Abstract
Oxidative stress is implicated in various kinds of neurological disorders, including human immunodeficiency virus (HIV) associated dementia (HAD). Our laboratory has been studying the murine retrovirus ts1, a pathogenic mutant of the Moloney murine leukemia virus (MoMuLV), as a model for HAD. Like HIV in humans, ts1 induces oxidative stress and progressive neurodegeneration in mice. We have shown previously that an antioxidant and anti-inflammatory drug GVT or MSL (monosodium luminol) suppresses ts1-induced oxidative stress, attenuates the development of spoorm encephalopathy, and delays hind limb paralysis in infected mice. It is known that upregulation of the nuclear transcription factor NF-E2-related factor 2 (Nrf2) is involved in upregulating cellular antioxidant defenses. Since Nrf2 is associated with elevation of antioxidant defenses in general, and since GVT suppresses ts1-induced neurodegeneration, our aim in this study was to determine whether GVT neuroprotection is linked to Nrf2 upregulation in the brain. We report here that GVT upregulates the levels of Nrf2, both in primary astrocyte cultures and in brainstem of ts1-infected mice. Significant upregulation of Nrf2 expression by GVT occurs in both the cytosolic and nuclear fractions of cultured astrocytes and brainstem cells. Notably, although GVT treatment increases Nrf2 protein levels in cultured astrocytes and brainstem tissues, Nrf2 mRNA levels are not altered. This suggests that the neuroprotective effects of GVT may be mediated by the stabilization of the Nrf2 protein, allowing continuous upregulation of Nrf2 levels in the astrocytes.
Keywords: Oxidative stress, GVT, Nrf2, Moloney murine leukemia virus-ts1, HAD
Introduction
The ts1 mutant of Moloney murine leukemia virus (MoMuLV) induces progressive neurodegenerative disease in susceptible strains of mice, when infected if shortly after birth (Stoica et al., 1992; Wong, 1990; Wong et al., 1991). The characteristic features of the ts1-induced neurodegeneration (ND) include spoorm encephalopathy, gliosis and neuronal loss leading to hind limb paralysis (Stoica et al., 2000). Misfolding of the ts1 precursor envelope protein gPr80env, as a result of a mutation in the ts1 env gene, reduces the efficiency of its transport from the endoplasmic reticulum (ER) to the plasma membrane (Szurek et al., 1990a; Szurek et al., 1990b), resulting in its accumulation in the endoplasmic reticulum, or ER (Liu et al., 2004; Liu et al., 2006; Szurek et al., 1990b). Although ts1 can infect various cell types, gPr80env accumulates only in astrocytes in the central nervous system (CNS) and in infected T cells in the immune system (Gonzalez-Scarano et al., 1995; Liu et al., 2006; Shikova et al., 1993). The specific killing of these target cells is associated with the neurodegeneration and immunosuppression caused in mice infected with ts1 (Gonzalez-Scarano et al., 1995; Wong et al., 1989).
In ts1-infected astrocytes, accumulation of the gPr80env in the ER initiates the unfolded protein response (UPR), which in turn results in oxidative stress and mitochondria-mediated cell death (Kim et al., 2004; Liu et al., 2004). Neurons are not directly affected by ts1, but they die alongside infected astrocytes, most likely because the thiol redox support normally supplied to neurons by astrocytes has been compromised. In infected astrocytes, this occurs as a result of increased levels of reactive oxygen species (ROS), and depletion of glutathione (GSH) as well as cysteine (Kuang et al., 2009; Lynn and Wong, 1995; Qiang et al., 2006; Stoica et al., 1992; Stoica et al., 1993). Since similar events occur in the CNS cells of HAD patients, neurodegeneration caused by the ts1 virus resembles HAD in its mechanisms (Clark et al., 2001; Gonzalez-Scarano et al., 1995; Mollace et al., 2001; Ronaldson and Bendayan, 2008; Sacktor N, 2004; Steiner et al., 2006).
Oxidative stress is a condition that occurs when there is an imbalance between increased ROS levels and cellular antioxidant defenses (Betteridge, 2000; Reddy et al., 2004). When ROS levels are increased in cells, and if their levels are not controlled, they can damage protein, RNA, and DNA. Healthy cells respond quickly to increased ROS, by a controlled sequence of responses. ROS elevation is first countered by the upregulation of the antioxidant enzymes such as superoxide dismutase and catalase, which inactivate singlet oxygen, or superoxide (O-) and hydrogen peroxide (H2O2). If the ROS load continues to increase, then a second line of defense is activated by the nuclear transcription factor Nrf2 (Johnson et al., 2008; Kim et al., 2009; Reddy et al., 2009a; Reddy et al., 2009b; Shih et al., 2003; Vargas and Johnson, 2009). Nrf2 is a transcription factor that binds to the antioxidant responsive element (ARE) promoter sequences, leading to coordinated upregulation of ARE-driven detoxification and antioxidant gene expression (Johnson et al., 2008; Lee et al., 2005; Moi et al., 1994; Vargas and Johnson, 2009). Nrf2 has been reported to be activated in CNS cells in Parkinson's disease (PD), Alzheimer's disease (AD), Huntington's disease (HD), and amyotrophic lateral sclerosis (ALS) (Calkins et al., 2008; Clements et al., 2006; Vargas and Johnson, 2009).
Previous studies from our laboratory have shown that ts1 infection of cultured astrocytes causes oxidative stress in the cells and that Nrf2 and its downstream target genes are activated in response to infection and cell survival (Qiang et al., 2004; Qiang et al., 2006). Further, ts1-induced damage to the CNS and thymus of infected mice is suppressed to some extent by the treatment with antioxidant N-acetylcysteine (NAC) (Lynn and Wong, 1998). While antioxidant NAC treatment effectively inhibits ts1-associated thymic atrophy, it has relatively limited effects against ts1-induced neurodegeneration (Lynn and Wong, 1998). This could be due to its limited ability to cross the blood brain barrier (BBB) (Grinberg et al., 2005; Offen et al., 2004). In addition, the antioxidant and anti-inflammatory drug minocycline protects ts1-infected mice against CNS oxidative stress and paralysis, although, as with NAC, the protection is not complete (Kuang et al., 2009).
We have also tested a novel antioxidant and anti-inflammatory drug called GVT for its ability to slow or prevent ND in ts1-infected mice (Kuang et al., 2009; Qiang et al., 2006). We found that GVT appears to be more effective than NAC and minocycline in slowing ts1-mediated neurodegeneration (Table 1). In addition, GVT has also been shown to reduce ROS levels and tissue damage in the lymphoid tissues (thymus and intestine) of ts1-infected mice (Scofield et al., 2009a; Scofield et al., 2009b). GVT is relatively non-toxic, well-absorbed and rapidly excreted upon systemic administration (Gross et al., 2009; Sanders et al., 2000). GVT can also be administered orally (Table 1). In the CNS, GVT easily enters cells and passes the BBB, and decreases ROS levels in infected primary astrocyte cultures (Qiang et al., 2006), where it reacts with free radicals such as ONOO-, and prevents protein nitration and oxidation in cells (unpublished data). In infected mice treated with GVT, CNS markers of lipid peroxidation are reduced in the CNS and thymus, compared with those for tissues of infected, untreated mice. Finally, GVT reverses signs of oxidative damage, without significant reduction of viral titer, in the CNS and thymus of ts1-infected mice. This suggests that GVT protects against ts1-induced ND by way of antioxidant effects, rather than by acting as a direct antiviral drug (Qiang et al., 2006).
Table 1.
