Abstract
Stressors are typically multidimensional, comprised of multiple physical and sensory components that rarely occur as single isolated events. In this study, the functional activation patterns of key corticolimbic structures in response to context exposure alone, its combination with restraint, and how prior experience with either of these modulates subsequent activation was measured using Fos expression. On day 1, rats were transported to a novel context and either restrained for 6 hours or left undisturbed. On day 2, these two groups were either restrained or not in the same context, then processed for Fos immunohistochemistry. Regardless of previous experience, rats in context and not restrained expressed more Fos-like immunoreactive (IR) labeling in CA1 and CA3 of dorsal hippocampus, and basolateral and central amygdala, while this pattern was reversed in the dentate gyrus infrapyramidal blade. Conversely for the infralimbic region of the medial prefrontal cortex (mPFC), the previous day's experience with restraint or immediate experience with restraint elevated Fos-like IR compared to rats placed in context on both days. These data show that exposure to context produces robust Fos induction in the hippocampus and amygdala, regardless of prior experience with restraint and compared to the immediate experience to restraint, with prior experience modulating Fos expression within the mPFC.
Keywords: acute stress, immediate early genes, hippocampus, amygdala, medial prefrontal cortex, Fos
It is well established that a real or perceived threat results in engagement of the neuroendocrine stress response, or hypothalamic-pituitary-adrenal (HPA) axis. Upon detection of a stressor, the paraventricular nucleus of the hypothalamus (PVN) participates in a cascade of events that reliably results in the adrenal cortex releasing glucocorticoids (GCs), which have widespread effects throughout the body and brain. An early misconception about the neuroendocrine stress response is that it is nonspecific, given that the output (i.e., GCs) appears similar across stressor types (Selye, 1998). We now know that the stress response is specific and not all stressors engage the HPA axis in the same way with each exposure (Pacak et al., 1998, Herman et al., 2005).
Immediate early genes (IEG) have provided a powerful resource to investigate functional activation in the brain following a stressor (Kovacs, 1998, Stamp and Herbert, 1999). In rodents, c-Fos is rapidly induced in response to novel contexts and exposure to restraint, two robust stressors commonly used in many behavioral paradigms (Pfister, 1979, Buynitsky and Mostofsky, 2009). However, the extent to which restraint and context exposure in combination influence the functional activation of corticolimbic brain regions is not well understood. This is especially important because stressors are typically not unidimensional events, but are rather comprised of multiple physical and sensory components. For instance, a rodent's encounter with a novel context might coincide with additional stressors, such as the visual or olfactory cues of a predator. In our paradigm, we implemented a widely used restraint manipulation to investigate the functional activation patterns of key corticolimbic structures in response to context exposure alone, its combination with restraint, and how prior experience with either of these modulates subsequent activation. In this study, we re-exposed rats to the context in which a previous experience of restraint occurred once before and measured Fos-like immunoreactive (IR) labeling in forebrain and limbic structures that are sensitive to stress, including the hippocampus, amygdala, and medial prefrontal cortex (mPFC). We predicted that context exposure and its combination with restraint will result in differential functional activation within limbic structures, with prior experience in either condition modulating subsequent Fos-like IR labeling.
Methods
Subjects
Thirty-two male Sprague-Dawley rats weighing approximately 225-250g upon arrival (Charles River Laboratories) were pair-housed (21-22°C) on a 12:12 reverse light cycle (lights off at 6am). Food and water were available ad libitum. All procedures occurred between 9AM to 4 PM. The procedures followed the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Science, National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee at Arizona State University.
