Abstract
C57BL/6J (C57) and DBA/2J (DBA) mice respond differently to drugs that affect dopamine systems, including alcohol. The current study compared effects of D1 and D2 receptor agonists and antagonists, and the interaction between D1/D2 antagonists and alcohol, on intracranial self-stimulation in male C57 and DBA mice to determine the role of dopamine receptors in the effects of alcohol on brain stimulation reward (BSR). In the initial strain comparison, dose effects on BSR thresholds and maximum operant response rates were determined for the D1 receptor agonist SKF-82958 (±-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine; 0.1–0.56 mg/kg) and antagonist SCH 23390 (+-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepinehydrochloride; 0.003–0.056 mg/kg), and the D2 receptor agonist quinpirole (0.1–3.0 mg/kg) and antagonist raclopride (0.01–0.56 mg/kg). For the alcohol interaction, SCH 23390 (0.003 mg/kg) or raclopride (0.03 mg/kg) was given before alcohol (0.6–2.4 g/kg p.o.). D1 antagonism dose-dependently elevated and SKF-82958 dose-dependently lowered BSR threshold in both strains; DBA mice were more sensitive to SKF-82958 effects. D2 antagonism dose-dependently elevated BSR threshold only in C57 mice. Low doses of quinpirole elevated BSR threshold equally in both strains, whereas higher doses of quinpirole lowered BSR threshold only in C57 mice. SCH 23390, but not raclopride, prevented lowering of BSR threshold by alcohol in DBA mice. Conversely, raclopride, but not SCH 23390, prevented alcohol potentiation of BSR in C57 mice. These results extend C57 and DBA strain differences to D1/D2 sensitivity of BSR, and suggest differential involvement of D1 and D2 receptors in the acute rewarding effects of alcohol in these two mouse strains.
Introduction
Intracranial self-stimulation (ICSS) is a valuable behavioral method to understand the role of dopamine in reward-related behaviors. ICSS measures the responding of an animal reinforced by electrical stimulation of mesolimbic reward circuitry, which ultimately increases activity of dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc). Brain stimulation reward (BSR) is exquisitely sensitive to manipulation of dopamine (DA) receptors (Kornetsky and Bain, 1992; Wise, 1996). By activating the dopaminergic system, drugs of abuse, including alcohol, potentiate BSR, whereas drugs that decrease dopaminergic activity decrease BSR.
C57BL/6J (C57) and DBA/2J (DBA) mice exhibit differences in dopamine systems (Crawley et al., 1997; Puglisi-Allegra and Cabib, 1997). Compared with C57 mice, DBA mice have more neurons expressing tyrosine hydroxylase (TH) in the VTA and substantia nigra pars compacta, increased dopamine transporter expression in the prefrontal cortex, a more discrete distribution of dopamine transporter and TH immunoreactive fibers in the NAc, higher density of TH-immunoreactive fibers in the prefrontal cortex, lower density of D1 and D2 receptors in the striatum, and greater density of D2 receptors in the midbrain (Ng et al., 1994; Zocchi et al., 1998; D'Este et al., 2007). Amphetamine and cocaine stimulate greater DA release in the NAc and more locomotor activity in C57 than in DBA mice (Cabib et al., 2000; Ventura et al., 2004; Orsini et al., 2005). DBA mice may also have increased D2 autoreceptor activity (Puglisi-Allegra and Cabib, 1997) given their greater sensitivity to apomorphine inhibition of DA metabolism (Cabib and Puglisi-Allegra, 1991) and quinpirole- and haloperidol-induced catalepsy (Kanes et al., 1993; Puglisi-Allegra and Cabib, 1997).
Two previous studies have directly compared ICSS in C57 and DBA mice. Both strains had similar BSR thresholds (Elmer et al., 2010; Fish et al., 2010) and no strain difference was found in amphetamine potency (Elmer et al., 2010); however, DBA mice were more sensitive to the threshold lowering effects of cocaine (Fish et al., 2010). Additionally, the two strains were differentially sensitive to morphine and alcohol. Morphine lowered BSR thresholds in C57 and elevated thresholds in DBA mice (Elmer et al., 2010), whereas high doses of alcohol lowered thresholds in DBA and elevated thresholds in C57 mice (Fish et al., 2010). This latter difference is consistent with other demonstrations that these strains respond uniquely to alcohol. For example, although DBA mice voluntarily drink much less alcohol due to an innate taste or olfactory aversion (Belknap et al., 1977; Grahame and Cunningham, 1997), they show increased locomotor activation and sensitization (Crabbe et al., 1982; Phillips et al., 1994), conditioned place preference (Cunningham et al., 1992b), and dopamine release (Kapasova and Szumlinski, 2008) following parenteral alcohol administration, and increased VTA cell firing in vitro (Brodie and Appel, 2000; McDaid et al., 2008). Differences in the mesolimbic dopamine system may underlie differences in the behavioral effects of alcohol in these two inbred strains.
C57 mice with reduced D1 or D2 receptor expression consume less alcohol (El-Ghundi et al., 1998; Phillips et al., 1998; Risinger et al., 2000; Bahi and Dreyer, 2012), whereas mice lacking D2 receptors also have reduced alcohol place preference (Cunningham et al., 2000). D2 antagonists can block alcohol self-administration in rats (Slawecki et al., 1997; Samson and Chappell, 1999), locomotor stimulation in several lines of mice (Shen et al., 1995; Broadbent et al., 2005; Pastor et al., 2005; Abrahao et al., 2012), and conditioned taste aversion in outbred Swiss-Webster mice (Risinger et al., 1999), but not conditioned place preference in DBA mice (Cunningham et al., 1992a; Risinger et al., 1992).
The present study had two objectives: to compare the sensitivity of BSR in C57 and DBA mice to D1 and D2 receptor agonists and antagonists, and to compare the potency of D1 or D2 receptor antagonists to reduce the reward-potentiating effects of alcohol in these strains. We hypothesized that BSR thresholds would be lowered by the D1 receptor agonist SKF-82958 (±-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine) and by higher doses of the D2 receptor agonist quinpirole, whereas the D1 receptor antagonist SCH 23390 (+-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepinehydrochloride) and the D2 receptor antagonist raclopride would elevate BSR thresholds. Based on previous demonstrations that DBA mice have enhanced D2 function, we hypothesized they would be more sensitive to the threshold-lowering effect of quinpirole and less sensitive to the threshold-elevating effect of raclopride than C57 mice. Because of the role of dopamine in alcohol reward, we hypothesized that D1 and D2 antagonists would attenuate the BSR threshold-lowering effects of alcohol.
Materials and Methods
Mice.
