Abstract
Proventricular dilatation disease (PDD) is a fatal infectious disease of birds that primarily affects psittacine birds. Although a causative agent has not been formally demonstrated, the leading candidate is a novel avian bornavirus (ABV) detected in post-mortem tissue samples of psittacids with PDD from the USA, Israel and, recently, Germany. Here we describe the presence of ABV in a parrot with PDD as well as in clinically normal birds exposed to birds with PDD. In two ABV-positive post-mortem cases, the tissue distribution of ABV was investigated by quantitative real-time reverse transcription-polymerase chain reaction. Viraemia was observed in a PDD-affected bird whereas a restriction of ABV to nerve tissue was found in the non- PDD-affected bird. Healthy birds from the same aviary as the affected birds were also found to harbour the virus; 19/59 (32.2%) birds tested positive for ABV RNA in cloacal swabs, providing the first evidence of ABV in clinically healthy birds. In contrast, 39 birds from the same geographic area, but from two different aviaries without PDD cases in recent years, had negative cloacal swabs. ABV RNA-positive, clinically healthy birds demonstrated the same serological response as the animal with confirmed PDD. These results indicate that ABV infection may occur without clinical evidence of PDD and suggest that cloacal swabs can enable the non-invasive detection of ABV infection.
Introduction
Proventricular dilatation disease (PDD) is a fatal dis- order of birds and a major threat to endangered parrot populations (Lublin et al., 2006; Doneley et al., 2007), including one of the world's most endangered bird species, the Spix's macaw (Cyanopsitta spixii). It is characterized by dilatation of the proventriculus, diges- tive malfunction, and cachexia. Definitive diagnosis depends on histologic demonstration of lymphocytic infiltrates in the brain, spinal cord, peripheral nerves and myenteric ganglia (Lutz & Wilson, 1991; Vice, 1992; Gregory, 1995; Sullivan et al., 1997; Gregory et al., 1998; Berhane et al., 2001; Lierz, 2005; Lublin et al., 2006; Perpiñ an et al., 2007). A viral aetiology of PDD has been presumed (Gough et al., 1996; Gregory et al., 1998) and several viruses were proposed, including adenovirus, coronavirus, herpesvirus, polyomavirus, eastern equine encephalitis virus and western equine encephalitis virus (Mannl et al., 1987; Sullivan et al., 1997; Vice, 1992; Gregory, 1995; Gregory et al., 1998; Grund et al., 2002; Gough et al., 2006; Lublin et al., 2006; Orosz & Dahlhausen, 2007); however, none was confirmed and most recently avian paramyxovirus 1 was ruled out (Deb et al., 2008). A leading candidate only emerged when two independent research groups recently identified a novel avian bornavirus (ABV) in PDD-affected birds (Honkavuori et al., 2008; Kistler et al., 2008). In one study of US parrots, 3/3 (100%) animals with PDD, and 0/4 (0%) without PDD, had ABV in brain, proventricu- lus and adrenal gland (Honkavuori et al., 2008). In the other study, ABV was detected in 5/8 (62.5%) PDD birds from the US and 5/7 (71%) such birds from Israel, but not in non-diseased controls (5 and 14 birds, respec- tively) (Kistler et al., 2008). Both studies showed less than 70% nucleotide conservation of the avian sequences to those of the mammalian Borna disease virus (BDV), and indicated that these viruses represent a novel species with multiple divergent genotypes, possibly forming a separate genus (Honkavuori et al., 2008; Kistler et al., 2008). In a subsequent report, ABV was described in six parrots in Europe with clinical PDD symptoms, in which ABV was not restricted to cells of the nervous system and the presence of ABV in the faeces led the authors to propose a possible faecal-oral transmission route (Rinder et al., 2009). In the same study, seven birds with clinical signs of PDD were tested negative. Bornavirus infection has been previously proposed for an outbreak of neurologic disease in ostriches based on serologic data (Malkinson et al., 1993). Bornavirus sequences have also been detected by polymerase chain reaction (PCR) in mallard (Anas plathyrhnchus) and jackdaw (Corvus monedula) droppings (Berg et al., 2001); however, in these instances viral sequences were closely related to those of mammalian BDV and distinct from the recently identified ABV. A reservoir for ABV, its geographic distribution, modes of transmission, and causative relationship to PDD remain to be determined.
