Abstract
Throughout Africa, Peer Mentors who are women living with HIV (WLH) are supporting pregnant WLH at antenatal and primary healthcare clinics (McColl in BMJ 344:e1590, 2012). We evaluate a program using this intervention strategy at 1.5 months post-birth. In a cluster randomized controlled trial in KwaZulu-Natal, South Africa, eight clinics were randomized for their WLH to receive either: standard care (SC), based on national guidelines to prevent mother-to-child transmission (4 clinics; n = 656 WLH); or an enhanced intervention (EI; 4 clinics; n = 544 WLH). The EI consisted of four antenatal and four postnatal small group sessions led by Peer Mentors, in addition to SC. WLH were recruited during pregnancy and 70 % were reassessed at 1.5 months post-birth. EI's effect was ascertained on 16 measures of maternal and infant well-being using random effects regressions to control for clinic clustering. A binomial test for correlated outcomes evaluated EI's overall effectiveness. Among EI WLH reassessed, 87 % attended at least one intervention session (mean 4.1, SD 2.0). Significant overall benefits were found in EI compared to SC using the binomial test. However, it is important to note that EI WLH were significantly less likely to adhere to ARV during pregnancy compared to SC. Secondarily, compared to SC, EI WLH were more likely to ask partners to test for HIV, better protected their infants from HIV transmission, and were less likely to have depressed mood and stunted infants. Adherence to clinic intervention groups was low, yet, there were benefits for maternal and infant health at 1.5 months post-birth.
Keywords: HIV, Maternal health, Child health, PMTCT
Introduction
Effective HIV interventions are urgently needed for the 12 million women living with HIV (WLH) in Sub-Saharan Africa [2]. South Africa has 3.2 million WLH [3]. Each year 200,000 WLH are pregnant [2]. The KwaZulu-Natal (KZN) Province, the site for this study, has the highest prevalence of HIV in South Africa [4]. While the national HIV prevalence has stabilized around 11 % [4], 40–60 % of pregnant women in rural KZN are living with HIV [5]. This study evaluates one potentially efficacious intervention using a peer mentor strategy, a strategy that has been broadly supported by global donors [1].
Learning that one is HIV positive changes a woman's identity almost immediately [6]. She has a virus that influences not only her physical health, but all of her social and community relationships. In addition, she has many new tasks to perform: maintaining her own health, the health of her baby and her family. In the moments following HIV diagnoses, this study offered support from a Peer Mentor who was also a WLH. The Peer Mentor was a role model whose support [7] and presence reassured the newly diagnosed WLH that HIV-related challenges can be successfully met. The Mothers 2 Mothers Program [M2M] (http://www.m2m.org/) inspired this intervention. The M2M program is currently providing Peer Mentor WLH in 589 clinics in seven countries and has substantial donor support. This study examines if the key concept of the M2M program having a Peer Mentor who is HIV-sero-positive is robust and influences outcomes for the WLH.
Pregnant WLH face a series of challenges in both preventing transmission and maintaining positive health outcomes [8]. Rather than prevention of a single disease outcome, that is, infant HIV acquisition, a social ecological model was adopted and designed to address HIV-related challenges from multiple perspectives [9]. WLH must prevent transmission to both partners and infants. Knowing her own status, she must decide whether and to whom to disclose her HIV status and ask her partner to be tested for HIV and use condoms consistently. To prevent mother-to-child transmission (PMTCT), adherence is required to a series of tasks during pregnancy and the first 6 weeks of life: (1) testing for HIV and receiving the test results; (2) adhering to antiretroviral medications (ARV) from week 28 of pregnancy and during labor; and (3) ensuring that the infant receives nevirapine (NVP) at birth and cotrimoxazole following birth [10, 11]. WLH are also challenged to address their own health and mental health needs: they must get tested for CD4 and tuberculosis (TB); adhere to ARV if they have a low CD4 count; cope with symptoms and feelings of depression and anxiety; and cope with potential stigma and intimate partner violence [12, 13]. For the long-term benefit of the family, not only the newborn infant, maternal adherence to each of these tasks is critical. Much more attention has been focused on technical aspects of PMTCT adherence, compared to concurrent interpersonal and social challenges. This study focuses on the multiple tasks and relationship challenges facing pregnant WLH.
The need for effective intervention is great, even though clinics in South Africa have implemented PMTCT services [14] for about 10 years. WLH's adherence to these tasks is variable, as is the case in the rest of Africa [15]. In KZN, HIV testing during pregnancy is 71 % [16]. Of the women testing for HIV, about a third do not receive their test results [4, 17]. In 2007, only 76 % of pregnant WLH who received their test results also received NVP during labor. Only 57 % of their infants received NVP at birth [2, 17]. Following childbirth, using a single feeding method is critical for the first 6 months of an infant's life [3, 10, 11]; yet, mixed feeding is the most common feeding method used [18]. In South Africa, there are wide-scale beliefs that infants cannot survive on mothers’ breast milk alone [11], leading to low rates of breastfeeding and early supplementation with infant formula [19]. Public health officials aim to have adherence rates above 90 % at each step in this cascade in order to reduce HIV transmission [3].
PMTCT adherence is also likely to be impacted by the perceived HIV stigma and isolation of WLH [20] through their avoidance of disclosure to sexual partners and family, as well as failure to care for their own health and mental health [7]. These factors are potentially influenced by having a Peer Mentor, as is suggested by the broad diffusion of the M2M program.
