Abstract
Covalent protein modifications by electrophilic acyl glucuronide (AG) metabolites are hypothetical causes of hypersensitivity reactions associated with certain carboxylate drugs. The complex rearrangements and reactivities of drug AG have been defined in great detail, and protein adducts of carboxylate drugs, such as diclofenac, have been found in liver and plasma of experimental animals and humans. However, in the absence of definitive molecular characterization, and specifically, identification of signature glycation conjugates retaining the glucuronyl and carboxyl residues, it cannot be assumed any of these adducts is derived uniquely or even fractionally from AG metabolites. We have therefore undertaken targeted mass spectrometric analyses of human serum albumin (HSA) isolated from diclofenac patients to characterize drug-derived structures and, thereby, for the first time, have deconstructed conclusively the pathways of adduct formation from a drug AG and its isomeric rearrangement products in vivo. These analyses were informed by a thorough understanding of the reactions of HSA with diclofenac AG in vitro. HSA from six patients without drug-related hypersensitivities had either a single drug-derived adduct or one of five combinations of 2–8 adducts from among seven diclofenac N-acylations and three AG glycations on seven of the protein’s 59 lysines. Only acylations were found in every patient. We present evidence that HSA modifications by diclofenac in vivo are complicated and variable, that at least a fraction of these modifications are derived from the drug’s AG metabolite, and that albumin adduction is not inevitably a causation of hypersensitivity to carboxylate drugs or a coincidental association.
Introduction
Acyl glucuronides (AG) are exceptional even among the numerous electrophilic metabolites of drugs (Stepan et al., 2011), including the multiple reactive intermediates of carboxylic acids (Kenny et al., 2004; Skonberg et al., 2008), because they can circulate in plasma, principally in noncovalent association with protein (Williams and Dickinson, 1994) and sometimes at concentrations matching or exceeding those of the parent compound (Dockens et al., 2000; Zhang et al., 2011). The circulation of AG, combined with extrahepatic expression of uptake transporters of O-glucuronides (Schiffer et al., 2003), allows for much wider systemic distribution and potentially much more widespread biologic effects than are usually associated with chemically reactive drug metabolites. From the earliest identifications of AG as unstable and protein-reactive conjugates these commonplace metabolites have been linked persistently, and at times almost generically, but always somewhat uncertainly, with the varied adverse reactions of carboxylate drugs (Regan et al., 2010; Sawamura et al., 2010). This hypothetical linkage of protein adduction and toxicity has been particularly enduring but no less contentious in the case of hypersensitivity reactions to nonsteroidal anti-inflammatory drugs (NSAIDs), such as diclofenac.
Hapten characterization is key to understanding hypersensitivity reactions. Protein adducts of carboxylate drugs have been located in liver and plasma by radiochemical tracing (Masubuchi et al., 2007), immunovisualization (Aithal et al., 2004), and hydrolytic deconjugation (Zia-Amirhosseini et al., 1994). Partial assignments of modified hepatic (Wade et al., 1997) and plasma (Bailey and Dickinson, 1996) proteins were achieved by immunoblotting. Antibodies to diclofenac-haptenated proteins circulate in some patients (Aithal et al., 2004). However, neither the metabolic origins nor structures of the covalent modifications could be defined completely. Correlations of plasma protein adduction with AG exposure in humans implicate direct combination (Castillo et al., 1995), but definitive identification of adducts derived uniquely from AG metabolites has proved elusive.