Materials and Methods
Animals and samples. In a parrot collection from a private breeder in Germany, consisting of 61 birds, two male salmon-crested cockatoos (Cacatua moluccensis) were euthanized (Z178/08) or died with PDD-like symptoms (Z1/09) within a period of 2 months. Z178/08 demonstrated wasting symptoms and shedding of undigested seeds in faeces before it was euthanized. From this bird a crop biopsy free of lymphoplasma- cytic ganglioneuritis, taken 2 weeks prior to death, was also available for investigation. Z1/09, kept in the neighbouring cage of Z178/08, died of ulcerative enteritis but no evidence of nutritional compromise. Tissue samples, blood and cloacal swabs from both birds were collected for histological and molecular biological investigations (see Table 1). For molecular biological analysis, tissues were placed immediately after sampling into RNAlater (Qiagen, Hilden, Germany) and stored within 2 h after sampling at −808C until processing.
Table 1.
Detection of ABV RNA by RT-qPCR, and observed histological alterations in different tissues of two Salmon-crested cockatoos (C. moluccensis)
| Tissue | Bird Z178/08 |
Bird Z1/09 | |||
|---|---|---|---|---|---|
| Histology | CT1367 | CT1034/1322 | Histology | CT1034/1322 | |
| Crop | Lymphoplasmacytic ganglioneuritis | 22.4 | 17.5 | Without pathological alteration | Negative |
| Ventriculus | Lymphoplasmacytic ganglioneuritis | 23.8 | 16.7 | Not done | 27.1 |
| Proventriculus | Lymphoplasmacytic ganglioneuritis | 21.5 | 17.1 | Without pathological alteration | 26.9 |
| Duodenum | Without pathological alteration | 24.3 | 17.2 | Ulcerative enteritis | 34.1 |
| Pancreas | 25.8 | 18.1 | Without pathological alteration | Negative | |
| Heart | Lymphoplasmacytic ganglioneuritis | 24.6 | 19.6 | Without pathological alteration | Not done |
| Chest-muscle | Without pathological alteration | 27.4 | 22.1 | Not done | Not done |
| Cerebrum | Lymphocytic encephalitis | 21.2 | 15.9 | Lymphocytic encephalitis | Not done |
| Cerebrellum | Lymphocytic encephalitis | 22.8 | 16.7 | Lymphocytic encephalitis | 25.6 |
| Lung | Slight anthracosis | 23.8 | 17.6 | Without pathological alteration | Not done |
| Spleen | Hyperplasia | 24 | 17.1 | Without pathological alteration | Not done |
| Nervus ischiadicus | Lymphocytic neuritis | 23 | 18.4 | Not done | Not done |
| Adrenal gland | Without pathological alteration | 22.6 | 16.6 | Lymphocytic adrenalitis | 30.8 |
| Liver | Slight hepatitis | 24.2 | 17.7 | Without pathological alteration | Negative |
| Kidney | Slight nephritis | 22.2 | 15.1 | Without pathological alteration | Not done |
| Plasma | Not done | 30.4 | 28.5 | Not done | Negativea |
| Cloacal swab | Not done | 23.7 | 17 | Not done | Negative |
Bird Z179/08 was euthanized due to PDD, Bird Z1/09 died from ulcerative enteritis. Samples of Z178/08 were analysed in parallel with primer/probe sets CT 1367 and CT 1034/1322, samples of Z1/09 with primer/probe set CT1034/1322 (Honkavuori et al., 2008). Ct >36 scored as negative.
Clotted heart blood as sample.
Other samples included cloacal swabs of the remaining 59 unaffected, clinically healthy birds in the same collection, and from 39 healthy birds in two different collections in the same geographic area of Germany that had not experienced any PDD cases in the past 6 years (control group). Swabs were processed within 3 h after sampling as described below. In several cases, serum samples were obtained and stored frozen at −208C until investigated.