Thus, an enhanced intervention (EI), labeled Masihambisane (we walk together in Zulu), was initiated to test the efficacy of Peer Mentor support of pregnant WLH in primary healthcare clinic programs in KZN, South Africa. We hypothesized that, compared to WLH receiving standard clinic care (the Standard Care (SC) condition), WLH at clinics in the EI condition would have improved maternal and infant well-being in the following domains: (1) prevention of HIV transmission to partners and infants; (2) infant health; (3) healthcare and health monitoring; (4) mental health; and (5) parenting tasks to ensure entitlements, including registering the child's birth and securing the child support grant. Given that we are examining outcomes in five domains, with multiple markers per domain, we utilized a binomial test for correlated outcomes as the primary evaluation measure after conducting a series of regression analyses [21]. At about 1.5 months, EI sessions were expected to be complete, and the follow-up assessment was a post-intervention analysis.
Methods
The Institutional Review Board of the University of California, Los Angeles (UCLA, G06-05-062), a Data Safety and Monitoring Board (DSMB; Drs. Mofeson, Couvadia, & Bryant), a local Community Advisory Board, and the Research Ethics Committee of the Human Sciences Research Council in South Africa (HSRC, REC 4/07/03/07) approved all study protocols [21]. The trial is registered at ClinicalTrials.gov # NCT00972699.
Setting, Clinic Selection and Matching, and Randomization
Eight clinics were selected based on proximity to the research office (within a radius of 70 km), the type of clinic (either a community health or primary healthcare clinic), the concurrent availability of antenatal and postnatal services at the same site, and an annual caseload of at least 300 pregnant women. Clinics were selected following a 6 month observation period, during which time patient flow was audited and the clinic staffs’ capacity to implement PMTCT procedures was standardized across clinics. This was implemented in cooperation with the provincial Department of Health. Based on the pilot data, four pairs of clinics were matched. Clinics were randomized within matched pairs by UCLA according to a simple randomization schedule. One EI clinic had two sites.
Sample Size Calculations
Power calculations were based on the randomization of clinics to either EI or SC, and assessments of WLH and their infants at least once between the ages of 6 weeks and 6 months post-birth. Power calculations were estimated in a two-step procedure. First, we calculated the necessary sample size N* to detect a significant difference between EI and SC on a single outcome using RMASS2 software [22]. Second, we multiplied N* by the variance inflation factor (VIF) to account for the clustering effect of randomization at the clinic level, where VIF = 1+ (m–1) × ICC, m is the average number of WLH per clinic, and ICC is the intra-cluster correlation coefficient. From other behavioral studies [23–25], we estimated ICC to be 0.01. The sample size goal was 1,200 WLH across eight clinics (150/clinic) to achieve 80 % power to detect an effect size of 0.25.
Recruitment, Retention, and Reassessment
Recruitment occurred from July 2008 to April 2010 (except for one EI clinic in which WLH could only be recruited for 6 months). All pregnant women in both EI and SC clinics learned of Masihambisane in the clinic waiting rooms and were informed again about the study by a nurse during their HIV testing interview. Figure 1 summarizes the movement of participants through the trial from recruitment to the baseline assessment and reassessment post-birth. We were not able to consistently monitor the flow of women within each clinic at each potential stage of engagement and assessment until about half of the women had been recruited, in March, 2009. Thus, data are available only for the final 602 WLH who were recruited. A more detailed figure with numbers of pregnant women entering clinics for the last half of recruitment of the baseline sample is available upon request. Among the eight clinics, recruitment rates of newly pregnant women ranged from 55 to 92 % per clinic; seven of the eight clinics recruited more than 74 % of eligible WLH. Overall, about 62 % of WLH were successfully recruited into the study. Stated reasons for not enrolling were transport difficulties, getting time off work, and inability to stay after clinic appointments due to family obligations.
Fig. 1.
Movement of participants through the trial at each assessment point for mothers in the standard care (SC) and the enhanced intervention (EI). 1Among mothers reassessed post-birth
Upon learning of their HIV+ serostatus, WLH gave voluntary informed consent to participate in Masihambisane and completed a baseline assessment interview lasting about one hour (n = 1,200 WLH total; EI: n = 544; SC: n = 656). Post-birth assessments were obtained from 70 % of participants when their infants were about 1.5 months old (range 1 day old to 14 months old), similar across conditions.
Clinic Conditions
SC
Each clinic, in both the SC and EI conditions, and all WLH in each clinic were offered the national PMTCT program. From enrolment through the first 1.5 months post-birth, SC for WLH included dual therapy for PMTCT [azidothymidine (AZT) and NVP], and referral to ARV for WLH with CD4 counts below 200 or WHO Stage 4 illness. A single feeding method was consistently recommended for the infant's first week of life. Tins of powdered milk were distributed at all delivery sites throughout the study.1
EI
We adapted the M2M program [21] based on a pilot study in Cape Town [26] and 6 months of piloting an adapted M2M program in KZN [21]. An eight-session intervention (four antenatal sessions, four postnatal sessions) was designed to support WLH through pregnancy and early motherhood. Peer Mentors were recruited from advertisements placed in the clinics, and WLH who were child-bearing and had good social skills were selected as Peer Mentors. The Peer Mentors were trained for about 2 months prior to implementation and were certified after being observed; in-person supervision was provided weekly.
After a woman tested as HIV seropositive, a nurse and then a Peer Mentor met with the pregnant WLH individually and invited her to the Masihambisane meetings. Prior to the first meeting, an assessment was conducted by a separate interviewing team.
The EI topics were: (1) destigmatizing HIV; (2) adhering to the PMTCT tasks; (3) establishing healthy daily routines; (4) using a single feeding method, encouraging breastfeeding, and abstaining from traditional medicines and gripe water; (5) obtaining a child grant for family economic support; (6) maintaining a strong social network and (7) encouraging couple HIV testing, disclosure of HIV serostatus, and regular condom use.
Fidelity to the intervention implementation was monitored by supervisors’ observations, reviewing attendance records and weekly small group supervision meetings.
