Abstract
Herein is reported the first asymmetric utilization of aryldiazonium cations as a source of electrophilic nitrogen. This is achieved through a chiral anion phase-transfer pyrroloindolinization reaction that forms C3-diazenated pyrroloindolines from simple tryptamines and aryldiazonium tetrafluoroborates. The title compounds are obtained in up to 99% yield and 96% ee. The air- and water-tolerant reaction conditions accommodate electronic and steric diversity of the aryldiazonium electrophile and of the tryptamine core.
Keywords: phase-transfer catalysis, cyclization, asymmetric catalysis, photolysis, amination
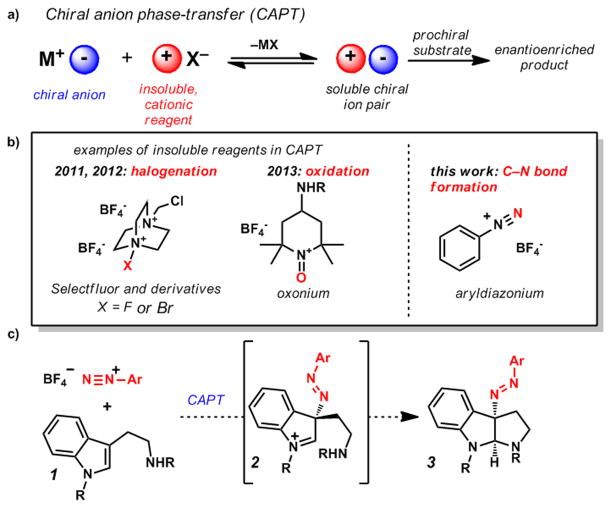
Chiral anion phase-transfer (CAPT) catalysis has recently arisen as an effective strategy in enantioselective catalysis (Figure 1a).[1] In particular, electrophilic halo-functionalization reactions of alkenes using Selectfluor (Figure 1b) and its derivatives have proven broadly effective, delivering a wide scope of valuable halogenated products in high yields and excellent enantioselectivities.[1f–m] Inspired by the substrate generality exhibited by CAPT halo-functionalization and other oxidative reactions,[1n–o] it has been a long-standing goal in our group to identify additional cationic electrophiles amenable to this strategy (Figure 1b).
Figure 1.
a) General chiral anion phase-transfer process, b) cationic reagents employed, and c) application of chiral anion phase-transfer catalysis to pyrroloindolinization.
We were specifically interested in cations comprised of electrophilic nitrogen atoms, as asymmetric electrophilic C–N bond formation remains a synthetic challenge.[2] Aryldiazonium salts were recognized as candidates, as their N-electrophilicity has been exploited to diazenate several classes of carbon nucleophiles in a non-stereoselective fashion, including aromatics,[3] enolates,[4] and heteroaromatics.[5] Reports of Gomberg–Bachmann–Hey biaryl syntheses[3b] and azo-coupling reactions[3a,c] that utilize aryldiazoniums under phase-transfer conditions further encouraged our efforts in this area. Furthermore, while azo compounds have been utilized extensively in materials science,[6] commodities,[7] and chemical biology[8] for their photochemical properties, studies of enantioenriched diazenes within these contexts are rare.
When considering transformations suitable for providing proof-of-principle for CAPT of diazonium cations, we were drawn to several enantioselective pyrroloindolinization reactions,[9,10] and recent total syntheses in which C3-diazenated pyrroloindolines were key intermediates (prepared in a 6-step diastereoselective sequence).[11] Furthermore, Antilla and coworkers have recently reported a highly efficient and enantioslective method for the preparation of C3-hydrazinated pyrroloindolines[9] utilizing azodicarboxylate electrophiles; however, no such transformation using diazonium cations to directly provide C3-diazenated products has been reported in either a racemic or asymmetric fashion.
We envisioned that CAPT of an insoluble aryldiazonium salt would provide a soluble chiral ion pair poised for attack at the terminal diazonium nitrogen by tryptamine 1 (Figure 1c). The resulting enantioenriched indolinium intermediate (2) could then cyclize to yield the desired pyrroloindoline structural motif 3. Herein we report the successful execution of this synthetic hypothesis, enabling the preparation of highly enantioenriched pyrroloindolines from simple tryptamine derivatives, thereby providing the first example of catalytic, enantioselective C–N bond formation utilizing aryldiazonium cations as an electrophilic nitrogen source.