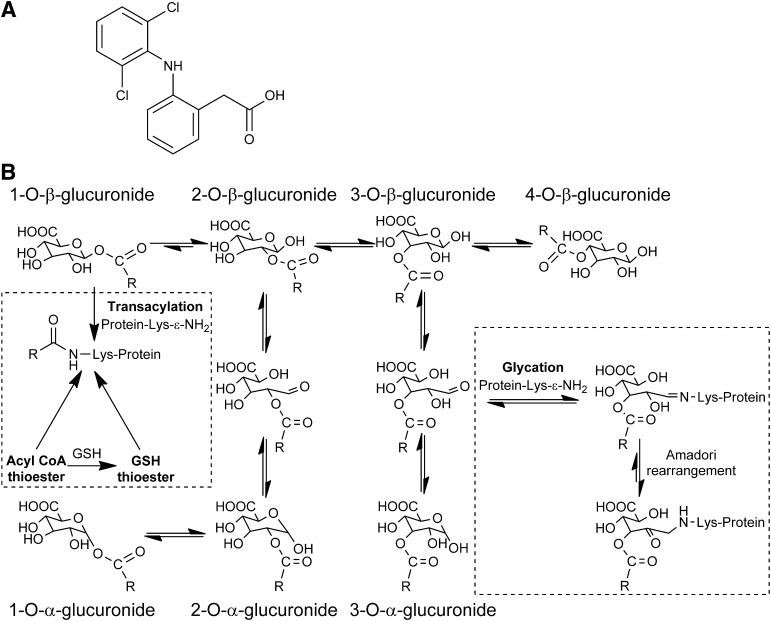
Any attempt to deconstruct protein haptenation by carboxylate drugs in vivo must differentiate between adduction products, some with identical substructures, of multiple bioactivation pathways: oxidation (Kenny et al., 2004), thioesterification (Grillo et al., 2003), and acyl glucuronylation (Stierlin and Faigle, 1979). Thioester and AG metabolites will produce indistinguishable N-acyl adducts (Skonberg et al., 2008). This dilemma is resolvable by exploiting the complex rearrangements of biosynthetic 1-β AG (Stachulski et al., 2006). Sequential nucleophilic attacks on the ester carbonyl by the glucuronyl hydroxyls cause progressive acyl migration (Fig. 1). The three AG regioisomers undergo deannulation and anomerization, yielding aldehydes that form hydroxyimine glycation adducts, and possibly tautomeric ketoamine structures, via condensation reactions with lysyl ε-amine groups (Ding et al., 1993). Glycation of human plasma proteins by AG in vivo is suggested by a higher correlation of adduction with exposure to positional isomers than exposure to 1-β AG (Hyneck et al., 1988). Therefore, carboxylate deconjugated from proteins hydrolytically might derive indiscriminately from side-chain acylations and the ester linkages within glycation adducts. The fundamental challenge to confirming protein adduction by drug AG in patients is identification of complete glycation structures.
Fig. 1.
(A) Diclofenac. (B) Proposed mechanisms of protein adduction at lysine residues by reactive AG and thioester metabolites of diclofenac in vivo. Synthetic diclofenac 1-β AG acylated and/or glycated up to ten of HSA’s 59 lysines in vitro depending on the molar ratio (Table 2). Between one and six modified HSA lysines were identified in each diclofenac patient (Table 4). The acylation adducts and complete glycation adducts were found in all and three of the six patients, respectively. In vivo, only complete glycation adducts, retaining the glucuronyl and drug carboxyl residues, are unambiguously formed from AG metabolites. The acylation adducts, as discussed in the text, might also be formed in vivo from thioester metabolites of coenzyme A (CoA) and glutathione (GSH). Only the 1-α AG, produced from 2-α AG by reverse acyl migration, was seen in vitro, but in principle all of the anomers can be formed. The 1-β AG, as shown here, is the predominant acylating isomer in vitro, but involvement of the three regioisomers and their anomers cannot be excluded. Lysine N-ε-glycation by the 3-β AG is purely representative; the regioisomers involved were not identified. The extent of any Amadori rearrangements of hydroxyimine adducts (Schiff bases) of the C-3 and C-4 esters to ketoamines is unknown.
Diclofenac is ideally suited to a search for AG-derived protein adducts in vivo: its conjugate, having a half-life of 0.51–0.7 hour in pH 7.4 buffer, is among the most reactive AG of currently prescribed pharmaceuticals (Stachulski et al., 2006; Sawamura et al., 2010). Diclofenac undergoes covalent binding to plasma proteins in rats (Masubuchi et al., 2007). Diclofenac AG circulates in mice (Sparidans et al., 2008) and reacts with human serum albumin (HSA) (Kenny et al., 2004) and hepatic microsomal protein (Kretz-Rommel and Boelsterli, 1994) in vitro.
Protein adduction by the reactive metabolites of diclofenac was analyzed using HSA from patients without drug-related hypersensitivities. HSA has numerous nucleophilic groups, including 59 ε-amines, an elimination half-time of about 19 days (Nicholson et al., 2000) favoring accumulation of modified protein (Zia-Amirhosseini et al., 1994), and is a physiologically relevant target for adduction by AG because these metabolites frequently circulate in plasma (Benet et al., 1993). It was used successfully in early mass spectrometric studies on protein adduction by NSAID AG in vitro (Ding et al., 1995; Qiu et al., 1998). Modification sites and structures were analyzed on tryptic peptides, using mass spectrometry to search lysines specifically for AG/thioester acylations and the glycation adducts derived exclusively from AG. These systematic searches were informed by detailed understanding of reactions between synthetic diclofenac AG and HSA.