Experimental Design
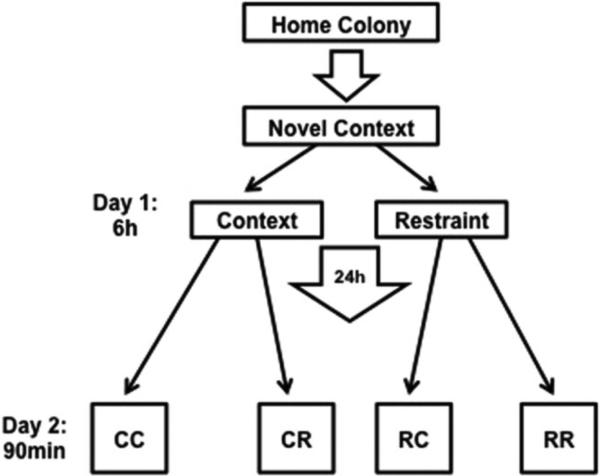
A 2 × 2 experimental design (n=8/group) for condition on Day 1 and Day 2 was implemented. On Day 1, rat pairs were transported in their home cages to a novel context (four different contexts that were unoccupied rooms with various behavioral testing and laboratory equipment, counterbalanced across conditions) and were either left undisturbed or restrained in wire mesh restrainers (16cm circumference, 24cm length) for 6h, then removed from restraint within that context and returned back to the home colony. On Day 2, rats were again transported in their home cages back to the same context, restrained or left undisturbed, depending on group assignment. Rats in the restraint condition on either day were given approximately 3min to form a representation of the context with lights on. Lights were then turned off to stay consistent with the reverse light cycle. Group assignments were: Day 1 and 2 context only (CC), Day 1 context and Day 2 restraint (CR), Day 1 restraint and Day 2 context (RC), and Day 1 and 2 restraint (RR), Figure 1.
Figure 1. Experimental Timeline.
Pair-housed rats were transported in their home cage to a novel context in which they were restrained (R) or left undisturbed in the context (C) for 6 h, then returned to the colony. The next day, rats again were transported to the previous day’s context and either restrained or left undisturbed, yielding four groups (first and second letter represent conditions on days 1 and 2, respectively; CC, CR, RC, RR). All groups were euthanized 90 min after placement in condition on day 2 for tissue processing.
Procedures on Day 2 lasted 90 min, to capture peak context- or restraint-induced Fos expression (Nikulina et al., 2004). At 90-min following placement in the respective Day 2 conditions, rats were overdosed (Euthasol, 100 mg/kg, i.p.) and transcardially perfused with heparinized phosphate buffered saline (pH 7.4) and 4% paraformaldehyde (pH 7.4). Brains were removed and post-fixed in 4% paraformaldehyde overnight (4°C). Brains were then cryoprotected in 15% and 30% sucrose over 2-d and stored (4°C) until sectioning.
Tissue Preparation and Fos Immunohistochemistry
Brains were sectioned (40 μm), mounted on subbed slides, and stored (-80°C) until tissue processing. Target sections were washed (3x, 0.05 M potassium-phosphate buffered saline, KPBS, pH 7.4) and incubated in 5% normal goat serum/0.05 M KPBS/0.4% Triton X (60-min, room temperature). Sections of dorsal hippocampus, amygdala, and mPFC were incubated with rabbit polyclonal antisera for Fos (Ab-5, Calbiochem, 1:15,000 in 5% normal goat serum/0.05 M KPBS/0.4% Triton X). Following incubation (48-h, 4°C), sections were processed using avidin-biotin-peroxidase (Vectastain ABC Elite kit, Vector Laboratories, Burlingame, CA), then developed with a DAB peroxidase kit (SK-4100; Vector Laboratories, 5-min, room temperature). This procedure was adapted from Nikulina, et al. (2004).