Male C57BL/6J (n = 35) and DBA/2J (n = 24) mice (The Jackson Laboratory, Bar Harbor, ME) arrived weighing at least 22 g and were housed in polycarbonate cages (28 × 17 × 14 cm) lined with cob bedding that was changed weekly, and covered with stainless steel wire lids for free access to dry chow and tap water. The vivarium was 21 ± 1°C, 30–40% humidity, and on a 12-hour dark/light cycle (lights off at 8:00 AM). All procedures were performed during the dark phase, approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Carolina, and conducted according to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication No. 85-23, revised 2011).
After a week of acclimation to the vivarium, the mice were anesthetized with intraperitoneal ketamine [(±)-2-2-(2-chlorophenyl)-2-(methylamino)cyclohexanone hydrochloride, 120 mg/kg] and xylazine [2-(2,6-dimethylphenylamino)-5,6-dihydro-4H-thiazine hydrochloride, 9 mg/kg] (Sigma-Aldrich, St. Louis, MO) and stereotaxically implanted with insulated monopolar stainless steel electrodes (0.28 mm diameter; Plastics One, Roanoke, VA) aimed at the right medial forebrain bundle at the level of the lateral hypothalamus (AP: −1.2; ML: −1.0; DV: −5.2), using coordinates from Paxinos and Franklin (2008). The electrode was connected to a stainless steel electrical ground screw and mounted to the skull with dental cement. Following surgery, the mice were housed individually.
Apparatus and Procedures.
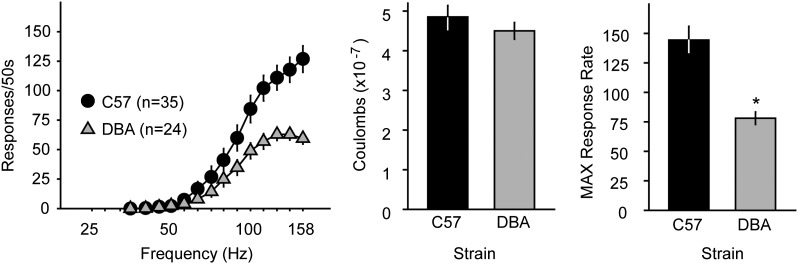
Similar to previous descriptions (Malanga et al., 2008; Fish et al., 2010), sound-attenuated operant conditioning chambers interfaced to computers running software (MED-PC for Windows, version 4.1; Med Associates, St. Albans, VT) that recorded wheel spins, controlled house lights, and issued current to electrodes connected through a swivel commutator and insulated wire (Plastics One). Each one-quarter turn of a wheel manipulandum was reinforced by a brief (500 milliseconds) unipolar cathodal square-wave current at a frequency of 158 Hz (pulse width = 100 microseconds) accompanied by illumination of the house light (500 milliseconds). Responses in the 500-millisecond stimulation period were recorded but did not earn additional stimulation. Current intensity was adjusted for each individual mouse and held constant throughout the experiment to maintain at least 40 responses/min (−40 to −180 µA). Mice responded for a series of 15 decreasing (0.05 log10) stimulation frequencies, each available for 1 minute beginning with a 10-second phase during which 5 noncontingent (“priming”) stimulations were presented. During conditioning, each frequency series was presented four times (60-minute session) and the range was adjusted such that mice responded only during the three to seven highest frequencies. The primary dependent variable, the threshold frequency to maintain responding (BSR threshold; θ0), was defined as the x-intercept of the least-squares regression line through frequencies that sustained 20, 30, 40, 50, and 60% of the maximal response rate as described by Coulombe and Miliaressis (1987), Rompré and Wise (1989), and reviewed by Carlezon and Chartoff (2007), and was calculated with custom-designed software. When thresholds varied less than 10% on three consecutive days, the mice were habituated to injections and drug-testing phases began. Comparison of electrical charge delivery (Q, in coulombs) at BSR threshold (θ0) between strains (Fig. 1) was performed before pharmacological experiments, where Q = A × t, or stimulus intensity (in amperes) × duration of current application (in seconds).
Fig. 1.
Baseline ICSS performance in C57BL/6J and DBA/2J mice. (Left) Both C57 (black circles) and DBA mice (gray triangles) responded for BSR in a frequency-dependent manner. Values are the mean number of responses per 50-second access to BSR at each stimulus frequency ±S.E.M. (Center) BSR sensitivity expressed as electrical charge delivery at BSR threshold frequency (θ0) did not differ between C57 (black bars) and DBA mice (gray bars). Values are the mean charge in coulombs ±S.E.M. (Right) Baseline maximum operant response rates were lower in DBA mice (gray bars) than in C57 mice (black bars). Values are the mean maximum number of responses ± S.E.M. *P < 0.05 versus C57.
Each test session began with a 45-minute preinjection period consisting of three series of 15 descending frequencies, and daily baseline parameters were calculated from the average of the second and third series. Results from each 15-minute series after drug or vehicle injection were expressed as a percentage of the daily preinjection baseline. For all dose-effect studies, the mice were removed from the chamber, injected, and returned immediately for four series of 15 descending stimulation frequencies. Eleven C57 and 10 DBA mice received each dose of SKF-82958 (saline, 0.1–0.56 mg/kg) and SCH 23390 (saline, 0.003–0.056 mg/kg). Thirteen C57 and nine DBA mice received each dose of quinpirole (saline, 0.1–3.0 mg/kg) and raclopride (saline, 0.01–0.56 mg/kg). The order of each drug dose was counterbalanced across the mice, and each drug dose was separated by a vehicle injection and at least 48 hours. The ethyl alcohol (ethanol; hereafter, alcohol) and DA receptor antagonist interaction studies were conducted in 17 C57 and 11 DBA mice. Six of the C57 and six of the DBA mice had previously completed the quinpirole and raclopride studies whereas the remaining 11 C57 and five DBA mice had no previous treatment history. After preinjection baseline responding, the mice were injected with saline, SCH 23390 (0.003 mg/kg), or raclopride (0.03 mg/kg), returned to their home cage for 15 minutes, orally gavaged with alcohol (0.3–2.4 g/kg), and placed immediately into the ICSS chamber as previously described (Fish et al., 2010, 2012). Six C57 and two DBA mice completed studies with both SCH 23390 and raclopride and alcohol. Two C57 and four DBA mice completed studies with alcohol and raclopride only. Nine C57 and five DBA mice completed studies with alcohol and SCH 23390 only.
Drugs.
Ethyl alcohol solutions were prepared (w/v) in tap water and injected via oral gavage through a stainless steel feeding tube in a volume of 1 ml/100 g body weight. The D1 receptor agonist, SKF-82958 (Sigma-Aldrich), D1 antagonist SCH 23390 (Sigma-Aldrich), D2 agonist quinpirole [(4aR-trans)-4,4a,5,6,7,8,8a,9-octahydro-5-propyl-1H-pyrazolo[3,4-g] quinolone hydrochloride; Tocris Bioscience, Bristol, UK], and D2 antagonist raclopride [3,5-dichloro-N-(1-ethylpyrrolidin-2-ylmethyl)-2-hydroxy-6-methoxybenzamide(+)-tartrate salt; Sigma-Aldrich] were dissolved in 0.9% saline and injected intraperitoneally through a 27-gauge needle. All doses were calculated as the free base.