Among the 377 EI WLH reassessed post-birth, 75 % attended at least one antenatal intervention session (mean 2.8 sessions, SD 1.1), 56 % attended at least one postnatal meeting (mean 2.5, SD 1.2), and 87 % attended any sessions (mean 4.1, SD 2.0).2 Only 5 % attended all 8 sessions.
Assessments and Outcome Measures
Female research assistants were trained to interview WLH at baseline and post-birth. Because interviewers were assigned by clinic, they were not blinded to condition. All interview responses and required clinic records were entered on mobile phones. Commercially available software (http://www.mobenzi.com/researcher/) allowed data to be uploaded to a secure central database after survey completion. The outcome measures assessed at the baseline (when available) and post-birth interview were:
HIV transmission-related behaviors, including PMTCT tasks: WLH self-reported disclosure of serostatus to sexual partners and requests for partner HIV testing. Few new mothers had reinitiated sex at the post-birth assessment, so condom use was not reported. Disclosure to friends was also reported. Adherence to the following PMTCT tasks was available at 1.5 months post-birth: (1) AZT from the 28th week of pregnancy or highly active anti-retroviral Therapy (HAART) if CD4 test results were less than 200; (2) AZT during labor or on HAART; (3) NVP during labor or on HAART; (4) infant NVP at birth; (5) infant AZT post-birth and (6) maintenance of one feeding method (either breastfeeding or formula) for the first week post-birth.
Infant health status post-birth was monitored by low birth weight (LBW, <2,500 g) as documented on the government-issued Road-to-Health card at birth, and weight-for-age, length/height-for-age, and weight-for-length z-scores calculated from post-birth weight and length measures based on WHO age- and sex-specific standards [27]. For weight, length/height, and weight-for-length, malnourishment was identified by a z-score below –2, and was defined as underweight, stunting, and wasting, respectively [28].
Maternal healthcare utilization included attendance at antenatal clinic visits.
Depression was assessed by the General Health Questionnaire (GHQ) (score ≥7 is depressed) [29].
Parenting tasks to ensure entitlements were registering the child's birth and applying and receiving the government child grant (R240/month, about 30 USD).
In addition to these outcomes, at the baseline assessment we monitored closely the alcohol, drug use, household items, social support and food insecurity. These are not targeted outcomes.
Data Analysis
Selection effects were examined between conditions at baseline and among those reassessed post-birth or not. To avoid multiple comparisons and measure EI's overall effect on well-being, our primary analysis of the impact of the intervention compared WLH in EI and SC using a binomial test of the number of significant effects favoring EI among 16 measures. For each measure, differences between conditions were tested at a one-sided upper-tail alpha = 0.025 using logistic random effects regressions adjusting for clinic clustering in SAS PROC GENMOD (version 9.2; SAS Institute Inc., Cary, North Carolina, USA). A one-tailed test was used because we initially hypothesized that the EI was superior to the SC. Models included an indicator variable for intervention status (1 = EI, 0 = SC). We can expect 16*0.025 = 0.4 significant tests (i.e., less than one of 16) on average if there are no differences between EI and SC. If outcomes are independent, the probability that there are two or fewer significant differences is 99.3 %, leading to a type 1 error of 0.007. However, outcomes are likely positively correlated, which does not affect the expected number of positive tests, but does affect the variance of the number of positive tests. To study the effects of global positive correlation among all outcomes on the number of positive tests assuming no intervention effect, we treated each of our 16 tests as a normal z-test (z-statistics were assumed to come from an equi-correlated multivariate normal distribution) and simulated 40,000 trials of the number of significant outcomes, for z-tests having mutual correlations rho for rho running from 0 to 0.9 in steps of 0.1. We declared significance for z >1.96. Simulations were performed in R (version 2.11.1).
From the results, using a decision rule of rejecting the null of no EI treatment effect given 3 or more significant tests of 16, the type 1 error will stay below 0.05 no matter what the outcomes’ correlations. We estimated the average absolute correlations among the outcomes using both Pearson (for “true dichotomies”, e.g. “Asked partner to test for HIV”) and tetrachoric correlations (for indicators created by dichotomizing continuous outcomes, e.g., height-for-age z-score ≥ –2), planning to use whichever method produced higher average absolute correlations.
Secondarily, we tested EI's impact on individual outcomes at a two-sided alpha = 0.05 using the regressions described above. We used the same models to estimate EI's effect on completing cumulative PMTCT tasks. We considered our secondary analyses to be exploratory and retained the model p values in lieu of a multiple-testing adjustment.
Results
Sample Characteristics
Table 1 summarizes WLH at the baseline assessment. EI and SC WLH were similar in age, education, marital status, food insecurity, alcohol and other substance use, depressive symptoms, HIV disclosure, and partner status.
Table 1.