Fos Protein Analysis
Tissue sections were examined for the presence of a grey reaction product indicating immobilized antigen. For each group, data were obtained from 4-6 sections/brain through each subregion on both hemispheres, and averaged across each region for each rat. Selected areas (30,000 μm2 for the hippocampal regions and 150,000 μm2 for the (mPFC) and amygdala regions) were captured and digitized using a camera (CX9000, MBF Biosciences, Burlington, VT) interfaced to a microscope (Olympus BX51) with a 20x objective. A cell profile was considered labeled if its pixel intensity was more than two standard deviations darker than the background, as calculated by Stereo Investigator software (MBF Biosciences). For hippocampal analyses, targeted subregions included CA1, CA3, and the infrapyramidal (or ventral, lower) and suprapyramidal (or dorsal, upper) blades of the dentate gyrus (DGinf and DGsup, respectively). Sampling areas within each subregion were consistent among each hippocampal slice. Once each subregion was identified at 20x, the subregion was outlined and Stereo Investigator calculated the area (mm2). All positively labeled profiles were quantified and that value was divided by the area value to determine a density value. For the mPFC and amygdala analyses, adjacent cresyl violet stained sections were used to localize regions or nuclei with high confidence because the borders of these regions are less distinct; for mPFC, analyzed regions included anterior cingulate cortex (ACG), prelimbic cortex (PL), and infralimbic cortex (IL); for the amygdala, analyzed nuclei included basolateral amygdala (BLA), central amygdala (CEA), and medial amygdala (MEA). Neuronal labeling was quantified using a systematic random approach to achieve unbiased counts. Stereo Investigator software partitioned each image into 20 equal counting frames (100 x 75μm each), half of which were randomly selected and analyzed. Labeling density was calculated by dividing the estimated total number of labeled profiles by the total area analyzed (adapted from Fanous et al., (2011). Intra-rater reliability measures were performed with 92.1% reliability. Inter-rater reliability measures were performed with 93.9% reliability.
Data Analysis
For immunohistochemistry data, specific inclusion criteria were implemented to ensure that sufficient data per animal were used for analyses and defined as having at least four quantifiable subregions per animal. Those excluded were due to technical complications from tissue processing. Group sizes ranged from n = 3 to 8, depending on the group and specific brain region. Data were analyzed by two-way analysis of variance (ANOVA). When significant interactions were detected at p ≤ 0.05, post hoc analyses were performed. Data are represented as mean ± SEM.
Results
Hippocampus
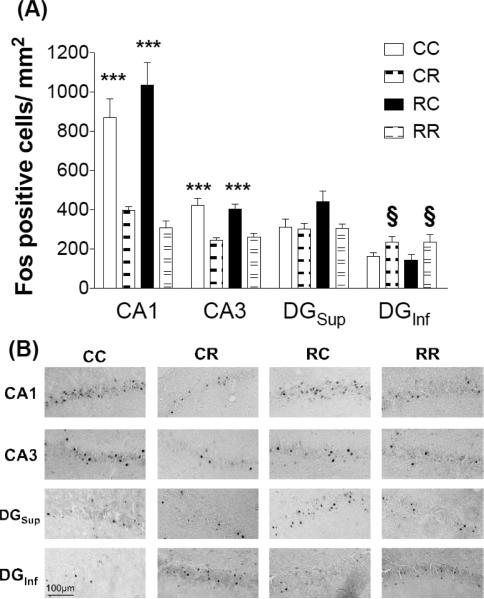
Separate omnibus ANOVAs were performed for each hippocampus subregion (CA1, CA3, DGInf, and DGSup ) to reveal that Fos expression differed depending upon restraint condition (CC, CR, RC, RR) and subregion. Two-way ANOVAs were performed based upon the manipulation on day 1 (restraint or context) and the manipulation on day 2 (restraint or context) for Fos-like IR labeling data (Fos-positive cells/mm2) for each subregion and revealed significant main effects of day 2 for CA1, CA3, and DGinf (CA1, F1,23 = 58.548, p < 0.001; CA3, F1,23 = 33.66, p < 0.001; DGInf, F1,22 = 9.19, p < 0.01, Fig. 2) and did not achieve significance for DGSup (main effect for day 2, F1,22 = 3.783, p = 0.06). These effects revealed a significant increase of Fos-like IR expression within CA1 and CA3 for groups that were placed in the context on day 2 and not restrained (CC, RC) compared to those restrained on day 2 (regardless of day 1 experience, CR, RR). In contrast, within the DGInf, there was a significant increase of Fos-like IR expression in groups that were restrained on day 2 (CR, RR) compared to those that were placed in the context and not restrained on day 2 (CC, RC). Therefore, conditions on day 2 predominately influenced Fos-IR labeling in the CA1, CA3 and DGInf, regardless of the experience on day 1. No other effects were significant.
