 ).
Percentage change in alveolar crest height - Comparative analysis of mean percentage change in AC for both the sites revealed a better result for test site with t value of −2.921 at 6 months (P = 0.009) and −3.659 at 9 months, indicating a statistically significant gain in AC height (P = 0.002) ( Graph 4 ).
There was a significant reduction in mean plaque scores and GI scores at various intervals during the study. A significant reduction in pocket probing depth was observed in experimental groups when compared to controls. The gain in CALs and GR was non-significant when both groups were compared. However, it was observed that PerioGlas® treated defects showed better results when evaluated radiographically to open debridement alone. The percentage fill of defect obtained with PerioGlas® in this study was 46.5% when compared to open debridement alone which was 15.3%.
Discussion
In the pursuit of quest for the ideal material to support bone repair or regeneration, the deficiencies of autogenous grafts
or allogenic bone have led to search for synthetic alloplast. Unfortunately, alloplasts available until recently have yielded inconsistent results and disappointing histological results, often evidenced by encapsulation of the graft particles. Considering only limited studies involving the analysis of PerioGlas® in intrabony defects in South Indian population, the present investigation was designed to clinically and radiographically evaluate particulate bioactive ceramic - PerioGlas® and compare it to the open debridement controls in the treatment of human infrabony vertical osseous defects (three and two walled). In the present study, three walled and two walled infrabony defects were selected as considered in the study of Low et al. 7
A split mouth study design was employed in this study similar to studies of Zamet et al., 4 Ong et al., 26 since; these designs have been used to evaluate a variety of preventive and therapeutic agents particularly in those studies where the procedure or treatment effects are localized.
Plaque index scores showed statistically significant reduction at various intervals (P < 0.001) These findings were in accordance with findings of Park et al. 25 GI scores also showed statistically significant reduction which could be attributed to the significant reduction in PI scores leading to decreased inflammation post-surgically. However comparative analysis of control and experimental sites revealed a non-significant difference between the sites indicating that the change was same for both experimental and control sites. Similar observations were made by Park et al., 25 However the findings in this study did not correlate with findings of Froum 24 who noted no significant differences in PI and GI from baseline to 12 months post-surgery in either debridement or test sites. This could be possibly because the baseline PI and GI scores was less when compared to PI and GI baseline scores in our study.
The mean reduction score of PPD revealed a significant change from baseline to 9 months irrespective of the sites. However comparative analysis of both the sites revealed a better pocket depth reduction in experimental site than control site. These findings were in accordance with findings of Low et al. 7 and Zamet et al., 4 but not in accordance with the findings of Ong et al., 26 who observed lesser probing depth reduction at PerioGlas® treated sites in their study. This could be attributed to the difference in evaluation method for this parameter (Use of an automated probe to measure the clinical parameter).
Clinical attachment level relative to a landmark, such as occlusal stent facilitates the assessment of periodontal regeneration and its measurement at sequential post-surgical examination allows the clinician to determine any improvement in the attachment level. Though gain in CAL was observed in both the group, the present study showed a non-significant difference between both the sites indicating change was same for both experimental and control sites. This finding is consistent with the findings of Ong et al., 26 However, these results do not correlate with study of Zamet et al., 4 wherein significant gain in CAL was achieved at the test site. This could be possibly due to higher baseline attachment loss in the study which showed greater gains in CAL compared to our study.
Apical shift of the gingival margin is likely to reduce the regenerative capacity at a site thus affecting the final outcome. Comparison between the two groups revealed that, the amount of GR reduction at 6 and 9 months was statistically not significant. These findings in accordance with observations of Park et al., 25 but fail to correlate with observations of Ong et al., 26 who observed increased recession at test sites when compared to control sites.
