Abstract
Background
Varenicline was developed to aid smoking cessation by reducing smoking reinforcement. The present study tests this reinforcement-reduction hypothesis among smokers preparing to quit.
Method
After a one-week baseline, treatment-seeking smokers were randomized to receive three weeks of varenicline or placebo (Weeks 2-4). During each of the four weeks of the study, smokers completed a hypothetical cigarette purchase task (CPT) via handheld device in their natural environment. Behavioral economic measures of simulated smoking if cigarettes were free (demand intensity), sensitivity of consumption to increasing price (elasticity), and price at which purchases would drop to 0 (breakpoint) were estimated.
Results
Exponential demand equations fit the purchase task data well across subjects and time. As predicted, demand intensity decreased and sensitivity to price (elasticity) increased over time. However, changes in demand intensity did not differ by treatment group. Contrary to our hypothesis that varenicline would increase sensitivity to price, the placebo group tended to become more elastic in their purchases during Weeks 2 and 3; the groups did not differ in elasticity at Week 4. Breakpoint did not vary by group, time, or their interaction.
Conclusion
Simulated smoking demand can be validly assessed in the natural environment of treatment-seeking smokers. Simulated demand indices of smoking reinforcement diminished as smokers approached their target quit date. However, there was no evidence that varenicline facilitated these changes over a three week period, leaving open the mechanisms by which varenicline reduces smoking rate prior to cessation and improves long-term abstinence.
Keywords: varenicline, behavioral economics, smoking reinforcement, smoking reward, cigarette purchase task
1. Introduction
Varenicline is an α4β2 nicotinic acetylcholine receptor (nAChR) partial agonist that was designed to attenuate the reinforcing effects of smoking. Consistent with a reinforcement-reduction mechanism, pre-clinical work has documented that varenicline decreases the degree to which rats will work (bar press) to obtain nicotine (Coe et al., 2005; Levin et al., 2012; Rollema et al., 2007).
In humans, several approaches have been taken to test the reinforcement-reduction hypothesis. Retrospective data from clinical trials suggest that varenicline decreases smoking satisfaction during post-quit lapses (for review see Cahill et al., 2012), and lab studies have reported a similar effect on subjective smoking satisfaction (Brandon et al., 2011; Patterson et al., 2009). In terms of smoking behavior, varenicline reduces the number of cigarettes smoked per day (and corresponding biochemical indices of smoking) in both non-treatment-seeking smokers (e.g., Ashare et al., 2012; Fucito et al. 2011; Poling et al., 2010) and in two small randomized clinical trials (Hajek et al., 2011; Hawk et al., 2012). Interestingly, these studies demonstrate that the effect of varenicline on smoking rate becomes more pronounced over several weeks. Although varenicline-related decrements in smoking rate are consistent with a reinforcement-reduction (i.e., extinction) mechanism, conclusions about reinforcement would be strengthened by evidence from paradigms that more closely parallel the operant tasks employed in pre-clinical operant work.
In a typical operant lab task with humans, smokers must make repeated responses (e.g., mouse clicks) to earn cigarette puffs (Donny, Houtsmuller, & Stitzer, 2007; McClure, Vandrey, Johnson, & Stitzer, 2013; Perkins, Epstein, Grobe, & Fonte, 1994; Perkins, Grobe, & Fonte, 1997). A progressive ratio task increases the cost (number of responses required) of each successive puff (e.g., from 4 clicks for the first puff to 512 clicks for the eighth puff). These paradigms yield measures of reinforcing value that include the total number of responses, puffs earned, and breakpoint, which is defined as the response requirement at which the person will no longer respond to earn puffs. Using a progressive ratio task, McClure et al. (2013) found that the reinforcing value of smoking decreased over a one-week period preceding a practice quit attempt, but varenicline did not result in a greater decrease than did placebo. McClure et al. suggested that the need to engage in a practice quit attempt beginning the next day may have artificially suppressed operant responding in both groups, obscuring any effect of varenicline.