Histology.
Brains from mice used for ICSS experiments were fixed by intracardiac perfusion with 4% paraformaldehyde in 0.1 M phosphate-buffered saline under deep pentobarbital anesthesia, removed, sectioned, and stained with cresyl violet for Nissl to determine electrode placements. The most ventral point of each electrode tract in the lateral hypothalamus was determined by visual inspection under low-power (10×) microscopy. All ICSS electrodes were implanted in the right lateral hypothalamus.
Data Analysis.
Baseline threshold and maximum response rates were compared between C57 and DBA mice using unpaired t tests. For the dose-effect studies, the threshold and the maximum operant response rate (MAX) from the final 45 minutes were averaged into a single value for each drug. Quinpirole had a biphasic effect on threshold and MAX, and the data from the first 15 minutes after injection were analyzed separately. The dose-effect studies for the D1 and D2 receptor agonists and antagonists were compared between C57 and DBA mice using two-way mixed-measures analysis of variance with strain as the between-subjects factor and drug dose as the within-subjects factor. The D1 and D2 receptor antagonist interaction with alcohol was analyzed separately in C57 and DBA mice using two-way repeated-measures analysis of variance with both alcohol dose and antagonist dose as within-subjects factors. Significant F tests were analyzed post hoc using Bonferroni-corrected tests compared with a common control.
Results
Baseline ICSS Responding.
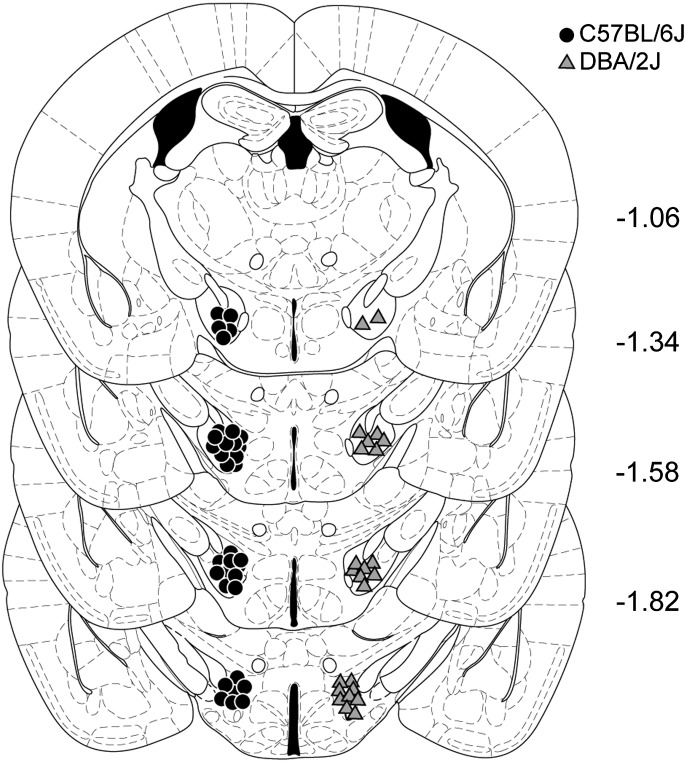
C57 and DBA mice responded for ICSS in a frequency-dependent manner, with higher frequencies supporting higher response rates (Fig. 1A). Although there was no difference in the charge sustaining responding at BSR threshold between strains (t57 = 0.89; P = 0.38; Fig. 1B), DBA mice had a significantly lower MAX than C57 mice (t57 = 4.5; P < 0.001; Fig. 1C). Placements of ICSS electrodes were similar in both mouse strains (Fig. 2).
Fig. 2.
Schematic representation of ICSS electrode tip placements in C57BL/6J and DBA/2J mice. All ICSS electrodes were implanted on the right. Tip positions are plotted on the left for C57 (black circles) and on the right for DBA mice (gray triangles) for clarity.
SKF-82958 and SCH 23390.
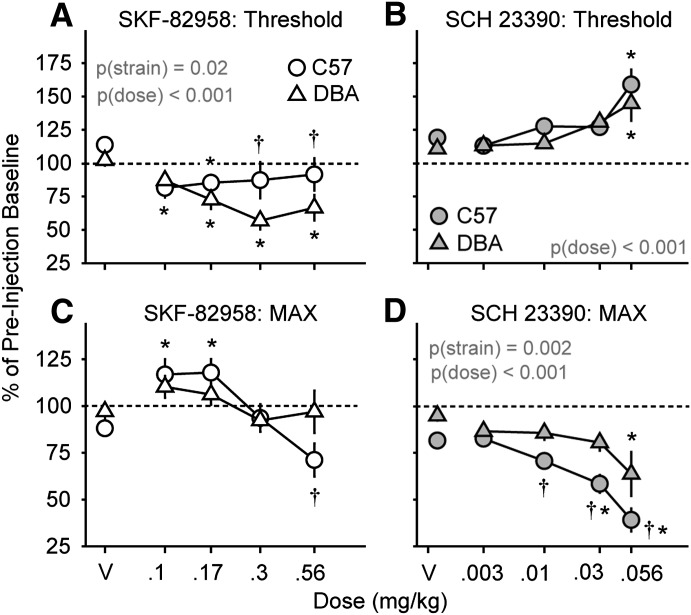
For the D1 receptor agonist SKF-82958, there were significant main effects of drug dose (F4,104 = 6.4; P < 0.001) and mouse strain (F1,104 = 6.1; P = 0.02) on BSR threshold (Fig. 3A). There was no significant interaction between SKF-82958 dose and mouse strain on threshold (P = 0.18). In C57 mice, the 0.1 and 0.17 mg/kg SKF-82958 doses lowered threshold, whereas in DBA mice, the 0.17, 0.3, and 0.56 mg/kg doses lowered threshold. The 0.3 and 0.56 mg/kg doses lowered threshold more in DBA mice than in C57 mice. There was a significant interaction between SKF-82958 dose and mouse strain on MAX (F4,104 = 2.7; P = 0.04; Fig. 3C). The 0.1 and 0.17 mg/kg doses increased MAX in C57 mice but not DBA mice. The 0.56 mg/kg dose suppressed MAX in C57 but not in DBA mice.
Fig. 3.
