Abstract
Neural progenitor cells (NPCs) have shown modest potential and some side effects (e.g. allodynia) for treatment of spinal cord injury (SCI). In only a few cases, however, have NPCs shown promise at the chronic stage. Given the 1.275 million people living with chronic paralysis, there is a significant need to rigorously evaluate the cell types and methods for safe and efficacious treatment of this devastating condition. For the first time, we examined the pre-clinical potential of NPCs derived from human induced pluripotent stem cells (hiPSCs) to repair chronic SCI. hiPSCs were differentiated into region-specific (i.e. caudal) NPCs, then transplanted into a new, clinically relevant model of early chronic cervical SCI. We established the conditions for successful transplantation of caudalized hiPSC-NPCs and demonstrate their remarkable ability to integrate and produce multiple neural lineages in the early chronic injury environment. In contrast to prior reports in acute and sub-acute injury models, survival and integration of hiPSC-derived neural cells in the early chronic cervical model did not lead to significant improvement in forelimb function or induce allodynia. These data indicate that while hiPSCs show promise, future work needs to focus on the specific hiPSC-derivatives or co-therapies that will restore function in the early chronic injury setting.
Keywords: spinal cord injury, induced pluripotent stem cells, neural progenitor cells
Introduction
Spinal cord injury (SCI) destroys neural and glial elements and severs the axonal connections between the motor and sensory systems leading to permanent and often devastating loss of function (Schwab and Bartholdi, 1996). In recent decades, the therapeutic promise of replacing lost neurons and glia by transplantation has gained significant momentum and even garnered the development of clinical trials (Fehlings and Vawda, 2011). Neural stem and progenitor cells comprise a promising resource for the treatment of traumatic spinal cord injury. Rationales for therapeutic use of stem cells in SCI include cell replacement, neuroprotection and trophic support, facilitation of axon outgrowth, and glial scar regulation. A number of studies have demonstrated the ability of human neural progenitor cells (NPCs) to promote functional recovery (Ogawa et al., 2002; Cummings et al., 2005; Hofstetter et al., 2005; Iwanami et al., 2005; Okada et al., 2005; Salazar et al., 2010). NPCs derived from human embryonic and induced pluripotent stem cells (hiPSCs) have also shown efficacy (Keirstead et al., 2005; Kumagai et al., 2009; Nori et al., 2011; Fujimoto et al., 2012). NPCs derived from hiPSCs (hiPSC-NPCs) offer particular advantages over those from other cell types and tissues, including a theoretically reduced need for immunosuppression (if cells are autologous) and obviating ethical concerns if cells are derived from adult tissues. The former is of particular importance to SCI patients, as they are prone to opportunistic infections (Nash, 2000), and an immunosuppressive regime would compound their vulnerability.
Interestingly, almost all of the studies cited above target the acute and subacute time points for intervention, transplanting at 9 days post-injury or sooner. Such models, however, are of limited value to the 1.275 million patients currently living with chronic spinal cord injury. There are only two published studies to date utilizing human neural cells in chronic spinal cord injury. The first study transplanted human embryonic stem cell (hESC)-derived oligodendrocyte precursor cells at 10 months after injury and found no improvement in functional recovery (Keirstead et al., 2005). The second study reported an improvement in locomotor recovery when human fetal brain neural stem cells (NSCs) were transplanted 30 days after injury (Salazar et al., 2010). This improvement, however, was only statistically significant when compared to vehicle injection, and no difference was observed between the human NSCs and human fibroblasts. Thus the appropriate cell population, conditions and co-therapies for the treatment of chronic spinal cord injury have yet to be identified.
The cervical region is the most commonly affected in spinal cord injury (approximately 50-60% of human SCI), and contusion is the most common type of injury (Sekhon and Fehlings, 2001). Further, regaining function in the hands and arms is the most critical determinant of quality of life for quadriplegic patients (Anderson, 2004). Thus for the present study we developed an early chronic cervical contusion injury that replicates many of the motor and sensory functional deficits seen in humans. We then examined the ability of hiPSC-NPCs to integrate into the injury environment and characterized their survival and ability to generate neuronal phenotypes. We conferred posterior identity to hiPSC-NPCs through the use of retinoic acid, which induced uniform expression of the hindbrain/spinal cord-specific homeobox gene HoxB4. Though hiPSC-NPCs survived for two months, integrated and differentiated into neurons and glia, this did not translate into measurable functional improvement.
Materials and Methods
Cell Culture
hiPSCs were generated from human fetal lung fibroblasts (IMR90) as previously described(Suhr et al., 2009). To generate hiPSC-NSCs, colonies were separated from their feeder layer and cultured in suspension for 10 days in DMEM:F12 and N2. The resultant embryoid bodies were then plated on poly-ornithine/laminin and observed for formation of neural tube-like rosettes.
Colonies containing rosettes were then detached mechanically and grown in suspension in medium consisting of DMEM:F12, N2, retinoic acid (RA, 1 μM) and basic fibroblast growth factor (bFGF, 10 ng/ml). On day 22, sonic hedgehog (100 ng/ml) was added, and on day 29 cAMP (1 μM) was added to the medium. From day 32 onward, cells were maintained in DMEM:F12, N2, cAMP, T3 (60 ng/ml), platelet-derived growth factor-AA (PDGF-AA, 10 ng/ml), insulin-like growth factor 1 (IGF-1, 10 ng/ml) and neurotrophin-3 (NT3, 10 ng/ml). IMR90 fibroblasts were cultured in DMEM containing 10% fetal bovine serum (FBS) and grown to confluence prior to transplant.
To obtain a single cell suspension, cells were treated with Accutase for 5-10 min, pelleted and resuspended in a PBS solution containing 5 mM glucose and 0.1 mg/ml DNase at a concentration of 100,000 cells/μl.
MRC5c3 hiPSCs were generated using MRC5 fibroblasts (ATCC) and Oct4-Sox2 and Nanog-Lin28 vectors (Addgene). Fibroblasts were exposed to 25 μl of each virus for 4-6 hours (day 0). Cells were plated on day 6 in hESC medium (DMEM:F12), 20% KnockOut(™) Serum Replacement, 4 ng/ml bFGF) on a feeder layer of mouse embryonic fibroblasts. Colonies were picked days 21-28. MRC5c3 hiPSCs were differentiated to the rosette stage as described for IMR90 hiPSCs.
TaqMan Low-Density Array Analysis
Total cellular RNA was isolated from each of four cell populations (hESCs, IMR90 hiPSCs, hiPSC-NPCs and IMR90 fibroblasts) with the RNeasy Mini Kit (Qiagen). RNA samples were reverse transcribed using random primers for mRNA expression analysis. mRNA expression of 92 validated genes associated with stem cell pluripotency and differentiation to all three germ layers were analyzed using the custom TaqMan low-density array fluidic card (TLDA) following the manufacturer's protocol (Applied Biosystems). The samples were run on an ABI Prism® 7900HT sequence Detection System (Applied Biosystems) in duplicate, and mean values used in subsequent analyses. Relative quantification was achieved using the formula 2-ΔΔCt, which relates the amount of the specific amplicon to the 18s internal control and the control cDNA.
Animals and Surgeries
Adult female Long-Evans rats were used in this study. Animals were 8 weeks old at initiation of training and approximately 5 months old at time of injury.
Animals received intraperitoneal injections of 80 mg/kg ketamine and 15 mg/kg xylazine. A laminectomy was performed at the C4 spinous process of the lamina ipsilateral to the animal's dominant paw (determined via behavioral tasks described below).
The animals were then placed in a spinal frame and a contusion injury was conducted using a fourth generation Ohio State Injury Device (Horner and Stokes, 1995). An electromagnetically controlled probe (0.7 mm end diameter, Ling Dynamics, Inc) was lowered to the surface of the cord just lateral to midline. The probe was oscillated on the surface of the spinal cord to achieve a common starting force of 3000 dynes for all animals. The spinal cord was displaced 0.8 mm for 14 msec to induce the injury. The surgical site was closed by suturing muscle in layers and closing skin with wound clips.