Baseline characteristics of sample (N = 1,200), grouped by intervention condition: Enhanced Intervention (EI, N = 544) vs. Standard Care (SC, N = 656)a
| EI (N = 544) n (%) | SC (N = 656) n (%) | Total (N = 1,200) n (%) | p Valuea | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Mean age (SD) | 26.5 (5.5) | 26.5 (5.5) | 26.5 (5.5) | 0.936 |
| Highest education level | 0.873 | |||
| No schooling/grades 1–6 (primary) | 81 (15.0) | 108 (16.5) | 189 (15.8) | |
| Grades 7–12 (secondary) | 429 (79.3) | 525 (80.0) | 954 (79.7) | |
| Tertiary | 31 (5.7) | 23 (3.5) | 54 (4.5) | |
| Married or lives with partner | 102 (18.8) | 153 (23.3) | 255 (21.3) | 0.656 |
| Had a sexual partner, past 3 months | 468 (86.2) | 523 (79.7) | 991 (82.7) | 0.247 |
| Employed | 279 (51.3) | 258 (39.3) | 537 (44.8) | 0.169 |
| Living in formal housing | 342 (63.0) | 373 (56.9) | 715 (59.7) | 0.997 |
| Water on site | 370 (68.3) | 392 (60.0) | 762 (63.8) | 0.783 |
| Flush toilet versus other types | 309 (57.0) | 291 (44.4) | 600 (50.1) | 0.583 |
| Have electricity | 387 (71.3) | 554 (84.5) | 941 (78.5) | 0.132 |
| Median days mother gone hungry past week (range) | 0.0 (0–6) | 0.0 (0–7) | 0.0 (0–7) | 0.611 |
| Median days children gone hungry past week (range) | 0.0 (0–6) | 0.0 (0–5) | 0.0 (0–6) | 0.871 |
| Maternal health | ||||
| Any chronic illness | 71 (13.1) | 61 (9.3) | 132 (11.0) | 0.549 |
| Tested positive for TB during this pregnancy | 3 (0.6) | 19 (2.9) | 22 (1.8) | 0.088 |
| Substance use in pregnancy | ||||
| Alcohol prior to pregnancy recognition | 105 (19.3) | 101 (15.4) | 206 (17.2) | 0.463 |
| Alcohol after pregnancy recognition | 32 (5.9) | 27 (4.1) | 59 (4.9) | 0.186 |
| Tobacco | 38 (7.0) | 69 (10.5) | 107 (8.9) | 0.742 |
| Cannabis | 7 (1.3) | 4 (0.6) | 11 (0.9) | 0.274 |
| Depression (GHQ ≥ 7; moderate–severe) | 80 (14.7) | 89 (13.6) | 169 (14.1) | 0.724 |
| Social support | ||||
| Number of close friends and relatives times frequency of contact past month > median of 25b | 297 (54.7) | 289 (44.1) | 586 (48.9) | 0.084 |
| HIV disclosure and partner HIV status | ||||
| Disclosed HIV status to partner (N = 679) | 209 (67.6) | 250 (67.6) | 459 (67.6) | 0.857 |
| Disclosed HIV status to friend (N = 679) | 119 (38.5) | 123 (33.2) | 242 (35.6) | 0.568 |
| Asked current partner to test for HIV (N = 497) | 165 (75.7) | 212 (76.0) | 377 (75.9) | 0.947 |
| Current partner HIV+ (N = 345) | 97 (52.4) | 93 (58.1) | 190 (55.1) | 0.770 |
No significant baseline differences. EI and SC compared using random effects regression models, controlling for clinic clustering
Number of close friends/relatives: EI (median 1, range 0–15); SC (median 1, range 0–10); total (median 1, range 0–15). Number of contacts with close friends/relatives, past month: EI (median 22, range 0–150); SC (median 14.5, range 0–262); total (median 17, range 0–262)
WLH reassessed were similar to those lost to follow-up except that they were slightly older (26.7 years vs. 25.9, p = 0.010), more likely to be employed (46.5 vs. 40.6 %, p = 0.008), have onsite water (64.8 vs. 61.4 %, p = 0.018) and a flush toilet (51.0 vs. 48.1 %, p = 0.030), and were less likely to report a chronic illness unrelated to HIV (8.9 vs. 15.8 %, p = 0.002). By the time of the post-birth assessment, six maternal deaths were reported (0.5 %) and 39 infant deaths or miscarriages were reported (3.3 %), similar across conditions.
Post-birth Outcome Measures
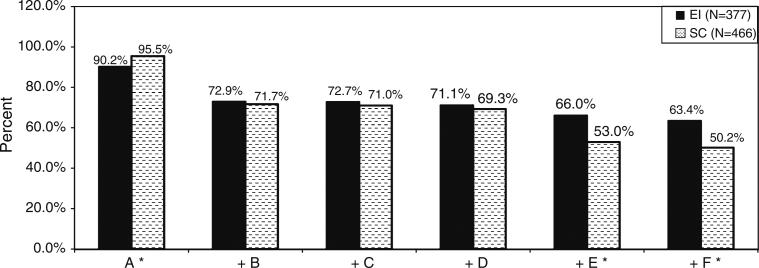
As shown in Table 2, EI performed better than SC on three of 16 outcomes, indicating significant overall benefits in EI compared to SC using the binomial test (correlation = 0.2, p = 0.028). On individual PMTCT outcomes, there were no significant benefits to EI versus SC. However, as shown in Fig. 2, cumulative adherence to PMTCT behaviors was significantly better for EI compared to SC. EI WLH were more likely to complete both maternal and infant ARV (OR = 1.72, p = 0.036) and adhere to all PMTCT tasks through 1.5 months post-birth (OR = 1.72, p = 0.023). EI WLH were less likely to adhere to ARV during pregnancy (OR = 0.44; p = 0.002).
Table 2.