A summary of survival results for various agents used for the treatment of ts1-induced neurodegeneration in the murine retrovirus ts1 model. This table provides the information on the modes of administration, doses of the drug, the times during which a particular drug was administered, and the routes of administration, either by intraperitoneal (i.p.) injection or orally in drinking water.
| Treatment | Delayed Days of onset of paralysis | Dose and duration of treatment | Reference |
|---|---|---|---|
| None | No Delay** | ||
| NAC | 25 | given i.p., not stopped | Lynn and Wong., 1998 |
| Minocycline | 30 | given i.p., not stopped | Kuang et al., 2009 |
| GVT | 72 | given i.p., 250mg/kg/d begun2dpi; and stopped @50dpi | Jiang et al., 2006 |
| GVT | 75 | given i.p., 200mg/kg/d begun5dpi; and continued | Jiang et al. 2006 |
| GVT | 80 | 250mg/kg/d; i.p., until weaning (14 dpi), followed by 1g/L in drinking water, continued | Unpublished data |
Median dpi delay relative to median for untreated mice
We hypothesize that GVT, in addition to acting as an ROS scavenger like NAC or minocycline, also upregulates and maintains Nrf2 levels in ts1-infected astrocytes. In this study, we therefore asked whether the Nrf2 protein is upregulated and stabilized in ts1-infected cultured astrocytes, or in CNS tissues of infected mice that are treated with GVT.
Materials and Methods
Virus
ts1, a spontaneous temperature-sensitive mutant of MoMuLV, was propagated in a thymus-bone marrow (TB) cell line. Virus titers were determined using a modified direct focus assay in the 15F cell line, a murine sarcoma-positive, leukemia-negative cell line, as described previously (Wong et al., 1985).
Cell Culture
Immortalized murine C1 astrocytes were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin) (Lin et al., 1997). The cells were passaged biweekly and used for experiments while in the exponential growth phase. Primary astrocytes were isolated from 1–2-day-old newborn mouse pups by a method described previously (Lee et al., 2001; Liu et al., 2005). Briefly, whole brains of newborn mice were removed and minced into ice-cold Dulbecco's modified Eagle's medium/F-12 medium. Single-cell suspensions were obtained by passing the minced tissue through a nylon mesh cell strainer. The cells were plated onto poly-L-lysine-coated flasks and grown in Dulbecco's modified Eagle's medium/F-12 medium, supplemented with 10% fetal bovine serum, 5 units/ml penicillin, 5 μg/ml streptomycin, and 2.5 g/ml Fungizone. The cultures were incubated for 4 days in a 5% CO2 atmosphere at 37°C, until reaching confluence. They were then passaged by trypsinization. Cells from passages 4 or 5 were used for all experiments. At the fourth or fifth passage, the cultures typically were >99% glial fibrillary acidic protein (GFAP)-positive (Shikova et al., 1993).
Protein Estimation
Primary astrocytes (1–5 × 106) were seeded into 10-cm dishes and grown to 70% confluence prior to treatments. Treated and control untreated cultures were then washed twice with ice-cold PBS and scraped directly into lysis buffer containing 150 mm NaCl, 0.5% w/v SDS, 0.5% v/v Nonidet P-40, 0.5% w/v sodium deoxycholate, 1 mm EGTA, and a mixture of protease inhibitors (Complete Mini tablets; Roche Applied Science). Protein concentrations were determined using a Bradford reagent (Bio-Rad).
Western Blotting
For Western blots, astrocyte cultures were solubilized in lysis buffer containing 150 mm NaCl, 0.5% w/v SDS, 0.5% v/v Nonidet P-40, 0.5% w/v sodium deoxycholate, 1 mm EGTA, and a mixture of protease inhibitors (Complete Mini tablets; Roche Applied Science). Tissue lysates from brainstems were prepared by homogenization of frozen tissues in the same lysis buffer. Nuclear and cytoplasmic extracts were prepared as described previously described (Kim et al., 2001). The lysates were centrifuged at 12,000 g for 12 min, prior to measurements of protein levels in the supernatants. Equal amounts of protein (30 μg of total protein per lane) were then subjected to SDS–PAGE, and electrophoretically transferred to polyvinylidene difluoride membranes at 100 V for 1h. The membranes were blocked with 5% nonfat milk in 3% Tris-buffered saline containing 0.1% Tween-20 for 40 min at room temperature. The membranes were then incubated with anti-Nrf2 antibody (1:1000) or anti-β-actin antibody (1:3000) at 4°C overnight, followed by washing and a second incubation in HRP-conjugated secondary antibodies (1:3000; 1 h at RT). Antibody binding to bands on the membranes was visualized using chemiluminescence, and the optical densities of individual bands were quantitated using the Chemi-Imager digital imaging system (Alpha Innotech, San Leandro, CA, USA).
RNA Extraction and Real-time PCR
Total cellular RNA was extracted by using the RNeasy kit, with optional DNaseI treatment (Qiagen, Valencia, CA, cat. no. 74104). Extractd RNA was analyzed for integrity using the Agilent 2100 Bioanalyzer (Agilent Technologies, Inc. Santa Clara, CA). Total RNA (1μg) was then used as template to synthesize cDNA with the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA), and qPCR was subsequently performed on the ABI 7900HT Fast Real Time PCR System (ABI) with assays on demand (AODs) specific to Nrf2. RNA levels were normalized to the endogenous control gene GAPDH by using the ABI Rodent Control Reagent kit (ABI, cat. no. 4352339E). Data analysis was performed using Sequence Detection System software from ABI, version 2.2.2. The experimental Ct (cycle thresh hold) was calibrated against a GAPDH control. All amplifications were performed in triplicate. The DDCt method was used to determine the amount of product relative to that expressed by (calibrator sample)-derived RNA.
Animals, virus inoculation and GVT treatment
FVB/N mice were obtained from Taconic Farms (Germantown, NY). The mice were divided into four groups: control, GVT-treated, ts1-infected, and GVT-treated-ts1-infected. The control group received 0.9% normal saline. Mice for the ts1-infected group were inoculated intraperitoneally with 0.1 ml of ts1 viral suspension containing 106 to 107 infection units/ml, as previously described (Kim et al., 2002) at 2 dpn (days postnatal). GVT and GVT-treated-ts1-infected groups received freshly prepared GVT by intraperitoneal injection (200 mg/kg of body weight/day; kindly provided by Bach Pharma, Inc., North Andover, MA), beginning at 3 dpi (days post infection), for 5 consecutive days followed by 2 days of rest. This treatment protocol was continued until 30 dpi. Mice from all groups were checked daily for clinical signs of disease, and all animals were sacrificed at 30 dpi. All animal procedures were performed according to the protocol approved by the University of Texas, M. D. Anderson Cancer Center Institutional Animal Care and Use Committee.
Immunohistochemistry
For immunohistochemistry, control (n=4), ts1-infected (n=4) and GVT-treated-ts1-infected (n=4) mice were anesthetized using an intraperitoneal injection of pentobarbital (150 mg/kg), and perfused with 10% buffered formalin, using a peristaltic pump. After a 12 h fixation in 10% buffered formalin, the brainstems were removed and embedded in paraffin. Paraffin sections (5 μm) were first deparaffinized and were subjected to an antigen retrieval protocol, in which sections were heated in 10 mM citrate buffer (pH 6.0) for 10 min, blocked with 5% normal serum in Tris- buffered saline (TBS, 100 mM Tris, 150 mM NaCl, pH 7.4), and then incubated overnight at 4°C with anti-Nrf2 antibody dilution of 1:50. After washing, the sections were then incubated with biotin-conjugated secondary IgGs and treated with reagents from a Vecta-Elite streptavidin-peroxidase kit, with a benzidine substrate for color development. The sections were counterstained with diluted hematoxylin. Sections without primary antibodies acted as controls for nonspecific binding by the secondary antibodies. In the control sections, no specific immunoreactivity was detected (data not shown).
Statistical Analysis
All experiments were conducted in triplicate or duplicate wells in the samples derived from three separate and independent experiments. The data were subjected to analysis of variance followed by Tukey's test for comparison. Data were represented as mean ± SEM. p<0.05 was considered significant.