Complementing traditional behavioral studies of reinforcement, behavioral economic studies examine consumption as a function of price (for review see Bickel and Madden, 1999a; Bickel and Madden, 1999b; Heinz, Lilje, Kassel, & de Wit, 2012). Real-world behavioral economics can be extended to simulation paradigms in which participants indicate how much of a commodity they would purchase under varying price conditions (see Jacobs & Bickel, 1999). Mackillop et al.'s (2008) cigarette purchase task (CPT) takes this approach, asking participants to indicate the number of cigarettes they would purchase and consume in a 24-hour period across prices ranging from “free” to US $1120 per cigarette. The CPT data are used to estimate three parameters of a smoking demand curve (i.e., the consumption-price function; for review see Hursh & Silberberg, 2008). Demand intensity (Q0), reflects the number of cigarettes “purchased” at zero price (i.e., “free”). Demand elasticity (α) quantifies the slope of the cigarette demand curve, or the sensitivity of consumption as a function of price (steeper slope = greater elasticity = less reinforcement). The reinforcement-reduction hypothesis predicts that varenicline will make cigarette purchases more sensitive to price as reflected in decreased demand intensity, increased demand elasticity, and/or a lower breakpoint.
The CPT has been increasingly used to examine smoking reinforcement (e.g., Bidwell et al., 2012; MacKillop et al., 2008; Madden & Kalman, 2010) to address questions ranging from reliability of the CPT (Few et al., 2011) to evaluating the impact of changes in tobacco control policy (MacKillop et al., 2012; O'Connor et al., 2012). In a recent clinical trial, Madden and Kalman (2010) found that greater increases in elasticity during the week prior to a quit attempt were associated with greater success in quitting. These data broadly support the predictive validity of the CPT; however, bupropion had no effect on CPT reinforcement parameters.
In McClure et al. (2013), participants completed both an operant paradigm (described above) and the CPT before and after one week of varenicline or placebo. In support of a reinforcement-reduction hypothesis, McClure et al. reported that elasticity was greater in the varenicline group compared to the placebo group at the second visit. However, the analytic framework in that paper differed from most other CPT studies by examining aggregate group-level curves (rather than individual curve-fitting values), an approach that also precluded a formal test of the group x time interaction and its effect size (E. McClure, personal communication, April 14, 2014). Thus, firm conclusions about the impact of varenicline on smoking reinforcement remain surprisingly tentative.
Informed by human operant laboratory paradigms, and guided by an interest in the application of ecological momentary assessment methodology to examine the effect of varenicline on smoking reinforcement, the current study analyzed behavioral economic reinforcement parameters derived from a hypothetical CPT, which was administered in the natural environments of treatment-seeking smokers preparing for a quit attempt.
1.1 The Present Study
The present study tested the reinforcement-reduction hypothesis for varenicline. Following a one-week baseline, treatment-seeking smokers were randomized to varenicline or placebo for three weeks prior to their target quit date (TQD). To enhance the ecological validity of the reinforcement data, the CPT was administered repeatedly across the four weeks via handheld device during morning assessments in smokers’ natural environments. We predicted that, relative to the placebo, varenicline would result in greater reductions in demand intensity and breakpoint, and a greater increase in demand elasticity (sensitivity to price).
2. Method
2.1 Participants
Participants were 60 adult treatment-seeking smokers enrolled in a randomized, two-group, double blind, placebo-controlled trial (see Hawk et al., 2012), conducted from March 2009 through April 2010. Participants that expressed a desire to quit smoking were recruited through local newspapers and flyers posted in the community that advertised a quit smoking study, and were screened by telephone to determine eligibility. Inclusion criteria included age 18-65 years, smoking at least 15 cigarettes per day (CPD) during the past year, and agreeing to refrain from using additional smoking cessation treatments (i.e., NRT) during the study. Exclusion criteria included self-reported serious medical condition(s) (e.g., diabetes, renal impairment, uncontrolled hypertension); current use of other tobacco products or smoking cessation aids; pregnancy or plans to become pregnant; depression requiring treatment in the past year; history of panic disorder, bipolar disorder, or psychosis; and a history of alcohol or substance abuse in the past year.
2.2 Study Procedures
The Roswell Park Cancer Institute (RPCI) Institutional Review Board approved all study procedures.
2.2.1 Clinic Visits
Study visits were conducted in an outpatient setting outside the main hospital. After providing informed consent, participants attended the baseline visit during which assessments included expired air carbon monoxide (CO), demographic information, smoking history, the Fagerström test of nicotine dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), vital signs, height, and weight, and urine pregnancy tests for females of childbearing potential.