Laser-capture microdissection. Samples of nerves, nerve ganglia, stroma and glandular structures of the proventriculus and adrenal gland of Z1/ 09 were isolated by laser-capture microdissection (LCM), using 8-mm- thick frozen sections mounted on glass slides covered with polyethylene naphthalate membrane (PALM Microlaser Technologies, Bernried, Germany) (Klopfleisch & Gruber, 2009). The size of the excised tissue sections ranged from 1.4 × 104 to 3.0 × 104 m2. The isolated tissue pieces were laser pressure catapulted into caps of 0.5-ml reaction tubes containing 40 ml lysis buffer (Nucleo Spin RNA XS; Macherey & Nagel, Dü ren, Germany) and stored at −808C.
Quantitative real-time reverse transcription-PCR. RNA was extracted from tissue samples using the RNeasy kit (Qiagen), and from cloacal swabs dissolved in phosphate-buffered saline using the QIAamp Viral RNA kit (Qiagen). Extraction of LCM samples was performed using the NucleoSpin kit (Macherey-Nagel). ABV-specific RNA was detected by quantitative real-time reverse transcription-polymerase chain reac- tion (RT-qPCR) with primer-probe sets 1367 or 1034/1322 that match different ABV strains (Honkavuori et al., 2008). Tissue samples from Z178/08 were investigated using both primer sets; for all other samples, primer set 1034/1322 was used. All PCRs were carried out with the QuantiTect Probe RT-PCR kit (Qiagen). Threshold cycle (Ct) values > 36 were scored negative, as determined using serial cloned standard dilutions (Honkavuori et al., 2008).
Sequence determination and analysis. Phylogenetic sequence analysis was performed using a 724-base-pair fragment spanning the C-terminal end of the N gene, and the N-terminal part of the P gene (GenBank accession numbers FJ770253, FJ932550, and FJ932551). Selected PCR products were size-fractionated on agarose gels, purified (QIAquick PCR purification kit; Qiagen), and directly dideoxy-sequenced in both directions with ABI PRISM Big Dye Terminator Cycle Sequencing kits on ABI PRISM DNA Analyzers (Applied Biosystems, Foster City, California, USA). Sequences were assembled and analysed using programs of the Wisconsin GCG Package (Accelrys, San Diego, California, USA). Phylogenetic analysis was performed using www.phy-logeny.fr (Dereeper et al., 2008), applying T-coffee for alignment (Notredame et al., 2003), Gblocks 0.91 b for curation (Castresana, 2000), and PhyML 3.0 for phylogenetic reconstruction (Guindon & Gascuel, 2003); all with standard settings.
Western blot analysis. ABV proteins P (strain 1322, Genbank accession number FJ169441; 30 kDa, including tag and vector sequence) and N (strain 1367, Genbank accession number FJ169440; 49 kDa, including tag and vector sequence) were expressed in prokaryotic systems (pDest17 Gateway vector; Invitrogen, Carlsbad, California, USA) and purified by affinity chromatography using Ni-NTA-Agarose (Qiagen). In addition, P (24 kDa) and N (40 kDa) of mammalian BDV were analysed in comparison (Briese et al., 1995). Protein concentrations were estimated by Bradford assay (Bio-Rad, Hercules, California, USA), and 100 ng each protein in Laemmli buffer were boiled for 5 min and size-fractionated by discontinuous 4 to 15% gradient sodium dodecyl sulphate-polyacrylamide gel electrophoresis (Laemmli & Favre, 1973). Proteins were transferred to nitrocellulose membranes (Bio-Rad) and blocked with 2% non-fat dry milk in 20 mM Tris-HCl, pH 7.6, 137 mM NaCl, 0.1% Tween 20 (TTBS) overnight at room temperature (Towbin et al., 1979). Membranes were incubated with bird sera (1:1000 in TTBS) for 1 h at room temperature; washed three times with TTBS, and then incubated with peroxidase-conjugated goat anti- bird immunoglobulin (1:2000 in TTBS; Bethyl Laboratories, Montgom- ery, Texas, USA) for 1 h at room temperature. After three washes with TTBS, the membranes were developed using the ECL western blot detection system (Amersham Biosciences, Arlington Heights, Illinois, USA) and scanned for chemiluminescence using a Storm 840 imager (Molecular Dynamics, Sunnyvale, California, USA).