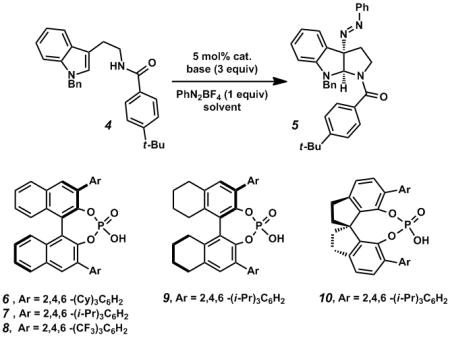
Due to the demonstrated success of the benzamide group in CAPT catalysis,[1h–k] our efforts began with the readily prepared tryptamine derivative 4 (Table 1). We were pleased to find that exposure of tryptamine 4 to 3 equivalents of Na3PO4, 1 equivalent of phenyldiazonium tetrafluoroborate, and 5 mol% of (S)–TCyP (6) in hexane solvent provided the desired pyrroloindoline 5 with good conversion and moderate enantioselectivity (35% ee) (Table 1, entry 1).
Table 1.
Optimization Experiments.
| |||||
|---|---|---|---|---|---|
| entry | cat. | solv. | base | conv. (%)[a] | ee (%)[b] |
| 1 | 6 | hexanes | Na3PO4 | >95 | 35 |
| 2 | 7 | hexanes | Na3PO4 | >95 | 62 |
| 3 | 8 | hexanes | Na3PO4 | >95 | 4 |
| 4 | 9 | hexanes | Na3PO4 | >95 | 44 |
| 5 | 10 | hexanes | Na3PO4 | >95 | 81 |
| 6 | 10 | pentane | Na3PO4 | >95 | 82 |
| 7 | 10 | pet. ether | Na3PO4 | >95 | 84 |
| 8 | 10 | acetone[c] | Na3PO4 | >95(21[d]) | 51 |
| 9 | 10 | Et2O | Na3PO4 | 52[d] | 88 |
| 10 | 10 | MTBE | Na3PO4 | 99[d] | 91 |
| 11 | 10 | MTBE[e] | Na3PO4 | >95 | 90 |
| 12 | 10 | MTBE | NEt3 | <5 | – |
| 13 | 10 | MTBE | K2CO3 | >95 | 90 |
| 14 | 10 | MTBE | K3PO4 | >95 | 91 |
| 15 | – | MTBE | Na3PO4 | <5 | – |
| 16 | 10 | MTBE | – | <5 | – |
Estimated from 1H NMR.
Determined by chiral phase HPLC.
The aryldiazonium salts are soluble in acetone.
Isolated yield.
10 equiv. H2O.
With this initial result, a phase-transfer catalyst screen was undertaken that focused on three distinct chiral phosphoric acid scaffolds (6–8, 9, and 10). BINOL-derived (S)–TRIP (7) delivered the desired compound in 62% ee. (entry 2). Partially saturated (S)–H8-TRIP (9) proved to be suboptimal, furnishing the product in 44% ee (entry 4). Finally, the SPINOL-derived (R)–STRIP[11] (10) was found to be the most selective catalyst, yielding the product in 81% ee (entry 5).
Having identified (R)–STRIP (10) as our optimal catalyst, an extensive screen of solvent and base was undertaken. Notably, hydrocarbon solvents such as pentane and petroleum ether provided the product with equivalent enantioselectivities (81%, 82%, and 84% ee, entries 5–7). The use of ethereal solvents provided a significant improvement in enantioselectivity (88% ee, entry 9), with methyl tert-butyl ether (MTBE) furnishing the product in 99% yield and 91% ee (entry 10).[13,14]
Soluble, organic bases such as triethylamine attenuated product formation, while inorganic bases performed well (entries 12–14). Notably, addition of excess water did not affect the selectivity or yield of the reaction (entry 11). Omission of base or catalyst under heterogeneous conditions prevented conversion, and both selectivity and efficiency were eroded under homogeneous conditions, supporting our hypothesis of a phase-transfer process (entries 8, 15, and 16).[15]
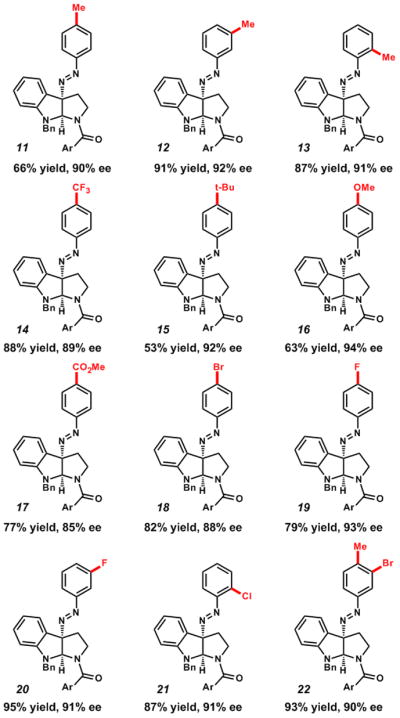
With our optimized conditions in hand, we explored the aryldiazonium scope (Table 2). The reaction conditions were tolerant of both electron-rich and electron-poor substitution of the p-, m-, and o-positions of the aryldiazonium (Table 2, 11–22). Notably, difunctionalized aryldiazonium salts were competent under the reaction conditions (Table 2, 22 and Table 3, 25).