At this visit, eligible participants were provided with a Palm Tungsten E2 (Palm Inc., Sunnyvale, CA) personal digital assistant (PDA). A research assistant trained each participant on how to use and complete daily measures on the PDA. Following the baseline visit, participants completed daily morning assessments on the PDA. Daily morning assessments required participants to record the number of cigarettes smoked the day prior, indicate if they had smoked upon waking, and record the time they woke up (see Gass, Wray, Hawk, Mahoney, & Tiffany, 2012 for details; Gass et al. focus on daily assessments of tonic and cue-specific craving).
Subsequent visits included visits at the end of the Week 1 (baseline week) and Weeks 2 and 4, as well as later visits outside the scope of the present report (the TQD was at the end of Week 5; see Hawk et al., 2012). At each visit, assessments included side effects, pill counts, vital signs, and expired-breath CO measurements. Participants also completed weekly self-report questionnaires (e.g., craving, withdrawal, side-effects checklist) and received new supplies of study medication and brief behavioral counseling.
PDA data were downloaded at each clinic visit (end of Week 1 baseline, Week 2, and Week 4) and reviewed for compliance to allow for remuneration ($20-$64 based on adherence) during each visit. To maximize compliance, participants were required to complete at least three morning assessments per week to remain in the study.
2.2.2 Power and Randomization
The sample size of 30 per group was chosen to provide power of 0.8, with two-tailed α= .05, to detect moderate-to-large group differences (~d = 0.7) in changes in smoking behavior and reinforcement (i.e., Group x Time interactions). A study statistician provided the research pharmacist with a randomization scheme designating small-block (2:2) randomization within gender.
2.2.3 Medication
Pfizer provided identical-looking varenicline and placebo. Participants were dispensed a 1-week supply of study medication (varenicline or placebo) at the end of the Week 1 (baseline week) and instructed on its use (0.5mg daily × 3 days, 0.5mg twice daily × 4 days, then 1.0mg twice daily, beginning on day 8); a 2-week supply was provided at the end of Week 2. At each clinic visit, participants returned any unused pills. Pill counts suggested that adherence was excellent across the 3-week period, with no differences between placebo and varenicline groups (means [SDs] = 97.5% [7.2%] and 98.3% [6.9%], respectively), F <1.
Participants and all study personnel, except the statistician and research pharmacist, were blind to group membership. However, assessment of participant guesses about treatment condition at the end of Week 4 suggested partial unblinding: the percentage of participants guessing they were taking varenicline was greater in the varenicline group (76%) compared to the placebo group (44%), p=.02.
2.2.4 Cigarette Purchase Task
The hypothetical CPT was embedded within the daily morning assessment and was administered after participants recorded the time they awoke, the number of cigarettes smoked the day prior, and indicated whether they had smoked since initiating the morning assessment. Participants were instructed to complete the morning assessment before consuming their first cigarette of the day. As illustrated in Table 1, each PDA was programmed to administer the hypothetical CPT on two randomly selected days during each week (participants received one additional CPT during Week 3 or Week 4; this prompt was randomly distributed across participants). At least one day elapsed between each CPT assessment.
Table 1.
Hypothetical CPT Administrations By Study Phase and Week
| Study Phase | Baseline | Drug Manipulation |
||
|---|---|---|---|---|
| Study Week | Week 1 | Week 2 | Week 3 | Week 4 |
| CPT Assessments | 2 | 2 | 2.5* | 2.5* |
| Varenicline Group | - | V | V | V |
| Placebo Group | - | P | P | P |
CPT= Cigarette Purchase Task; V=Varenicline, P=Placebo
5 CPTs were spread across Weeks 3 and 4. Participants either completed 2 CPTs during Week 3 and 3 during Week 4, or 3 CPTs during Week 3 and 2 during Week 4.
Instructions were modeled after MacKillop et al. (2008). Participants used a stylus pen to indicate how many cigarettes they would purchase across nineteen ascending prices on the PDA: $0.00 (Free), $0.01, $0.05, $0.13, $0.25, $0.50, $1, $2, $3, $4, $5, $6, $11, $35, $70, $140, $280, $560, and $1,120.