Results
Clinically affected birds. Post-mortem tissue samples as well as plasma and a cloacal swab from each of two birds, Z178/08 and Z1/09, were collected and analysed by RT-qPCR. The euthanized Bird Z178/08 was ema- ciated and had an enlarged thin-walled proventriculus filled with feed. Histological examination revealed lym- phocytic infiltrates in the epicardial ganglia, the Purkinje fibres, ventriculus, proventriculus (indicating PDD), crop, ischiatic nerve and less pronounced in brain. No abnormalities were found in the adrenals, cloaca, or intestine. All tissues, including those where no histologic abnormalities were found, as well as the plasma and cloacal swab were positive for ABV RNA (Table 1), suggesting a viraemia. A crop biopsy taken 2 weeks prior to death, which showed no histologic abnormality, was also positive (Ct =24.7, primer set 1034/1322). Bird Z1/ 09 had an enlarged proventriculus filled with feed and adhesions consistent with slight peritonitis. Histopatho- logical examination revealed a lymphocytic encephalitis and adrenalitis. Nerve tissue from the heart, proventri- culus, intestine, crop, liver and spleen was not affected, demonstrating that the bird did not suffer from PDD. The intestine showed severe ulcerative enteritis in con- junction with acute purulent peritonitis as the cause of death. Viral burden was lower than in Z178/08 and measured in the two tissues with lymphocytic infiltrates, the cerebrum and adrenal gland, and also in the proventriculus, ventriculus and duodenum, but was absent in the crop, liver, pancreas, cloacal swab and blood (Table 1).
Two sets of nerve ganglia tissue obtained by LCM from the proventriculus of Bird Z1/09 yielded a positive PCR signal (Ct =35.24/34.93), whereas two sets each of stroma and glandular tissue were negative. LCM samples of the adrenal gland revealed a positive signal from two sets of nerve ganglia tissue (Ct =33.94/31.93); six sam- ples of inter-renal and chromaffin portions with diffuse infiltrations by lymphocytes and plasma cells were negative.
Clinical healthy birds. Nineteen out of 59 parrots (32.2%) from the same aviary as the two dead birds had ABV- RNA in their cloacal swabs (Table 2). The presence of ABV was not correlated with age, sex or cage location, including cases with positive and negative birds in the same cage. Positive birds were distributed across all age groups (juvenile to 20 years old). No ABV-positive cloacal swabs were detected in any of 39 birds from two PDD-free independent aviaries located in the same geographic area (Table 2).
Table 2.
Species distribution of examined parrots from Germany and their RT-qPCR results from cloacal swabs
| Species | Total | Negative | Positive | CT1034/1322 |
|---|---|---|---|---|
| PDD-affected parrot collection (n=59) | ||||
| Lilac-crowned amazon (Amazona finschi) | 1 | 0 | 1 | 28.5 |
| Hispaniolan amazon (Amazona ventralis) | 4 | 2 | 2 | 19.7/21.5 |
| Turquoise-fronted amazon (Amazona aestiva) | 1 | 1 | 0 | – |
| Yellow-headed amazon (Amazona oratrix) | 2 | 2 | 0 | – |
| Yellow-naped amazon (Amazona auropalliata) | 6 | 4 | 2 | 28.7/35.5 |
| Red-lored amazon (Amazona autumnalis) | 2 | 1 | 1 | 35.3 |
| Red-browned amazon (Amazona rhodocorytha) | 11 | 3 | 8 | 24.2/25/23/22.8/21.3/21/23/21.5 |