Four weeks after injury, scores on the forelimb reaching task (FRT, see below) stabilized, allowing for tight control for injury severity prior to transplantation. Thus, animals were assigned to treatment groups based on FRT such that all groups had equal average scores. All animals received transplants or control procedures at four weeks after injury. One group receiving hiPSC-NPCs (n=7) was followed for four weeks. The remaining animals were assigned to receive hiPSC-NPCs (n=11), IMR90 fibroblasts (n=9), PBS (n=8) or sham transplantation (n=10) and were followed for eight weeks.
In preparation for transplantation, animals were anesthetized and the injury site exposed. After placement in a spinal frame, a pulled glass capillary (OD 70-100 μm) was placed on the lateral border of the gray matter and stereotactically lowered to a depth of 1.0 mm, targeting the gray-white border of the dorsolateral funiculus. Animals in the hiPSC-NPC and fibroblast groups received two injections of 100,000 cells each (1.0 μl injected over 60 s), one rostral and one caudal to the injury site. The PBS group received two 1 μl injections, while sham animals were suspended in the frame but received no injection. Muscles were subsequently sutured in two layers and the skin closed. All animals received daily subcutaneous injections of cyclosporine (10 mg/kg, Sandimmune) from the day of transplant to the time of sacrifice.
Tissue Processing
Animals were anesthetized and perfused transcardially with saline followed by 4% paraformaldehyde (PFA) in 0.1M phosphate buffer. Spinal columns were post-fixed overnight, cryoprotected with 30% sucrose, and then cut into five 1-mm coronal sections surrounding the lesion, embedded in OCT medium and flash frozen. 20 μm sections were cut on a Leica CM1850 cryostat in a one-in-six series and stored at -80°C.
Immunofluorescence
Tissue was permeabilized with 0.4% Triton, then blocked with 5% donkey serum (DKS) and 0.4% Triton. Primary antibodies were applied to sections at 4°C overnight. Slides were then rinsed 3 times in PBS, and incubated in the appropriate secondary antibody solution overnight at 4°C. Sections were rinsed twice more with PBS, then incubated with DAPI before mounting. Antibodies, sources and dilutions are listed in Table 1.
Table 1.
| Antibody | Source | Dilution |
|---|---|---|
| Dcx | Santa Cruz | 1:100 |
| GFAP | Millipore | 1:2000 |
| GSTpi | Chemicon (Millipore) | 1:500 |
| hGFAP | StemCells, Inc. | 1:1000 |
| HoxB4 | Developmental Studies Hybridoma Bank | 1:100 |
| HuNu | Millipore | 1:300 |
| Ki-67 | Abcam | 1:500 |
| MBP | Millipore | 1:500 |
| Sox9 | R&D Systems | 1:500 |
| Tuj1 | Covance | 1:1000 |
To determine cell survival, stereology was conducted using a Zeiss Axioplan microscope and StereoInvestigator software (MBF Biosciences). Survival of human cells was quantified by HuNu (Millipore) immunolabeling with the optical disector probe and systematic random sampling according to stereological principles.
For quantification of hiPSC-NPC phenotypes in vivo, imaging of 15 sections per animal was performed on a Nikon Confocal microscope. A minimum of 100 HuNu+ cells per animal were counted, as were the numbers of human cells expressing GSTpi, GFAP, or Dcx, and percentages of HuNu-positive cells for each were calculated.
Limb-Use Asymmetry Test
The limb-use asymmetry test (LUAT) was used to assess forelimb preference during vertical exploration in a clear Plexiglas cylinder. Animals were scored by blinded observers on independent and simultaneous use of their left and right forelimbs when rearing to make wall contacts(Schallert et al., 2000; Gensel et al., 2006). Each session lasted until an animal made 20 wall contacts. To determine the degree to which animals preferred their unaffected forelimb after injury, an asymmetry score was calculated for wall contacts for each test session, asymmetry = [(affected forelimb) + .5(both forelimbs)]/20, with a score below 0.5 indicating preference for the unaffected forelimb. No training is required for this test, and all animals prior to injury were close to a score of 0.5, indicating no paw preference (average 0.4875 ± 0.08 SD)
Forelimb Reaching Task
The forelimb reaching task was used to assess skilled reaching ability (Alaverdashvili and Whishaw, 2010). Animals were trained to extend their forelimb through a slot in a clear Plexiglas box to grasp and eat a chocolate-flavored 35 mg precision-ground food pellet (Bioserv).
In a series of 10-minute sessions, animals were first introduced to the food pellets and testing box, and gradually trained to reach through the slots to grasp pellets. Once animals showed a clear preference for reaching with either the left or right paw, the slot corresponding to the other paw was blocked off and animals were allowed to reach with only their preferred paw. Animals were trained until they were reaching consistently with a success rate ∼70% (approximately 3 months training for all animals).
Throughout the experiment, animals were tested for 10 minutes or until they completed 20 trials. A trial was defined as any advance of the paw towards the pellet, concluding when the animal moved the pellet off of the indentation with its paw or left the front of the box. Experimenters blind to treatment groups recorded total successes, successes on the first attempt, and failures. A trial was considered successful when an animal grasped the pellet with the appropriate paw, transported it into the box, and placed it into its mouth without allowing the pellet to touch the floor.
Allodynia Testing
Control and injured animals were assessed for signs of allodynia with up-down Von Frey monofilament testing. Testing with filaments up to 6.10 was used weekly during the termination of the transplantation experiment in order to detect changes in tactile sensory thresholds.
Statistical Analysis
For each treatment group, the difference in LUAT scores at 3 weeks post-injury and 8 weeks post-transplant was compared using a paired two-tailed t-test. All errors are SEM unless otherwise indicated.
Study Approval
All animal-related procedures were approved by the Institutional Animal Care and Use Committee of the University of Washington and were conducted in accordance with the guidelines of the NIH.
Results
Human induced pluripotent stem cells generate neural stem cells and can be further differentiated into specific neural lineages
We tested the ability of hiPSCs to first form neuroepithelial stem cells, and then further differentiate into cells of neural lineages. The differentiation protocol is outlined in Fig. 1A. After two weeks, hiPSC colonies contained radially-oriented columnar cells that formed neural tube-like rosettes (Fig. 1B). After 45 days, adherent aggregates (Fig. 1C) produced TuJ1-positive neurons (70.2 ± 10.7%, Fig. 1E) and GFAP-positive astrocytes (Fig. 1F). Prior to transplantation, hiPSC-NPCs formed a monolayer displaying complex progenitor morphologies (Fig. 1D). The caudalizing factor retinoic acid (RA) was included during differentiation to induce the expression of the hindbrain/spinal cord-specific transcription factor HoxB4 (Bass and Baker, 1997; Hu and Zhang, 2009) (Fig. 1H). A small percentage (9.8 ± 2.2%) of hiPSC-NSCs expressed Sox9 (Fig. 1I), a marker of neural stem cells(Cheng et al., 2009; Scott et al., 2010) and an essential component of the developmental switch from neurogenesis to gliogenesis (Stolt et al., 2003; Kang et al., 2012). In contrast, another hiPSC line differentiated only to the neuronal epithelial stage was uniformly HoxB4-positive, but expressed much higher levels of Sox9 (94.7 ± 2.1%) (Fig. S1).
Figure 1. hiPSCs form region-specific neural progenitor cells.