Maternal and infant health and well-being outcomes at 1.5 months post-birth (N = 843), grouped by intervention condition: enhanced intervention (EI, N = 377) vs. standard care (SC, N = 466)
| EI (N = 377) n (%) | SC (N = 466) n (%) | Estimated odds ratio, EI versus SC (95 % CI)a | 2-sided p valuea | 1-sided, upper tail p valuea | |
|---|---|---|---|---|---|
| HIV transmission-related behaviours | |||||
| Told sexual partner about HIV status | 298 (79.0) | 339 (72.7) | 1.32 (0.54, 3.21) | 0.546 | 0.273 |
| Asked sexual partner to go for HIV test (N = 476) | 167 (77.3) | 168 (64.6) | 1.84 (1.13, 3.00) | 0.014 | 0.007* |
| PMTCT | |||||
| Mother took AZT from the 28th week of pregnancy, or on HAART | 340 (90.2) | 445 (95.5) | 0.44 (0.26, 0.74) | 0.002 | 0.999x |
| Mother took AZT during labor, or on HAART | 282 (74.8) | 334 (71.7) | 1.16 (0.44, 3.02) | 0.768 | 0.384 |
| Mother took NVP tablet at onset of labor, or on HAART | 361 (95.8) | 456 (97.9) | 0.53 (0.20, 1.41) | 0.203 | 0.899 |
| Infant given NVP syrup within 24 h of birth | 364 (96.6) | 451 (96.8) | 1.00 (0.36, 2.79) | 0.996 | 0.498 |
| AZT dispensed for infant and medicated as prescribed | 348 (92.3) | 374 (80.3) | 2.98 (0.78, 11.30) | 0.109 | 0.055 |
| One feeding method first week post-birth: formula or breastfeeding | 366 (99.5) | 448 (98.5) | 2.23 (0.68, 7.31) | 0.184 | 0.092 |
| Infant health status | |||||
| Birth weight ≥2,500 g | 310 (82.9) | 414 (89.8) | 0.55 (0.27, 1.14) | 0.108 | 0.946 |
| Weight-for-age z-score ≥ –2 (N = 707) | 274 (81.8) | 296 (79.6) | 1.10 (0.59, 2.02) | 0.771 | 0.386 |
| Height-for-age z-score ≥ –2 (N = 178) | 76 (80.9) | 45 (53.6) | 3.30 (1.41, 7.74) | 0.006 | 0.003* |
| Weight-for-length z-score ≥ –2 (N = 150) | 54 (71.1) | 63 (85.1) | 0.84 (0.19, 3.61) | 0.810 | 0.595 |
| Healthcare and health monitoring | |||||
| 4 or more antenatal clinic visits (4 is standard practice) | 328 (87.2) | 355 (76.2) | 2.17 (0.96, 4.88) | 0.062 | 0.031 |
| No depression (GHQ <7) | 357 (94.7) | 409 (87.8) | 2.55 (1.37, 4.76) | 0.003 | 0.002* |
| Post birth parenting tasks | |||||
| Registered infant's birth | 298 (79.9) | 318 (68.2) | 2.02 (0.98, 4.16) | 0.057 | 0.029 |
| Receiving child support grant | 57 (15.1) | 73 (15.7) | 1.16 (0.59, 2.25) | 0.670 | 0.335 |
EI significantly better than SC using 1-sided, upper tail p < 0.025
SC significantly better than EI using 2-sided p < 0.05
Random effects logistic regression, adjusted for clinic clustering. One-sided p value used in the binomial test; Two-sided p value used in the secondary analysis of individual outcomes
Fig. 2.
Adherence to cumulative behaviours in the PMTCT cascade among participants at 1.5 months post-birth (N = 843), grouped by intervention condition: Enhanced Intervention (EI, N = 377) versus Standard Care (SC, N = 466). Key: A Maternal AZT from the 28th week of pregnancy, or on HAART. B Maternal AZT during labour, or on HAART. C Maternal NVP at onset of labour, or on HAART. D Infant NVP within 24 h of birth. E Infant AZT dispensed and medicated as prescribed. F One feeding method first week post-birth. Note: “+” indicates that the behaviour listed includes itself and all behaviours listed to the left: cumulative adherence. EI and SC compared using random effects logistic regression, adjusting for clinic clustering. A*: OR EI versus SC (95 % CI) = 0.44 (0.26, 0.74), p = 0.002; E: OR = 1.72 (1.04, 2.86), p = 0.036; F: OR = 1.72 (1.08, 2.75), p = 0.023
Table 2 summarizes individual measures’ results post-birth. Compared to SC WLH, EI were more likely to ask partners to test for HIV (OR = 1.84; two-sided p = 0.014), have infants with height-for-age z-score ≥ –2 (OR = 3.30; p = 0.006) and were less likely to report depressed mood (OR = 2.55; p = 0.003). Healthcare utilization was similar across conditions.
Discussion
This study evaluated the benefits of having a WLH who is a Peer Mentor support a newly diagnosed pregnant WLH immediately after receiving their HIV test results and in small groups during the perinatal period. The model is one that has been broadly diffused throughout Africa. The urgency in the HIV epidemic often prohibits the rigorous evaluation of intervention approaches prior to broad implementation. The intervention strategy being broadly diffused (e.g., in the Mother-2-Mother Programme) is based on theories of social support [30, 31]. Peer support is consistently found to be helpful during stressful life transitions [32]. We evaluated how and whether the theory improved maternal and infant health outcomes in response to HIV in rural Africa.
Significant benefits were found using an overall measure of improved preventive and health outcomes, despite low attendance at the small group intervention meetings. In addition, cumulative adherence to tasks in the PMTCT cascade was significantly better. The gains were not solely in the tasks traditionally evaluated in PMTCT programs, i.e., adherence to tasks to prevent vertical transmission.
This study is among the first to examine multiple domains of functioning for WLH who are pregnant, in addition to the PMTCT tasks. As HIV has become a chronic disease, functioning in multiple domains and multiple relationships must be considered in designing interventions. Rather than adopting an educational or informational approach solely, the M2M program focuses on an empowerment and support model [1]. Partner relationships and depression were improved by the intervention. These factors are consistently related to better long-term outcomes. It is important to note that reductions in depressive symptoms were for cut-off scores indicating relatively severe levels of depressed mood. Reduction in depression is critical for WLH to effectively bond with and take care of young infants. Given the high rates of depression in South Africa [33, 34], Peer Mentors may offer a useful intervention model to improve infants’ well-being. Importantly, infants were less likely to be malnourished and stunted post-birth, important predictors of long-term outcomes for children [35]. Future studies will need to expand beyond PMTCT tasks for pregnant women to meet HIV-related challenges that emerge with their family and community. Placing the Peer Mentors in clinics is a structural intervention which may become a force for community mobilization and mainstreaming HIV over time.