Results
GVT treatment induces accumulation of Nrf2 in cultured primary astrocytes
Our earlier studies with cultured astrocytes, have shown that ts1 infection causes increased ROS levels and activates Nrf2 and its targeted genes (Qiang et al., 2004). Oxidative stress caused about half of the ts1-infected cells to die. For those that survive the cytopathic effects, they show continuous upregulation of Nrf2 and its target gene products, e.g. xCT antiporter which is downstream of Nrf2 and protects from oxidative stress by increasing cystine import (Qiang et al., 2006). We also reported in ts1-infected primary astrocyte cultures, the intracellular levels of ROS are downregulated by GVT treatment. More importantly, GVT prevents ND in the infected mice (Jiang et al., 2006). As mentioned above, of the several agents that we have tested in mice for protection against ts1-induced ND, we have found that GVT is the best (Table 1). We hypothesize that GVT, in addition to acting as a ROS scavenger, also upregulates and maintains high, stable Nrf2 levels in ts1-infected astrocytes. As a proof-of-concept test of the hypothesis that GVT alone upregulates Nrf2, we first asked whether GVT increases Nrf2 levels in a dose-dependent manner in cultured astrocytes. We treated cultured C1 astrocytes with GVT or tert-butyl hydroquinone (tBHQ), a known Nrf2 activator, at different concentrations and at different doses. The Western blots in Figure 1 show that treatment of C1 cells with GVT significantly enhanced the levels of Nrf2 in these cells, in GVT dose-dependent manner. In addition, Figure 1 shows that treatment of the cells with tBHQ also caused Nrf2 upregulation. Like C1 cells, in primary astrocytes, GVT treatment resulted in elevated Nrf2 levels, which occured as early as 2h post treatment, and this increase was maintained for up to 16h (Fig.2).
Fig.1. Upregulation of Nrf2 by GVT and tBHQ in C1 astrocytes.
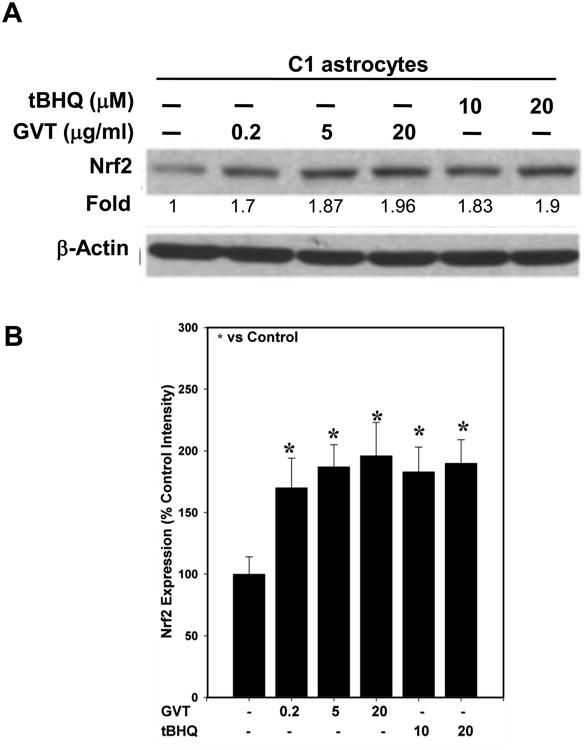
C1 astrocytes were treated with various concentrations of GVT (0.2, 5 and 20 μg/ml) for 16h after which the levels of Nrf2 protein were analyzed. The western blots show that GVT treatment resulted in a upregulation and accumulation of Nrf2. Treatment with tertiary-butyl hydroquinone (tBHQ), a known positive regulator of Nrf2 (10 and 20 μg/ml), also resulted in upregulation of Nrf2 (A). Quantification of the band intensities of Nrf2 normalized against actin (B). Data in (B) were obtained from three independent experiments and analysis of variance was done by employing Tukey test. *p<0.05 vs. control was considered statistically significant. Error bars represent mean ± SEM.
Fig.2. GVT induces Nrf2 upregulation in primary astrocyte cultures.
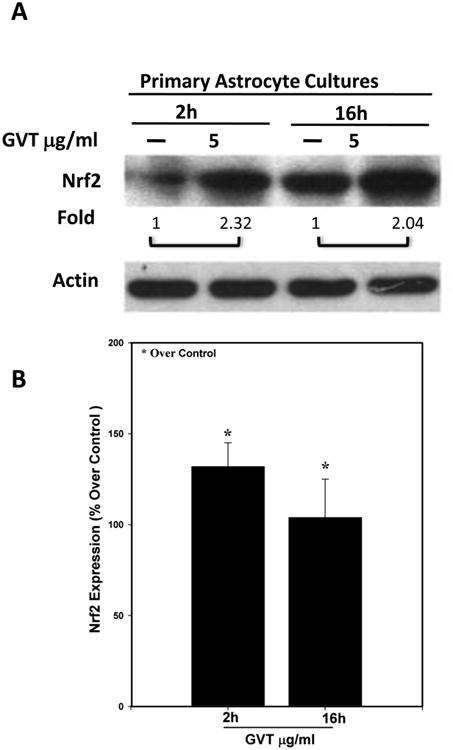
Primary cultures of astrocytes derived from mouse pups were exposed to GVT for 2-16 h and then immunoblotted to compare their Nrf2 content. GVT treatment resulted in increased expression of Nrf2 as early as 2h and this increase in Nrf2 levels was maintained up to 16h of post treatment (A). Nrf2 band was quantified and normalized against actin (B). Data were obtained from three independent experiments and analysis of variance was done by employing Tukey test. *p<0.05 vs. control was considered statistically significant. Error bars represent mean ± SEM.
GVT treatment causes Nrf2 accumulation in both the cytosol and the nuclei of cultured astrocytes
As noted above, oxidatively stressed cells activate their Nrf2 and translocate it into the nucleus, resulting in increased transcription of its target genes. We had reported previously that Nrf2 protects cultured cells from the adverse effects caused by ts1 infection (Qiang et al., 2006), and we had also shown that GVT prevents ts1-induced oxidative stress in the CNS and thymus of in ts1-infected mice (Qiang et al., 2006) (Scofield et al., 2009a). To find out whether GVT protection of infected cells involves Nrf2 activation, we used Western blotting analysis to follow levels of Nrf2 after infection of primary astrocytes. Figure 3 shows that treatment of primary cultured astrocytes with GVT increased the levels of Nrf2, not only in the cytosol (Fig.3A), but also in the nuclear fractions (Fig.3B), over a period of 4h.
Fig.3. GVT increases Nrf2 levels in the cytosolic and nuclear fractions of cultured astrocytes.
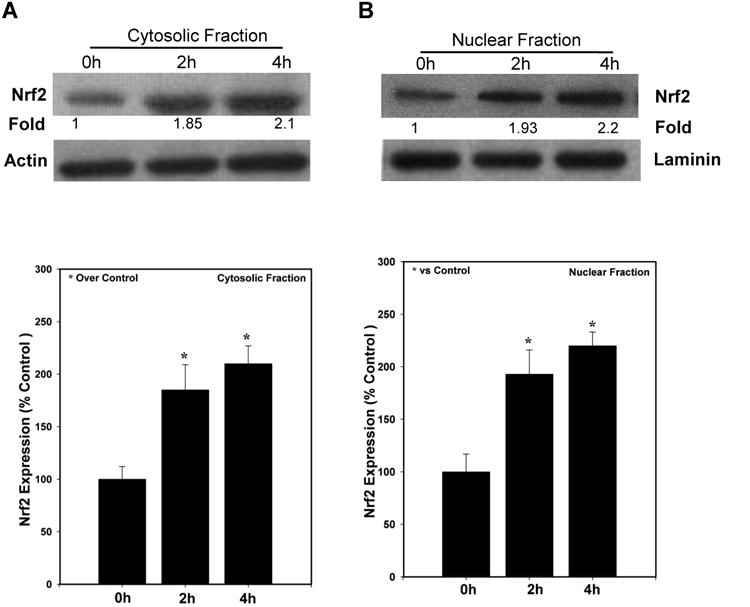
Primary astrocytes were treated with GVT for 0, 2 and 4 h. Following treatment, the cells were harvested and lysates of cytosolic and nuclear fractions were isolated. Immunoblots run against anti-Nrf2 showed a significant upregulation in the Nrf2 protein levels both in cytosol (A) as well as in nucleus (B). Quantification of the band intensities of Nrf2 normalized against actin and lamin in cytosol (lower panel of A) and nuclear fractions (lower panel of B) Data were obtained from three independent experiments and analysis of variance was done by employing Tukey test. *p<0.05 vs. control was considered statistically significant. Error bars represent mean ± SEM.