Effects of a dopamine D1 receptor agonist, SKF-82958, and D1 receptor antagonist, SCH 23390, on ICSS performance in C57BL/6J and DBA/2J mice. Changes in BSR threshold (A and B) and MAX (C and D) after injection of SKF-82958 (open symbols) or SCH 23390 (filled symbols) are shown for C57 (circles) and DBA mice (triangles). DBA mice were generally more sensitive to the reward-potentiating effects of the D1 agonist than C57 mice (A). The dose-response curve of SCH 23390 on MAX (D), but not BSR threshold (B), was shifted to the left with an increase in maximum response over the dose range tested (0.003–0.56 mg/kg i.p.) in C57 mice relative to DBA mice. Values are expressed as the mean percentages of preinjection baselines (± S.E.M.) averaged across the final 45 minutes after drug injection. P values for significant main effects of strain and drug dose are inset as text. *Significance versus vehicle (V) (<0.05); †significance versus DBA mice (<0.05).
For the D1 receptor antagonist SCH 23390, there was a significant main effect of drug dose on BSR threshold (F4,99 = 10.1; P < 0.001; Fig. 3B). In both mouse strains, the 0.056 mg/kg dose of SCH 23390 significantly elevated threshold. There was no main effect of mouse strain and no interaction between mouse strain and SCH 23390 dose. There were significant main effects of SCH 23390 dose (F4,99 = 21.0; P < 0.001) and mouse strain (F1,99 = 13.5; P = 0.002), but no significant interaction between SCH 23390 dose and strain (P = 0.18) on MAX (Fig. 3D). In C57 mice, the 0.01, 0.03, and 0.056 mg/kg doses of SCH 23390 reduced MAX response rates, whereas only the 0.056 mg/kg dose reduced MAX response rates in DBA mice. The 0.1, 0.3, and 0.56 doses suppressed MAX more in C57 mice than in DBA mice.
Quinpirole and Raclopride.
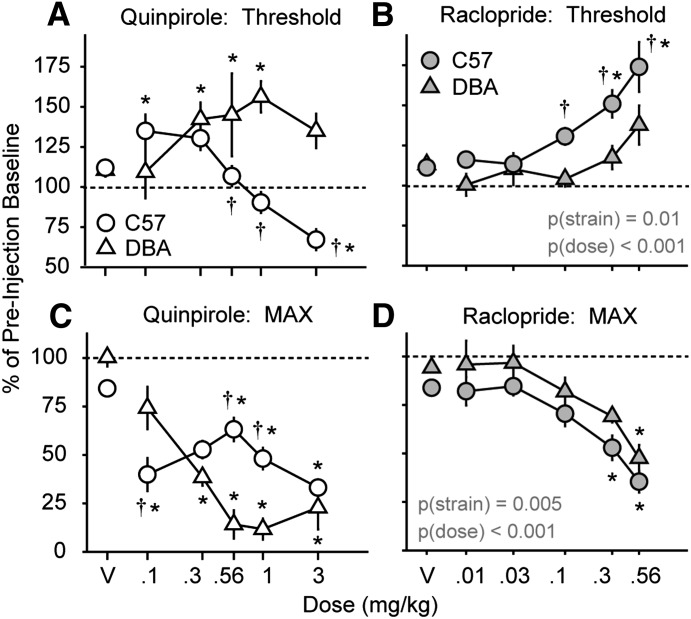
For the D2 receptor agonist quinpirole, there was a significant main effect of drug dose on BSR threshold in the first 15 minutes after administration (F5,121 = 6.7; P < 0.001; Table 1). In C57 mice, the 0.3, 1.0, and 3.0 mg/kg doses elevated threshold, whereas in the DBA mice, the 0.3 and 1.0 mg/kg doses elevated threshold. There was no main effect of mouse strain and no interaction between quinpirole dose and mouse strain. There was a significant interaction between quinpirole dose and mouse strain on MAX in the first 15 minutes after administration (F5,121 = 5.0; P < 0.001; Table 1). In both mouse strains, the 1.0 and 3.0 mg/kg doses suppressed MAX response rate. After the first 15 minutes of quinpirole administration, there was a significant interaction between drug dose and mouse strain on BSR threshold (F5,121 = 10.1; P < 0.001; Fig. 4A). In C57 mice, the 0.1 mg/kg dose elevated threshold and the 3.0 mg/kg dose lowered threshold, whereas in DBA mice, the 0.3, 0.56, 1.0, and 3.0 mg/kg doses elevated threshold. The effects of the 0.56, 1.0, and 3.0 mg/kg doses on threshold were significantly different between C57 and DBA mice. There was also a significant interaction between quinpirole dose and mouse strain on MAX (F5,121 = 9.1; P ≤ 0.001). In C57 mice, all quinpirole doses suppressed MAX, and in DBA mice, all quinpirole doses except 0.1 mg/kg suppressed MAX (Fig. 4C). The suppression of MAX was greater in C57 mice after the 0.1 mg/kg dose and greater in DBA mice after the 0.56 and 1.0 mg/kg doses.
TABLE 1.
Effects of quinpirole on ICSS responding in minutes 0–15 after injection
All data are the mean percentage of preinjection baseline (± S.E.M.).
| Dose | ||||||
|---|---|---|---|---|---|---|
| VEH | 0.1 | 0.3 | 0.56 | 1.0 | 3.0 | |
| mg/kg | ||||||
| BSR threshold | ||||||
| C57 | 111 ± 3.8 | 140 ± 11 | 174 ± 19* | 144 ± 10 | 155 ± 12* | 162 ± 5.6* |
| DBA | 115 ± 5.3 | 96.6 ± 9.4 | 160 ± 17* | 144 ± 21 | 159 ± 13* | 137 ± 13 |
| MAX response rate | ||||||
| C57 | 79.6 ± 3.8 | 59.0 ± 7.6 | 66.7 ± 3.7 | 60.0 ± 12 | 49.6 ± 5.2* | 28.7 ± 2.8* |
| DBA | 86.5 ± 3.0 | 112 ± 8.6† | 62.8 ± 8.6 | 45.5 ± 6.9 | 31.9 ± 6.6* | 29.7 ± 10.2* |
VEH, vehicle.
Post hoc significance versus vehicle (P < 0.05).
Post hoc significance versus C57 (P < 0.05).
Fig. 4.
Effects of a dopamine D2 receptor agonist, quinpirole, and D2 receptor antagonist, raclopride, on ICSS performance in C57BL/6J and DBA/2J mice. Changes in BSR threshold (A and B) and MAX (C and D) after injection of quinpirole (open symbols) or raclopride (filled symbols) are shown for C57 (circles) and DBA mice (triangles). Biphasic effects of quinpirole on BSR threshold were found, with elevations at lower doses (0.1 mg/kg i.p.) and reduction of threshold at the highest dose tested (3.0 mg/kg i.p.) in C57 mice, whereas consistent elevations of BSR threshold to quinpirole were seen in DBA mice (A). In contrast, dose-dependent elevations in BSR threshold after raclopride were found in C57 but not DBA mice (B). Reduction in MAX was found across most quinpirole doses (C) and at the highest dose of raclopride tested (0.56 mg/kg i.p.) (D) in both mouse strains. Values are expressed as the mean percentages of preinjection baselines (± S.E.M.) averaged across the final 45 minutes after drug injection. P values for significant main effects of strain and drug dose are inset as text. *Significance versus vehicle (V) (<0.05); †significance versus DBA mice (<0.05).
