Abstract
Changes in NAD+ and its metabolites contribute to longevity and age-associated diseases. The role of NAD+ metabolism in bone diseases has however not been investigated, despite the fact that osteoporosis is a leading cause of morbidity in old age. TRAP(+) osteoclast formation from C57 Bl/6J mice was assessed after the addition of varying concentrations of NAD+ metabolites or exogenous ADPribosyl cyclase and NADase enzymes. The NAD+ metabolite cyclic ADPribose (cADPr) or exogenous addition of the enzyme ADPribosyl cyclase stimulated osteoclast formation. Blocking cADPr action with the antagoinst 8-Br-cADPr potently inhibited osteoclast formation. In contrast to cADPr, its non cyclized derivative ADPribose (ADPr) or the exogenous addition of NADase both inhibited osteoclastogenesis. As CD38 is the major NAD+-degrading enzyme present in the bone marrow, these results suggest that CD38-mediated inhibition of osteoclastogenesis is related to its NADase activity, not its ADPribosyl cyclase activity.
Keywords: NAD+, osteoclast, cyclic ADPribose, ADPribose, NAADP+, lymphocyte, metabolism, ADPr, cADPr, NADase, ADPribosyl cyclase
Introduction
Changes in NAD+ and its metabolites, such as nicotinamide, have received attention for their modulation of cellular and organismal longevity, and thus their contribution to age-associated diseases [1]. However, there are no reports investigating the relationship between NAD+ metabolism and bone, despite the fact that osteoporosis is the leading cause of morbidity in old age [2]. Indeed, the only connection between bone and NAD+ was the appreciation 40 years ago that NAD+ is needed for optimal collagen synthesis [3]. Thereafter, we reported the first link between NAD+ metabolism and osteoclastogenesis; nonetheless, our investigation was only undertaken to understand how Ca2+-regulating metabolites of NAD+ may affect osteoclast Ca2+-sensing [3].
Since that initial report, we have subsequently found that the NAD+-degrading enzyme CD38 plays an in vivo role in osteoclastogenesis and bone metabolism [4]. Moreover, a polymorphorphism in the CD38 promoter has recently been linked to the regulation of bone mass in post-menopausal women [5]. While evidence is accumulating for a role of CD38 in regulating bone mass, the exact mechanism whereby CD38 affects bone mass remains unclear. CD38 can catalyze the breakdown of NAD+ into cyclic ADPribose (cADPr) or ADPribose (ADPr), but there are additional CD38 actions that appear to be NAD+-independent. For example, CD38-mediated tyrosine kinase signalling in lymphocytes is independent of NAD+ breakdown [6].
Here we examine the effects of NAD+ and its metabolites on osteoclast formation. While the addition of NAD+ or NAADP+ did not appreciably affect osteoclastogenesis, we unexpectedly identified cADPr as a stimulant of osteoclast formation. In contrast, we provide evidence that the cleavage of NAD+ into ADPr negatively modulates osteoclast formation.
Results and Discussion
The major Ca2+-releasing metabolites of NAD+ were tested for effects on osteoclast formation. While NAD+ and NAADP+ were without effect, cyclic ADPribose (cADPr) unexpectedly enhanced osteoclast formation at low doses (Figure 1). This was unexpeceted because the genetic deletion of CD38 results in enhanced osteoclastogenesis [4]. The effect of cADPr on osteoclast formation was further examined by analyzing the effects of the cADPr antagonist 8-Br-cADPr. Application of 8-Br-cADPr potently inhibited osteoclast formation (Figure 2), suggesting that the pathways positively affected by cADPr are crucial in osteoclast formation.
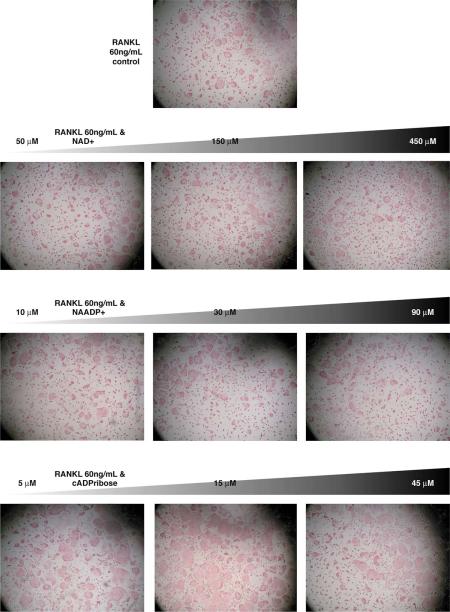
Figure 1. The effect of NAD+ and its Ca2+-releasing metabolites NAADP+ and cyclic ADPribose on osteoclast formation.