GVT treatment does not alter mRNA levels of Nrf2 in uninfected or infected primary cultured astrocytes
In normal cells, the Nrf2 protein has a short half-life, since it is degraded in cytoplasmic proteasomes ∼8 h after its synthesis. To determine how Nrf2 levels are increased in GVT-treated cultured astrocytes, we first asked whether GVT treatment of cultured astrocytes prevents the scheduled degradation of Nrf2 that is characteristic of normal cells, and whether this stabilization of Nrf2 levels is correlated with its protection of ts1-infected cells from oxidative stress. To do this, we first followed the effect of GVT treatment on Nrf2 mRNA levels, using real-time PCR. Our results show that treatment of uninfected cultured primary astrocytes with GVT for 2, 4 and 8 h did not cause any significant changes in the mRNA levels (Fig.4). These results suggest GVT-induced upregulation of Nrf2 protein levels is not due to increased protein synthesis.
Fig.4.
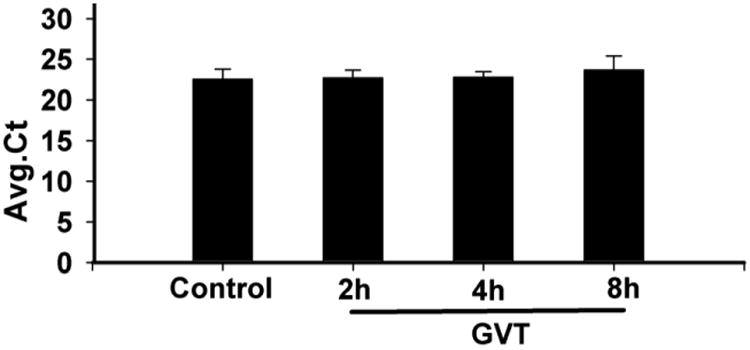
Primary cultured astrocytes were treated with GVT for 2, 4 and 8h, after which time their mRNA was isolated and the levels of Nrf2 mRNA quantitated by RT-PCR. There was no significant alteration in average ct values, indicative of levels of Nrf2-specific mRNAs, after GVT treatment. Three independent experiments and analysis of variance was done by employing Tukey test. *p<0.05 vs. control was considered statistically significant. Error bars represent mean ± SEM.
GVT stabilizes the Nrf2 protein in primary astrocyte cultures
To confirm that GVT treatment does not elevate Nrf2 by elevating its transcription in treated cells, we followed Nrf2 levels over time in cultures of primary astrocyes that were treated with the protein synthesis inhibitor cycloheximide (CHX), either alone or after pretreatment of the cells with GVT. The immunoblots in Figure 5A show that the degradation or reduction of the already existing Nrf2 protein, in cultures treated with CHX to prevent de novo protein synthesis, starts as early as 2h after treatment. However, when the astrocytes were pretreated with GVT prior to the exposure to the CHX, degradation of Nrf2 protein was significantly reduced, even though new protein synthesis has been prevented (Fig. 5B and C). From these findings we conclude that GVT upregulates and stabilizes levels of Nrf2 in astrocytes.
Fig.5. GVT treatment stabilizes existing Nrf2 protein, but does not increase de novo production of Nrf2.
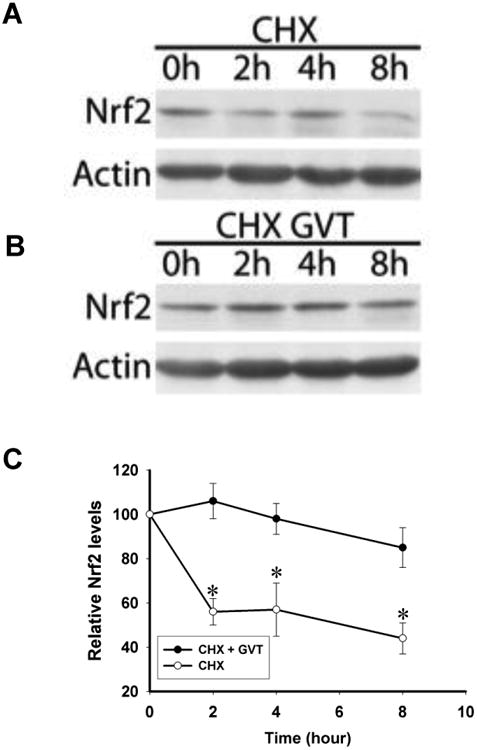
Primary cultured astrocytes were either left untreated or pretreated with GVT, followed by CHX (10μg/ml). In cells treated with CHX alone, loss of Nrf2 protein, presumably by proteasomal degradation in the absence of new protein synthesis, occurred within 2h (A). However, in cells pretreated with GVT, Nrf2 was levels remained elevated (B). The relative intensities of the Nrf2 protein bands were compared using densitometry followed by normalization of the band values (C). Data from (C) were obtained from three independent experiments (n=3). *p<0.05 vs. control was considered statistically significant. Error bars represent mean ± SEM.
MG132, a proteasomal inhibitor, further enhances levels of Nrf2
To determine whether GVT treatment inhibits scheduled proteasomal degradation of Nrf2, we asked whether treatment of primary cultured astrocytes with MG132, a known inhibitor of proteasomal activity, causes Nrf2 protein stabilization in the cells. Figure 6 shows that Nrf2 elevation in GVT-treated cells is amplified by MG132, suggesting that GVT may delay the degradation of Nrf2 by directly inhibiting proteasomal activity, by inhibiting the ubiquitination steps required for targeting of Nrf2 to the proteasomes, or by other mechanisms that stabilize Nrf2 in the cells.
Fig.6.
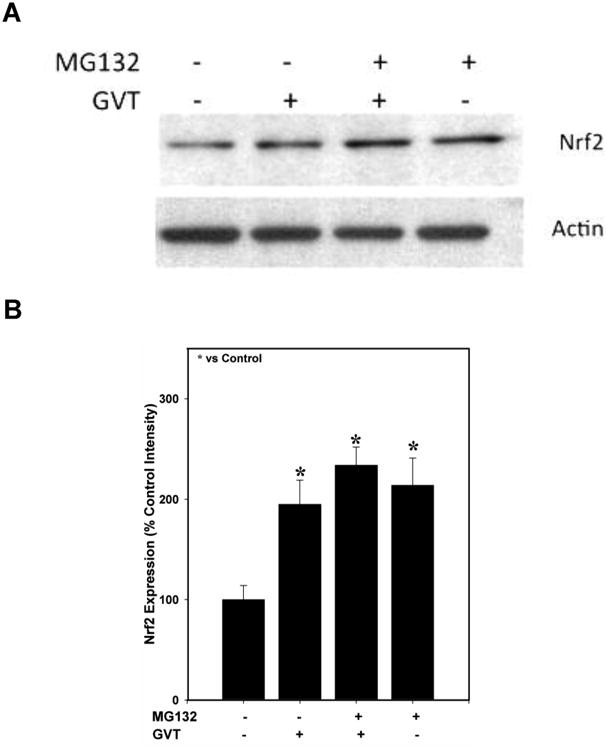
Primary cultured astrocytes were pretreated with GVT followed by treatment with a proteasomal inhibitor MG-132. Treatment with GVT alone increased the Nrf2 protein to certain extent. However the MG132 treated cells expressed increased levels of Nrf2 protein (A). Quantification of the band intensities of Nrf2 normalized against actin (B). Data were obtained from three independent experiments and analysis of variance was done by employing Tukey test. *p<0.05 vs. control was considered statistically significant. Error bars represent mean ± SEM.