For the D2 receptor antagonist raclopride, there were significant main effects of drug dose (F5,125 = 11.0; P < 0.001) and mouse strain (F1,125 = 7.5; P = 0.01) on BSR threshold. There was a trend for an interaction between mouse strain and raclopride dose on BSR threshold that did not reach significance (P = 0.08). In C57 mice, the 0.3 and 0.56 mg/kg doses of raclopride elevated threshold (Fig. 4B), and the 0.1, 0.3, and 0.56 mg/kg doses elevated threshold more in C57 mice than in DBA mice. There were significant main effects of raclopride dose (F5,125 = 17.8; P < 0.001) and mouse strain (F1,125 = 10.1; P = 0.005) on MAX (Fig. 4D). There was no interaction between raclopride dose and mouse strain on MAX (P = 0.99). In C57 mice, the 0.3 and 0.56 mg/kg raclopride doses suppressed MAX, whereas the 0.56 mg/kg dose suppressed MAX in DBA mice.
SCH 23390 and Alcohol.
In C57 mice, there were significant main effects of alcohol on BSR threshold (F4,149 = 4.5; P = 0.003; Fig. 5A) and MAX (F4,149 = 6.9; P < 0.001; Fig. 5C), but no main effect of SCH 23390 pretreatment and no interaction between SCH 23390 pretreatment and alcohol dose. Regardless of pretreatment with SCH 23390, the 0.6 g/kg alcohol dose lowered threshold, whereas the 1.7 and 2.4 g/kg doses suppressed MAX. In DBA mice, there was a significant interaction between SCH 23390 pretreatment and alcohol dose on BSR threshold (F4,69 = 4.1; P = 0.01; Fig. 5B). The 1.7 and 2.4 g/kg alcohol doses significantly lowered threshold following saline pretreatment, but not following SCH 23390 pretreatment. The effects of these alcohol doses were significantly different following saline or SCH 23390 pretreatment. There was a significant main effect of 1.0 g/kg alcohol on MAX (F4,69 = 2.9; P = 0.04; Fig. 5D).
Fig. 5.
Interaction between a low dose of a dopamine D1 receptor antagonist, SCH 23390, and different doses of alcohol on ICSS performance in C57BL/6J and DBA/2J mice. Changes in BSR threshold (A and B) and MAX (C and D) after gavage with water (vertical bars) or alcohol (symbols) following i.p. injection with saline (Sal; open symbols) or the 0.003 mg/kg dose of SCH 23390 (SCH; filled symbols) are shown for C57 (circles) and DBA mice (triangles). The D1 antagonist SCH 23390 had no significant effect on alcohol in C57 mice (A), but completely blocked the reward-potentiating effect of alcohol in DBA mice (B). Values are expressed as the mean percentages of preinjection baselines (± S.E.M.) in the first 15 minutes after alcohol or water. In panels in which no significant interaction effects of SCH 23390 and alcohol are seen, P values for significant main effects of alcohol are inset as text. Numbers within the vertical bars indicate the number of C57 and DBA mice tested. *Significance versus water (H2O) (<0.05); †significance versus saline pretreatment (<0.05).
Raclopride and Alcohol.
In C57 mice, there was a significant interaction between raclopride pretreatment and alcohol dose on BSR threshold (F4,79 = 7.0; P < 0.001; Fig. 6A) and MAX (F4,79 = 2.9; P = 0.04; Fig. 6C). The 0.6 g/kg alcohol dose significantly lowered threshold following saline pretreatment. Following raclopride pretreatment, the 1.0 g/kg alcohol dose significantly elevated threshold and trended toward elevating threshold after the 0.6 g/kg dose (P = 0.059). Raclopride significantly altered the effects of the 0.6 and 1.0 g/kg alcohol doses, but did not alter the effects of the 1.7 and 2.4 g/kg alcohol doses. Whereas no dose of alcohol significantly affected MAX in C57 mice, raclopride pretreatment significantly reduced the effects of the 1.0 g/kg alcohol dose and trended toward reducing the effects of the 0.6 g/kg dose (P = 0.053). In DBA mice, there was a significant main effect of alcohol on BSR threshold (F4,59 = 5.0; P = 0.006; Fig. 6B). There was no significant interaction between raclopride pretreatment and alcohol dose (P = 0.28); however, post-hoc analysis of the main effect showed that the 1.0 and 1.7 g/kg alcohol doses lowered threshold. There was a significant interaction between raclopride pretreatment and alcohol dose on MAX (F4,59 = 3.4; P = 0.03; Fig. 6D). Whereas no alcohol dose significantly affected MAX in DBA mice, raclopride pretreatment significantly reduced the effects of the 1.7 and 2.4 g/kg alcohol doses.
Fig. 6.
Interaction between a low dose of a dopamine D2 receptor antagonist, raclopride, and different doses of alcohol on ICSS performance in C57BL/6J and DBA/2J mice. Changes in BSR threshold (A and B) and MAX (C and D) after gavage with water (vertical bars) or alcohol (symbols) following i.p. injection with saline (Sal; open symbols) or the 0.03 mg/kg dose of raclopride (Rac; filled symbols) are shown for C57 (circles) and DBA mice (triangles). The D2 antagonist raclopride blocked and reversed the reward-potentiating effect of low-dose (0.6 mg/kg) alcohol in C57 mice (A), but did not affect alcohol potentiation of BSR in DBA mice (B). Values are expressed as the mean percentages of preinjection baselines (± S.E.M.) in the first 15 minutes after alcohol or water. In panels in which no significant interaction effects of raclopride and alcohol are seen, P values for significant main effects of alcohol are inset as text. *Significance versus water (H2O) (<0.05); †significance versus saline pretreatment (<0.05).
Discussion
These data provide evidence for a difference in the contributions of dopamine D1 and D2 receptor activity to BSR in the C57 and DBA mouse strains. DBA mice were more sensitive to effects of the D1 agonist SKF-82958 and less sensitive to effects of the D2 receptor antagonist raclopride and the D2 agonist quinpirole. These data confirm previous strain differences in dopaminergic modulation of locomotor activity, prepulse inhibition (PPI), learning, and memory (Puglisi-Allegra and Cabib, 1997; Ralph and Caine, 2005) and extend them to a direct behavioral measure of reward. These data also replicate our previously observed strain difference in alcohol effects on ICSS (Fish et al., 2010), in that alcohol has greater potency to lower BSR threshold in DBA than C57 mice. A low dose of the D1 antagonist SCH 23390 blocked the reward-potentiating effects of alcohol on BSR in the DBA strain, but had no effect in the C57 strain. In contrast, a low dose of the D2 antagonist raclopride blocked the reward-potentiating effects of alcohol on BSR in the C57 strain, but not in the DBA strain. This suggests that D1 and D2 receptor signaling may contribute differently to the rewarding effects of alcohol in C57 and DBA mice.