Table 2.
|
Ar = 4–(t-Bu)C6H4.
Conditions: 4 (1 equiv), (R)–STRIP (10) (5 mol%), Na3PO4 (3 equiv), ArN2BF4 (1 equiv), MTBE, rt, 2–8 h.
Isolated yields.
ee determined by chiral phase HPLC.
Relative and absolute stereochemistry assigned by analogy to 5.
Table 3.
|
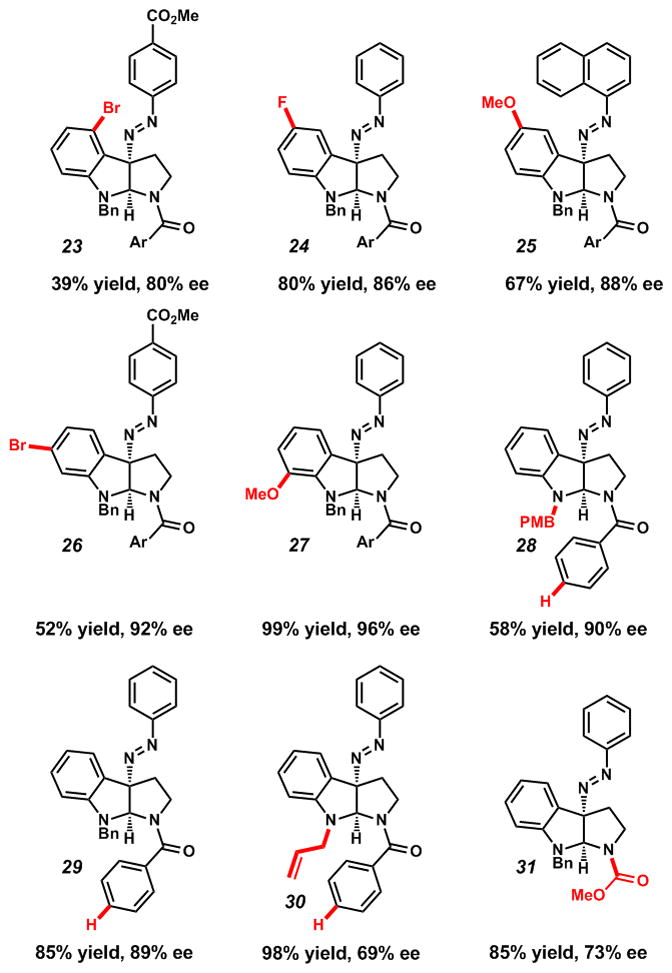
Ar = 4–(t-Bu)C6H4.
Conditions: 4 (1 equiv), (R)–STRIP (10) (5 mol%), Na3PO4 (3 equiv), ArN2BF4 (1 equiv), MTBE, rt, 2–8 h.
Isolated yields.
ee determined by chiral phase HPLC.
Relative and absolute stereochemistry assigned by analogy to 5.
To probe tryptamine scope, several derivatives of 5 were prepared and examined under our optimized conditions (Table 3). Substitution of the indole 5-, 6- or 7-positions allowed for highly selective product formation (Table 3, 24–27). Electron-poor diazonium cations were employed for substrates containing bromine substitution to provide improved reactivity, and good stereoselectivities were achieved, albeit with diminished yields (4-CO2Me in 23 and 26).[16] Electron-withdrawing substituents on the indole nitrogen prevented reactivity. Moreover, replacement of the benzyl group with a p-methoxy benzyl (28) had no deleterious effect on ee, nor did removal of the t-butyl group from the benzamide (28, 29). Protection of the exocyclic tryptamine-N as a carbamate (31) or the endocyclic indole-N with an allyl group (30) resulted in diminished, but synthetically useful enantioselectivities.