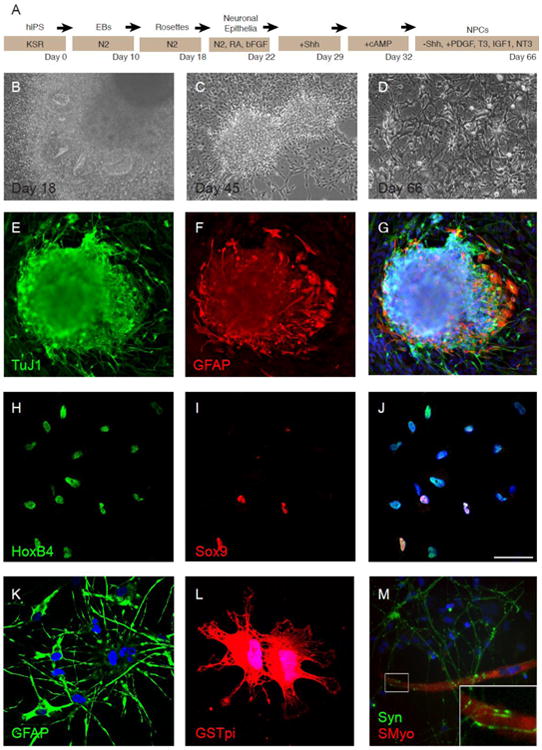
(A) Outline of differentiation protocol. (B-D) Morphological characteristics of differentiated IMR90 hiPSCs. At day 18 of differentiation, IMR90 hiPSCs formed neural rosettes (B), while at day 45, hiPSCs displayed a multipolar morphology and coalesced into neurobuds consistent with NPCs (C). Caudalized hiPSC-NPCs at day 66, just prior to transplant, tended toward a more complex morphology, as indicated in (D). (E-G) At day 45, differentiated hiPSCs expressed the pan-neuronal marker Tuj1 (E), and the astrocyte marker GFAP (F). (H-J) hiPSC-NPCs uniformly express HoxB4(H), and demonstrate expression of Sox9 (I). (K) Most hiPSC-NPCs express GFAP. (L) A small subset of hiPSC-NPCs express the oligodendrocyte marker GSTpi. (M) When co-cultured with RMT fibers, hiPSC-NPCs express synapsin and are observed in close association with muscle fibers (inset). Nuclei in (G, J, K-M) are counterstained with DAPI. Error bars are SEM. Scale bars = 50 μm.
A very high percentage of hiPSC-NPCs displayed a stellate morphology and weakly expressed the astrocytic marker GFAP (Fig. 1K). A smaller proportion expressed the mature oligodendrocyte marker GSTpi (Fig. 1L). These cells exhibited morphologic characteristics of oligodendrocytes, including membranous sheets with lacy appearances (Dyer and Benjamins, 1989; Dyer and Benjamins, 1989). A subset of cells also expressed mature neuronal markers including neurofilament (56.4 ± 9.7%) and vesicular acetylcholine (62.6 ± 7.8%) (data not shown). Further, when co-cultured with sMyo-positive RMT myofibers, synapsin-positive hiPSC-NPCs were observed in close association with the muscle fibers (Fig. 1M), suggesting the ability to make the physical contact necessary to form a neuromuscular junction.
hiPSC-NPCs express decreased levels of pluripotency genes and increased levels of neural genes
To further characterize hiPSC-NPCs, TaqMan low-density array (LDA) analysis was performed, with hESCs, undifferentiated IMR90 hiPSCs and IMR90 fibroblasts (FIB) serving as controls. Compared to both hESCs and hiPSCs, hiPSC-NPCs expressed significantly lower levels of several pluripotency markers, including LIN28, NANOG, POU5F1(OCT4), and UTF1 (Fig. 2A). Additionally, immunocytochemical analysis indicated an absence of OCT4 expression (data not shown). hiPSC-NPCs also expressed lower levels of telomerase, a reverse transcriptase important in maintaining telomere length, compared to pluripotent cells. Meanwhile, hiPSC-NPCs displayed upregulated levels of neural stem cell markers PAX6 and SOX9. As expected, high levels of SOX2 were observed in hESCs, hiPSCs and hiPSC-NPCs compared to fibroblasts. SOX2 is a transcription factor essential in maintaining self-renewal and pluripotency of undifferentiated hESCs (Adachi et al., 2010) and one of the key transcription factors required in hiPSCs (Zhao and Daley, 2008). It is also a marker for multipotent neural stem cells (Ellis et al., 2004).
Figure 2. Caudalized hiPSC-NPCs downregulate pluripotency genes and broadly upregulate neuron and glial specific genes.
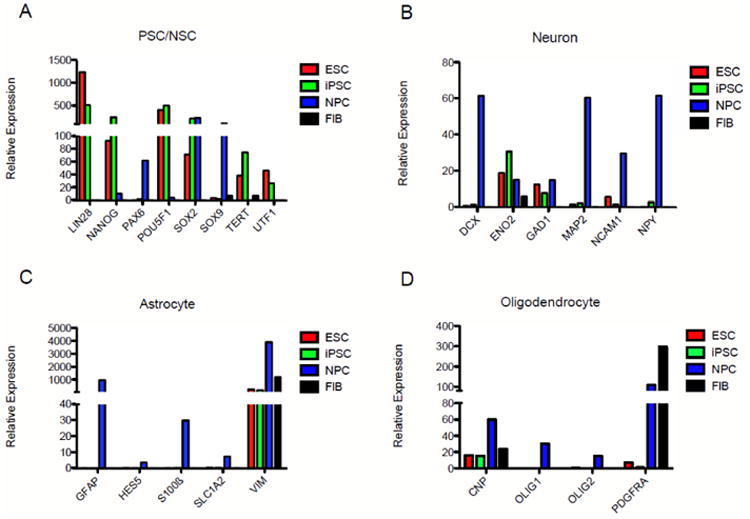
TaqMan low-density array data is arranged by pathway. (A) Pluripotent stem cell and neural stem cell markers. ESCs and hiPSCs expressed high levels of pluripotency markers, while hiPSC-NPCs downregulated these same genes and dramatically elevated expression of the neural stem cell markers PAX6 and SOX9. (B) Neuronal markers. hiPSC-NPCs expressed elevated levels of neuron-specific transcripts including DCX, MAP2, NCAM1 and NPY compared to pluripotent ESC or iPSC controls. (C) Astrocyte markers. hiPSC-NPCs expressed dramatically higher levels of GFAP and S100β and modest elevation of SLC1A2. (D) Oligodendrocyte markers. hiPSC-NPCs expressed many genes of the oligodendrocyte lineage, including CNPase and PDGRα. OLIG1 and OLIG2 were uniquely expressed in hiPSC-NPCs when compared to pluripotent stem cell controls.
hiPSC-NPCs generally expressed elevated levels of genes specific to cells of neuronal and glial lineages as well. For neurons, these included doublecortin (DCX), microtubule-associated protein 2 (MAP2), neural cell adhesion molecule 1 (NCAM1), and neuropeptide Y (NPY) (Fig. 2B). hiPSC-NPCs also expressed neuron-specific enolase (ENO2) and glutamic acid decarboxylase (GAD1), though levels were comparable to pluripotent cell types.
Neural induction of hiPSCs resulted in upregulation of several astrocyte markers (Fig. 2C), most notably glial fibrillary acidic protein (GFAP), consistent with the expression seen in Fig. 1N. Caudalized hiPSC-NPCs were also the only cell type to detectably express the immature astrocyte marker S100β, the astrocyte-specific glutamate transporter SLC1A2, and HES5, a transcription factor thought to regulate astrocyte v. neuron fate of neural stem cells. The intermediate filament vimentin (VIM) was expressed at the highest levels by hiPSC-NPCs, though all four cell types expressed high levels of this marker. Within the nervous system, vimentin is typically used as an immature or reactive astrocyte marker, though it is also expressed by many other cell types throughout the body and thus is not expected to be CNS-specific.
Oligodendrocyte lineage markers expressed by hiPSC-NPCs included CNPase (CNP), olig1, and olig2 (Fig. 2D). The platelet-derived growth factor receptor PDGFRα, an oligodendrocyte precursor cell marker, was also expressed at very high levels compared to pluripotent cell types, though somewhat lower than fibroblasts, which are also known to express this receptor.
Unilateral cervical contusion results in cavitation, extensive myelin loss and gliosis
To create a clinically relevant model of cervical spinal cord injury, we adapted a fourth generation Ohio State SCI Device (Fig. 3A). Animals received a unilateral C4 contusion injury on the side of the spinal cord corresponding to the dominant forelimb, as determined by training on the forelimb reaching task.