The results are mixed however, and SC WLH were significantly more likely than EI WLH to adhere to ARV during pregnancy. It is difficult to know why the SC was significantly better at adherence to ARV during pregnancy. Perhaps WLH self-selected into the intervention, with those most acutely upset more motivated to attend the intervention. Previous work in psychotherapy and preventive interventions with depressed and homeless have found patients to self-select to attend interventions from among those randomized to an EI condition [36].
Limitations
The binomial test analyses significant benefits of EI compared to SC. However, SC was significantly better than EI on one outcome. While this outcome does not impact the validity of EI's benefits compared to SC, a concern is raised about how EI should be adapted in the future.
There were no significant differences between conditions at recruitment. However, the follow-up rate was lower than desired and there were significant differences between participants reassessed post-birth and those lost to follow-up. WLH who were more stable and better-off were more likely to be retained. In addition, the duration of time that elapsed between baseline and follow-up assessments was variable.
An important limitation of the intervention is that not all eligible women were enrolled into the government-led PMTCT program and only about two-thirds of eligible women were recruited into the trial. Only about 74 % of eligible women were successfully tested for HIV and received their results across conditions, and only 62 % were recruited into the study [37]. Laher and colleagues found that WLH fear the stigma of being identified by others at the clinic as HIV seropositive and, therefore, may not test for HIV nor attend the intervention groups. The low uptake does contrast to recent studies of WLH in South Africa visited at home by paraprofessional community health workers [18]. Home visitors reach more than 98 % of mothers and the follow-up rates are more than 91 % at 6 months.
Moreover, session uptake was relatively low: 25 % of EI WLH did not attend any antenatal intervention sessions, and 44 % did not attend any postnatal sessions. While 87 % attended at least one session, only 5 % attended all eight EI sessions. Further work needs to be undertaken to assess whether the benefits of the intervention could be achieved with fewer sessions. Perhaps the intervention benefits occur because the Peer Mentor support is given at a critical moment, close to learning that one is seropositive. Alternatively, WLH who need support may be those most likely to attend the intervention. Given our current adherence data, the power of the Peer Mentor may be achieved based on the Peer Mentor's presence immediately following HIV testing.
The lives of low-income South African women are difficult. While WLH typically attended four antenatal clinic visits to monitor their health, and EI sessions were timed to coincide with these visits, it was not always possible for women to stay for EI sessions. For example, some pregnant women were transported in groups by their employers and had to leave when all women were ready. Distance, waiting periods, cost and transport are significant barriers to attending clinic-based interventions [38]. Alternative intervention strategies are needed to complement clinic interventions. One such strategy may be to provide home-based Peer Mentor support [18]. A second possible strategy worth further research is to build upon the ubiquity of mobile phones in South Africa and package EI sessions, along with real-time peer support, into an application installed on WLH mobile phones. This would reduce costs associated with clinic visits and connect WLH to a Peer Mentor in their home [39, 40].
By the time of the post-birth interview, WLH would not have completed infant HIV testing, so we did not ask about infant HIV status in this assessment. In addition, this study was not powered for, nor did we realistically expect to see any differences in the infant HIV rate. Three outcomes were not monitored until 6 months post-birth (condom use between partners, infant HIV testing, and using one feeding method until 6 months) and will be reported in a future manuscript. Preliminary results show that, similar to other studies in Africa [41], only about 2 % of infants are diagnosed HIV seropositive at 6 months.
The current study utilized mobile phone technology to collect baseline and follow-up data from a large sample of WLH, technology widely used in South Africa. Entering data on phones was very familiar and a relatively easy and reliable process for interviewers. Mobile technologies eased the process of data collection and the downloading and cleaning of data. This study demonstrates the feasibility of mobile phone use in healthcare interventions in low and middle income countries.
Conclusions
An intervention modeled on an existing program, the M2M program, was found to have modest uptake and significant, but modest improvements in maternal and child outcomes at 6 weeks post-birth.
Acknowledgments
This study was funded by the National Institute of Mental Health (1R01MH077553) and supported by NIH Grants P30MH58107, 5P30AI028697, and UL1TR000124. We are grateful to all the families who took part in the study, and to members of the study team, Lungie Mkhize, Nonhle Mtungwa, Lindo Ndlovu, and Lungie Ntombela. Linda Richter and Mark Tomlinson are supported by the National Research Foundation (South Africa). Trial Registration: ClinicalTrials.gov. NCT00972699; South African trial registration: DOH-27-0812-2348.
Footnotes
The guidelines of the National Department of Health have since shifted to consistent breastfeeding (2005).
While we reassessed 69 % of EI WLH post-birth, we documented intervention attendance among the full baseline sample. Among EI WLH assessed at baseline, 64 % attended at least one antenatal intervention session (mean 2.7 sessions, SD 1.1), 41 % attended at least one postnatal meeting (mean 2.5, SD 1.2), and 73 % attended any sessions (mean 3.8, SD 2.0).
Conflict of interest The authors declare that they have no competing interests.
Contributor Information
Linda Richter, Human Sciences Research Council, Durban, KwaZulu-Natal, South Africa; Developmental Pathways to Health Research Unit, University of the Witwatersrand, Johannesburg, Gauteng, South Africa.
Mary Jane Rotheram-Borus, University of California at Los Angeles (UCLA), 10920 Wilshire Blvd., Suite. 350, Los Angeles, CA 90024, USA.
Alastair Van Heerden, Human Sciences Research Council, Durban, KwaZulu-Natal, South Africa.
Alan Stein, Human Sciences Research Council, Durban, KwaZulu-Natal, South Africa; Department of Psychiatry, Oxford University, Oxford, UK.
Mark Tomlinson, Department of Psychology, University of Stellenbosch, Stellenbosch, South Africa.