Liquid chromatography–tandem mass spectrometry (LC-MS/MS) can identify many peptide modifications from a single milligram-scale HSA sample. We have used this technology to characterize benzylpenicillin haptens in patients (Meng et al., 2011). Now, for the first time, protein adducts derived from an AG metabolite have been identified in vivo.
Materials and Methods
Reagents.
Diclofenac sodium, zomepirac sodium, dithiothreitol, iodoacetamide, and HSA (approximately 99% pure, essentially fatty acid free and globin free; product A3782) were purchased from Sigma-Aldrich (Poole, Dorset, UK). Protein concentrations were determined throughout using Bradford assay dye reagent purchased from Bio-Rad (Hemel Hempstead, Hertfordshire, UK). Sequencing-grade modified trypsin was obtained from Promega (Southampton, Hampshire, UK). LC-MS–grade (acetonitrile, ethanol, isopropanol, and methanol) and high-performance liquid chromatography–grade (diethyl ether and ethyl acetate) organic solvents were purchased from Fisher Scientific (Loughborough, Leicestershire, UK). Standard inorganic chemicals and organic acids were products of either Sigma-Aldrich or Fisher Scientific.
Synthesis of Diclofenac 1-β AG.
The chemical synthesis of diclofenac 1-β AG via a modified form of the method of Bowkett et al. (2007) is described in Supplemental Scheme 1.
Human Blood Collection.
Blood taken for preparing the single pool of blank plasma used in the LC-MS/MS assays of diclofenac and its AG and for the incubations with diclofenac 1-β AG was obtained from three healthy unmedicated male volunteers, aged 21–25 years, who gave informed consent according to a procedure approved by the University of Liverpool Committee of Research Ethics. The blood was collected into 9-ml lithium heparin–coated Vacuette tubes (Greiner Bio-One GmbH, Kremsmünster, Austria). Plasma was separated by centrifugation at 2000g for 10 minutes and stored at −80°C.
Isomerization and Hydrolysis of Diclofenac 1-β Acyl Glucuronide in Phosphate Buffer, HSA Solution, and Human Plasma In Vitro.
The incubation conditions and analytical techniques used for investigating these reactions, including the methods for identifying the regioisomeric degradation products of diclofenac 1-β AG, are detailed in Supplemental Methods.
Covalent Binding of Diclofenac Residues to HSA Incubated with 1-β AG In Vitro.
The incubation conditions and analytical techniques used for measuring the covalent binding of diclofenac residues to HSA are detailed in Supplemental Methods and Supplemental Table 1.
Recruitment of Diclofenac Patients, Blood Sampling, and Sample Stabilization.
The clinical study was designed for analysis of circulating drug-derived HSA adducts. Ethical approval was obtained from the National Research Ethics Service Committee East Midlands-Derby. All the patients were recruited from a rheumatology clinic in the Nottingham University Hospitals and gave informed written consent. They were of Northern European ethnic origin and had been on a stable daily dose of diclofenac (100–150 mg in two or three divided doses) for at least 1 year (Table 1; additional details in Supplemental Table 3). This extended period of diclofenac therapy was selected to attain the highest level of plasma protein adduction by reactive drug metabolites through multiple dosing (Zia-Amirhosseini et al., 1994). The patients were undergoing regular review, including blood monitoring for their disease every 3 months. None of the patients, based upon routine liver enzyme assays (serum alanine aminotransferase, aspartate aminotransferase, and γ-glutamyl transferase) and clinical observation, demonstrated evidence of either liver injury or drug-related hypersensitivity reactions at the time of the study. Some had in the past experienced mild, transient, alanine aminotransferase rises, but those episodes were not clinically relevant and did not lead to drug or dose modifications. No restrictions were placed on the patients’ food intake. For patients N01–N03, who had taken two tablets daily, single blood samples (18 ml) removed by venipuncture were collected into lithium heparin–coated Vacuette tubes 1 hour after the first tablet of the day. Samples were collected similarly from patients N08–N10 2.5–3 hours after the first tablet of the day. Blood was collected over melting ice to minimize AG degradation and protein adduction ex vivo and centrifuged immediately at 2000g for 10 minutes at 4°C. A drug AG in human plasma can be stabilized effectively through cooling alone (Matthews and Woolf, 2008). Plasma aliquots (60 µl) intended for mass spectrometric analyses of drug-derived HSA adducts were frozen immediately at −80°C. Plasma containing drug AG is conventionally acidified to stabilize the conjugates (Hyneck et al., 1988; Matthews and Woolf, 2008), but these aliquots remained unacidified to avoid selective hydrolytic loss of AG-derived glycation adducts from the serum albumin (Smith et al., 1990) and interference with the isolation of HSA by affinity chromatography. Aliquots for quantitative LC-MS/MS analyses of diclofenac and its AG metabolite (100 µl) were acidified immediately through addition of 2 M acetic acid (final concentration, 4% v/v), a procedure known to stabilize diclofenac AG (Kenny et al., 2004; Sparidans et al., 2008). They were frozen at −80°C. All of the plasma aliquots were frozen within 1 hour of blood collection, transported from the clinic to the laboratory buried in dry ice, and stored at −80°C. Control plasma for the quantitative analyses was obtained from the healthy unmedicated volunteers. The plasma samples used as controls for the analyses of drug-derived HSA adducts were obtained independently from male and female adult subjects who had not taken diclofenac but, in common with the diclofenac patients, were taking a variety of prescribed comedications. None of them exhibited hypersensitivity reactions to those medications.