Figure 3. A chronic cervical hemi-contusion model that recapitulates human pathology.
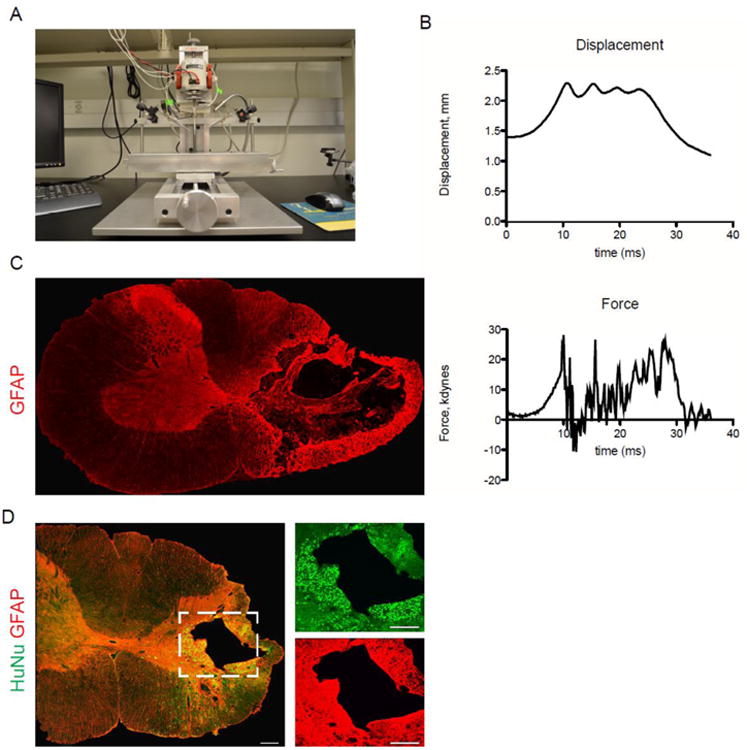
(A) Fourth generation of the OSU injury device. (B) Contusion injury data: representative displacement and force tracings from one injury. (C) Four weeks after injury, GFAP staining reveals substantial cavitation and reactive gliosis on the side of contusion. (D) Eight weeks after transplant, hiPSC-NPCs have survived and integrated in the chronic contusion model. Most cells remain close to the site of injection (i.e. near the gray-white border of the dorsolateral funiculus and juxtaposed to the lesion cavity), but many migrated throughout the ipsilateral white and gray matter.
Histologic signs of traumatic injury in this model occur primarily in the dorsolateral funiculus, where the lesion epicenter includes substantial cavitation, widespread loss of myelin in the lateral white matter, and a rim of reactive astrocytosis surrounding the lesion cavity (Fig. 3C-D) In contrast to midline thoracic contusions, which typically display considerable mediolateral extension, cavitation and signs of cellular loss in this model are limited to the side of injury. Up to 8 weeks after transplantation, caudalized hiPSC-NPCs had survived and integrated into the injured spinal cord. The average number of cells per animal was 169,126 ± 65,359. Most cells were found in juxtaposition to the lesion cavity, however, cells could be observed throughout the injured hemicord (Fig. 3D). Caudalized hiPSC-NPCs were observed in the gray matter, in the dorsal columns and the ventral white matter, with some cells in close proximity to the ventral pia mater. No cells were observed on the contralateral side of the spinal cord, though some could be found near the anterior median fissure.
hiPSC-NPCs generate specific neuronal subtypes and influence host neuron distribution
Staining for neuron-specific NeuN/FOX-3 labeled a small proportion of grafted hiPSC-NPCs, indicating mature neuron formation (Fig. 3A). hiPSC-NPC projections were also observed tightly surrounding host neurons, suggesting integration of the cells into host networks. Transplanted cells did not express the glutamate receptor GluR1 (Fig. 3B). Many host cells were observed to express the AMPA receptor GluR1, but none appeared to interact with the grafted cells (data not shown). hiPSC-NPCs also did not express the neurotransmitter serotonin (Fig. 3C), but did stain positively for the inhibitory neurotransmitter GABA (Fig. 3D). Additionally, many GABAergic host neurons were detected in the host white matter corresponding to the corticospinal tract (Fig. 3D, right) in animals receiving hiPSC-NPCs, while such neurons were rarely observed in the contralateral corticospinal tract (Fig. 3D, right), or in the same location in control groups (data not shown). In all rats studied, thick choline acetyltransferase-positive (ChAT+) projections from host neurons were frequently observed surrounding or immediately adjacent to transplanted cells (Fig. 3E). There was an increase in the number and the thickness of host ChAT processes compared to the corresponding contralateral region (Fig. 3E, right).
hiPSC-NPCs continue to divide and form neurons and astrocytes in the chronically injured cord 4 weeks after transplant
Four weeks after transplantation, some transplanted cells expressed Ki-67 (5.1 ± 0.7%) (Fig. 5A), a protein strictly associated with proliferation (Scholzen and Gerdes, 2000). Many caudalized hiPSC-NPCs stained positively for a human-specific GFAP marker (hGFAP) (30.2 ± 3.3%), while none were observed to express MBP (Fig. 5B). In fact, hGFAP+ cells were often well-demarcated from MBP+ regions of the cord, demonstrating relatively little physical contact between transplanted cells and myelin.
Figure 5. Caudalized hiPSC-NPCs four weeks after transplant divide and express Sox9.
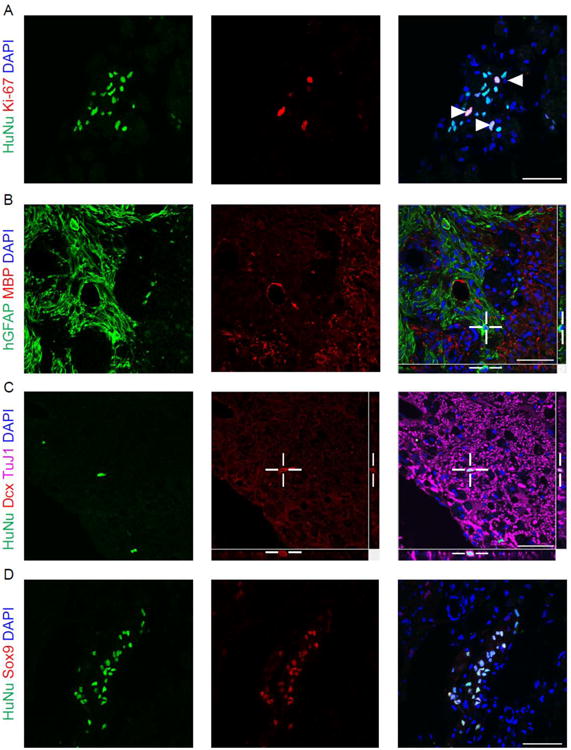
(A) NPCs continue to divide after transplantation. A percentage of HuNu-positive cells also express the proliferation marker Ki-67. Arrowheads indicate HuNu/Ki-67 double-positive cells. (B) NPCs are positive for a human-specific GFAP antigen, but do not express MBP. Merge indicates little association of hGFAP-positive cells with MBP-positive regions of the injured cord. (C) Exogenous formation of immature neurons is rare after four weeks. HuNu-positive cells infrequently express doublecortin. Z-stack merge confirming colocalization of Dcx with HuNu. (D) A higher percentage of NPCs express Sox9 in vivo than in vitro. Scale bars = 50μm.
We observed some HuNu+ cells expressing doublecortin (15.4 ± 4.6%) (Fig. 5C, 6C), a microtubule-associated protein expressed by neuronal precursor cells and retained during early neuronal maturation (Brown et al., 2003). Orthogonal views in Figure 5G confirm the association of doublecortin and the neurogenic capacity of transplanted hiPSC-NPCs in the chronically injured cord. HuNu+/Dcx+ cells also expressed the pan-neuronal marker beta-tubulin, as did many HuNu+/Dcx- cells (data not shown), providing further evidence for neurogenesis by caudalized hiPSC-NPCs.