| Cuban amazon (Amazona leucocephala) | 5 | 3 | 2 | 27.1/29.5 |
| Red-spectacled amazon (Amazona pretrei) | 2 | 2 | 0 | – |
| Vinaceous amazon (Amazona vinacea) | 7 | 6 | 1 | 22.7 |
| Orange-winged amazon (Amazona amazonica) | 2 | 2 | 0 | – |
| Scarlet macaw (Ara macao) | 3 | 3 | 0 | – |
| Grey parrot (Psittacus erithacus) | 2 | 2 | 0 | – |
| Major Mitchel?s Cockatoo (Cacatua leadbeateri) | 2 | 1 | 1 | 25.2 |
| Lesser sulphur-crested cockatoo (Cacatua sulphurea parvula) | 2 | 1 | 1 | 24.9 |
| Citron-crested cockatoo (Cacatua sulphurea citrinocristata) | 2 | 2 | 0 | – |
| Salmon-crested cockatoo (Cacatua moluccensis) | 5 | 5 | 0 | – |
| Total number of birds | 59 | 40/67.8% | 19/32.2% | |
| Control parrot collections (n =39) | ||||
| Yellow-headed amazon (Amazona oratrix) | 4 | 4 | 0 | – |
| Orange-winged amazon (Amazona amazonica) | 2 | 2 | 0 | – |
| Yellow-naped amazon (Amazona auropalliata) | 4 | 4 | 0 | – |
| Haycinth macaw (Anodorhynchus hyacinthinus) | 4 | 4 | 0 | – |
| Blue-winged macaw (Ara maracana) | 1 | 1 | 0 | – |
| Blue-and-yellow macaw (Ara ararauna) | 8 | 8 | 0 | – |
| Spix's macaw (Cyanopsitta spixii) | 2 | 2 | 0 | – |
| Maroon-fronted parrot (Rhynchopsitta terrisi) | 6 | 6 | 0 | – |
| Thick-billed parrot (Rhynchopsitta pachyrhynchus) | 8 | 8 | 0 | – |
| Total number of birds | 39 | 39/100% | 0 | – |
Ct >36 are scored as negative.
PCR detection of ABV RNA in healthy contacts was corroborated by western blot analysis. A healthy Hispa- niolan Amazon (Amazona ventralis, Z14/09-13; Ct =19.7; Table 2) and a healthy lesser sulphur crested cockatoo (Cacatua sulphurea parvula, Z14/09-05; Ct =24.9; Table 2) both showed a similar antibody response when compared with PDD-affected Bird Z178/08 (Figure 1). A strong reaction to ABV N with limited cross-reactivity to BDV N was noted; reaction to the P antigen appeared to be weaker and more variable.
Figure 1.
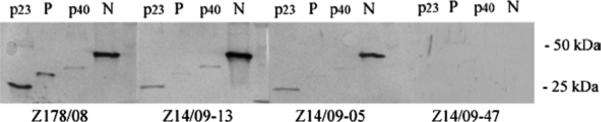
Sera from PDD-affected Bird Z178/08, asymptomatic ABV PCR-positive Birds Z14/09-13 and Z14/09-05, and healthy PCR- negative Bird Z14/09-47, were evaluated by western blot analysis using recombinant ABV P and N, as well as BDV P and N (p23, p40), demonstrating a strong reaction to ABV N with limited cross-reactivity to BDV N.
Phylogenetic analysis. Samples obtained from the crop, cerebellum, and proventriculus of Bird Z178/08 re- vealed no sequence variation (GenBank accession number FJ770253). The same was true for the sequence obtained from the brain and gizzard of Bird Z1/09 (GenBank accession number FJ932550). Comparison with four mammalian BDV isolates from horses in Germany and three ABV, for which sequence in the respective genome region is available, grouped strain Z178/08 and Z1/09 sequences (100% sequence identity) in the ABV clade and clearly separate from German horse BDV (Figure 2). Strains Z178/08 and Z1/09 from Germany were phylogenetically more similar to strain 1034/1322 from the USA, than were strains 1034/1322 and bil from the USA to one another. Almost identical sequences were also obtained from cloacal swabs of healthy PCR-positive contacts, Bird Z14/09-13 (100% identical to Z178/08 and Z1/09) and Z14/09-28 (97% identical to Z178/08 and Z1/09, GenBank accession number FJ932551; Figure 2).
Figure 2.