2.3 Data Reduction1
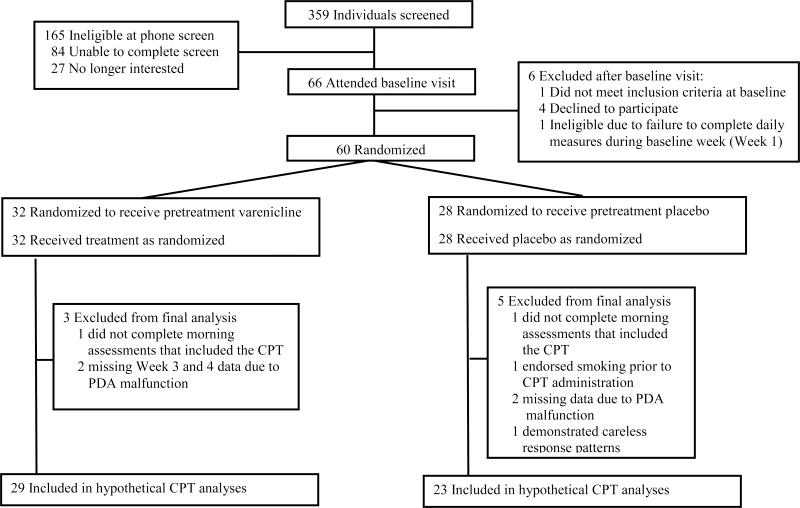
Hypothetical CPT data from each participant were examined individually to ensure protocol adherence. Data for individual participants were included if the following criteria were met: 1) smoking abstinence was endorsed on the PDA the morning of CPT administration, and 2) the participant completed at least one CPT assessment during the baseline week and each week of the three-week drug manipulation phase. Data from eight participants (5 placebo) were excluded due to missing data as shown in Figure 1, resulting in a final sample of 52 participants (n=29 varenicline) with complete data. Instances in which participants indicated they would purchase a cigarette after previously indicating zero purchases at two lower prices were replaced with a value of zero (n=3 Varenicline, n=4 Placebo).
Figure 1.
Participant Disposition
2.4 Data Analytic Plan
CPT data were aggregated within week (Week 1, Week 2, Week 3, Week 4) for each subject and analyzed following the equation from Hursh and Silberberg (2008):
Within the exponential demand equation, Q refers to the number of cigarettes smoked per day, expressed in logarithmic units. Demand intensity (Q0) represents the number of cigarettes purchased at zero price (i.e., free). Demand elasticity (α), represents the slope of demand curve or sensitivity of consumption to price. Ps represents price, and is standardized in order to analyze α while accounting for differences in price (Q0 x C; Hursh & Silberberg, 2008). The k parameter is a rate constant that reflects the range of cigarettes smoked (Q0-Q) in log units. The k parameter was derived from Hursh's exponential model in GraphPad Prism 6.0 software (La Jolla, California), and was set to 2.948 based on model fit of mean consumption across all participants at baseline (Week 1). The first instance of zero consumption was replaced with an arbitrary nonzero value (0.009), as the log of zero is undefined (Jacobs & Bickel, 1999; MacKillop, et al., 2008). Demand intensity (Q0) and demand elasticity (α) were derived from the exponential demand equation. Breakpoint was computed as the first price that completely suppressed consumption. Inspection of breakpoint data at baseline (Week 1) and the drug manipulation phase (Week 2, Week 3, Week 4) indicated that a price of $11 per cigarette completely suppressed consumption for the majority of participants (> 79% of the sample). To minimize outliers, individual breakpoint values that exceeded $11 were coded as one unit above $11 (i.e., $12).
Demand intensity, demand elasticity, and breakpoint were analyzed with separate 2 Group (varenicline, placebo) × 4 Week repeated measures ANOVA. Orthogonal polynomial contrasts were used in all analyses to evaluate linear and quadratic trends. Sex was included in preliminary models but interactions involving sex were all non-significant; therefore, sex was removed from the final data analyses.
3. Results
3.1 Participant Demographics and Smoking Variables
Figure 1 displays the flow of participants, and Table 2 presents participant demographics and smoking variables for the final sample. There were no significant group differences on any demographic or smoking variables.2
Table 2.