D1 receptor agonists modulate BSR, although inconsistently across species and procedure (Nakajima and O'Regan, 1991; Hunt et al., 1994; Ranaldi and Beninger, 1994; Baldo et al., 1999; Malanga et al., 2008). Mice lacking D1 receptors have deficient ICSS performance (Tran et al., 2005). Across a dose range similar to previous studies in outbred Swiss-Webster mice, SKF-82958 lowered BSR threshold in both C57 and DBA mice, but had greater efficacy in DBA mice. Consistent with previous rat studies (Panagis and Spyraki, 1996; Sundstrom et al., 2002; Cheer et al., 2007), the D1 antagonist SCH 23390 elevated threshold equally in both strains, but the potency of SCH 23390 to decrease maximum response rates was lower in DBA mice, indicating that the strain difference was specific to a measure of operant performance. It is generally regarded by most investigators that, although some degree of overlap in the neural circuits mediating motor activation and reward perception exists, operant response rate per se is more closely tied to activation or inhibition of motor output and BSR threshold to the perceived value of the reward. Although statistically significant, the overall D1-related pharmacological differences between C57 and DBA mice were modest, suggesting that D1 receptor activity may have a relatively smaller role in the dopaminergic differences of the two strains. Radioligand binding studies have shown small strain differences in D1 receptors that suggest more D1-like receptors in the striatum of C57 mice (Ng et al., 1994; Puglisi-Allegra and Cabib, 1997). A strain comparison of D1 effects on PPI, a measure of sensorimotor gating, showed that the agonist R-6-Br-APB decreased PPI in C57 mice and had no effect in DBA mice (Ralph and Caine, 2005), whereas it more potently stimulated locomotor activity in DBA mice (Thomsen et al., 2011). Together with our ICSS data, it is reasonable to conclude that strain differences in response to D1 receptor activity are behaviorally specific.
In contrast to D1 sensitivity, the C57 and DBA strain difference was more pronounced after treatment with the D2 agonist, quinpirole, and the D2 antagonist, raclopride. Quinpirole had dose- and time-dependent effects on threshold and MAX consistent with previous observations (Hatcher and Hagan, 1998; Malanga et al., 2008). In both C57 and DBA strains, quinpirole elevated threshold and suppressed MAX in the first 15 minutes after administration. After this initial phase, higher quinpirole doses lowered BSR threshold in C57 but not in DBA mice, which also showed a greater suppression of MAX. This strain difference in response to quinpirole is consistent with differences in locomotor activity, catalepsy, and social behavior (Puglisi-Allegra and Cabib, 1997). Quinpirole is thought to act in a concentration-dependent manner, with low concentrations acting at presynaptic D2 autoreceptors and higher concentrations activating postsynaptic D2 receptors (White and Wang, 1986). Quinpirole elevated but did not lower BSR threshold in DBA mice, suggesting that this strain may have enhanced D2 autoreceptor and/or decreased postsynaptic D2 sensitivity, consistent with previous findings that D2-like receptor binding and mRNA expression are greater in the VTA and substantia nigra pars compacta and lower in the striatum of DBA compared with C57 mice (Ng et al., 1994; Cabib et al., 1998). In both strains, raclopride elevated thresholds and reduced MAX in a dose range similar to previous studies in C57 mice (Riday et al., 2012), and more potently in C57 than DBA mice, which is inconsistent with data on haloperidol-induced catalepsy (Kanes et al., 1993) and suppression of sucrose drinking by raclopride (Dym et al., 2009). Taken together, the differences in responses to quinpirole and raclopride suggest that, regarding reward-related behavior in both C57 and DBA strains, D2 receptors may contribute more than D1 receptors.
The effects of alcohol differed in the C57 and DBA strains, and were consistent with our previous comparison (Fish et al., 2010); the 0.6 g/kg dose produced a small but significant reduction in the threshold of C57 mice, whereas the 1.7 and 2.4 g/kg doses reduced threshold in DBA mice. MAX tended to be suppressed by the highest alcohol doses in C57 mice, but was modestly increased by the higher alcohol doses in the DBA mice. Based on initial dose-effect determinations, a low dose of SCH 23390 or raclopride, which had no behavioral effects on its own, was then administered before alcohol. In C57 mice, SCH 23390 did not alter the effects of any dose of alcohol tested on BSR threshold. However, raclopride significantly altered the effects of 0.6 and 1.0 g/kg alcohol. In the presence of raclopride, these alcohol doses elevated, rather than lowered, BSR thresholds in C57 mice. In contrast, SCH 23390 prevented the threshold-lowering effects of 1.7 and 2.4 g/kg alcohol in DBA mice, whereas raclopride did not alter the effects of these alcohol doses. Although raclopride appeared to enhance the threshold-lowering effects of 1.0 g/kg alcohol, there was no significant overall interaction between raclopride and alcohol. Taken together, these results suggest that D2 receptor activity contributes relatively more than D1 activity to the rewarding effects of alcohol in C57 mice; conversely, D1 activity contributes relatively more than D2 activity to alcohol reward in DBA mice. This does not, however, translate directly to the effects of dopamine receptor activity on voluntary oral alcohol intake in the two strains, comparison of which is unavoidably confounded by an innate taste aversion to alcohol in DBA mice (Belknap et al., 1977; Grahame and Cunningham, 1997) that is bypassed in our study.
An important consideration is that only very low doses of SCH-23390 (0.003 mg/kg) and raclopride (0.03 mg/kg) were given in combination with alcohol. The rationale was that these doses had no independent effects on either BSR thresholds or MAX. Higher doses of each antagonist may have altered the reward-potentiating effects of alcohol in both strains, but would be more difficult to interpret due to confounding effects, such as locomotor suppression. Also, systemic administration does not address the role of DA receptors in specific brain regions, which may be particularly important for D2 receptors and may be relevant to strain differences. As observed by Elmer et al. (2010), ICSS is not as reliable in the DBA strain as in other mouse strains, even at comparable stimulation sites. The previously described functional anatomic differences in the mesocorticolimbic dopaminergic systems of C57 and DBA mice (D'Este et al., 2007) may have a pronounced impact on reward-related behavior measured by ICSS.
Our current findings support the hypotheses that DA acting at both D1 and D2 receptors contributes to brain stimulation reward, and that mouse strains differ in the contribution of signaling through these two receptors. D1 and D2 receptors are also involved in BSR potentiation by alcohol, and it is likely that the mesolimbic DA system contributes to the rewarding and reinforcing effects of acute alcohol. Blunting the rewarding and reward-enhancing effects of acute alcohol remains a viable pharmacotherapeutic strategy to treat alcohol use disorders, particularly for individuals who are most sensitive to these alcohol effects (Heilig et al., 2010). Although the efficacy of classic neuroleptics remains limited by undesirable side effects, newer DA receptor antagonists that are proposed to modulate the balance between tonic and phasic DA levels, such as aripiprazole, or other classes of drugs that impact dopaminergic neurotransmission indirectly, such as ondansetron, may be more effective and behaviorally specific (Edwards et al., 2011).