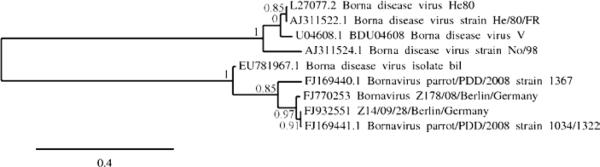
Maximum likelihood tree based on nucleotide sequences of five ABV strains (strain Z178/08, accession number FJ770253, identical to strain Z1/09, accession number FJ932550; strain Z14/09/28 accession number FJ932551; strain 1367, accession number FJ169440.1; strain 1034_1322, accession number FJ169441.1; strain bil, accession number EU781967.1) and four BDV strains from mammals (strain No/98, accession number AJ311524.1; strain V, accession number U04608.1; strain He80, accession number L27077.2; strain He/80/FR, accession number AJ311522.1). Numbers near the nodes indicate the branch support. Unit of the scale bar is substitutions per site.
Discussion
Borna disease, a neurological syndrome of ungulates due to infection with BDV, has been endemic in Germany for more than a century (Ludwig et al., 1988; Lipkin & Briese, 2007). Although cross-species transfer between ungulate and bird has been achieved experimentally (Ludwig et al., 1988), its occurrence in nature is unclear. Furthermore, Borna disease of ungulates is not recorded in the USA or Israel despite the presence of a novel Bornavirus in PDD-affected birds in both countries (Honkavuori et al., 2008; Kistler et al., 2008). These observations together with the phylogenetic distance between ABV and mammalian BDV strains suggest that, if it occurs, cross-species infection appears to be an uncommon event.
Although ABV represents a plausible aetiologic can- didate for PDD (Honkavuori et al., 2008; Kistler et al., 2008; Rinder et al., 2009), much of its biology remains to be characterized. In the present study we confirmed the presence of ABV in two birds from the same collection in Germany. Whereas Bird Z178/08 had classical symp- toms, gross lesions and histological alterations of PDD, Bird Z1/09 did not have a clinical presentation consistent with PDD. Despite having a characteristic proventricular enlargement, Bird Z1/09 lacked typical lymphocytic infiltrates in nerve ganglia of the digestive tract. Similarly, whereas ABV-RNA was demonstrated in all organs and even in plasma of the PDD-affected Bird Z178/08, ABV RNA was restricted to nerve tissue in non-PDD Bird Z1/09, consistent with the pattern of BDV infection found in immunocompetent mammals (Ludwig et al., 1988; Briese et al., 1999). However, it remains obscure why the lymphoplasmacytic ganglio- neuritis was not detectable in all organs positive for ABV in Bird Z178/08 and also missing in the proventriculus of Bird Z1/09 despite the fact that ABV was detected in the nerve ganglia of this organ.
Rinder et al. (2009) also found ABV extension beyond neural tissues. It is conceivable that early infection or carrier status is characterized by confinement to nerve tissue and that wider distribution only occurs in the late stages of PDD. The presence in healthy birds of ABV RNA in cloacal swabs in association with ABV-specific serum antibodies, which was demonstrated in the present study for the first time, indicates either that ABV is not causally related to PDD or that disease is multifactorial. Differentiating these possibilities awaits formal testing of Koch's postulates. Although shedding of infectious virus remains to be demonstrated, this finding is consistent with a possible transmission mode by subclinical individuals, especially as birds of collections not facing PDD were tested ABV negative.
Taken together our results support previous concerns regarding the reliability of histological examination of crop biopsies (Gregory et al., 1996; Berhane et al., 2001) as the recommended method for PDD screening (Sullivan et al., 1997); Bird Z178/08 tested negative by crop biopsy 2 weeks prior euthanasia due to clinical PDD. The applied RT-qPCR presents an adequate diagnostic addition, although an individual diagnosis based on cloacal swab alone may fail as indicated by Bird Z1/09; despite the presence of ABV in brain and adrenal gland, the cloacal swab tested negative. None- theless, a cloacal-oral route of transmission is plausible. Furthermore, the observation that ABV sequences can be detected in cloacal swabs by PCR offers the prospect for efficient non-invasive surveillance and diagnosis. Until larger data-sets become available, collection of biopsy and cloacal swab for histologic as well as RT- qPCR analyses is recommended for PDD/ABV diagnosis in psittacine birds. Furthermore, ABV-antibody detection by western blot analysis may prove useful in diagnosis as well as a screening tool, especially when screening flocks in conjunction with ABV-RNA detec- tion in cloacal swabs.