Participant Demographics
| Varenicline Group | Placebo Group | p Value | |
|---|---|---|---|
| Female:Male (n) | 17:12 | 15:8 | 0.63 |
| Age (in years) | 48.3 (8.4) | 48.3 (10.9) | 0.99 |
| Minority (%) | 6.9 % | 13.0% | 0.46 |
| Income (% below 40k) | 41.4% | 30.4% | 0.42 |
| FTND | 5.5 (2.0) | 4.9 (2.0) | 0.32 |
| Years Smoking | 27.3 (13) | 26.6 (13) | 0.83 |
| # Quit Attempts | 6.0 (4.8) | 6.0 (10.5) | 0.99 |
| CPD (Baseline) | 17.7 (4.8) | 17.4 (4.8) | 0.83 |
Values are mean and standard deviations (SD), except where noted. p values are from independent samples t tests (df1,50) and chi-square tests (df=1) FTND=Fagerstrom Test for Nicotine Dependence, CPD=Cigarettes per Day, Income was assessed in 8 bins ranging from “Less than $10,00” to “$100,000 or mor”.
3.2 Hypothetical CPT Completion Rates
The total number of completed CPTs across all four weeks (maximum number possible=9) was high (M=8.3, S.D.=1.1), and did not significantly vary between groups t(50)=-0.53, p=0.60.
3.3 Hypothetical CPT Reinforcement Parameters
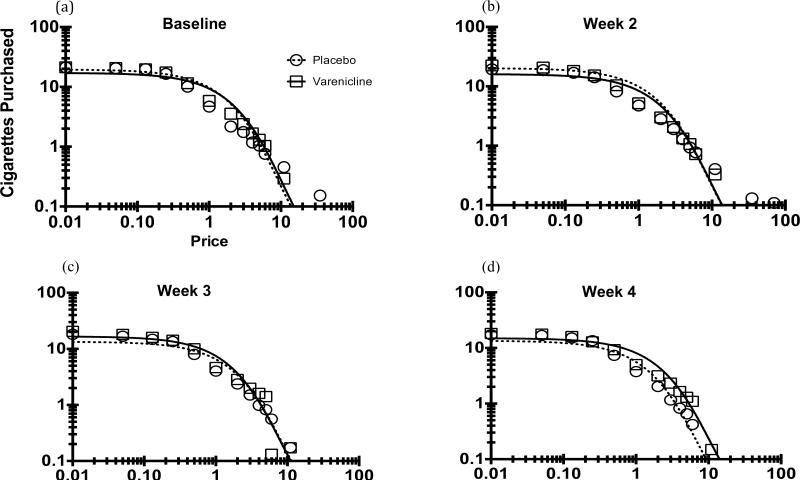
Figure 2 illustrates CPT demand curves (mean number of hypothetical cigarettes) at baseline and the three-week drug manipulation phase. Hursh's exponential demand equation provided excellent fit to mean CPT consumption across participants for all four weeks (R2s=.89 to .99).
Figure 2.
Cigarette purchase demand curves for placebo (circles) and varenicline (squares) groups at baseline (panel a) and each week of the drug manipulation (panels b-d). Data are plotted in log units: x-axis is log (US dollars); y-axis is log (cigarettes purchased)
Demand intensity (Q0), the parameter reflecting purchases when cigarettes are free, did not significantly differ as a function of group at baseline, F(1,50) < 1, p=0.97, d=.01, (Figure 3, panel a). Although demand intensity decreased in linear fashion over time, Week linear F(1,50)=17.03, p < 0.001, d=.59, this change in intensity was not greater among the varenicline group, Group x Week linear and quadratic Fs < 2.1, p's > 0.15, d's=0.15 and 0.41, respectively.
Figure 3.
Mean demand intensity (panel a), demand elasticity (panel b), and breakpoint (panel c). Bars represent standard error of the mean (SEM).
Demand elasticity (α), or sensitivity of consumption to increasing price, did not differ between groups at baseline, F(1,50)=1.62, p=.21, d=.33. As illustrated in Figure 3 (panel b), demand elasticity increased across weeks; Week linear F(1,50)=9.37, p=0.004, d=.41. Contrary to the reinforcement-reduction hypothesis, demand elasticity tended to increase more quickly across weeks among the placebo group compared to the varenicline group, resulting in a marginally significant Group x Week quadratic interaction F(1,50)=2.82, p=0.10, d=.41 (linear F < 1.23, p=.27, d=.29); pairwise follow-up tests were not significant at any week – p's =.06, .09, and .12 at Weeks 2, 3, and 4, respectively.
Breakpoint did not differ between groups at baseline, F(1,50) < 1, p=0.41, d=.23, and breakpoint did not vary as a function of group, week, or their interaction, all Fs < 1.53, ps > 0.20 (see Figure 3, panel c).