Jessica M. Harwood, University of California at Los Angeles (UCLA), 10920 Wilshire Blvd., Suite. 350, Los Angeles, CA 90024, USA
Tamsen Rochat, Africa Centre for Health and Population Studies, Mtubatuba, South Africa.
Heidi Van Rooyen, Human Sciences Research Council, Durban, KwaZulu-Natal, South Africa.
W. Scott Comulada, University of California at Los Angeles (UCLA), 10920 Wilshire Blvd., Suite. 350, Los Angeles, CA 90024, USA.
Zihling Tang, University of California at Los Angeles (UCLA), 10920 Wilshire Blvd., Suite. 350, Los Angeles, CA 90024, USA.
References
- 1.McColl K. Mentor mothers to prevent mother-to-child transmission of HIV. BMJ. 2012;344:e1590. doi: 10.1136/bmj.e1590. [DOI] [PubMed] [Google Scholar]
- 2.UNICEF [20 Feb 2013];Children and AIDS: Fourth Stocktaking Report. 2009 Available at: http://www.unicef.org/publications/files/Children_and_AIDS_Fourth_Stocktaking_Report_EN_120209.pdf.
- 3.AVERTing HIV and AIDS [20 Feb 2013];Sub-Saharan Africa HIV & AIDS Statistics. 2009 Available at: http://www.avert.org/africa-hiv-aids-statistics.htm.
- 4.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Pillay-van-Wyk V, et al. South African national HIV prevalence, incidence, behaviour and communication survey 2008: a turning tide among teenagers? HSRC Press; Cape Town: 2009. [Google Scholar]
- 5.Karim QA, Kharsany AB, Frohlich JA, Werner L, Mashego M, Mlotshwa M, et al. Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu-Natal, South Africa. Int J Epidemiol. 2011;40(4):922–30. doi: 10.1093/ije/dyq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgartner LM, David KN. Accepting being poz: the incorporation of the HIV identity into the self. Qual Health Res. 2009;19(12):1730–43. doi: 10.1177/1049732309352907. [DOI] [PubMed] [Google Scholar]
- 7.Dohrenwend BS, Dohrenwend BP, Dodson M, Shrout PE. Symptoms, hassles, social supports, and life events: problem of confounded measures. J Abnorm Psychol. 1984;93(2):222. doi: 10.1037//0021-843x.93.2.222. [DOI] [PubMed] [Google Scholar]
- 8.Hackl KL, Somlai AM, Kelly JA, Kalichman SC. Women living with HIV/AIDS: the dual challenge of being a patient and care-giver. Health Soc Work. 1997;22(1):53–62. doi: 10.1093/hsw/22.1.53. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention [20 Feb 2013];The Social-ecological Model: A Framework for Prevention. n.d. Available at: http://www.cdc.gov/violenceprevention/overview/social-ecologicalmodel.html.
- 10.World Health Organization [20 Feb 2013];HIV and Infant Feeding: Revised Principles and recommendations. 2009 Available at: http://whqlibdoc.who.int/publications/2009/9789241598873_eng.pdf.
- 11.Doherty T, Sanders D, Goga A, Jackson D. Implications of the new WHO guidelines on HIV and infant feeding for child survival in South Africa. Bull World Health Organ. 2011;89(1):62–7. doi: 10.2471/BLT.10.079798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coovadia HM, Rollins NC. Impact of HIV on the health of women, children, and families in less developed countries. In: Ehiri J, editor. Maternal and child health: global challenges, programs, and policies. Springer; 2009. pp. 271–285. [Google Scholar]
- 13.Hampanda K. Vertical transmission of HIV in Sub-Saharan Africa: applying theoretical frameworks to understand social barriers to PMTCT. ISRN Infect Dis. 2013 doi:10.5402/2013/420361. [Google Scholar]
- 14.South Africa National Department of Health [20 Feb 2013];Policy and guidelines for the implementation of the PMTCT programme. 2008 Available at: http://southafrica.usembassy.gov/root/pdfs/2008-pmtct.pdf.
- 15.Stringer EM, Ekouevi DK, Coetzee D, Tih PM, Creek TL, Stinson K, et al. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA. 2010;304(3):293–302. doi: 10.1001/jama.2010.990. [DOI] [PubMed] [Google Scholar]
- 16.Johnson L. Access to prevention of mother-to-child transmission (PMTCT) programmes: HIV testing. Cape Town, South Africa: 2009. [20 Feb 2013]. Available at: http://www.childrencount.ci.org.za/uploads/NSP-PMTCT-access-to-HIV-testing-in-pregnant-women.pdf. [Google Scholar]
- 17.Department of Health, Medical Research Council, OrcMacro . South Africa Demographic and Health Survey 2003. Pretoria: Department of Health; 2007. [21 Feb 2013]. 2007 Available at: http://www.measuredhs.com/pubs/pdf/FR206/FR206.pdf. [Google Scholar]
- 18.le Roux IM, Tomlinson M, Harwood JM, O'Connor MJ, Worthman CM, Mbewu N, Rotheram-Borus MJ. Outcomes of home visits for pregnant mothers and their infants: a cluster randomized controlled trial. AIDS. 2013;27(9):1461–71. doi: 10.1097/QAD.0b013e3283601b53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomlinson M, Doherty T, Jackson D, Lawn JE, Ijumba P, Colvin M, et al. An effectiveness study of an integrated, community-based package for maternal, newborn, child and HIV care in South Africa: study protocol for a randomized controlled trial. Trials. 2011;12(1):236. doi: 10.1186/1745-6215-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandelowski M, Lambe C, Barroso J. Stigma in HIV-positive women. J Nurs Scholarsh. 2004;36(2):122–8. doi: 10.1111/j.1547-5069.2004.04024.x. [DOI] [PubMed] [Google Scholar]