Figure 6. Caudalized hiPSC-NPCs eight weeks after transplant stop dividing and differentiate into neurons and glia.
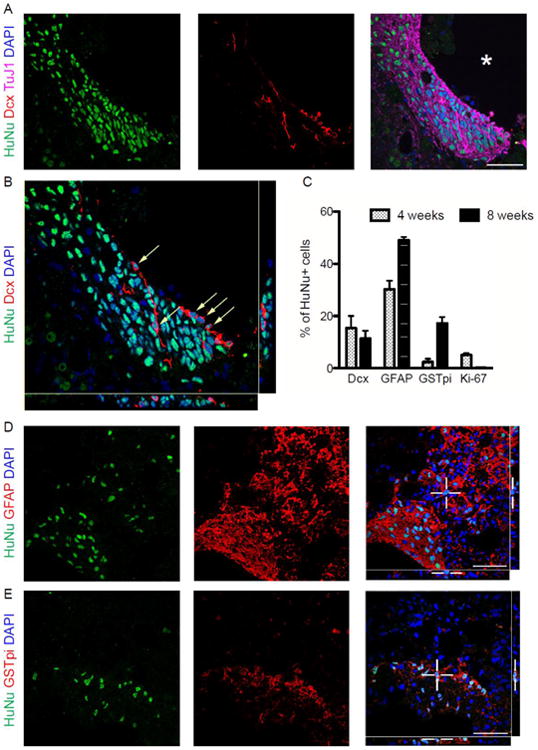
(A) HuNu-positive cells adjacent to the lesion cavity (asterisk) express the immature neuronal marker doublecortin and the pan-neuronal marker Tuj1. (B) Z-stack imaging of Dcx-positive hiPSC-NPCs (arrows) confirms neurogenesis in the injured spinal cord. (C) Phenotypic analysis of hiPSC-NPCs shows the percentages of Dcx-positive neurons, GFAP-positive astrocytes and GSTpi-positive oligodendrocytes at 4 and 8 weeks after transplant. (D) A portion of NPCs retain expression of GFAP. (E) Some NPCs have differentiated into GSTpi-positive oligodendrocytes. Error bars are SEM. Scale bars = 50 μm.
While less than 10% of hiPSC-NPCs were Sox9+ in vitro, a much higher percentage expressed the marker in vivo (Fig. 5D), suggesting either a selection for - or acquisition of - this phenotype in the spinal cord.
hiPSC-NPCs form neurons, astrocytes and oligodendrocytes 8 weeks after transplant
Eight weeks after transplantation, a high percentage of transplanted cells near the injury cavity expressed the neuronal marker beta-tubulin (Fig. 6A). A subset of these also expressed doublecortin (11.4 ± 3.0%) (Fig. 6A-C), and some HuNu+/Dcx+ cells displayed bipolar morphologies consistent with those of newly generated migrating neurons.
A large percentage of transplanted cells expressed glial markers as well, including GFAP (49.1 ± 1.2%) (Fig. 6D) and the oligodendrocyte marker GSTpi (17.2 ± 2.4%) (Fig. 6E). No Ki-67+ cells were detected at this time point, suggesting that hiPSC-NPCs were no longer dividing 8 weeks after transplant.
Behavioral analysis indicates limited improvement in grasping or weight-bearing ability after hiPSC-NPC transplantation compared to sham controls
Despite thorough integration and differentiation into both neurons and glia, assessment of behavioral recovery indicates that transplantation of hiPSC-NPCs did not confer significant improvement on either the forelimb reaching task (Fig. 7A) or the limb-use asymmetry test (LUAT) (Fig. 7B). Neither the hiPSC-NPC group nor any of the control groups exhibited improvement on the FRT. Animals in the hiPSC-NPC and sham group showed statistically significant improvement in their LUAT scores (hiPSC-NPC: p=0.0092, sham: p=0.0032) (Fig. 7C). Animals receiving either PBS or IMR90 fibroblasts demonstrated little change in their paw preferences. Comparisons across groups are not valid for the LUAT, as treatment assignments were based on FRT scores, thus the average performance on the LUAT was not similar across groups prior to transplant. Finally, no change in tactile sensory thresholds was detected during weekly assessment with Von Frey filaments up to 6.10.
Figure 7. Transplants of caudalized hiPSC-NPCs do not significantly improve motor function compared to control conditions.
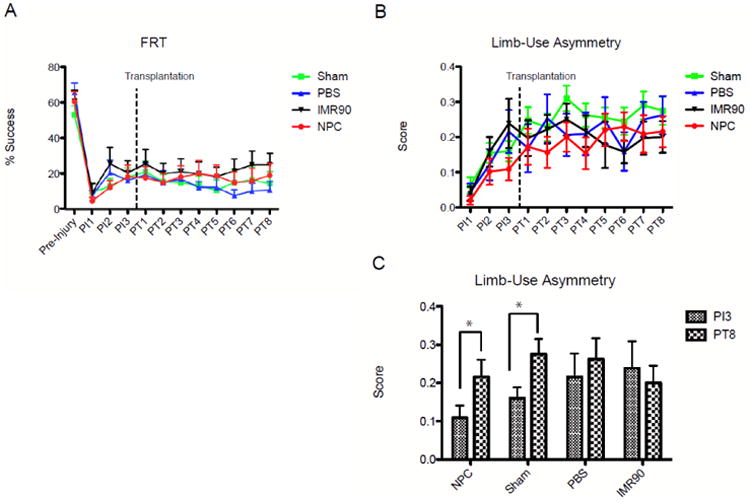
(A) Weekly performance on the forelimb reaching task, an indicator of volitional functional recovery. No significant difference was observed among NPC and control groups. (B) Weekly performance on limb-use asymmetry test (LUAT), an indicator of paw preference and weight-bearing ability. (C) Comparison of pre-transplant to final LUAT scores, indicating improvement in both the NPC and sham treatment groups. PIx indicates x weeks after injury. PTx indicates x weeks after transplant. * = p < 0.01. Error bars are SEM.
Discussion
Neural transplantation shows great promise for promoting cell replacement and plasticity, particularly in the acute and sub-acute period following spinal cord injury. Treatment of the chronically injured spinal cord has proven significantly less tractable. Research efforts have been limited, in part due to the scientific challenges of working with chronic animal models of scarring and a decreased inherent plasticity of the aging nervous system. But there are also practical limitations that have left this clinical population underserved, including the exceptionally demanding laboratory time and cost of conducting large-scale, blinded studies in chronic animal models. Here we developed an early chronic model of cervical SCI which mimics many of the clinical characteristics of human cervical injury. We focused on the development of an exciting new cell engineering technology, hiPSCs, to create a potentially autologous source of NPCs to use as transplantable resource for neural cell replacement. To our knowledge, this is the first study describing the transplantation of hiPSC-NPCs into the early chronically injured spinal cord. These studies indicate that caudalized hiPSC-NPCs develop into multiple neural lineages that survive for up to two months post-transplant without risk of overgrowth or loss of function.