(Top picture) Ficol-purified bone marrow cells were plated at 3×10^4 cells/ well with 60 ng/mL murine RANK-L and 30 ng/mL murine M-CSF. After 5 days, the cells were stained for TRAP and a photograph taken at 4× power on an inverted microscope. The second row shows the effects of increasing concentration of NAD+ on RANK-L-induced osteoclast formation; there were no significant effects of NAD+ at the concentrations tested. The third row displays the effects of increasing doses of NAADP+ on osteoclast formation; there were no significant effects of NAADP+ at the concentrations tested. The last row shows the effects of increasing doses of cyclic ADPribose; cyclic ADPribose increased osteoclast formation at 5 μM, with effects peaking at 15 μM and dropping off by 45 μM.
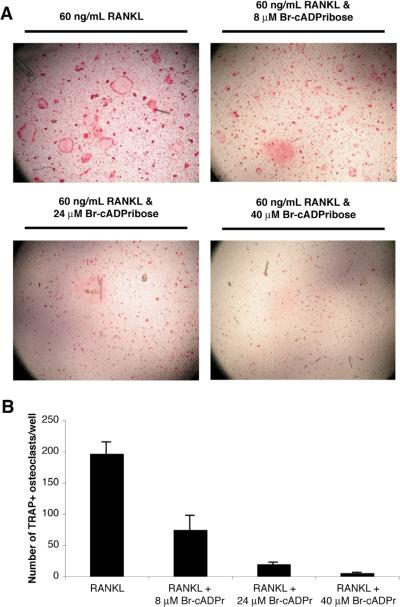
Figure 2. The cADPr antagonist 8-Br-cyclic ADPribose inhibits osteoclast formation.
(A) The effects of increasing doses of Br-cADPribose on RANK-L-induced osteoclast formation are shown at 4× power. (B) The effects of Br-cADPribose on osteoclast formation was quantitated by counting the number of TRAP(+) osteoclasts/well. The results are presented as the mean (n = 4) with the positive error bars representing the S.E.M. All three doses produced a significant decrease in osteoclast formation (P < 0.01, assessed by T-testing) compared to the RANK-L only group.
We recently discovered that CD38 upregulation by TNF serves to induce the breakdown of NAD+ [7]. While both cADPr and NAADP+ are potent Ca2+-releasing agents, the majority of NAD+ is broken down into ADPribose (ADPr). Thus, we examined whether ADPr could be the agent responsible for osteoclast inhibition by CD38. Indeed, Figure 3 shows that ADPr inhibited osteoclast formation.
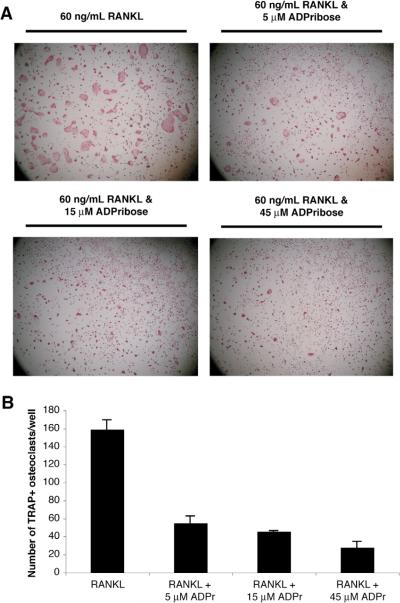
Figure 3. The NAD+ metabolite ADPribose negatively regulates osteoclast formation.
(A) The effects of increasing doses of ADPribose on RANK-L-induced osteoclast formation are shown at 4× power. (B) The effects of ADPribose on osteoclast formation was quantitated by counting the number of TRAP(+) osteoclasts/well. The results are presented as the mean (n=4) with the positive error bars representing the S.E.M. All three doses produced a significant decrease in osteoclast formation (P < 0.05, assessed by T-testing) compared to the RANK-L only group.