- 21.Rotheram-Borus MJ, Richter L, Van Rooyen H, van Heerden A, Tomlinson M, Stein A, Greco E. Project Masihambisane: a cluster randomised controlled trial with peer mentors to improve outcomes for pregnant mothers living with HIV. Trials. 2011;12(2) doi: 10.1186/1745-6215-12-2. doi:10.1186/1745-6215-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedeker D, Gibbons RD, Waternaux C. Sample size estimation for longitudinal designs with attrition: comparing time-related contrasts between two groups. J Educ Behav Stat. 1999;24(1):70–93. [Google Scholar]
- 23.Todd J, Carpenter L, Li X, Nakiyingi J, Gray R, Hayes R. The effects of alternative study designs on the power of community randomized trials: evidence from three studies of human immunodeficiency virus prevention in East Africa. Int J Epidemiol. 2003;32(5):755–62. doi: 10.1093/ije/dyg150. [DOI] [PubMed] [Google Scholar]
- 24.NIMH Collaborative HIV/STD Prevention Trial Group Results of the NIMH collaborative HIV/sexually transmitted disease prevention trial of a community popular opinion leader intervention. J Acquir Immune Defic Syndr. 2010;54(2):204–14. doi: 10.1097/QAI.0b013e3181d61def. doi:10.1097/QAI.0b013e3181d61def. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wawer MJ, Sewankambo NK, Serwadda D, Quinn TC, Paxton LA, Kiwanuka N, Gray RH. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Lancet. 1999;353(9152):525–35. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 26.Futterman D, Shea J, Besser M, Stafford S, Desmond K, Comulada WS, Greco E. Mamekhaya: a pilot study combining a cognitive-behavioral intervention and mentor mothers with PMTCT services in South Africa. AIDS Care. 2010;22(9):1093–100. doi: 10.1080/09540121003600352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Onis M. WHO child growth standards based on length/height, weight and age. Acta Paediatr. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 28.Cogill B. Anthropometric indicators measurement guide. Food and Nutrition Technical Assistance Project, Academy for Educational Development; Washington: D.C.: 2003. [Google Scholar]
- 29.Chibanda D, Mangezi W, Tshimanga M, Woelk G, Rusakaniko P, Stranix-Chibanda L, et al. Validation of the Edinburgh Postnatal Depression Scale among women in a high HIV prevalence area in urban Zimbabwe. Arch Womens Ment Health. 2010;13(3):201–6. doi: 10.1007/s00737-009-0073-6. [DOI] [PubMed] [Google Scholar]
- 30.Besser M. Mothers 2 Mothers. South African J Obstet Gynaecol. 2008;12(3):122. [Google Scholar]
- 31.Teasdale CA, Besser MJ. Enhancing PMTCT programmes through psychosocial support and empowerment of women: the mothers2mothers model of care. Southern African J HIV Med. 2008;9(1):60–4. [Google Scholar]
- 32.Fisher E, Boothroyd RI, Baumann L, Mbanya JC, Rotheram-Borus MJ, Sanguanprasit B, et al. Peer support for self-management of diabetes improved outcomes in international settings. Health Aff. 2012;31(1):130–9. doi: 10.1377/hlthaff.2011.0914. doi:10.1377/hlthaff.2011.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartley M, Tomlinson M, Greco E, Comulada WS, Stewart J, Le Roux I, et al. Depressed mood in pregnancy: prevalence and correlates in two Cape Town peri-urban settlements. Reprod Health. 2011;8:9. doi: 10.1186/1742-4755-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper PJ, Tomlinson M, Swartz L, Woolgar M, Murray L, Molteno C. Post-partum depression and the mother–infant relationship in a South African peri-urban settlement. Brit J Psychiatry. 1999;175(6):554–8. doi: 10.1192/bjp.175.6.554. doi:10.1192/bjp.175.6.554. [DOI] [PubMed] [Google Scholar]
- 35.Dewey KG, Begum K. Long-term consequences of stunting in early life. Maternal Child Nutr. 2011;7(s3):5–18. doi: 10.1111/j.1740-8709.2011.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Amico EJ, Anderson KG, Metrik J, Frissell KC, Ellingstad T, Brown SA. Adolescent self-selection of service formats: implications for secondary interventions targeting alcohol use. Am J Addict. 2006;15(s1):s58–66. doi: 10.1080/10550490601003722. doi:10.1080/10550490601003722. [DOI] [PubMed] [Google Scholar]
- 37.Laher F, Cescon A, Lazarus E, Kaida A, Makongoza M, Hogg RS, et al. Conversations with mothers: exploring reasons for prevention of mother-to-child transmission (PMTCT) failures in the era of programmatic scale-up in Soweto, South Africa. AIDS Behav. 2012;16(1):91–8. doi: 10.1007/s10461-010-9875-9. [DOI] [PubMed] [Google Scholar]
- 38.Sprague C, Chersich MF, Black V. Health system weaknesses constrain access to PMTCT and maternal HIV services in South Africa: a qualitative enquiry. AIDS Res Ther. 2011;8:10. doi: 10.1186/1742-6405-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomlinson M, Solomon W, Singh Y, Doherty T, Chopra M, Ijumba P, et al. The use of mobile phones as a data collection tool: a report from a household survey in South Africa. BMC Med Inform Decis Mak. 2009;9(1):51. doi: 10.1186/1472-6947-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rotheram-Borus MJ, Tomlinson M, Swendeman D, Lee A, Jones E. Standardized functions for smartphone applications: examples from maternal and child health. Int J Telemed Appl. 2012 doi: 10.1155/2012/973237. doi:10.1155/2012/973237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mofenson LM. Prevention in neglected subpopulations: prevention of mother-to-child transmission of HIV infection. Clin Infect Dis. 2010;50(Suppl 3):S130–48. doi: 10.1086/651484. [DOI] [PubMed] [Google Scholar]