GVT upregulates Nrf2 levels in the cytoplasm and nuclei of CNS neurons and glial cells
To determine whether Nrf2 levels in CNS cells are affected in ts1-infected mice, and to find out whether GVT treatment modifies these effects, we used Western blot analysis to determine levels of Nrf2 in CNS tissues of untreated ts1-infected mice vs. GVT-treated ts1-infected mice. We also asked whether GVT treatment affects relative levels of Nrf2 in the cytoplasmic vs. nuclear fractions of CNS cells for both groups of animals. Figure 7 shows that cytoplasmic Nrf2 protein levels are slightly decreased in the brainstems of both ts1-infected untreated and ts1-infected GVT-treated mice, compared with the brainstem tissues from uninfected control animals. In addition, the data also show increased amounts of Nrf2 protein in the nuclear fractions of brainstem cells from ts1-infected and ts1-infected-GVT treated mice compared with control (Fig.7). The ratio of Nrf2 in nuclear/Nrf2 in cytoplasm increases 2-fold in ts1-infected GVT-treated brainstems when compared with control.
Fig.7.
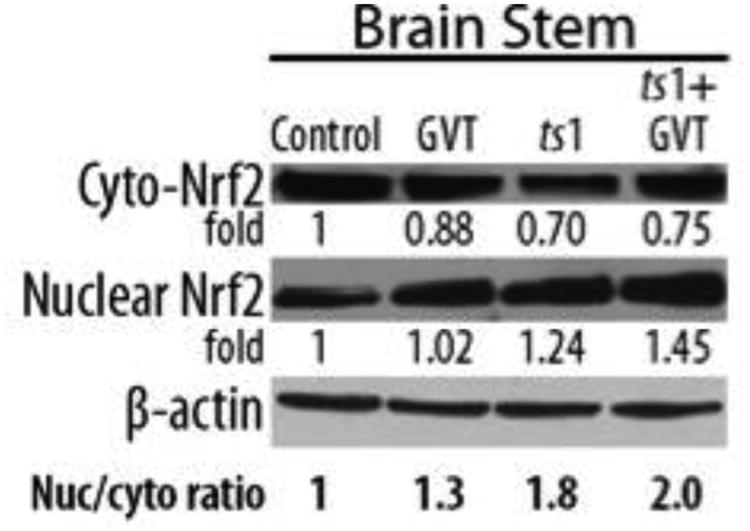
In lysates of brain tissue from ts1-infected untreated and ts1-infected GVT-treated mice, Western blots show a slight decrease in levels of cytoplasmic Nrf2 protein, compared to brainstem lysates from uninfected control mice. While increased levels of Nrf2 protein were present in the nuclear fractions isolated from the GVT-treated uninfected, ts 1-infected, and ts 1-infected-GVT treated mice, nuclear localization of Nrf2 was most evident in the ts1-infected, GVT-treated brainstem lysates. For these tissues, the ratio of Nrf2 in nuclear/Nrf2 in cytosol is increased by 2 fold in ts1-infected GVT-treated brainstems, relative to levels for control uninfected mouse tissues.
To visualize these effects of GVT in the tissues, we performed immunohistochemistry (IHC) to localize Nrf2 protein in the cells of control, ts1-infected and GVT-treated–ts1-infected brainstems. The photomicrographs in Figure 8 show intense anti-Nrf2 labeling of cells that morphologically resemble neurons and glial cells in the brainstems of ts1-infected, and of GVT-treated-ts1-infected mice, compared with brainstem tissues from uninfected control mice (Fig.8). In both neurons and glial cells of ts1-infected and GVT-treated-ts1-infected mouse brainstems, high levels of Nrf2 are present both in the strong immunoreactivity for Nrf2 in both_cytoplasm and nuclei of the cells, compared with brainstems from control uninfected mice, where anti-Nrf2 immunoreactivity was weaker, and detected only in the cytoplasm.
Fig.8. Increased levels of Nrf2 in the brainstem of ts1-infected vs. GVT-treated-ts1-infected mice.
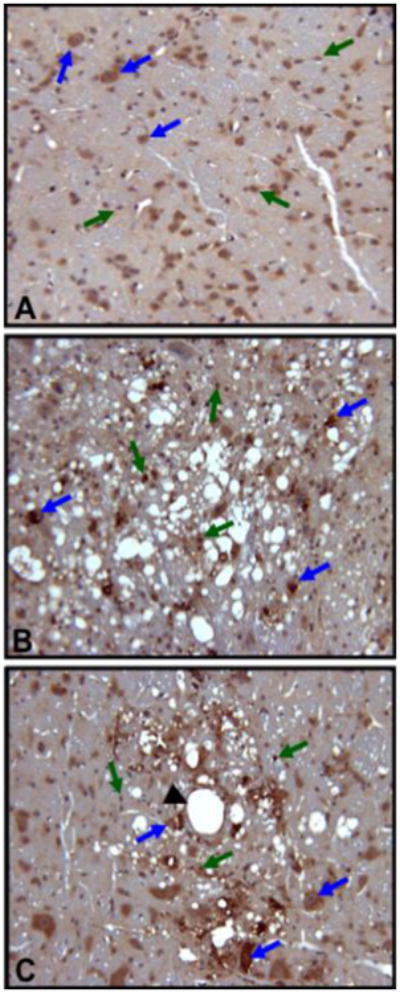
The photomicrographs show Nrf2 immunoreactivity (brown) in the cytoplasm and nucleus of neurons and glial cells in sectioned brainstem tissues from control (A), ts1-infected untreated (B) and GVT-treated-ts1-infected mice (C). Note the intense cytoplasmic and nuclear Nrf2 immunoreactivity in the neurons (blue arrows) and glial cells (green arrows) in the ts1-infected and GVT-treated-ts1-infected mice brainstems, compared with the same cells in sectioned uninfected mouse brainstem tissue. The black arrowhead is pointing at a blood vessel.
Discussion
Earlier studies from this laboratory have shown that while administration of the antioxidant NAC to ts1-infected mice has a potent protective effect in the thymus, this treatment is only marginally effective in the CNS (Lynn and Wong, 1998). Studies by others have also shown that NAC has a restricted ability to cross the blood brain barrier (Grinberg et al., 2005). In addition, NAC must be administered at higher doses for therapeutic effects, and these doses can be toxic. As noted above, we have also shown that the minocycline delays ts1-mediated neurodegeneration (Kuang et al., 2009). However, neuroprotection by minocycline is only slightly better than that resulting from NAC treatment (Table 1). Of the different agents we have tested for neuroprotection in the ts1-infected mice, the therapeutic effect of GVT greatly surpasses protection by either NAC or minocycline (Table 1). The results reported here show that GVT acts as an Nrf2 stabilizing agent. Although we have not tested NAC for its effect on Nrf2 levels in cultured astrocytes or CNS cells, it seems likely this drug act primarily as radical scavengers and as thiol-replenishing agents, while GVT acts both as a radical-scavenging chemical and as a potent activator of sustained thiol antioxidant protection via Nrf2 stabilization.