In closing, we have demonstrated the utility of aryldiazonium cations as electrophilic nitrogen sources in enantioselective transformations. A chiral anion phase-transfer reaction process provides C3-diazenated pyrroloindolines in good to excellent enantioselectivities and yields, with diverse functionality. Further, through highly enantioselective electrophilic C–N bond formation, this technology represents a significant expansion of phase transfer methodologies. Efforts to further utilize these novel compounds are currently underway.[17]
Experimental Section
A suspension of the tryptamine (0.05 mmol), Na3PO4 (24 mg, 0.15 mmol, 3 equiv), and (R)-STRIP (1.8 mg, 0.0025 mmol, 5 mol%) in MTBE (0.5 mL) was stirred vigorously at 20 °C for 15 minutes. To this suspension, the aryldiazonium salt (0.05 mmol, 1 equiv) was added rapidly in one portion. The reactions were stirred until TLC analysis indicated completion (1–12 h). The bright yellow reaction mixtures were filtered through cotton wool and the volatiles were removed by rotary evaporation. The crude product was dissolved in hexanes and loaded onto a 1 cm column and eluted in 5:95 EtOAc:hex to yield yellow foams. The products were generally stable for several months neat, in protic solvents, or in pyridine.
Supplementary Material
Footnotes
We gratefully acknowledge NIHGMS (R01 GM104534) for financial support. H.M.N. would like to acknowledge the UNCF and Merck for generous funding. S.H.R. would like to acknowledge the Amgen Foundation for generous funding. The authors would like to thank Pascal Tripet for useful discussions.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
References
- 1.a) Brak K, Jacobsen EN. Angew Chem. 2013;125:558. doi: 10.1002/anie.201205449. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2013;52:534. [Google Scholar]; b) Carter C, Fletcher S, Nelson A. Tetrahedron: Asymmetry. 2003;14:1995. [Google Scholar]; c) Hamilton GL, Kanai T, Toste FD. J Am Chem Soc. 2008;130:14984. doi: 10.1021/ja806431d. [DOI] [PubMed] [Google Scholar]; d) Honjo T, Phipps RJ, Rauniyar V, Toste FD. Angew Chem. 2012;124:9822. doi: 10.1002/anie.201205383. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2012;51:9684. [Google Scholar]; e) Phipps RJ, Hamilton GL, Toste FD. Nature Chem. 2012;4:603. doi: 10.1038/nchem.1405. [DOI] [PubMed] [Google Scholar]; f) Phipps RJ, Hiramatsu K, Toste FD. J Am Chem Soc. 2012;134:8376. doi: 10.1021/ja303959p. [DOI] [PubMed] [Google Scholar]; g) Phipps RJ, Toste FD. J Am Chem Soc. 2013;135:1268. doi: 10.1021/ja311798q. [DOI] [PubMed] [Google Scholar]; h) Rauniyar V, Lackner AD, Hamilton GL, Toste FD. Science. 2011;334:1681. doi: 10.1126/science.1213918. [DOI] [PubMed] [Google Scholar]; i) Shunatona HP, Früh N, Wang YM, Rauniyar V, Toste FD. Angew Chem. 2013;125:7878. doi: 10.1002/anie.201302002. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2013;52:7724. [Google Scholar]; j) Wang YM, Wu J, Hoong C, Rauniyar V, Toste FD. J Am Chem Soc. 2012;134:12928. doi: 10.1021/ja305795x. [DOI] [PubMed] [Google Scholar]; k) Wu J, Wang YM, Drljevic A, Rauniyar V, Phipps RJ, Toste FD. Proc Natl Acad Sci. 2013;110:13729. doi: 10.1073/pnas.1304346110. [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Romanov-Michailidis F, Guénée L, Alexakis A. Angew Chem. 2013;125:9436. doi: 10.1002/anie.201303527. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:9266. [Google Scholar]; m) Xie W, Jiang G, Liu H, Hu J, Pan X, Zhang H, Wan X, Lai Y, Ma D. Angew Chem. 2013;125:13162. doi: 10.1002/anie.201306774. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2013;52:12924. [Google Scholar]; n) Lackner AD, Samant AV, Toste FD. J Am Chem Soc. 2013;135:14090. doi: 10.1021/ja4082827. [DOI] [PMC free article] [PubMed] [Google Scholar]; o) Neel AJ, Hehn JP, Tripet PF, Toste FD. J Am Chem Soc. 2013;135:14044. doi: 10.1021/ja407410b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.While to the best of our knowledge, aryldiazonium salts have not been employed in enantioselective C–N bond forming reactions, several strategies for catalytic asymmetric amination utilizing addition reactions of azodicarboxylates have been reported. For relevant reviews, see: Kosmrlj J, Kocevar M, Polanc S. Synlett. 2009:2217.Janey JM. Angew Chem, Int Ed. 2005;44:4292. doi: 10.1002/anie.200462314.Angew Chem. 2005;117:4364.