The injury model developed corresponds closely to the most common forms of spinal cord injury; contusions are the most commonly observed type of spinal cord injury, and the cervical region is the most often injured. A recent systematic review (Tetzlaff et al., 2011) reported the paucity of data on the transplantation of human cells into chronic cervical models of SCI. This gap in the SCI community's knowledge base is reflected in a recent survey of opinion in which SCI researchers expressed strong support for the demonstration of preclinical evidence of efficacy in cervical injury models prior to conducting human SCI trials (Kwon et al., 2010). Further clinical relevance arises from the chronic time point chosen for transplantation, one that is far less frequently studied than acute and sub-acute transplants despite the fact that there are currently 1.275 million people in the U.S. living with chronic SCI. There has been a recent shift among SCI researchers in the criteria for what constitutes a chronic time point, when relative stability in both the injury environment and behavioral recovery is achieved. For humans, this is generally considered to be 12 months post-injury. The corresponding time point in rats is still under debate. Previously, this was thought to occur by about 30 days post-injury (Houle and Tessler, 2003; Fawcett et al., 2007), but recently the field has moved toward defining the threshold for ‘chronic’ at 6 weeks after injury (Anderson et al., 2005; Kwon et al., 2010). Thus, by transplanting at 4 weeks post-injury, our model may be better characterized as ‘early chronic.’ Whereas most studies assessing the efficacy of transplanted cells in spinal cord injury use locomotion to monitor recovery, we chose to observe forelimb function. For human SCI patients, arm use and grasping ability are the important determinants of overall quality of life and independence (Anderson, 2004). Even a small recovery in hand function could result in significant benefits. Skilled reaching is useful for assessing injury to motor systems in a more objective manner than locomotion scales. It is also a volitional task that requires greater descending input from the brain compared to locomotor movements, where local spinal circuits may dominate (de Leon et al., 1999; Tillakaratne et al., 2000; Tillakaratne et al., 2002). While we did not observe a significant difference in performance on the FRT, it is possible that with chronic injuries there is a greater temporal requirement for observation of improvement. Future studies should include longer observation periods to assess the long-term effects, if any, of hiPSC-NPC transplant on behavior. Performance on the LUAT demonstrated a modest but statistically significant improvement in weight-bearing ability in the NPC group, though a similar improvement was also seen in the sham group. Still, even this limited enhancement of function is somewhat promising for early chronic SCI, in which no cell-based therapy to date has shown improvement. While previous studies have shown efficacy in the acute and subacute injury environment (Nori et al., 2011), it has previously been shown that therapeutic benefits in these settings does not guarantee similar effects at later time points (Keirstead et al., 2005). Still it would be informative to test whether the caudalized hiPSC-NPCs employed here are more efficacious at acute or subacute time points, indicating a possible influence of environment rather than intrinsic characteristics that limit the cells' ability to induce recovery.
There are many potential approaches to repair of the injured spinal cord through cell therapy. Investigators have transplanted immature neural stem cells, committed progenitors and purified populations of astrocytes, oligodendrocytes and neurons (Ikegami et al., 2005; Mitsui et al., 2005; Karimi-Abdolrezaee et al., 2010; Tetzlaff et al., 2011; Yuan et al., 2011). In the current work, we established a multipotent neural progenitor population with the capacity to accomplish multiple lineage replacement. We show that these caudalized hiPSC-NPCs form cells of both neuronal and the two macroglial lineages, astrocytes and oligodendrocytes in vitro and in vivo. The key defining features of our cells include loss of pluripotent stem cell markers, region specificity, and a multipotent NPC phenotype. TaqMan LDA analysis demonstrated the loss of telomerase, the pluripotency marker UTF1, and three of the four reprogramming factors. The hiPSC-NPCs transplanted in our injury model uniformly expressed the hindbrain/spinal cord-specific marker HOXB4, a homeobox protein restricted to the region of the neural tube caudal to the border between rhombomeres 6 and 7 during development. Inclusion of retinoic acid in the differentiation of pluripotent cells is important in conferring this regional specificity (Hu and Zhang, 2009). In our experience and in work published by other groups, neuralization of hiPSCs without retinoic acid does not confer HoxB4 expression (Hu and Zhang, 2009; Krencik et al., 2011), instead resulting in widespread expression of Otx2, indicating forebrain specificity (Li et al., 2005; Pankratz et al., 2007). Only a small fraction (14.6%) of caudalized hiPSC-NPCs expressed the motor neuron marker Hb9. While motor neurons have frequently been targeted in cell replacement strategies for SCI, replacement of other neuronal phenotypes can also be beneficial, including generation of interneurons to form new functional relay circuits (Lu et al., 2012). hiPSC-NPCs were grafted in part to stimulate such plasticity, as this may also be more practical than long tract regeneration.
Caudalized hiPSC-NPCs were also largely GFAP-positive and expressed reduced levels of the neural stem cell (NSC) marker Sox9 as compared to cells at earlier stages of differentiation. However, the cells displayed multipotency in vitro and in vivo, indicating a neural progenitor phenotype, rather than mature astrocytes. Thus, our caudalized hiPSC-NPCs were well-suited to generate neurons and glia in the adult spinal cord without risk of contamination by undifferentiated cells.
Key concerns regarding transplantation into the injured spinal cord are the relative survival of grafted cells (Anderson et al., 2011) and the potential for overgrowth or tumor formation (Tsuji et al., 2010; Nori et al., 2011). There is a dearth of defined practices for striking a balance of controlled survival. Using the protocol we have described here, we found that grafted hiPSC-derived neural progenitor cells survived remarkably well when injected juxtaposed to the lesion epicenter of the chronically injured spinal cord. Early attempts at transplantation in a medium of PBS alone, or in PBS+DNase proved unsuccessful (unpublished observations) due to poor cell viability. In the presented work we utilized a solution of DNase and glucose in PBS that proved effective. Cells were well-distributed throughout mediolateral and dorsoventral axes of the ipsilateral hemicord, and found in the white and gray matter. On average, approximately 169,000 cells were detected at 8 weeks after transplant (out of 200,000 injected). As a percentage of cells transplanted, this differs markedly from the study by Salazar et al cited earlier, where transplanting 75,000 human cells at 30 days post-injury resulted in 215,000 cells after 16 weeks, indicating substantial proliferation without apparent deleterious effects. The number of cells injected may have important implications for functional recovery, as it is possible that 200,000 cells is insufficient to generate a detectable improvement in reaching performance. The Keirstead study cited earlier injected far more (1.5 million cells). Thus, a dose-response experiment with caudalized hiPSC-NPCs would be a useful future experiment to test this possibility. In our work the relative safety of caudalized hiPSC-NPCs is also likely, as no masses were detected and Ki-67 expression reduced to zero by 8 weeks after transplant. We also performed weekly testing for allodynia with Von Frey filaments, as this adverse effect has been noted in previous experiments with transplantation of stem cells in SCI, and this phenomenon was never observed, further supporting the relative safety of hiPSC-NPCs.
While hiPSC-NPCs could be found throughout the gray and white matter, most were confined to the perilesional area. The relative lack of migration in these cells could be due to a number of factors, both intrinsic (e.g., terminal differentiation) and extrinsic, such as the influence of glial scar on migration and proliferation. Hyaluronic acid, for example, is an important element of the extracellular matrix after injury and has been shown to reduce progenitor proliferation (Back et al., 2005; Khaing et al., 2011). Other scar signals affecting migration and differentiation include chondroitin sulfate proteoglycans (Silver and Miller, 2004; Busch and Silver, 2007). Together, these factors could contribute to the phenotypes and cessation in proliferation observed at 8 weeks after transplant.
It has been shown that oligodendrocyte progenitor cells are ineffective in chronic injury (Keirstead et al., 2005). Neural progenitor cells, however, have shown promise (Cummings et al., 2005; Salazar et al., 2010) and here we implanted a multipotent cell capable of creating the three major neural lineages. Interestingly, despite the majority of cells expressing the classic astrocyte marker GFAP prior to transplant, hiPSC-NPCs showed multipotentiality in vivo, generating neurons, astrocytes and oligodendrocytes. This plasticity is consistent with recent findings in the field, as adult rodent NSCs are GFAP-positive. In addition, several groups have noted the stem cell potential of astrocytes, a capacity augmented by injury (Doetsch, 2003; Vaccarino et al., 2007; Chong and Chan, 2010; Robel et al., 2011).
While <10% of caudalized hiPSC-NPCs were positive for the NSC marker Sox9 prior to transplant, a substantially greater percentage stained positively for Sox9 in vivo. The increase in percentage of Sox9+ cells among hiPSC-NPCs after transplant could be attributed to either a selective survival among cells already expressing Sox9 prior to injection, or to acquisition of positivity after transplant. Other groups have observed dramatically reduced survival of fully differentiated hiPSC-derived neural cells as compared to hiPSC-derived stem and progenitor cells (Rhee et al., 2011; Yuan et al., 2011), indicating that selective survival is more likely to be the mechanism for our observed change in Sox9 reactivity. The work reported here, however, did not explicitly test this possibility. Future studies including lineage tracing could be helpful in determining which mechanism (selective survival or Sox9 acquisition) is more relevant.