4. Discussion
To evaluate the hypothesis that varenicline reduces smoking reinforcement, the present study examined the influence of three weeks of varenicline on responses to a hypothetical cigarette purchase task (CPT) administered in the natural environment of treatment-seeking smokers preparing to make a quit attempt. Demand intensity, elasticity, and breakpoint reinforcement parameters were estimated from the exponential demand equation of Hursh and Silberberg (2008) for a baseline week and each of the three weeks of study medication. Across the four-week period, there were expected decreases in demand intensity (i.e., the number of hypothetical cigarettes an individual would purchase and consume at zero cost) and increases in demand elasticity (sensitivity of consumption as a function of price). These reductions in smoking reinforcement, however, were not greater in the varenicline group compared to the placebo group. Indeed, there was a trend in the opposite direction for elasticity.
The absence of a varenicline effect on demand for cigarettes when “free” (demand intensity) is consistent with prior work with both varenicline (McClure et al., 2013) and bupropion (Madden & Kalman, 2010). Although demand intensity may be of interest from a theoretical perspective, the complete absence of price constraint may render it insensitive as a measure of smoking reinforcement. In future work, it may be useful to examine hypothetical consumption at the price a smoker pays for their actual cigarettes. Because cigarette prices vary widely across brands and outlets (in our geographic area, each cigarette may cost from US $0.10 when purchased by the carton at a Native American reservation, to US $0.50 when purchased by the pack at a convenience store), we recommend future studies document each participant's typical cost per cigarette.
The demand elasticity parameter, which reflects the impact of increasing price on consumption, may better parallel traditional progressive-ratio operant measures. Our finding that varenicline did not result in greater elasticity is not likely due to low power; the group means were actually in the opposite direction. In contrast, McClure et al. (2013) found greater elasticity after one week of varenicline than after one week of placebo. Although there may be many reasons for the discrepancy across studies (e.g. >50% attrition in McClure et al.; presentation of the CPT in a less controlled but more naturalistic environment in the present study), it is also important to note that McClure et al. did not observe parallel effects of varenicline on their traditional progressive-ratio task measured concurrently with the CPT. Overall, these data provide only modest evidence for a reinforcement-reduction mechanism.
Data from the current study cannot resolve the disconnect between the CPT and operant paradigms, and the finding that varenicline reduces the rate of cigarette smoking and subjective satisfaction. Looking forward, perhaps smoking reinforcement may be best assessed in a theoretically rich and clinically meaningful way by developing hybrid paradigms that incorporate the practicality of hypothetical tasks with the ecological validity of real purchases. For example, future work might require participants to spend their own money on usual-brand cigarettes in a “laboratory store” in which prices systematically vary over time or across visits. Similarly, MacKillop and colleagues (2012) recently gave participants real money to spend on cigarettes that varied in cost (Mackillop, Brown, et al., 2012). A significant strength of this newer approach is the translation of cigarette purchases to subsequent smoking behavior.
Breakpoint (the price that completely suppressed consumption) in the present study was insensitive to time and medication. Similarly McClure et al. (2013) found no effect of varenicline on their behavioral task (they did not report breakpoint from their CPT). The insensitivity of breakpoint may be influenced by the wide range of prices examined, which typically covers from $0 to $1,120 per cigarette (MacKillop, et al., 2008; MacKillop & Tidey, 2011; Madden & Kalman, 2010; McClure, et al., 2013; Murphy, et al., 2011; O'Connor, et al., 2012). After $6, prices escalate rapidly, leaving the task insensitive to breakpoints between $6 and $11 and between $11 and $35. As can be seen in Figure 3 (panel c), breakpoint values tended to cluster between $5 and $6, perhaps as an artifact of the choices available. Recent work has begun to address this issue by including smaller price options and price intervals (see Mackillop, Few, et al., 2012).
4.1 Strengths and Limitations
The present study has several strengths, which include the use of a randomized, placebo-controlled, between-subjects design. The three-week duration of the medication manipulation provided ample time for a varenicline effect to emerge (see e.g. Ashare et al., 2012; Hajek et al., 2011; Hawk et al., 2012).