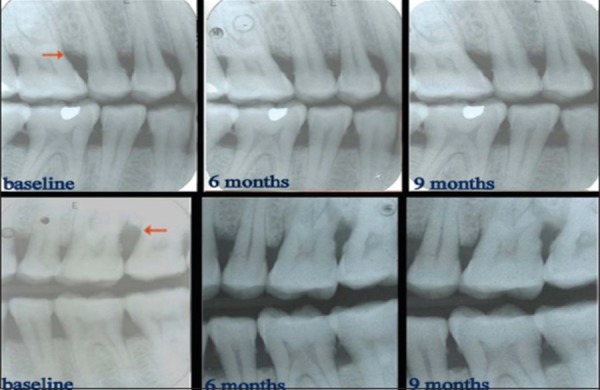
Radiographic evaluation provides the non-invasive method for assessing hard tissue changes and hence it was employed. Though, periapical radiograph is the technique of choice to visualize the tooth and its surrounding structures. In recent years, some studies have recommended the use of vertical bitewings, which allow more extensive coverage of alveolar bone in the apical direction. 27 Bitewing images record the distance between the emento-enamal junction and the crest of the interradicular alveolar bone more accurately. And with bitewing views the beam is oriented at the right angle to the long axis of the teeth, thus providing an accurate view of the relation of the height of the alveolar bone to the roots ( Figure 3 ).
Figure 3: Radiographs of control site and experimental site.
Comparison of the arithmetic determination in this study with those reported in other similar studies is difficult as studies in the past have used different radiographic analysis for measurements or adopted surgical reentry methods.
Defect fill is a desirable outcome of any periodontal regenerative therapy. The mean amount of DF on comparative analysis revealed a better result for experimental site (1.73 mm) when compared to control (0.60 mm). The amount of DF in this study was 1.73 mm at 9 months. This finding is not consistent with findings of Froum., 24 who have reported significant fill 2.60 mm over a 12 months period.
The mean DF obtained in our study could have attained significance if the study had been carried out for a longer duration. The mean percentage DF in this study was 46.5% for PerioGlas® treated sites and 15.3% for control site. The results are not in consistent with study by Ong et al., 26 who have obtained significantly greater percentage DF in the test sites 62% (3.28 mm) and 33.6% (1.45 mm) over 12 months duration.
The mean percentage of ODR when compared between control and experimental sites, the latter showed a higher percentage than control site with a t value of −2.978 at 6 months which was statistically significant and a t value of −1.389 at 9 months which was statistically non-significant.
As the bone regenerates, the position of AC or the angle of the defect may change, thereby influencing the different measurements, hence it becomes an important parameter while assessing regeneration. The AC height when compared between control and experimental sites, the later showed a
t value of −3.125 at 6 months and −3.658 at 9 months which was indicating gain in AC height in experimental site. In this study, PerioGlas® caused no adverse clinical side effects. The tolerance of the material from a clinical standpoint was excellent. There was no unusual finding with regard to the postoperative healing which is consistent with the findings of studies in the past. 8 , 26 , 28 In the present study, experimental group (PerioGlas®) showed better results than the control group (open flap debridement only) in terms of radiographic assessment.
Although PerioGlas® has shown better results on clinical and radiological evaluation in the present study, it must be noted that this was a relatively small study group. Further studies of longer duration and increased sample size is required to obtain more clinical evidence as well as a sound basis for more regular use of this material.
Conclusion
The following conclusions were drawn from the present study:
There was a significant reduction in mean plaque and GI scores in both experimental and control sites. On comparison between the two groups there was no statistically significant difference observed. PerioGlas® treated sites showed increased reduction in probing depth when compared to controls. Gain in CAL in both groups was non-significant. There was a slight amount of GR that was observed in both groups. Radiographic assessment showed greater DF at the experimental site compared to control site indicating the efficacy of graft material. PerioGlas® bone graft was well tolerated by human tissues.
However, the study has certain limitations. Limited number of patients in the present study may have contributed to the lack of any detectable significance between the two groups. Longer follow up is necessary to study the efficiency of the material to obtain a predictable outcome in the treatment of osseous defects. Surgical reentry and histologic observation was not performed in the study which helps to better evaluate the regenerative potential of the material. The measurement of osseous fill in the current study was more difficult to compare to previous studies because of difference in the method of measurements and as most of the studies used reentry measurements.
Footnotes
Source of Support: Nil
Conflict of Interest: None
Contributor Information
Neelathil Lisa Chacko, Department of Periodontology, SMBT Dental College and Hospital, Amrutnagar, Sangamner, Ahmednagar, Maharashtra, India.