In terms of preclinical utility, although C57 mice voluntarily consume alcohol, by most behavioral metrics they appear less sensitive to its effects. Conversely, due to an inherent taste aversion, DBA mice do not drink significant amounts of alcohol but are more sensitive to its effects. For purposes of preclinical therapeutics development, differences in behavioral effects of alcohol on these two commonly used mouse strains are important not only to considerations of drug mechanisms but also to the development of personalized therapy for alcohol use disorders in genetically distinct patient populations.
Acknowledgments
The authors thank Megan M. McGuigan for technical assistance with the D1 dose-effect studies and Thorfinn T. Riday for advice on the raclopride dose-effect studies.
Abbreviations
- BSR
brain stimulation reward
- C57
C57BL/6J mice
- DA
dopamine
- DBA
DBA/2J mice
- ICSS
intracranial self-stimulation
- MAX
maximum operant response rate
- NAc
nucleus accumbens
- PPI
prepulse inhibition
- SCH 23390
+-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepinehydrochloride
- SKF-82958
±-6-chloro-7,8-dihydroxy-3-allyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
Authorship Contributions
Participated in research design: Fish, Robinson, Malanga.
Conducted experiments: Fish, DiBerto, Krouse.
Performed data analysis: Fish, DiBerto, Robinson.
Wrote or contributed to the writing of the manuscript: Fish, Robinson, Malanga.
Footnotes
This research was supported by grants from the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants R01-AA018335 to C.J.M., F30-AA021312 to J.E.R., and P60-AA007573 to the UNC Bowles Center for Alcohol Studies].
References
- Abrahao KP, Quadros IM, Andrade AL, Souza-Formigoni ML. (2012) Accumbal dopamine D2 receptor function is associated with individual variability in ethanol behavioral sensitization. Neuropharmacology 62:882–889 [DOI] [PubMed] [Google Scholar]
- Bahi A, Dreyer JL. (2012) Involvement of nucleus accumbens dopamine D1 receptors in ethanol drinking, ethanol-induced conditioned place preference, and ethanol-induced psychomotor sensitization in mice. Psychopharmacology (Berl) 222:141–153 [DOI] [PubMed] [Google Scholar]
- Baldo BA, Jain K, Veraldi L, Koob GF, Markou A. (1999) A dopamine D1 agonist elevates self-stimulation thresholds: comparison to other dopamine-selective drugs. Pharmacol Biochem Behav 62:659–672 [DOI] [PubMed] [Google Scholar]
- Belknap JK, Belknap ND, Berg JH, Coleman R. (1977) Preabsorptive vs. postabsorptive control of ethanol intake in C57BL/6J and DBA/2J mice. Behav Genet 7:413–425 [DOI] [PubMed] [Google Scholar]
- Broadbent J, Kampmueller KM, Koonse SA. (2005) Role of dopamine in behavioral sensitization to ethanol in DBA/2J mice. Alcohol 35:137–148 [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. (2000) Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res 24:1120–1124 [PubMed] [Google Scholar]
- Cabib S, Giardino L, Calzá L, Zanni M, Mele A, Puglisi-Allegra S. (1998) Stress promotes major changes in dopamine receptor densities within the mesoaccumbens and nigrostriatal systems. Neuroscience 84:193–200 [DOI] [PubMed] [Google Scholar]
- Cabib S, Orsini C, Le Moal M, Piazza PV. (2000) Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science 289:463–465 [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. (1991) Genotype-dependent effects of chronic stress on apomorphine-induced alterations of striatal and mesolimbic dopamine metabolism. Brain Res 542:91–96 [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. (2007) Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2:2987–2995 [DOI] [PubMed] [Google Scholar]
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. (2007) Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron 54:237–244 [DOI] [PubMed] [Google Scholar]
- Coulombe D, Miliaressis E. (1987) Fitting intracranial self-stimulation data with growth models. Behav Neurosci 101:209–214 [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Jr, Johnson NA, Gray DK, Kosobud A, Young ER. (1982) Biphasic effects of ethanol on open-field activity: sensitivity and tolerance in C57BL/6N and DBA/2N mice. J Comp Physiol Psychol 96:440–451 [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, et al. (1997) Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132:107–124 [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Howard MA, Gill SJ, Rubinstein M, Low MJ, Grandy DK. (2000) Ethanol-conditioned place preference is reduced in dopamine D2 receptor-deficient mice. Pharmacol Biochem Behav 67:693–699 [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Malott DH, Dickinson SD, Risinger FO. (1992a) Haloperidol does not alter expression of ethanol-induced conditioned place preference. Behav Brain Res 50:1–5 [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. (1992b) Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 107:385–393 [DOI] [PubMed] [Google Scholar]
- D’Este L, Casini A, Puglisi-Allegra S, Cabib S, Renda TG. (2007) Comparative immunohistochemical study of the dopaminergic systems in two inbred mouse strains (C57BL/6J and DBA/2J). J Chem Neuroanat 33:67–74 [DOI] [PubMed] [Google Scholar]
- Dym CT, Pinhas A, Robak M, Sclafani A, Bodnar RJ. (2009) Genetic variance contributes to dopamine receptor antagonist-induced inhibition of sucrose intake in inbred and outbred mouse strains. Brain Res 1257:40–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Kenna GA, Swift RM, Leggio L. (2011) Current and promising pharmacotherapies, and novel research target areas in the treatment of alcohol dependence: a review. Curr Pharm Des 17:1323–1332 [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, George SR, Drago J, Fletcher PJ, Fan T, Nguyen T, Liu C, Sibley DR, Westphal H, O’Dowd BF. (1998) Disruption of dopamine D1 receptor gene expression attenuates alcohol-seeking behavior. Eur J Pharmacol 353:149–158 [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Hamilton LR, Wise RA. (2010) Qualitative differences between C57BL/6J and DBA/2J mice in morphine potentiation of brain stimulation reward and intravenous self-administration. Psychopharmacology (Berl) 208:309–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Riday TT, McGuigan MM, Faccidomo S, Hodge CW, Malanga CJ. (2010) Alcohol, cocaine, and brain stimulation-reward in C57Bl6/J and DBA2/J mice. Alcohol Clin Exp Res 34:81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Robinson JE, Krouse MC, Hodge CW, Reed C, Phillips TJ, Malanga CJ. (2012) Intracranial self-stimulation in FAST and SLOW mice: effects of alcohol and cocaine. Psychopharmacology (Berl) 220:719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ, Cunningham CL. (1997) Intravenous ethanol self-administration in C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res 21:56–62 [PubMed] [Google Scholar]