Other study features reflect tradeoffs between internal and external validity. Study participants were treatment-seeking smokers; although studying medication effects during actual quit attempts can raise several concerns, these would seem to be offset by the benefits of studying the drug in the intended context. The hypothetical CPT was repeatedly assessed in smokers’ natural environments, using ecological momentary assessment (EMA; for review see Shiffman, Stone, & Hufford, 2008; Stone & Shiffman, 1994). Although this increases the external validity of the study, the tradeoff is that we cannot be certain that smokers complied with our instructions to complete the measure prior to the first cigarette of the day. Demand characteristics are a concern for both traditional operant tasks and the CPT (e.g., McClure et al., 2013). However, this does not appear to be a major concern in the present study. For example, we would predict that demand characteristics would be the greatest during the week closest to the target quit date (Week 4). Even during Week 4, however, 96% of participants had a breakpoint of $0.50 or more.
4.2 Conclusions
Three weeks of pre-quit varenicline had no reinforcement-reducing effect on behavioral economic smoking parameters assessed among treatment-seeking smokers in their natural environment. However, smoking reinforcement did decrease over time, which may hold important clinical implications and clinical utility (e.g., Heinz et al., 2012; Madden and Kalman, 2010). Moreover, results of the present study lead to several suggestions for future work examining smoking reinforcement and its role in the efficacy of varenicline and other cessation approaches.
Highlights.
> We examined the effect of varenicline using behavioral economic demand parameters.
> Treatment-seeking smokers were randomized to receive varenicline or placebo
> A hypothetical cigarette purchase task was administered in the natural environment.
> Varenicline did not reduce behavioral economic indices of smoking reinforcement.
Acknowledgements
This research was completed in partial fulfillment of the requirements for the doctoral degree of NJS under the supervision of LWH. Portions of this research were presented at the 2013 Annual Meeting of the Society for Research on Nicotine and Tobacco. The authors wish to thank Julie C. Gass and Sarah C. Mye for assistance with PDA data reduction.
Role of Funding Sources
This manuscript was funded in part by a 2008 Global Research Award for Nicotine Dependence (GRAND), an independent, investigator-initiated research program awarded to Martin C. Mahoney, M.D., Ph.D., sponsored by Pfizer, and a grant from the National Institute on Drug Abuse (R21 DA019653) awarded to Stephen T. Tiffany, Ph.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Contributors
Larry W. Hawk, Jr. and Martin C. Mahoney designed the study. Nicolas J. Schlienz, Larry W. Hawk, Jr., Stephen T. Tiffany, Richard J. O'Connor, and Martin C. Mahoney contributed to the writing of the manuscript. Nicolas J. Schlienz and Larry W. Hawk, Jr. conducted all statistical analyses. All authors contributed to and have approved the final manuscript.
The second (of two) Week 1 CPT assessments was used for one participant as the data for the first administration was the only instance across all participant data where the individual made purchases at each price point.
Participant income (assessed in $15,000 increments) was unrelated to demand intensity (Q0), demand elasticity (α), and breakpoint (r's = .04 to .17, all p's > .22).
Conflict of Interest
Martin C. Mahoney has served on the Speaker's Bureau for Pfizer and as the medical director of the New York State Smokers Quit Line.