Some important differences in phenotype and proliferation were observed between the 4-week and 8-week time points, indicating that the profile of transplanted cells evolves over a time course on the order of weeks and months. One difference is that Ki-67+ cells were detected at 4 weeks, but not at 8, indicating that proliferation occurred for some time after transplant, but eventually ceased. This is an important consideration, as concerns about the tumorigenicity of hiPSC transplants remain. Though our cells were differentiated for months prior to transplant, contamination with undifferentiated cells and/or dedifferentiation of NPCs remained potential avenues for tumorigenesis, though no signs of masses or other deleterious effects of overgrowth were observed. Studies in other models of CNS disease such as Parkinson's and stroke have shown masses, neural tube-like rosettes and/or Ki-67+ cells after transplant of hiPSC-derived neural cells (Jensen et al., 2011; Rhee et al., 2011), emphasizing the importance of the absence of these features in our study.
hiPSC-NPCs showed a limited capacity for neurogenesis in vivo, and most of the neurons formed were of the inhibitory GABAergic phenotype. This may be related to the relative youth of these neurons, for in many CNS regions in rodents and primates expression of GABA receptors precedes that of glutamate receptors in newborn neurons. The influence of grafted cells on host GABA expression may also be consistent with hiPSC-NPCs forming young neurons, as newly generated neurons form GABAergic synapses before receiving glutamatergic synapses in other regions of the CNS (Tyzio et al., 1999; Hennou et al., 2002; Ben-Ari, 2006). The increase in host ChaT+ process number and thickness indicates sprouting of axon-like processes that meet some morphological and molecular criteria of axons, but whether they meet the functional capacity of an axon is unknown. Similarly, whether such reorganization can be translated into a mechanism for functional recovery remains to be seen.
Interestingly, the presence of transplanted neural progenitor cells did not translate into myelin repair. By four weeks after transplant, no oligodendrocytes were detected, and grafted cells were well-demarcated from myelinated regions of the cord, despite the fact that almost all NPCs were located in the white matter at this time point. By 8 weeks, 17.1% of NPCs formed GSTpi+ oligodendrocytes, though none could convincingly be demonstrated to form myelin. The lack of myelin repair may correlate with the lack of chronic demyelination (Powers et al., 2012) in that there is not a large population of otherwise intact axons to remyelinate at chronic time points. It is also possible that the inclusion of both sonic hedgehog and cAMP in the differentiation protocol predisposed some cells to a neuronal phenotype rather than an oligodendrocyte lineage, as these signals are implicated in the specification and survival of both cell types.
Conclusion
These studies demonstrate that hiPSCs can be used to deliver multiple neural cell phenotypes to the early chronically injured spinal cord. While we only observed a modest recovery of function, we established a preliminary safety margin of this particular cell type, did not see overgrowth or delayed proliferation and did not detect any decline in function. We followed our transplants for two months, but future studies may need to extend this timeframe and also consider complementary therapies such as physical therapy, electrical stimulation, or growth factor delivery. Interestingly, the number of oligodendrocytes and Dcx+ immature neurons increased from 4 weeks to 8 weeks in our model. Hence, it is reasonable to suspect that hiPSC-NPCs may still have been migrating and differentiating two months after transplant. If this is an indication of continued cell plasticity, and LUAT scores were still trending upward, then longer follow-up periods or combinatorial therapies to direct transplanted cells are clearly warranted. It is also reasonable to presume that recovery may take longer in the early chronic injury setting than in the acute phase, as the injury environment is more dynamic shortly after injury, and it is likely that any endogenous repair mechanisms have concluded after 4 weeks since behavioral recovery has plateaued. In conclusion, hiPSC-derived neural progenitor cells show promise as a potential method to introduce cellular plasticity in the early chronically injured spinal cord.
Supplementary Material
Figure 4. hiPSC neuronal subtypes and influence of hiPSC-NPCs on host neurons.
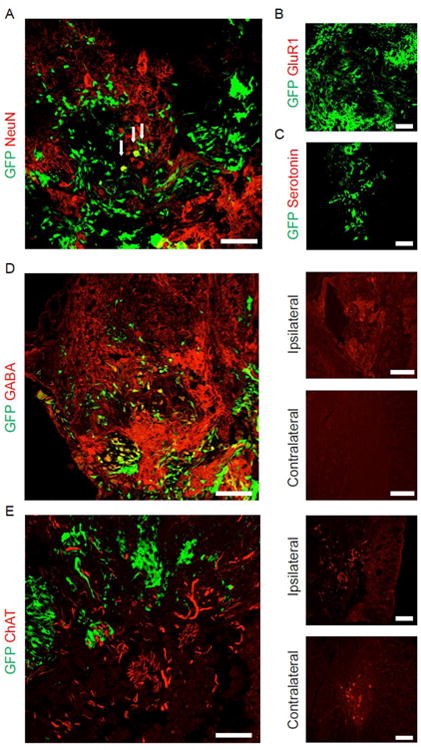
(A) Projections from grafted hiPSC-NPCs surrounding host NeuN+/GFP- neurons. A number of hiPSC-NPCs formed NeuN+/GFP+ neurons. (B) hiPSC-NPCs did not form excitatory GluR1+ cells. (C) No hiPSC-NPC serotonergic cells were observed. (D) GABAergic hiPSC-derived neurons in the ipsilateral corticospinal tract. There was an increase in GABA signal compared to the contralateral region of the same section. (E) Increase in number and thickness of host ChAT+/GFP- neuronal projections surrounding GFP+ hiPSC-NPCs. Ipsilateral and contralateral panels are taken from the same coronal section. Scale bar = 200 μm, except in “Ipsilateral”/“Contralateral” panels, where scale bar = 400 μm.
Highlights for Nutt et al.
A consistent, chronic preclinical model of cervical spinal cord injury with measurable limb and hand motor function tests.
Human iPSC can be caudalized with retinoic acid and remain multipotent in vivo.
Treatment of chronic injury with an iPSC-derived neural stem cell transplant leads to limited recovery.
Acknowledgments
This work was supported by NIH grant NS066357 (PJH & CTM), a grant from the Mike Utley Foundation (PJH & SEN), and a generous gift from the Irv Naylor Foundation (PJH & JBC). MRC5 hiPSCs were generated by Angelique M. Nelson at the University of Washington Tom & Sue Ellison Stem Cell Core. Confocal images were generated in the University of Washington Lynn and Mike Garvey Cell Imaging Lab. PJH is a member of the University of Washington Center on Human Development and Disability.
Footnotes
Authorship Note: Samuel E. Nutt and Eun-Ah Chang contributed equally to this work.
Author contributions: S.E.N: conception and design, collection and assembly of data, data analysis and interpretation, and manuscript writing; E.A.C: collection and assembly of data, data analysis and interpretation, and manuscript writing; S.T.S: collection and assembly of data, data analysis and interpretation; L.O.S: collection and assembly of data, data analysis and interpretation; C.T.M.: provision of study materials, financial support; J.B.C: conception and design; P.J.H: conception and design, manuscript writing, and final approval of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Prevalence of Paralysis. 2009 Retrieved May 17, 2012, from http://www.christopherreeve.org/site/c.mtKZKgMWKwG/b.5184255/k.6D74/Prevalence_of_Paralysis.htm.
- Spinal Cord Injury Facts and Figures at a Glance. Retrieved May 17, 2012, from https://www.nscisc.uab.edu/PublicDocuments/nscisc_home/pdf/Facts%202011%20Feb%20Final.pdf.