Together with other data from our laboratory, this study shows that GVT restores redox equilibrium in ts1-infected cells. The stabilization of Nrf2 by GVT is on par with its activation by tBHQ, which is a known positive regulator of Nrf2 (Li et al., 2005). Upregulation of Nrf2 by tBHQ has been shown to reduce the toxic effects of the amyloid protein in Alzheimer's disease (Kanninen et al., 2008). Activation and stabilization of Nrf2 has previously been shown to be a protective mechanism adapted by the endothelial cells against ROS/NOS induced by sheer stress (Warabi et al., 2007) as well as in cadmium-induced oxidative stress (He et al., 2008). We have shown recently that GVT prevents ts1-induced damage in T cells in the thymus, and in intestinal T cells, by upregulating and stabilizing Nrf2 levels in these cells (Scofield et al., 2009a; Scofield et al., 2009b). In infected T cells, GVT-induced restoration of redox equilibrium prevents gPr80env accumulation, which we know to be the primary trigger for ts1-induced cytopathology and cell death in astrocytes (Scofield et al., 2009a; Scofield et al., 2009b).
In healthy cells, Nrf2 resides in the cytoplasm as part of a complex with the redox-sensitive protein Keap-1. After a short period, this Nrf2 is ubiquitinated and released for proteasomal degradation (Itoh et al., 1999; Lee et al., 2003). If ROS levels are elevated in the cell, Keap-1 releases Nrf2, allowing it to translocate to the nucleus. In the nucleus, Nrf2 binds to ARE-containing promoter regions of genes associated with GSH synthesis, including NADPH quinone oxidoreductase, glutamate cysteine ligase, glutathione peroxidase, thioredoxin, thioredoxin reductase, and xCT antiporter, which protects the cells from oxidative stress by increasing cystine import to the cell. These enzymes and molecules act together to reduce the ROS load and to restore and maintain redox balance (Moi et al., 1994; Qiang et al., 2004; Qiang et al., 2006; Rushmore and Kong, 2002; Rushmore and Pickett, 1990).
Work by other laboratories, using various drugs and treatments, has shown that drug-induced upregulation of Nrf2 can occur either by prevention of its proteasomal degradation, or by upregulation of the de novo synthesis of Nrf2, through transcriptional activation. Ceftriaxone is one example of whose neuroprotective effect is mediated by the transcriptional activation of Nrf2 (Lewerenz et al., 2009). Our results from the present study show that GVT does not increase Nrf2 levels by elevated transcription of Nrf2. However, our results show that Nrf2 elevation mediated by GVT treatment is due to its effect on stabilization of Nrf2. For example, data presented here show that the proteasomal inhibitor MG132 can amplify the stabilization of Nrf2 in GVT-treated astrocytes. However, studies from other laboratories have demonstrated that Nrf2 stabilization, during oxidative stress, may occur by mechanisms that allow its release from Keap-1 in a nonubiquitinated state, thereby preventing its targeting to proteasomes. In this metastable state, Nrf2 can be maintained in the cytoplasm, ready for rapid phosphorylation and recruitment to the nucleus for its transcriptional activation activities. One candidate mediator of this effect is the DJ-1 protein, which binds to free Nrf2 released by Keap-1 during oxidative stress, and which maintains high cytoplasmic levels of Nrf2 without proteasomal inhibition (Clements et al., 2006).
Oxidative stress has been implicated in the pathogenesis of a variety of neurological disorders, such as ataxia talengiectasia or A-T (Chen et al., 2003; Kim and Wong, 2009a, b; Liu et al., 2005), AD (Smith et al., 1991; Smith et al., 1996), HD (Maksimovic et al., 2001; Polidori et al., 1999), ALS (Ghadge et al., 1997) and PD (Spina and Cohen, 1989). In addition to retroviruses, Epstein-Barr virus, Hepatitis B virus, Hepatitis C virus, respiratory syncytial virus, Japanese encephalitis virus, cytomegalovirus, and the herpes simplex virus have all been shown to induce oxidative stress in their infected cells as a result of elevated ROS generation (Cerimele et al., 2005; Cho et al., 2009; Kumar et al., 2009a; Kumar et al., 2009b; Mishra et al., 2009; Valyi-Nagy and Dermody, 2005).
Studies by others have demonstrated that the activation of the Nrf2 pathway in the brain plays a vital role in the neuroprotection mediated by astrocytes (Calkins et al., 2005; Shih et al., 2003). Nrf2-mediated gene products that regulate the activity of GSH synthesizing enzymes are largely located in astrocytes (Johnson et al., 2002; Kraft et al., 2004; Lee et al., 2003; Qiang et al., 2004). Interestingly, a recent report demonstrates that expression of Nrf2 in astrocytes and neurons improves spatial learning in a mouse model of AD (Kanninen et al., 2009).
In conclusion, our results show that GVT is a powerful neuroprotective drug due to its ability to scavenge ROS, and due to its ability to cause Nrf2 stabilization and accumulation in treated cells. Current treatments of diseases often focus on striving to eliminate the inciting agent or the clinical symptoms. These, however, could result in injuring healthy tissue in the process. Treatment with GVT in our animal model of retrovirus-mediated neurological disease presented here focuses instead on GVT's effect in supporting the oxidatively stressed cells in controlling or stabilizing the cellular defense by upregulating Nrf2 levels. As mentioned above, Nrf2 upregulation has been shown to be an important protective mechanism against oxidative stress induced neurological disorders in a number of studies by others (Calkins et al., 2008; Clements et al., 2006; Johnson et al., 2008; Li et al., 2007; Vargas and Johnson, 2009). Consistent with these results our earlier study shows that GVT attenuates hindlimb paralysis, wasting and also inhibit free radical generation. Furthermore, this study demonstrates that GVT, in vivo as well as in vitro, upregulates Nrf2 levels. Thus, the effects of GVT on Nrf2 may be useful and further manipulated to develop effective and safe preventive and therapeutic agents not only against oxidative stress mediated neurological diseases but also against other systems that are affected.