References
- Ashare RL, Tang KZ, Mesaros AC, Blair IA, Leone F, Strasser AA. Effects of 21 days of varenicline versus placebo on smoking behaviors and urges among non-treatment seeking smokers. Journal of Psychopharmacology. 2012;26(10):1383–1390. doi: 10.1177/0269881112449397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Madden GJ. The behavioral economics of smoking. In: Chaloupka FJ, editor. The economic analysis of substance use and abuse : an integration of econometric and behavioral economic research. University of Chicago Press; Chicago: 1999a. [Google Scholar]
- Bickel WK, Madden GJ. A comparison of measures of relative reinforcing efficacy and behavioral economics: cigarettes and money in smokers. Behavioural Pharmacology. 1999b;10(6-7):627–637. doi: 10.1097/00008877-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Bidwell LC, MacKillop J, Murphy JG, Tidey JW, Colby SM. Latent factor structure of a behavioral economic cigarette demand curve in adolescent smokers. Addictive Behaviors. 2012;37(11):1257–1263. doi: 10.1016/j.addbeh.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, et al. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews. 2012;(4) [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. Journal of Medicinal Chemistry. 2005;48(10):3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Few LR, Acker J, Murphy C, MacKillop J. Temporal stability of a cigarette purchase task. Nicotine & Tobacco Research. 2012;14(6):761–765. doi: 10.1093/ntr/ntr222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology. 2011;215(4):655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JC, Wray JM, Hawk LW, Mahoney MC, Tiffany ST. Impact of varenicline on cue-specific craving assessed in the natural environment among treatment-seeking smokers. Psychopharmacology. 2012;223(1):107–116. doi: 10.1007/s00213-012-2698-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, McRobbie HJ, Myers KE, Stapleton J, Dhanji AR. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Archives of Internal Medicine. 2011;171(8):770–777. doi: 10.1001/archinternmed.2011.138. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Jr., Ashare RL, Lohnes SF, Schlienz NJ, Rhodes JD, Tiffany ST, et al. The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clinical Pharmacology and Therapeutics. 2012;91(2):172–180. doi: 10.1038/clpt.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heinz AJ, Lilje TC, Kassel JD, de Wit H. Quantifying reinforcement value and demand for psychoactive substances in humans. Current Drug Abuse Reviews. 2012;5(4):257–272. doi: 10.2174/1874473711205040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychological Review. 2008;115(1):186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Jacobs EA, Bickel WK. Modeling drug consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Experimental and Clinical Psychopharmacology. 1999;7(4):412–426. doi: 10.1037//1064-1297.7.4.412. [DOI] [PubMed] [Google Scholar]
- Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Sved AF, Donny EC. Varenicline dose dependently enhances responding for nonpharmacological reinforcers and attenuates the reinforcement-enhancing effects of nicotine. Nicotine & Tobacco Research. 2012;14(3):299–305. doi: 10.1093/ntr/ntr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Brown CL, Stojek MK, Murphy CM, Sweet L, Niaura RS. Behavioral economic analysis of withdrawal- and cue-elicited craving for tobacco: An initial investigation. Nicotine & Tobacco Research. 2012;14(12):1426–1434. doi: 10.1093/ntr/nts006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Few LR, Murphy JG, Wier LM, Acker J, Murphy C, et al. High-resolution behavioral economic analysis of cigarette demand to inform tax policy. Addiction. 2012;107(12):2191–2200. doi: 10.1111/j.1360-0443.2012.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG, Ray LA, Eisenberg DT, Lisman SA, Lum JK, et al. Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Experimental and Clinical Psychopharmacology. 2008;16(1):57–65. doi: 10.1037/1064-1297.16.1.57. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology. 2011;216(1):91–99. doi: 10.1007/s00213-011-2185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Kalman D. Effects of bupropion on simulated demand for cigarettes and the subjective effects of smoking. Nicotine & Tobacco Research. 2010;12(4):416–422. doi: 10.1093/ntr/ntq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Vandrey RG, Johnson MW, Stitzer ML. Effects of varencline on abstinence and smoking reward following a programmed lapse. Nicotine & Tobacco Research. 2013;15(1):139–148. doi: 10.1093/ntr/nts101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Tidey JW, Brazil LA, Colby SM. Validity of a demand curve measure of nicotine reinforcement with adolescent smokers. Drug and Alcohol Dependence. 2011;113(2-3):207–214. doi: 10.1016/j.drugalcdep.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor RJ, Bansal-Travers M, Carter LP, Cummings KM. What would menthol smokers do if menthol in cigarettes were banned? Behavioral intentions and simulated demand. Addiction. 2012;107(7):1330–1338. doi: 10.1111/j.1360-0443.2012.03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Grobe J, Fonte C. Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacology, Biochemistry, and Behavior. 1994;47(1):107–112. doi: 10.1016/0091-3057(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biological Psychiatry. 2009;65(2):144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Grobe J, Fonte C. Influence of acute smoking exposure on the subsequent reinforcing value of smoking. Experimental and Clinical Psychopharmacology. 1997;5(3):277–285. doi: 10.1037//1064-1297.5.3.277. [DOI] [PubMed] [Google Scholar]
- Poling J, Rounsaville B, Gonsai K, Severino K, Sofuoglu M. The safety and efficacy of varenicline in cocaine using smokers maintained on methadone: A pilot study. The American Journal on Addictions. 2010;19(5):401–408. doi: 10.1111/j.1521-0391.2010.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. [In Vitro]. Neuropharmacology. 2007;52(3):985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman SS. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16(3):199–202. [Google Scholar]