Figure 2. Fos-IR labeling in hippocampus.
(A) Regardless of day 1 experience, groups placed in context and not restrained on day 2 (CC, RC) showed elevated Fos-IR labeling in CA1 and CA3 of the hippocampus compared to those restrained on day 2 (***p < 0.001 vs. CR, RR in CA1; ***p < 0.001 vs. CR, RR in CA3). By contrast, groups that were restrained on day 2 (CR, RR) exhibited greater Fos-IR labeling in the infrapyramidal blade of the dentate gyrus (DGInf) compared to those that were placed in the context but not restrained (§p < 0.05 vs. CC, RC; n=6-8/group) (B) Representative photomicrographs of Fos immunolabeling across groups in the selected subregions of the hippocampus. Scale bar: 100 μm.
Amygdala
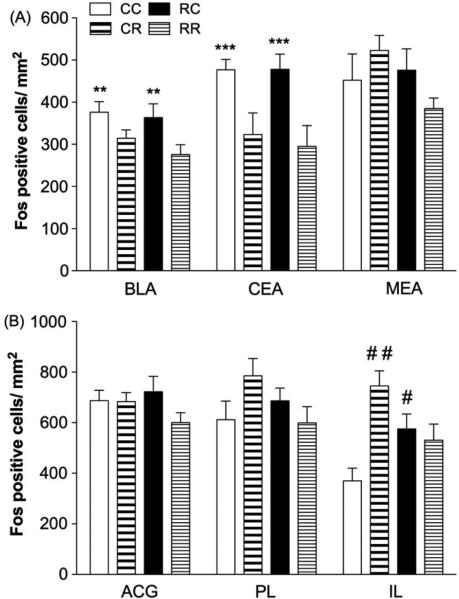
Within the amygdalar BLA and CEA, significant main effects were revealed on the basis of the day 2 manipulation (restraint or context) for Fos expression (BLA, F1,24 = 8.546, p < 0.01; CEA, F1,24 = 14.655, p = 0.001, Fig. 3A). These analyses revealed significantly greater BLA and CEA Fos-IR labeling in the groups that were placed in context only on day 2 (CC, RC) compared to the group that were restrained, regardless of their experience on day 1(CR, RR). No other effects were significant.
Figure 3. Fos-IR labeling in amygdala and mPFC.
(A) Groups that were placed in a novel context but not restrained on day 2 exhibited elevated Fos expression in BLA and CEA compared to those that were restrained (**p < 0.01 vs. CR, RR in BLA; ***p = 0.001 vs. CR, RR in CEA; n=5-8/group).(B) Restraint and context conditions altered Fos labeling within the IL without significantly affecting the PL or the ACG. Specifically, rats placed in the previous day's restraint context (RC) but not restrained showed elevated Fos-IR labeling within the IL compared to those that were never restrained (#p < 0.05 vs. CC). Rats that were acutely restrained (CR) also exhibited greater Fos-IR labeling compared to those that were never restrained (##p < 0.01 vs. CC; n=3-8/group).
Medial prefrontal cortex (mPFC)
In contrast to the hippocampus and amygdala subregions, conditions on day 1 impacted Fos expression within the mPFC depending on day 2 conditions. A two-way ANOVA for conditions on day 1 and day 2 for Fos IR labeling revealed a significant day 1 x day 2 interaction in the IL (F1,15=10.827, p<.01) and did not reach statistical significance for the day 1 x day 2 interaction in the PL (F1,20=3.894, p=.06; Figure 3B). Post hoc analyses revealed that rats previously placed in the restraint context (RC) exhibited greater IL Fos-like IR labeling compared to those that were never restrained (CC; p < 0.05). Additionally, rats that were acutely restrained (CR) had significantly greater Fos-like IR labeling compared to those that were never restrained (CC; p < 0.01). Similar patterns were observed within the PL, albeit not statistically significant. No other effects were significant.