We next sought to understand how the pharmacological stimulation of osteoclastogenesis with cADPr and inhibition with ADPr occurs within the physiological context of CD38. CD38 is an extracellularly-facing enzyme most highly expressed on lymphocytes [8]. We therefore examined whether exogenous ADPribosyl cyclase or NADase would recapitulate the effects of cADPr or ADPr, respectively. As shown in Figure 4, the introduction of Aplaysia ADPribosyl cyclase augmented osteoclastogenesis when low units were added. In contrast to the ADPribosyl cyclase, the introduction of snake venom NADase led to a unit-dependant decrease in osteoclast formation, with no osteoclasts seen at 30 mU or higher (Figure 4).
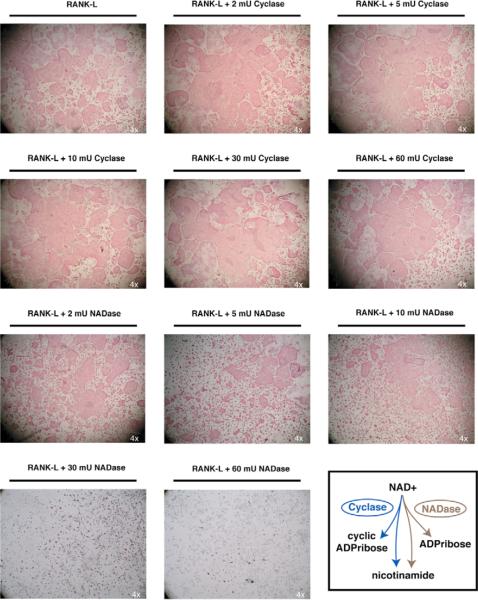
Figure 4. Exogenous addition of ADPribosyl cyclase augments, while NADase inhibits osteoclast formation.
Ficol-purified bone marrow cells were plated with either RANK-L and M-CSF alone, or with the addition of 2 to 60 mU Aplaysia ADPribosyl cyclase or 2 to 60 mU of snake venom NADase enzymes. The exogenous addition of ADPribosyl cyclase (Cyclase) augmented osteoclast formation with doses detectable at doses as low as 2 mU. In contrast, the addition of NADase inhibited osteoclast formation, with modest inhibition between 2 to 10 mU, and complete inhibition at 30 mU or greater. The depiction of the differences in enzymatic activity between the Cyclase and NADase is shown in the lower right corner.
Thus, we show that changes in NAD+ metabolism can significantly impact osteoclast formation. Interestingly, the same molecule can either stimulate or inhibit osteoclast formation depending upon its stereochemical configuration; specifically, cyclic ADPr stimulates, while the non-cyclic form ADPr inhibits osteoclast formation. While it was initially hypothesized that CD38's production of cADPr was the mechanism through which the enzyme inhibited osteoclast formation [4], it is shown here that ADPr, which represents >97% of the product of CD38-catalyzed NAD+ breakdown, is likely the major negative regulator of osteoclast formation.
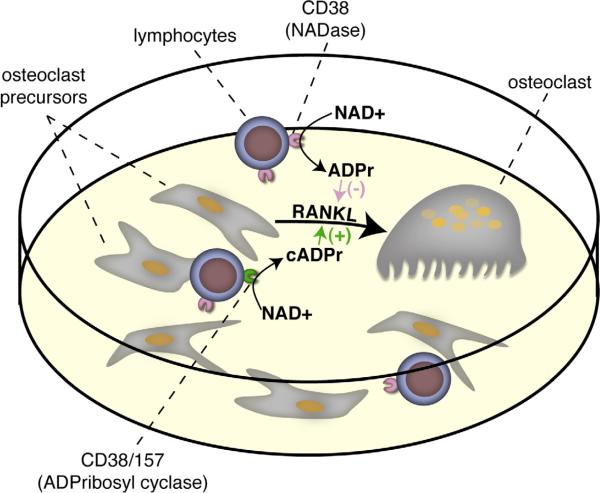
The demonstration that exogenous addition of NADase or ADPribosyl cyclase can exert appreciable effects shows that the extracellular membrane localization of CD38 is permissive to its effects on osteoclast formation. It was recently demonstrated that the major cell type expressing CD38 in Ficol-purified osteoclastic cultures are contaminating lymphocytes [9]. It remains to be tested whether CD38 expressed on the surface of lymphocytes serves as floating NADase to exert effects similar to the exogenous addition of snake venom NADase. Figure 5 graphically depicts this speculative hypothesis showing how the presence of CD38 on contaminating lymphocytes might negatively modulate osteoclast formation. Future investigations will address the osteoclastogenic contribution of CD38 expressed on B-cells and T-cells.