- Hatcher JP, Hagan JJ. (1998) The effects of dopamine D3/D2 receptor agonists on intracranial self stimulation in the rat. Psychopharmacology (Berl) 140:405–410 [DOI] [PubMed] [Google Scholar]
- Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, Hommer D, Barr CS. (2010) Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neurosci Biobehav Rev 35:334–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GE, Atrens DM, Jackson DM. (1994) Reward summation and the effects of dopamine D1 and D2 agonists and antagonists on fixed-interval responding for brain stimulation. Pharmacol Biochem Behav 48:853–862 [DOI] [PubMed] [Google Scholar]
- Kanes SJ, Hitzemann BA, Hitzemann RJ. (1993) On the relationship between D2 receptor density and neuroleptic-induced catalepsy among eight inbred strains of mice. J Pharmacol Exp Ther 267:538–547 [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. (2008) Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res 32:617–631 [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Bain G. (1992) Brain-stimulation reward: a model for the study of the rewarding effects of abused drugs. NIDA Res Monogr 124:73–93 [PubMed] [Google Scholar]
- Malanga CJ, Riday TT, Carlezon WA, Jr, Kosofsky BE. (2008) Prenatal exposure to cocaine increases the rewarding potency of cocaine and selective dopaminergic agonists in adult mice. Biol Psychiatry 63:214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, McElvain MA, Brodie MS. (2008) Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: involvement of barium-sensitive potassium currents. J Neurophysiol 100:1202–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, O’Regan NB. (1991) The effects of dopaminergic agonists and antagonists on the frequency-response function for hypothalamic self-stimulation in the rat. Pharmacol Biochem Behav 39:465–468 [DOI] [PubMed] [Google Scholar]
- Ng GY, O’Dowd BF, George SR. (1994) Genotypic differences in brain dopamine receptor function in the DBA/2J and C57BL/6J inbred mouse strains. Eur J Pharmacol 269:349–364 [DOI] [PubMed] [Google Scholar]
- Orsini C, Bonito-Oliva A, Conversi D, Cabib S. (2005) Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology (Berl) 181:327–336 [DOI] [PubMed] [Google Scholar]
- Panagis G, Spyraki C. (1996) Neuropharmacological evidence for the role of dopamine in ventral pallidum self-stimulation. Psychopharmacology (Berl) 123:280–288 [DOI] [PubMed] [Google Scholar]
- Pastor R, Miquel M, Aragon CM. (2005) Habituation to test procedure modulates the involvement of dopamine D2- but not D1-receptors in ethanol-induced locomotor stimulation in mice. Psychopharmacology (Berl) 182:436–446 [DOI] [PubMed] [Google Scholar]
- Paxinos GT, Franklin KB. (2008) The Mouse Brain in Stereotaxic Coordinates, Ed. 3rd Academic Press, San Diego [Google Scholar]
- Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly MA, Rubinstein M, Grandy DK, Low MJ. (1998) Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci 1:610–615 [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S, Burkhart-Kasch S. (1994) Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci 108:789–803 [DOI] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cabib S. (1997) Psychopharmacology of dopamine: the contribution of comparative studies in inbred strains of mice. Prog Neurobiol 51:637–661 [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. (2005) Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague-Dawley rats to Swiss-Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. J Pharmacol Exp Ther 312:733–741 [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Beninger RJ. (1994) The effects of systemic and intracerebral injections of D1 and D2 agonists on brain stimulation reward. Brain Res 651:283–292 [DOI] [PubMed] [Google Scholar]
- Riday TT, Dankoski EC, Krouse MC, Fish EW, Walsh PL, Han JE, Hodge CW, Wightman RM, Philpot BD, Malanga CJ. (2012) Pathway-specific dopaminergic deficits in a mouse model of Angelman syndrome. J Clin Invest 122:4544–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger FO, Brown MM, Oakes RA, Love JA. (1999) Effects of haloperidol or SCH-23390 on ethanol-induced conditioned taste aversion. Alcohol 18:139–145 [DOI] [PubMed] [Google Scholar]
- Risinger FO, Dickinson SD, Cunningham CL. (1992) Haloperidol reduces ethanol-induced motor activity stimulation but not conditioned place preference. Psychopharmacology (Berl) 107:453–456 [DOI] [PubMed] [Google Scholar]
- Risinger FO, Freeman PA, Rubinstein M, Low MJ, Grandy DK. (2000) Lack of operant ethanol self-administration in dopamine D2 receptor knockout mice. Psychopharmacology (Berl) 152:343–350 [DOI] [PubMed] [Google Scholar]
- Rompré PP, Wise RA. (1989) Opioid-neuroleptic interaction in brainstem self-stimulation. Brain Res 477:144–151 [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell AM. (1999) Effects of microinjection of the D2 dopamine antagonist raclopride into the ventral tegmental area on ethanol and sucrose self-administration. Alcohol Clin Exp Res 23:421–426 [PubMed] [Google Scholar]
- Shen EH, Crabbe JC, Phillips TJ. (1995) Dopamine antagonist effects on locomotor activity in naive and ethanol-treated FAST and SLOW selected lines of mice. Psychopharmacology (Berl) 118:28–36 [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Hodge CW, Samson HH. (1997) Dopaminergic and opiate agonists and antagonists differentially decrease multiple schedule responding maintained by sucrose/ethanol and sucrose. Alcohol 14:281–294 [DOI] [PubMed] [Google Scholar]
- Sundstrom JM, Hall FS, Stellar JR, Waugh EJ. (2002) Effects of isolation-rearing on intracranial self-stimulation reward of the lateral hypothalamus: baseline assessment and drug challenges. Life Sci 70:2799–2810 [DOI] [PubMed] [Google Scholar]
- Thomsen M, Ralph RJ, Caine SB. (2011) Psychomotor stimulation by dopamine D₁-like but not D₂-like agonists in most mouse strains. Exp Clin Psychopharmacol 19:342–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran AH, Tamura R, Uwano T, Kobayashi T, Katsuki M, Ono T. (2005) Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc Natl Acad Sci USA 102:2117–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Alcaro A, Cabib S, Conversi D, Mandolesi L, Puglisi-Allegra S. (2004) Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology 29:72–80 [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. (1986) Electrophysiological evidence for the existence of both D-1 and D-2 dopamine receptors in the rat nucleus accumbens. J Neurosci 6:274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. (1996) Neurobiology of addiction. Curr Opin Neurobiol 6:243–251 [DOI] [PubMed] [Google Scholar]
- Zocchi A, Orsini C, Cabib S, Puglisi-Allegra S. (1998) Parallel strain-dependent effect of amphetamine on locomotor activity and dopamine release in the nucleus accumbens: an in vivo study in mice. Neuroscience 82:521–528 [DOI] [PubMed] [Google Scholar]