Acknowledgments
The authors thank Soo Jin Kim and Drs. Virginia Scofield and Mingshan Yan, Jeesun Kim for their helpful support and discussions. We are also most grateful to Lifang Zhang for her invaluable technical assistance. This work was supported by NIH grants MH071583 and NS043984 (awarded to Dr. Paul Wong) the University of Texas M. D. Anderson Cancer Center Support Grant CA16672 and the NIEHS Center Grant ES07784. We are also thankful to Bach Pharma, Inc., North Andover, MA for kindly providing us with GVT. Support was also provided by the Longevity Foundation of Austin, Texas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Betteridge DJ. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci U S A. 2005;102:244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, Williamson TP, Kraft AD, Lee JM, Li J, Johnson JA. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerimele F, Battle T, Lynch R, Frank DA, Murad E, Cohen C, Macaron N, Sixbey J, Smith K, Watnick RS, Eliopoulos A, Shehata B, Arbiser JL. Reactive oxygen signaling and MAPK activation distinguish Epstein-Barr Virus (EBV)-positive versus EBV-negative Burkitt's lymphoma. Proc Natl Acad Sci U S A. 2005;102:175–179. doi: 10.1073/pnas.0408381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Peng C, Luff J, Spring K, Watters D, Bottle S, Furuya S, Lavin MF. Oxidative stress is responsible for deficient survival and dendritogenesis in purkinje neurons from ataxia-telangiectasia mutated mutant mice. J Neurosci. 2003;23:11453–11460. doi: 10.1523/JNEUROSCI.23-36-11453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, Polack FP, Kleeberger SR. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am J Respir Crit Care Med. 2009;179:138–150. doi: 10.1164/rccm.200804-535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S, Duggan J, Chakraborty J. Tsl and LP-BM5: a comparison of two murine retrovirus models for HIV. Viral Immunol. 2001;14:95–109. doi: 10.1089/088282401750234475. [DOI] [PubMed] [Google Scholar]
- Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer-and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadge GD, Lee JP, Bindokas VP, Jordan J, Ma L, Miller RJ, Roos RP. Mutant superoxide dismutase-1-linked familial amyotrophic lateral sclerosis: molecular mechanisms of neuronal death and protection. J Neurosci. 1997;17:8756–8766. doi: 10.1523/JNEUROSCI.17-22-08756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Nathanson N, Wong P. Retroviruses and the nervous system. Plenum Press; New York: 1995. [Google Scholar]
- Grinberg L, Fibach E, Amer J, Atlas D. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radic Biol Med. 2005;38:136–145. doi: 10.1016/j.freeradbiomed.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Gross S, Gammon ST, Moss BL, Rauch D, Harding J, Heinecke JW, Ratner L, Piwnica-Worms D. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat Med. 2009;15:455–461. doi: 10.1038/nm.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Chen MG, Ma Q. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem Res Toxicol. 2008;21:1375–1383. doi: 10.1021/tx800019a. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Scofield VL, Yan M, Qiang W, Liu N, Reid AJ, Lynn WS, Wong PK. Retrovirus-induced oxidative stress with neuroimmunodegeneration is suppressed by antioxidant treatment with a refined monosodium alpha-luminol (Galavit) J Virol. 2006;80:4557–4569. doi: 10.1128/JVI.80.9.4557-4569.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DA, Andrews GK, Xu W, Johnson JA. Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. J Neurochem. 2002;81:1233–1241. doi: 10.1046/j.1471-4159.2002.00913.x. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanninen K, Heikkinen R, Malm T, Rolova T, Kuhmonen S, Leinonen H, Yla-Herttuala S, Tanila H, Levonen AL, Koistinaho M, Koistinaho J. Intrahippocampal injection of a lentiviral vector expressing Nrf2 improves spatial learning in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:16505–16510. doi: 10.1073/pnas.0908397106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanninen K, Malm TM, Jyrkkanen HK, Goldsteins G, Keksa-Goldsteine V, Tanila H, Yamamoto M, Yla-Herttuala S, Levonen AL, Koistinaho J. Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol Cell Neurosci. 2008;39:302–313. doi: 10.1016/j.mcn.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Kim HT, Qiang W, Wong PK, Stoica G. Enhanced proteolysis of IkappaBalpha and IkappaBbeta proteins in astrocytes by Moloney murine leukemia virus (MoMuLV)-ts1 infection: a potential mechanism of NF-kappaB activation. J Neurovirol. 2001;7:466–475. doi: 10.1080/135502801753170327. [DOI] [PubMed] [Google Scholar]
- Kim HT, Tasca S, Qiang W, Wong PK, Stoica G. Induction of p53 accumulation by Moloney murine leukemia virus-ts1 infection in astrocytes via activation of extracellular signal-regulated kinases 1/2. Lab Invest. 2002;82:693–702. doi: 10.1097/01.lab.0000017373.82871.45. [DOI] [PubMed] [Google Scholar]
- Kim HT, Waters K, Stoica G, Qiang W, Liu N, Scofield VL, Wong PK. Activation of endoplasmic reticulum stress signaling pathway is associated with neuronal degeneration in MoMuLV-ts1-induced spoorm encephalomyelopathy. Lab Invest. 2004;84:816–827. doi: 10.1038/labinvest.3700104. [DOI] [PubMed] [Google Scholar]
- Kim J, Cha YN, Surh YJ. A protective role of nuclear erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2009 doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Kim J, Wong PK. Loss of ATM impairs proliferation of neural stem cells through oxidative stress-mediated p38 MAPK signaling. Stem Cells. 2009a;27:1987–1998. doi: 10.1002/stem.125. [DOI] [PubMed] [Google Scholar]
- Kim J, Wong PK. Oxidative stress is linked to ERK1/2-p16 signaling-mediated growth defect in ATM-deficient astrocytes. J Biol Chem. 2009b;284:14396–14404. doi: 10.1074/jbc.M808116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang X, Scofield VL, Yan M, Stoica G, Liu N, Wong PK. Attenuation of oxidative stress, inflammation and apoptosis by minocycline prevents retrovirus-induced neurodegeneration in mice. Brain Res. 2009;1286:174–184. doi: 10.1016/j.brainres.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kalita J, Saxena V, Khan MY, Khanna VK, Sharma S, Dhole TN, Misra UK. Some observations on the tropism of Japanese encephalitis virus in rat brain. Brain Res. 2009a;1268:135–141. doi: 10.1016/j.brainres.2009.02.051. [DOI] [PubMed] [Google Scholar]
- Kumar S, Misra UK, Kalita J, Khanna VK, Khan MY. Imbalance in oxidant/antioxidant system in different brain regions of rat after the infection of Japanese encephalitis virus. Neurochem Int. 2009b;55:648–654. doi: 10.1016/j.neuint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? Faseb J. 2005;19:1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- Lee Y, Chong MJ, McKinnon PJ. Ataxia telangiectasia mutated-dependent apoptosis after genotoxic stress in the developing nervous system is determined by cellular differentiation status. J Neurosci. 2001;21:6687–6693. doi: 10.1523/JNEUROSCI.21-17-06687.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Calkins MJ, Johnson DA, Johnson JA. Role of Nrf2-dependent ARE-driven antioxidant pathway in neuroprotection. Methods Mol Biol. 2007;399:67–78. doi: 10.1007/978-1-59745-504-6_6. [DOI] [PubMed] [Google Scholar]
- Li J, Johnson D, Calkins M, Wright L, Svendsen C, Johnson J. Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxicol Sci. 2005;83:313–328. doi: 10.1093/toxsci/kfi027. [DOI] [PubMed] [Google Scholar]
- Lin YC, Chow CW, Yuen PH, Wong PK. Establishment and characterization of conditionally immortalized astrocytes to study their interaction with ts1, a neuropathogenic mutant of Moloney murine leukemia virus. J Neurovirol. 1997;3:28–37. doi: 10.3109/13550289709015790. [DOI] [PubMed] [Google Scholar]
- Liu N, Kuang X, Kim HT, Stoica G, Qiang W, Scofield VL, Wong PK. Possible involvement of both endoplasmic reticulum- and mitochondria-dependent pathways in MoMuLV-ts1-induced apoptosis in astrocytes. J Neurovirol. 2004;10:189–198. doi: 10.1080/13550280490448043. [DOI] [PubMed] [Google Scholar]
- Liu N, Scofield VL, Qiang W, Yan M, Kuang X, Wong PK. Interaction between endoplasmic reticulum stress and caspase 8 activation in retrovirus MoMuLV-ts1-infected astrocytes. Virology. 2006;348:398–405. doi: 10.1016/j.virol.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Liu N, Stoica G, Yan M, Scofield VL, Qiang W, Lynn WS, Wong PK. ATM deficiency induces oxidative stress and endoplasmic reticulum stress in astrocytes. Lab Invest. 2005;85:1471–1480. doi: 10.1038/labinvest.3700354. [DOI] [PubMed] [Google Scholar]