Acknowledgements
The present study was supported by funds provided by the Association for the Conservation of Threatened Parrots, Berlin, Germany, by Google.org, and National Institutes of Health awards AI051292 and AI57158 (Northeast Biodefense Center - Lipkin).
References
- Berg M, Johansson M, Montell H, Berg ASL. Wild birds as a possible natural reservoir of Borna Diseases Virus. Epidemiology and Infection. 2001;127:173–178. doi: 10.1017/s0950268801005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhane Y, Smith DA, Newman S, Taylor M, Nagy E, Binnington B, Hunter B. Peripheral neuritis in psittacine birds with proventricular dilatation disease. Avian Pathology. 2001;30:563–570. doi: 10.1080/03079450120078770. [DOI] [PubMed] [Google Scholar]
- Briese T, Hatalski CG, Kliche S, Park YS, Lipkin WI. Enzyme-linked immunosorbent assay for detecting antibodies to Borna disease virus-specific proteins. Journal of Clinical Microbiol-ogy. 1995;33:348–351. doi: 10.1128/jcm.33.2.348-351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briese T, Hornig M, Lipkin WI. Bornavirus immuno-pathogenesis in rodents: models for human neurological diseases. Journal for Neurovirology. 1999;5:604–612. doi: 10.3109/13550289909021289. [DOI] [PubMed] [Google Scholar]
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- Deb A, Borjal RJ, Bürkle M, Watson R, Hammer S. Proceedings of the 7th Scientific Meeting of the European Association of Zoo and Wildlife Veterinarians (EAZWW) Leipzig; Germany: 2008. Evaluation of Avian Paramyxovirus-1 serology and crop biopsy for the diagnosis of proventricular dilatation disease in captive Spix's macaws (Cyanopsitta spixii). pp. 239–242. [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research. 2008;36(WSI):W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doneley RJT, Miller RI, Fanning TE. Proventricular dilatation disease: an emerging exotic disease of parrots in Australia. Australian Veterinary Journal. 2007;85:119–123. doi: 10.1111/j.1751-0813.2007.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough RE, Drury SE, Harcourt-Brown NA, Higging RJ. Virus like particles associated with macaw wasting disease. The Veterinary Record. 1996;139:24. [PubMed] [Google Scholar]
- Gough RE, Drury SE, Culver F, Britton P, Cavanagh D. Isolation of a coronavirus from a green-cheeked Amazon parrot (Amazon viridigenalis cassin). Avian Pathology. 2006;35:122–126. doi: 10.1080/03079450600597733. [DOI] [PubMed] [Google Scholar]
- Gregory CR. Proventricular dilatation disease. In: Ritchie BW, editor. Avian Viruses, Function and Control. Wingers Publishing; Lake Worth, FL: 1995. pp. 439–448. [Google Scholar]
- Gregory CR, Latimer K, Campagnoli R, Ritchie B. Histologic evaluation of the crop for diagnosis of proventricular dilatation syndrome in psittacine birds. Journal of Veterinary Diagnostic Investigation. 1996;8:76–80. doi: 10.1177/104063879600800112. [DOI] [PubMed] [Google Scholar]
- Gregory CR, Ritchie BW, Latimer KS, Steffens WL, Campagnoli RP, Pesti D, Lukert PD. Experimental transmission of psittacine proventricular dilatation disease (PDD) and preliminary characterization of a virus recovered from birds with naturally ocurring and experimentally induced PDD. University of Georgia; Athens, GA: 1998. Available online at: http://www.toolady.com/library/avian/bird_health/bird_disease/pdd/experiment.htm (accessed 23 February 2009) [Google Scholar]