- Adachi K, Suemori H, et al. Role of SOX2 in maintaining pluripotency of human embryonic stem cells. Genes Cells. 2010;15(5):455–470. doi: 10.1111/j.1365-2443.2010.01400.x. [DOI] [PubMed] [Google Scholar]
- Alaverdashvili M, Whishaw IQ. Compensation aids skilled reaching in aging and in recovery from forelimb motor cortex stroke in the rat. Neuroscience. 2010;167(1):21–30. doi: 10.1016/j.neuroscience.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Anderson AJ, Haus DL, et al. Achieving stable human stem cell engraftment and survival in the CNS: is the future of regenerative medicine immunodeficient? Regen Med. 2011;6(3):367–406. doi: 10.2217/rme.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DK, Beattie M, et al. Recommended guidelines for studies of human subjects with spinal cord injury. Spinal Cord. 2005;43(8):453–458. doi: 10.1038/sj.sc.3101746. [DOI] [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Back SA, Tuohy TM, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11(9):966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- Bass AH, Baker R. Phenotypic specification of hindbrain rhombomeres and the origins of rhythmic circuits in vertebrates. Brain Behav Evol. 1997;50(Suppl 1):3–16. doi: 10.1159/000113351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Basic developmental rules and their implications for epilepsy in the immature brain. Epileptic Disord. 2006;8(2):91–102. [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17(1):120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Cheng LC, Pastrana E, et al. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12(4):399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SY, Chan JR. Tapping into the glial reservoir: cells committed to remaining uncommitted. J Cell Biol. 2010;188(3):305–312. doi: 10.1083/jcb.200905111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102(39):14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon RD, Tamaki H, et al. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J Neurophysiol. 1999;82(1):359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6(11):1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Dyer CA, Benjamins JA. Organization of oligodendroglial membrane sheets: II. Galactocerebroside:antibody interactions signal changes in cytoskeleton and myelin basic protein. J Neurosci Res. 1989;24(2):212–221. doi: 10.1002/jnr.490240212. [DOI] [PubMed] [Google Scholar]
- Dyer CA, Benjamins JA. Organization of oligodendroglial membrane sheets. I: Association of myelin basic protein and 2′,3′-cyclic nucleotide 3′-phosphohydrolase with cytoskeleton. J Neurosci Res. 1989;24(2):201–211. doi: 10.1002/jnr.490240211. [DOI] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26(2-4):148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Curt A, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45(3):190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Vawda R. Cellular treatments for spinal cord injury: the time is right for clinical trials. Neurotherapeutics. 2011;8(4):704–720. doi: 10.1007/s13311-011-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y, Abematsu M, et al. Treatment of a Mouse Model of Spinal Cord Injury by Transplantation of Human iPS Cell-derived Long-term Self-renewing Neuroepithelial-like Stem Cells. Stem Cells. 2012 doi: 10.1002/stem.1083. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Tovar CA, et al. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J Neurotrauma. 2006;23(1):36–54. doi: 10.1089/neu.2006.23.36. [DOI] [PubMed] [Google Scholar]
- Hennou S, Khalilov I, et al. Early sequential formation of functional GABA(A) and glutamatergic synapses on CA1 interneurons of the rat foetal hippocampus. Eur J Neurosci. 2002;16(2):197–208. doi: 10.1046/j.1460-9568.2002.02073.x. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Holmstrom NA, et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8(3):346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Stokes BT. Fetal transplantation following spinal contusion injury results in chronic alterations in CNS glucose metabolism. Exp Neurol. 1995;133(2):231–243. doi: 10.1006/exnr.1995.1026. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tessler A. Repair of chronic spinal cord injury. Exp Neurol. 2003;182(2):247–260. doi: 10.1016/s0014-4886(03)00029-3. [DOI] [PubMed] [Google Scholar]
- Hu BY, Zhang SC. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc. 2009;4(9):1295–1304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Nakamura M, et al. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth-associated protein-43-positive fibers after rat spinal cord injury. Eur J Neurosci. 2005;22(12):3036–3046. doi: 10.1111/j.1460-9568.2005.04492.x. [DOI] [PubMed] [Google Scholar]
- Iwanami A, Kaneko S, et al. Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res. 2005;80(2):182–190. doi: 10.1002/jnr.20436. [DOI] [PubMed] [Google Scholar]
- Jensen MB, Yan H, et al. Survival and Differentiation of Transplanted Neural Stem Cells Derived from Human Induced Pluripotent Stem Cells in A Rat Stroke Model. J Stroke Cerebrovasc Dis. 2011 doi: 10.1016/j.jstrokecerebrovasdis.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P, Lee HK, et al. Sox9 and NFIA Coordinate a Transcriptional Regulatory Cascade during the Initiation of Gliogenesis. Neuron. 2012;74(1):79–94. doi: 10.1016/j.neuron.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30(5):1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25(19):4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaing ZZ, Milman BD, et al. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J Neural Eng. 2011;8(4):046033. doi: 10.1088/1741-2560/8/4/046033. [DOI] [PubMed] [Google Scholar]
- Krencik R, Weick JP, et al. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29(6):528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai G, Okada Y, et al. Roles of ES cell-derived gliogenic neural stem/progenitor cells in functional recovery after spinal cord injury. PLoS One. 2009;4(11):e7706. doi: 10.1371/journal.pone.0007706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Hillyer J, et al. Translational research in spinal cord injury: a survey of opinion from the SCI community. J Neurotrauma. 2010;27(1):21–33. doi: 10.1089/neu.2009.1048. [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23(2):215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Lu P, Wang Y, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150(6):1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui T, Shumsky JS, et al. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J Neurosci. 2005;25(42):9624–9636. doi: 10.1523/JNEUROSCI.2175-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash MS. Known and plausible modulators of depressed immune functions following spinal cord injuries. J Spinal Cord Med. 2000;23(2):111–120. doi: 10.1080/10790268.2000.11753518. [DOI] [PubMed] [Google Scholar]
- Nori S, Okada Y, et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108(40):16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Sawamoto K, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69(6):925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- Okada S, Okada K, et al. In vivo imaging of engrafted neural stem cells: its application in evaluating the optimal timing of transplantation for spinal cord injury. FASEB J. 2005;19(13):1839–1841. doi: 10.1096/fj.05-4082fje. [DOI] [PubMed] [Google Scholar]
- Pankratz MT, Li XJ, et al. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25(6):1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers BE, Lasiene J, et al. Axonal Thinning and Extensive Remyelination without Chronic Demyelination in Spinal Injured Rats. J Neurosci. 2012;32(15):5120–5125. doi: 10.1523/JNEUROSCI.0002-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee YH, Ko JY, et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J Clin Invest. 2011;121(6):2326–2335. doi: 10.1172/JCI45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel S, Berninger B, et al. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12(2):88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- Salazar DL, Uchida N, et al. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS One. 2010;5(8):e12272. doi: 10.1371/journal.pone.0012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, et al. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39(5):777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76(2):319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- Scott CE, Wynn SL, et al. SOX9 induces and maintains neural stem cells. Nat Neurosci. 2010;13(10):1181–1189. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26(24 suppl):S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, et al. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17(13):1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhr ST, Chang EA, et al. Telomere dynamics in human cells reprogrammed to pluripotency. PLoS One. 2009;4(12):e8124. doi: 10.1371/journal.pone.0008124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28(8):1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillakaratne NJ, de Leon RD, et al. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J Neurosci. 2002;22(8):3130–3143. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillakaratne NJ, Mouria M, et al. Increased expression of glutamate decarboxylase (GAD(67)) in feline lumbar spinal cord after complete thoracic spinal cord transection. J Neurosci Res. 2000;60(2):219–230. doi: 10.1002/(SICI)1097-4547(20000415)60:2<219::AID-JNR11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Tsuji O, Miura K, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 2010;107(28):12704–12709. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Represa A, et al. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci. 1999;19(23):10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino FM, Fagel DM, et al. Astroglial cells in development, regeneration, and repair. Neuroscientist. 2007;13(2):173–185. doi: 10.1177/1073858406298336. [DOI] [PubMed] [Google Scholar]
- Yuan SH, Martin J, et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6(3):e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Daley GQ. From fibroblasts to iPS cells: induced pluripotency by defined factors. J Cell Biochem. 2008;105(4):949–955. doi: 10.1002/jcb.21871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