Discussion
In the current study, we used a two-day exposure paradigm to understand the experience-dependent relationship between context and restraint on functional activation patterns by cellular labeling of Fos-like IR protein in subregions of the hippocampus, amygdala, and mPFC. Overall, context exposure on day 2 increased Fos expression in the hippocampus (CA1 and CA3) and amygdala (BLA and CEA), regardless of the previous day's experience. The DGInf exhibited contrasting patterns, with groups restrained on day 2 showing elevated expression of Fos. Interestingly, we found interactions between conditions on day 1 and day 2 for Fos expression in the mPFC. Specifically in the IL, restraint on day 1 followed by context exposure only on day 2 (RC) and context on day 1 followed by restraint on day 2 (CR) expressed significantly more Fos compared to no experience with restraint (CC). While many studies have documented that the immediate experience of restraint or context engages the corticolimbic system (Melia et al., 1994, Pace et al., 2005, VanElzakker et al., 2008), the present data revealed a significant role in how context might engage the limbic system to a greater extent than does restraint or the combination of these two. Furthermore, the mPFC has a role in the modulation of experience-dependent corticolimbic engagement to context or its combination with restraint.
In the present study, groups that were exposed to context on day 2, but not restrained (CC, RC), exhibited consistent patterns of elevated Fos expression in the hippocampus (CA1 and CA3) and amygdala (BLA and CEA), compared to groups that were restrained on day 2 (CR, RR). Specifically, the previous day's experience did not alter the immediate experience in response to context. One interpretation is that context exposure alone engaged the neurocircuitry of the CA1, CA3, BLA and CEA regions, as the dorsal hippocampus is important in context integration (Fanselow, 2000) and sends projections to the amygdala (Pitkanen et al., 2000). Novel contexts are also stressors in rodents (Pfister, 1979, van den Buuse et al., 2001) and the combined functional activation of the hippocampus and amygdala suggests that a second exposure to context still maintained novelty. Our data corroborate findings that environmental novelty produces robust Fos expression in the hippocampus (VanElzakker et al., 2008) and that experimental testing cages result in Fos induction within the hippocampus and amygdala (Campeau et al., 1997). Another interpretation may be that restraint suppressed context-induced Fos induction in these regions. Some evidence reveals that acute stress increases GABAergic inhibitory transmission within the hippocampus (Bowers et al., 1998), which might suppress neighboring pyramidal cell Fos induction in those restrained on day 2. Future double-labeling studies could address this possibility.
Within the DG, DGinf Fos expression was enhanced with restraint compared to context on day 2 (compare CR, RR with CC, RC) and unaltered in the DGsup. The literature reveals that a variety of novel experiences reduce DGInf activation (Fevurly and Spencer, 2004, Pace et al., 2005, VanElzakker et al., 2008). Moreover, some IEG products are differentially expressed in the two blades of the DG after various stressor challenges (Fevurly and Spencer, 2004, Pace et al., 2005, VanElzakker et al., 2008), corroborating our findings.
Interestingly, the mPFC (IL) was the only area demonstrating experience-dependent modulation of Fos induction in the regions investigated. Previous research on dendritic arborization found the mPFC to be exquisitely sensitive to subtle environmental challenges, as dendritic retraction occurred with vehicle injections (Wellman, 2001) or after one week restraint manipulations (Brown et al., 2005). This contrasts with the several weeks required for hippocampal dendritic changes (McLaughlin et al., 2007). It is noteworthy to speculate that the mPFC is the first to undergo modifications based on experiences that contribute to adjustments in neuroplasticity. However, more studies are needed on the time course of experience-dependent effects of context and restraint on IEG induction.