- Lynn WS, Wong PK. Neuroimmunodegeneration: do neurons and T cells use common pathways for cell death? Faseb J. 1995;9:1147–1156. doi: 10.1096/fasebj.9.12.7672507. [DOI] [PubMed] [Google Scholar]
- Lynn WS, Wong PK. Neuroimmunopathogenesis of ts1 MoMuLV viral infection. Neuroimmunomodulation. 1998;5:248–260. doi: 10.1159/000026345. [DOI] [PubMed] [Google Scholar]
- Maksimovic ID, Jovanovic MD, Colic M, Mihajlovic R, Micic D, Selakovic V, Ninkovic M, Malicevic Z, Rusic-Stojiljkovic M, Jovicic A. Oxidative damage and metabolic dysfunction in experimental Huntington's disease: selective vulnerability of the striatum and hippocampus. Vojnosanit Pregl. 2001;58:237–242. [PubMed] [Google Scholar]
- Mishra MK, Ghosh D, Duseja R, Basu A. Antioxidant potential of Minocycline in Japanese Encephalitis Virus infection in murine neuroblastoma cells: correlation with membrane fluidity and cell death. Neurochem Int. 2009;54:464–470. doi: 10.1016/j.neuint.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollace V, Nottet HS, Clayette P, Turco MC, Muscoli C, Salvemini D, Perno CF. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 2001;24:411–416. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- Offen D, Gilgun-Sherki Y, Barhum Y, Benhar M, Grinberg L, Reich R, Melamed E, Atlas D. A low molecular weight copper chelator crosses the blood-brain barrier and attenuates experimental autoimmune encephalomyelitis. J Neurochem. 2004;89:1241–1251. doi: 10.1111/j.1471-4159.2004.02428.x. [DOI] [PubMed] [Google Scholar]
- Polidori MC, Mecocci P, Browne SE, Senin U, Beal MF. Oxidative damage to mitochondrial DNA in Huntington's disease parietal cortex. Neurosci Lett. 1999;272:53–56. doi: 10.1016/s0304-3940(99)00578-9. [DOI] [PubMed] [Google Scholar]
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol. 2004;78:11926–11938. doi: 10.1128/JVI.78.21.11926-11938.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W, Kuang X, Liu J, Liu N, Scofield V, Stoica G, Lynn WS, Wong PKY. Astrocytes survive chronic infection and cytopathic effects of the ts1 mutant of the retrovirus Moloney murine leukemia virus by upregulation of antioxidant defenses. J Virol. 2006;80:3273–3284. doi: 10.1128/JVI.80.7.3273-3284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy NM, Kleeberger SR, Kensler TW, Yamamoto M, Hassoun PM, Reddy SP. Disruption of Nrf2 impairs the resolution of hyperoxia-induced acute lung injury and inflammation in mice. J Immunol. 2009a;182:7264–7271. doi: 10.4049/jimmunol.0804248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy NM, Suryanarayana V, Kalvakolanu DV, Yamamoto M, Kensler TW, Hassoun PM, Kleeberger SR, Reddy SP. Innate immunity against bacterial infection following hyperoxia exposure is impaired in NRF2-deficient mice. J Immunol. 2009b;183:4601–4608. doi: 10.4049/jimmunol.0901754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PV, Murthy Ch R, Reddanna P. Fulminant hepatic failure induced oxidative stress in nonsynaptic mitochondria of cerebral cortex in rats. Neurosci Lett. 2004;368:15–20. doi: 10.1016/j.neulet.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. J Neurochem. 2008;106:1298–1313. doi: 10.1111/j.1471-4159.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Kong AN. Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr Drug Metab. 2002;3:481–490. doi: 10.2174/1389200023337171. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- Sacktor N, H N, Cutler R, Tamara A, Turchan J, Pardo C, Vargas D, Nath A. Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol. 2004;157:176–184. doi: 10.1016/j.jneuroim.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Chen LJ, Burka LT, Matthews HB. Metabolism and disposition of luminol in the rat. Xenobiotica. 2000;30:263–272. doi: 10.1080/004982500237659. [DOI] [PubMed] [Google Scholar]
- Scofield VL, Yan M, Kuang X, Kim SJ, Crunk D, Wong PK. The drug monosodium luminol (GVT) preserves thymic epithelial cell cytoarchitecture and allows thymocyte survival in mice infected with the T cell-tropic, cytopathic retrovirus ts1. Immunol Lett. 2009a;122:159–169. doi: 10.1016/j.imlet.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Scofield VL, Yan M, Kuang X, Kim SJ, Wong PK. The drug monosodium luminol (GVT) preserves crypt-villus epithelial organization and allows survival of intestinal T cells in mice infected with the ts1 retrovirus. Immunol Lett. 2009b;122:150–158. doi: 10.1016/j.imlet.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikova E, Lin YC, Saha K, Brooks BR, Wong PK. Correlation of specific virus-astrocyte interactions and cytopathic effects induced by ts1, a neurovirulent mutant of Moloney murine leukemia virus. J Virol. 1993;67:1137–1147. doi: 10.1128/jvi.67.3.1137-1147.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer's. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- Spina MB, Cohen G. Dopamine turnover and glutathione oxidation: implications for Parkinson disease. Proc Natl Acad Sci U S A. 1989;86:1398–1400. doi: 10.1073/pnas.86.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Haughey N, Li W, Venkatesan A, Anderson C, Reid R, Malpica T, Pocernich C, Butterfield DA, Nath A. Oxidative stress and therapeutic approaches in HIV dementia. Antioxid Redox Signal. 2006;8:2089–2100. doi: 10.1089/ars.2006.8.2089. [DOI] [PubMed] [Google Scholar]
- Stoica G, Floyd E, Illanes O, Wong PK. Temporal lymphoreticular changes caused by ts1, a paralytogenic mutant of Moloney murine leukemia virus TB. Lab Invest. 1992;66:427–436. [PubMed] [Google Scholar]
- Stoica G, Illanes O, Tasca SI, Wong PK. Temporal central and peripheral nervous system changes induced by a paralytogenic mutant of Moloney murine leukemia virus TB. Lab Invest. 1993;69:724–735. [PubMed] [Google Scholar]
- Stoica G, Tasca SI, Wong PK. Motor neuronal loss and neurofilament-ubiquitin alteration in MoMuLV-ts1 encephalopathy. Acta Neuropathol. 2000;99:238–244. doi: 10.1007/pl00007433. [DOI] [PubMed] [Google Scholar]
- Szurek PF, Floyd E, Yuen PH, Wong PK. Site-directed mutagenesis of the codon for Ile-25 in gPr80env alters the neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990a;64:5241–5249. doi: 10.1128/jvi.64.11.5241-5249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurek PF, Yuen PH, Ball JK, Wong PK. A Val-25-to-Ile substitution in the envelope precursor polyprotein, gPr80env, is responsible for the temperature sensitivity, inefficient processing of gPr80env, and neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990b;64:467–475. doi: 10.1128/jvi.64.2.467-475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyi-Nagy T, Dermody TS. Role of oxidative damage in the pathogenesis of viral infections of the nervous system. Histol Histopathol. 2005;20:957–967. doi: 10.14670/HH-20.957. [DOI] [PubMed] [Google Scholar]
- Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warabi E, Takabe W, Minami T, Inoue K, Itoh K, Yamamoto M, Ishii T, Kodama T, Noguchi N. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic Biol Med. 2007;42:260–269. doi: 10.1016/j.freeradbiomed.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Wong PK. Moloney murine leukemia virus temperature-sensitive mutants: a model for retrovirus-induced neurologic disorders. Curr Top Microbiol Immunol. 1990;160:29–60. doi: 10.1007/978-3-642-75267-4_3. [DOI] [PubMed] [Google Scholar]
- Wong PK, Floyd E, Szurek PF. High susceptibility of FVB/N mice to the paralytic disease induced by ts1, a mutant of Moloney murine leukemia virus TB. Virology. 1991;180:365–371. doi: 10.1016/0042-6822(91)90041-9. [DOI] [PubMed] [Google Scholar]
- Wong PK, Knupp C, Yuen PH, Soong MM, Zachary JF, Tompkins WA. ts1, a Paralytogenic mutant of Moloney murine leukemia virus TB, has an enhanced ability to replicate in the central nervous system and primary nerve cell culture. J Virol. 1985;55:760–767. doi: 10.1128/jvi.55.3.760-767.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PK, Prasad G, Hansen J, Yuen PH. ts1, a mutant of Moloney murine leukemia virus-TB, causes both immunodeficiency and neurologic disorders in BALB/c mice. Virology. 1989;170:450–459. doi: 10.1016/0042-6822(89)90436-4. [DOI] [PubMed] [Google Scholar]