Figure 5. A schematic of the proposed mechanism whereby CD38 negatively regulates osteoclast formation.
Contaminating lymphocytes, which highly express CD38, act as a NADase to cleave NAD+ present in the media into ADPribose. It is possible that a signal that increases the cyclase activity of CD38, or possibly CD157, may produce cyclic ADPribose, which will serve to enhance osteoclast formation.
Methods
Cell preparation
To obtain total bone marrow, mice were sacrificed by an institutionally approved protocol and both femurs and tibea were surgically extracted and their bone marrow flushed. The bone marrow pellet was resuspended in α-MEM (Invitrogen, CA) containing 5% FBS (Select USA stock, Invitrogen, CA), and 1% penicillin/streptomycin.
Osteoclast Formation
5 ng/mL recombinant murine M-CSF (R&D Systems, NJ) was added and the cells incubated for one day. Non-adherent cells were collected and layered over 6 mL of Ficoll-Hypaque (Amersham/GE Health Sciences, NJ) for density centrifugation. The cells from the interface layer were collected and resuspended in α-MEM containing 10% FBS and 1% P/S. Cells were plated at 3×104/well in a 96-well plate with 30 ng/mL M-CSF and various concentrations of RANK-L (R&D Systems, NJ). After 5 days, the cells were stained according to BD Biosciences Technical Bulletin #445.
Acknowledgements
We gratefully acknowledge support from the NIH: AG14907, DK70526, AG23176 (to MZ), an Endocrinology Training grant (to Mount Sinai), and an MSTP Institutional grant (to Mount Sinai).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: No conflicts declared.
References
- 1.Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Stepnick LS. The frequency of bone disease. In: McGowan LGRASNJA, Elderkin AL, editors. Bone Health and Osteoporosis: A Report of the Surgeon General, Office of the U.S. Surgeon General. Washington D.C.: 2004. pp. 68–87. [Google Scholar]
- 3.Fullmer HM, Martin GR. Activity of D(−)-Beta-Hydroxyputyric Dehydrogenase in Scurvy. Nature. 1964;202:302. doi: 10.1038/202302a0. [DOI] [PubMed] [Google Scholar]
- 4.Sun L, Iqbal J, Dolgilevich S, Yuen T, Wu XB, Moonga BS, Adebanjo OA, Bevis PJ, Lund F, Huang CL, Blair HC, Abe E, Zaidi M. Disordered osteoclast formation and function in a CD38 (ADP-ribosyl cyclase)-deficient mouse establishes an essential role for CD38 in bone resorption. Faseb J. 2003;17:369–375. doi: 10.1096/fj.02-0205com. [DOI] [PubMed] [Google Scholar]
- 5.Drummond FJ, Mackrill JJ, O'Sullivan K, Daly M, Shanahan F, Molloy MG. CD38 is associated with premenopausal and postmenopausal bone mineral density and postmenopausal bone loss. J Bone Miner Metab. 2006;24:28–35. doi: 10.1007/s00774-005-0642-3. [DOI] [PubMed] [Google Scholar]
- 6.Lund FE, Muller-Steffner H, Romero-Ramirez H, Moreno-Garcia ME, Partida-Sanchez S, Makris M, Oppenheimer NJ, Santos-Argumedo L, Schuber F. CD38 induces apoptosis of a murine pro-B leukemic cell line by a tyrosine kinase-dependent but ADP-ribosyl cyclase- and NAD glycohydrolase-independent mechanism. Int Immunol. 2006 doi: 10.1093/intimm/dxl037. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal J, Zaidi M. TNF regulates cellular NAD+ metabolism in primary macrophages. Biochem Biophys Res Commun. 2006;342:1312–1318. doi: 10.1016/j.bbrc.2006.02.109. [DOI] [PubMed] [Google Scholar]
- 8.Lund FE, Cockayne DA, Randall TD, Solvason N, Schuber F, Howard MC. CD38: a new paradigm in lymphocyte activation and signal transduction. Immunol Rev. 1998;161:79–93. doi: 10.1111/j.1600-065x.1998.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 9.Iqbal J, Kumar K, Sun L, Zaidi M. Selective Upregulation of the ADPribosyl cyclases CD38 and CD157 by TNF but not by RANK-L Reveals Differences in Downstream Signaling. Am J Physiol Renal Physiol. 2006 doi: 10.1152/ajprenal.00066.2006. [DOI] [PubMed] [Google Scholar]