Sathish Abraham, Department of Conservative Dentistry and Endodontics, SMBT Dental College and Hospital, Amrutnagar, Sangamner, Ahmednagar, Maharashtra, India.
H N Shama Rao, Department of Periodontology, MS Ramaiah Dental College and Hospital, Bangalore, Karnataka, India.
N Sridhar, Department of Periodontology, RV Dental College and Hospital, Bangalore, Karnataka, India.
Ninad Moon, Department of Periodontology, RKDF Dental College, Bhopal, Madhya Pradesh, India.
Dhananjay H Barde, Department of Oral and Maxillofacial Surgery, SMBT Dental College and Hospital, Amrutnagar, Ahmednagar, Maharashtra, India.
References
- 1.SS Socransky, AD Haffajee. The nature of periodontal diseases. Ann Periodontol. 1997;2:3–10. doi: 10.1902/annals.1997.2.1.3. [DOI] [PubMed] [Google Scholar]
- 2.S Garrett. Periodontal regeneration around natural teeth. Ann Periodontol. 1996;1:621–666. doi: 10.1902/annals.1996.1.1.621. [DOI] [PubMed] [Google Scholar]
- 3.AH Melcher. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–260. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 4.JS Zamet, UR Darbar, GS Griffiths, JS Bulman, U Brägger, W Bürgin, et al. Particulate bioglass as a grafting material in the treatment of periodontal intrabony defects. J Clin Periodontol. 1997;24:410–418. doi: 10.1111/j.1600-051x.1997.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 5.EJ Schepers, P Ducheyne, L Barbier, S Schepers. Bioactive glass particles of narrow size range: A new material for the repair of bone defects. Implant Dent. 1993;2(3):151–156. doi: 10.1097/00008505-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 6.H Oonishi, S Kushitani, H Iwaki, K Saka, H Ono, A Tamura, et al. Comparative Bone Formation in Several Kinds of Bioceramic Granules. 8th International Symposium on Ceramics in Medicine. 1995 [Google Scholar]
- 7.SB Low, CJ King, J Krieger. An evaluation of bioactive ceramic in the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent. 1997;17:358–367. [PubMed] [Google Scholar]
- 8.T Turunen, J Peltola, H Helenius, A Yli-Urpo, RP Happonen. Bioactive glass and calcium carbonate granules as filler material around titanium and bioactive glass implants in the medullar space of the rabbit tibia. Clin Oral Implants Res. 1997;8:96–102. doi: 10.1034/j.1600-0501.1997.080204.x. [DOI] [PubMed] [Google Scholar]
- 9.H Oonishi, LL Hench, J Wilson, F Sugihara, E Tsuji, S Kushitani, et al. Comparative bone growth behavior in granules of bioceramic materials of various sizes. J Biomed Mater Res. 1999;44:31–43. doi: 10.1002/(sici)1097-4636(199901)44:1<31::aid-jbm4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Product Profile of PerioGlas®. Available from: http://www.novabone.com /NB/perioglas.html [Google Scholar]
- 11.N Price, SP Bendall, C Frondoza, RH Jinnah, DS Hungerford. Human osteoblast-like cells (MG63) proliferate on a bioactive glass surface. J Biomed Mater Res. 1997;37:394–400. doi: 10.1002/(sici)1097-4636(19971205)37:3<394::aid-jbm10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 12.UE Pazzaglia, C Gabbi, B Locardi, A Di Nucci, G Zatti, P Cherubino. Study of the osteoconductive properties of bioactive glass fibers. J Biomed Mater Res. 1989;23(11):1289–1297. doi: 10.1002/jbm.820231106. [DOI] [PubMed] [Google Scholar]
- 13.Y Fujishiro, LL Hench, H Oonishi. Quantitative rates of in vivo bone generation for Bioglass and hydroxyapatite particles as bone graft substitute. J Mater Sci Mater Med. 1997;8:649–652. doi: 10.1023/a:1018527621356. [DOI] [PubMed] [Google Scholar]