- 3.a) Bredereck K, Karaca S. Tetrahedron Lett. 1979;20:3711. [Google Scholar]; b) Beadle JR, Korzeniowski SH, Rosenberg DE, Garcia-Slanga BJ, Gokel GW. J Org Chem. 1984;49:1594. [Google Scholar]; c) Ellwood M, Griffiths J, Gregory P. J Chem Soc, Chem Commun. 1980:181. [Google Scholar]
- 4.a) Japp FR, Klingemann F. Ber. 1887;20:2942. [Google Scholar]; b) Garst ME, Lukton D. Synth Commun. 1980;10:155. [Google Scholar]; c) Sakakura T, Hara M, Tanaka M. J Chem Soc, Perkin Trans 1. 1994:289. [Google Scholar]; d) Sakakura T, Tanaka M. J Chem Soc, Chem Commun. 1985:1309. [Google Scholar]; e) Shchepin VV, Sazhneva YK, Bagara MV, Russkikh NY. Russ J Gen Chem. 2003;73:1261. [Google Scholar]; f) Sakakura T, Tanaka M. 4772714. US. 1988
- 5.a) Adbullah MI, Jackson AH, Lynch PP, Record KAF. Heterocycles. 1990;30:317. [Google Scholar]; b) Albar HA, Shawali AS, Abdaliah MA. Can J Chem. 1993;71:2144. [Google Scholar]; c) Colonna M, Greci L, Poloni M. J Chem Soc, Perkin Trans 2. 1982:455. [Google Scholar]; d) Colonna M, Poloni M. Gazz Chim Ital. 1984;114:495. [Google Scholar]; e) Daly S, Hayden K, Malik I, Porch N, Tang H, Rogelj S, Frolova LV, Lepthien K, Kornienko A, Magedov IV. Bioorg Med Chem Lett. 2011;21:4720. doi: 10.1016/j.bmcl.2011.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Hsinmin H, Mann FG. J Chem Soc. 1949:2903. [Google Scholar]; g) Iwasaki K, Kanno R, Morimoto T, Yamashita T, Yokoshima S, Fukuyama T. Angew Chem. 2012;124:9294. doi: 10.1002/anie.201204726. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2012;51:9160. [Google Scholar]; h) Jackson AH, Lynch PP. J Chem Soc, Perkin Trans 2. 1987:1483. [Google Scholar]
- 6.a) Natansohn A, Rochon P. Chem Rev. 2002;102:4139–4176. doi: 10.1021/cr970155y. [DOI] [PubMed] [Google Scholar]; b) Sourisseau C. Chem Rev. 2004;104:3851. doi: 10.1021/cr030042g. [DOI] [PubMed] [Google Scholar]
- 7.a) Husain A, Sawaya W, Al-Omair A, Al-Zenki S, Al-Amiri H, Ahmed N, Al-Sinan M. Food Additives and Contaminants. 2006;23:245. doi: 10.1080/02652030500429125. [DOI] [PubMed] [Google Scholar]; b) Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco-Paulo A, Gübitz GM. Appl Environ Microbiol. 2000;66:3357. doi: 10.1128/aem.66.8.3357-3362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wedzicha BL, Rumbelow SJ. J Sci Food Agric. 1981;32:699. [Google Scholar]; d) Pinheiro HM, Touraud E, Thomas O. Dyes Pigm. 2004;61:121. [Google Scholar]; e) O’Neill C, Hawkes FR, Hawkes DL, Lourenco ND, Pinheiro HM, Delée W. J Chem Technol Biotechnol. 1999;74:1009. [Google Scholar]
- 8.a) Samanta S, Beharry AA, Sadovski O, McCormick TM, Babalhavaeji A, Tropepe V, Woolley GA. J Am Chem Soc. 2013;135:9777. doi: 10.1021/ja402220t. [DOI] [PubMed] [Google Scholar]; b) Beharry AA, Wong L, Tropepe V, Woolley GA. Angew Chem, Int Ed. 2011;50:1325. doi: 10.1002/anie.201006506. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2011;123:1361. [Google Scholar]; c) Liang X, Takenaka N, Nishioka H, Asanuma H. Nucleic acids symposium series. 2004;2007:169–170. doi: 10.1093/nass/nrm085. [DOI] [PubMed] [Google Scholar]; d) Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Nat Chem Biol. 2006;2:47. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Antilla JC. Angew Chem. 2012;124:11948. doi: 10.1002/anie.201203553. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2012;51:11778. [Google Scholar]