- Grund CH, Werner O, Gelderbrom HR, Grimm F, Kösters J. Avian Paramyxovirus serotype 1 isolates from the spinal cord of parrots display a very low virulence. Journal of Veterinary Medicine, Series B. 2002;49:445–451. doi: 10.1046/j.1439-0450.2002.00596.x. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Systems Biology. 2003;52:696–694. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Honkavuori KS, Schivaprasad HL, Williams BL, Quan PL, Hornig M, Street C, et al. Novel Borna virus in psittacine birds with proventricular dilatation disease. Emerging Infectious Diseases. 2008;14:1883–1886. doi: 10.3201/eid1412.080984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler AL, Gancz A, Clubb S, Skewes-Cox P, Fischer K, Sorber K, et al. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virology Journal. 2008;5:88. doi: 10.1186/1743-422X-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfleisch R, Gruber AD. Differential expression of cell cycle regulators p21, p27 and p53 in metastasizing canine mammary adenocarcinomas versus normal mammary glands. Research Veterinary Science. 2009;87:91–96. doi: 10.1016/j.rvsc.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Laemmli UK, Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. Journal of Molecular Biology. 1973;80:575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lierz M. Proventricular dilatation disease. In: Harcourt-Brown N, Chitty J, editors. BSAVA Manual of Psittacine Birds 2nd edn. British Small Animal Association; Gloucester, UK: 2005. pp. 161–162. [Google Scholar]
- Lipkin WI, Briese T. Bornaviridae. In: Knipe DM, Howley RD, editors. The Virology. 5th edn Williams & Wilkins; Philadelphia: Lippincott: 2007. pp. 1829–1851. [Google Scholar]
- Lublin A, Mechani S, Farnoushi I, Perl S, Bendheim U. An outbreak of proventricular dilatation disease in a psittacine breading farm in Israel. Israel Journal of Veterinary Medicine. 2006;61:16–19. [Google Scholar]
- Ludwig H, Bode L, Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Progress in Medical Virology. 1988;35:107–151. [PubMed] [Google Scholar]
- Lutz ME, Wilson RB. Psittacine proventricular dilatation syndrome in an Umbrella cockatoo. Journal of the American Veterinary Medical Association. 1991;198:1962–1964. [PubMed] [Google Scholar]
- Malkinson M, Weisman Y, Ashash E, Bode L, Ludwig H. Borna disease in ostriches. The Veterinary Record. 1993;133:304. doi: 10.1136/vr.133.12.304-b. [DOI] [PubMed] [Google Scholar]
- Mannl A, Gerlach H, Leipold R. Neuropathic gastric dilatation in psittaciformes. Avian Diseases. 1987;31:214–221. [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. Journal of Molecular Biology. 2003;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- Orosz SE, Dahlhausen RD. Proceedings of the 9th European AAV Conference*7th Scientific Meeting. Zürich; Switzerland: 2007. Proventricular dilatation syndrome in an Amazon parrot: possible role of PCR diagnosis of paramyxovirus-1 for presumptive diagnosis. pp. 27–28. [Google Scholar]
- Perpiñan D, Fernández-Bellon H, López C, Ramis A. Lymphoplasmacytic myenteric, subepicardial, and pulmonary gang-lioneuritis in four nonpsittacine birds. Journal of Avian Medicine and Surgery. 2007;21:210–214. doi: 10.1647/1082-6742(2007)21[210:LMSAPG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rinder M, Ackermann A, Kempf H, Kaspers B, Korbel R, Staeheli P. Broad tissue and cell tropism of avian bornavirus in parrots with proventricular dilatation disease. Journal of Virology. 2009;83:5401–5407. doi: 10.1128/JVI.00133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan ND, Mackie JT, Miller RI, Giles A. First case of psittacine proventricular dilatation syndrome (macaw wasting disease) in Australia. Australian Veterinary Journal. 1997;75:674. doi: 10.1111/j.1751-0813.1997.tb15371.x. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vice CA. Myocarditis as a component of psittacine proven-tricular dilatation syndrome in a Patagonian conure. Avian Diseases. 1992;36:1117–1119. [PubMed] [Google Scholar]