- 14.JP Zhong, GP LaTorre, LL Hench. The kinetics of bioactive ceramics part VII: Binding of collagen to hydroxyapatite and bioactive glass. Bioceramics. 1994;7:62–66. [Google Scholar]
- 15.DC Greenspan, JP Zhong, GP LaTorre. The evaluation of surface structure of bioactive glasses. In vitro study. Bioceramics. 2000;7:59–61. [Google Scholar]
- 16.LL Hench, JW Hench, DC Greenspan. Bioglass®: A short history and bibliography. J Aust Ceram Soc. 2004;1(40):1–42. [Google Scholar]
- 17.C Knabe, M Stiller, G Berger, D Reif, R Gildenhaar, CR Howlett, et al. The effect of bioactive glass ceramics on the expression of bone-related genes and proteins in vitro. Clin Oral Implants Res. 2005;16(1):119–127. doi: 10.1111/j.1600-0501.2004.01066.x. [DOI] [PubMed] [Google Scholar]
- 18.M Bosetti, L Zanardi, L Hench, M Cannas. Type I collagen production by osteoblast-like cells cultured in contact with different bioactive glasses. J Biomed Mater Res A. 2003;64(1):189–195. doi: 10.1002/jbm.a.10415. [DOI] [PubMed] [Google Scholar]
- 19.IA Silver, J Deas, M Erecińska. Interactions of bioactive glasses with osteoblasts in vitro: Effects of 45S5 Bioglass, and 58S and 77S bioactive glasses on metabolism, intracellular ion concentrations and cell viability. Biomaterials. 2001;22(2):175–185. doi: 10.1016/s0142-9612(00)00173-3. [DOI] [PubMed] [Google Scholar]
- 20.R Mengel, M Soffner, L Flores-de-Jacoby. Bioabsorbable membrane and bioactive glass in the treatment of intrabony defects in patients with generalized aggressive periodontitis: Results of a 12-month clinical and radiological study. J Periodontol. 2003;74:899–908. doi: 10.1902/jop.2003.74.6.899. [DOI] [PubMed] [Google Scholar]
- 21.JH Villaca, AB Novaes, Jr., SL Souza, M Taba, Jr., GO Molina, TL Carvalho. Bioactive glass efficacy in the periodontal healing of intrabony defects in monkeys. Braz Dent J. 2005;16(1):67–74. doi: 10.1590/s0103-64402005000100012. [DOI] [PubMed] [Google Scholar]
- 22.P Stoor, E Soderling, JI Salonen. Antibacterial effects of a bioactive glass paste on oral microorganisms. Acta Odontol Scand. 1998;56(3):161–165. doi: 10.1080/000163598422901. [DOI] [PubMed] [Google Scholar]
- 23.I Allan, H Newman, M Wilson. Particulate Bioglass reduces the viability of bacterial biofilms formed on its surface in an in vitro model. Clin Oral Implants Res. 2002;13:53–58. doi: 10.1034/j.1600-0501.2002.130106.x. [DOI] [PubMed] [Google Scholar]
- 24.SJ Froum, MA Weinberg, D Tarnow. Comparison of bioactive glass synthetic bone graft particles and open debridement in the treatment of human periodontal defects. A clinical study. J Periodontol. 1998;69:698–709. doi: 10.1902/jop.1998.69.6.698. [DOI] [PubMed] [Google Scholar]
- 25.JS Park, JJ Suh, SH Choi, IS Moon, KS Cho, CK Kim, et al. Effects of pretreatment clinical parameters on bioactive glass implantation in intrabony periodontal defects. J Periodontol. 2001;72:730–740. doi: 10.1902/jop.2001.72.6.730. [DOI] [PubMed] [Google Scholar]
- 26.MM Ong, RM Eber, MI Korsnes, RL MacNeil, Glickman GN, Y Shyr, et al. Evaluation of a bioactive glass alloplast in treating periodontal intrabony defects. J Periodontol. 1998;69:1346–1354. doi: 10.1902/jop.1998.69.12.1346. [DOI] [PubMed] [Google Scholar]
- 27.K Almas. Imaging in periodontology.An update. Indian J Dent Res. 1995;6:47–53. [PubMed] [Google Scholar]
- 28.S Karatzas, A Zavras, D Greenspan, S Amar. Histologic observations of periodontal wound healing after treatment with PerioGlas in nonhuman primates. Int J Periodontics Restorative Dent. 1999;19:489–499. [PubMed] [Google Scholar]