- 10.a) Cai Q, Liu C, Liang XW, You SL. Org Lett. 2012;14:4588. doi: 10.1021/ol302043s. [DOI] [PubMed] [Google Scholar]; b) Cera G, Chiarucci M, Mazzanti A, Mancinelli M, Bandini M. Org Lett. 2012;14:1350. doi: 10.1021/ol300297t. [DOI] [PubMed] [Google Scholar]; c) Espejo VR, Li XB, Rainier JD. J Am Chem Soc. 2010;132:8282. doi: 10.1021/ja103428y. [DOI] [PubMed] [Google Scholar]; d) Govek SP, Overman LE. Tetrahedron. 2007;63:8499. [Google Scholar]; e) Kieffer ME, Chuang KV, Reisman SE. Chem Sci. 2012;3:3170. doi: 10.1039/C2SC20914D. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Kieffer ME, Chuang KV, Reisman SE. J Am Chem Soc. 2013;135:5557. doi: 10.1021/ja4023557. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Newhouse T, Baran PS. J Am Chem Soc. 2008;130:10886. doi: 10.1021/ja8042307. [DOI] [PubMed] [Google Scholar]; h) Ni J, Wang H, Reisman SE. Tetrahedron. 2013;69:5622. doi: 10.1016/j.tet.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Repka LM, Ni J, Reisman SE. J Am Chem Soc. 2010;132:14418. doi: 10.1021/ja107328g. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Spangler JE, Davies HML. J Am Chem Soc. 2013;135:6802. doi: 10.1021/ja4025337. [DOI] [PubMed] [Google Scholar]; k) Zhu S, MacMillan DWC. J Am Chem Soc. 2012;134:10815. doi: 10.1021/ja305100g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xing CH, Liao YX, Zhang Y, Sabarova D, Bassous M, Hu QS. Eur J Org Chem. 2012;6:1115. [Google Scholar]
- 12.a) Movassaghi M, Ahmad OK, Lathrop SP. J Am Chem Soc. 2011;133:13002. doi: 10.1021/ja2057852. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lathrop SP, Movassaghi M. Chem Sci. 2014;5:333. doi: 10.1039/C3SC52451E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The absolute stereochemistry and connectivity were determined unambiguously by x-ray diffraction, see supporting information for details.
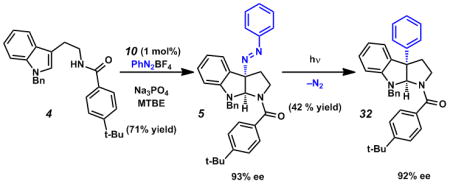
- 14.Reduction of catalyst loading to 1 mol% resulted in decreased efficiency (71% yield), without loss of stereoselectivity (93% ee). See scheme in footnote 17.
- 15.It is noteworthy that while no significant reaction occurs in the absence of a CAPT catalyst under heterogeneous conditions (Table 1, entry 15), the observed enantioselectivity in the homogeneous reaction (entry 8) introduces the possibility of additional counterion effects.
- 16.We found that sterically bulky diazoniums helped to improve enantioselectivity of 4-methoxy tryptamines as in entry 25 (Table 3).
-
17.Photolysis of a deoxygenated sample of diazene 5 smoothly provided C3-arylated pyrroloindoline 32 in 78% conversion (42% isolated yield) and 92% e
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.