Our c-Fos data reveal differential effects of context and its combination with restraint within the hippocampus, amygdala, and mPFC, suggesting that these stress-sensitive structures integrate environmental experiences differently. We show that exposure to context drives functional activation in comparison to its combination with restraint in the majority of the hippocampus and amygdala, with restraint engaging these structures to a lesser degree. Only the IL mPFC and perhaps the PL, given its pattern of expression although it did not reach statistical significance, show experience-dependent influences from restraint exposure altering subsequent functional activation to context. One caveat is that Fos is not constitutively expressed—it is rapidly induced by many stimuli, including acute restraint (Melia et al., 1994), but then quickly returns to baseline and is undetectable. Consequently, any effects that suppress activity or produce lasting effects would not be detected and the main reason for omitting a quiet control for comparison. Future directions would include using an IEG that can be up or down regulated. To conclude, the experience-dependent relationship between context and restraint on functional activation patterns corroborates the expectation that experiences do not occur in isolation, but are complex and may build upon each other, with perhaps the mPFC being one of the first regions revealing experience-dependent outcomes.
Acknowledgements
This research was supported by funding from the Arizona Biomedical Research Commission (Conrad), NIH grant DA0226451 (Nikulina), the ASU School of Life Sciences and the Howard Hughes Medical Institute through the Undergraduate Science Education Program, and Barrett Honors College (Anouti). The authors would also like to thank Miles Orchinik and Heather A. Bimonte-Nelson.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bowers G, Cullinan WE, Herman JP. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J Neurosci. 1998;18:5938–5947. doi: 10.1523/JNEUROSCI.18-15-05938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: Recent developments. Neurosci Biobehav Rev. 2009;33:1089–1098. doi: 10.1016/j.neubiorev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Fanous S, Lacagnina MJ, Nikulina EM, Hammer RP., Jr Sensitized activation of Fos and brain-derived neurotrophic factor in the medial prefrontal cortex and ventral tegmental area accompanies behavioral sensitization to amphetamine. Neuropharmacology. 2011;61:558–564. doi: 10.1016/j.neuropharm.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fevurly RD, Spencer RL. Fos expression is selectively and differentially regulated by endogenous glucocorticoids in the paraventricular nucleus of the hypothalamus and the dentate gyrus. J Neuroendocrinol. 2004;16:970–979. doi: 10.1111/j.1365-2826.2004.01257.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14:5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, 3rd, Ganschow L, Hammer RP, Jr., Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: a test of Selye's doctrine of nonspecificity. Am J Physiol. 1998;275:R1247–1255. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- Pace TW, Gaylord R, Topczewski F, Girotti M, Rubin B, Spencer RL. Immediate-early gene induction in hippocampus and cortex as a result of novel experience is not directly related to the stressfulness of that experience. Eur J Neurosci. 2005;22:1679–1690. doi: 10.1111/j.1460-9568.2005.04354.x. [DOI] [PubMed] [Google Scholar]
- Pfister HP. The glucocorticosterone response to novelty as a psychological stressor. Physiol Behav. 1979;23:649–652. doi: 10.1016/0031-9384(79)90154-9. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiat Clin Neurosci. 1998;10:230–231. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Van Acker SA, Fluttert M, De Kloet ER. Blood pressure, heart rate, and behavioral responses to psychological “novelty” stress in freely moving rats. Psychophysiology. 2001;38:490–499. doi: 10.1017/s0048577201990687. [DOI] [PubMed] [Google Scholar]
- VanElzakker M, Fevurly RD, Breindel T, Spencer RL. Environmental novelty is associated with a selective increase in Fos expression in the output elements of the hippocampal formation and the perirhinal cortex. Learn Mem. 2008;15:899–908. doi: 10.1101/lm.1196508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]