TABLE 1.
Patients dosed with diclofenac
The complete lists of comedications are recorded in Supplemental Table 3. A blank entry signifies that none of the comedications is known to form an AG metabolite in humans.
| Patient | Diclofenac Doses and Formulationsa | Blood Sampling after Last Diclofenac Dose | Comedications Metabolized to AG in Humansb |
|---|---|---|---|
| hrs | |||
| N01 (male, 42 yr) | 50 mg, b.i.d. (EC) | 1 | |
| N02 (female, 65 yr) | 50 mg, b.i.d. (EC) | 1 | |
| N03 (female, 52 yr) | 50/75 mg AM/PM (EC/MR) | 1 | |
| N08 (male, 63 yr) | 50 mg, t.i.d. (EC) | 3 | aspirin, ramipril rosuvastatin |
| N09 (male, 48 yr) | 50 mg, t.i.d. (EC) | 2.5 | simvastatin |
| N10 (female, 77 yr) | 50 mg, t.i.d. (Voltarol dispersible tablets) | 2.5 |
EC, enteric-coated; MR, modified release.
Diclofenac sodium formulations except where indicated. All the patients had taken diclofenac for at least 1 year.
AG metabolites of comedications are formed in vivo and/or in vitro.
LC-MS/MS Analysis of Diclofenac and Diclofenac AG in Clinical Plasma Samples.
The sample processing and analytical techniques used for assaying diclofenac and diclofenac AG in human plasma are detailed in Supplemental Methods and Supplemental Table 2.
Mass Spectrometric Assessment of Covalent Modification of HSA by Diclofenac AG In Vitro.
The concentration- and time-dependent covalent modifications of HSA in vitro were investigated by mass spectrometric analysis of modified tryptic peptides. Diclofenac 1-β AG (400 nM, 4, 40, and 400 µM, and 2 mM) in 0.1 M potassium phosphate buffer, pH 7.4, was incubated with HSA (40 µM) at 37°C for 16 hours. The molar ratios of AG:HSA (0.01–50) were not chosen to replicate ratios achieved in plasma in vivo, which were unknown at that time, but expected to be substantially lower than any ratio that yielded detectable adducts in vitro. Rather, the higher AG concentrations were intended to ensure production and characterization of all the HSA adducts that might be formed in vivo and consequently to facilitate a thorough, systematic, targeted search for HSA adducts in patients. In a separate experiment, the 1-β AG (2 mM) was incubated with HSA (40 µM) under the same conditions, and aliquots were removed at intervals between 30 minutes and 16 hours. Additionally, the relative contributions of the 1-β AG and its positional (acyl migration) isomers collectively to the N-acylation of HSA were assessed by first allowing 1-β AG (2 mM) to isomerize in phosphate buffer, pH 7.4, at 37°C for 3 hours. Separately, it was shown that only 5.5 ± 1.4% (mean ± S.D., n = 3) of the 1-β AG remained at the end of an incubation under these conditions, yielding a mixture of 2-, 3-, and 4-isomers and small amounts of diclofenac (Fig. 2B). HSA (40 µM; final AG:HSA molar ratio, 0.01–50:1) was incubated in this predegraded solution at 37°C for 16 hours and simultaneously for comparison in freshly prepared phosphate-buffered solutions of 1-β AG. Aliquots of the various incubations (100 μl) were added to nine volumes of ice-cold methanol, mixed by vortexing, and centrifuged at 24,000g and 4°C for 15 minutes. The supernatant was removed, and the protein pellet was washed with ice-cold methanol (60 µl × 3) to extract noncovalently bound AG and diclofenac. The protein was dissolved in 50 µl of 0.1 M phosphate buffer, pH 7.4, reduced with dithiothreitol (10 mM) for 15 minutes at room temperature, and alkylated (carboxyamidomethylated) with iodoacetamide (55 mM) for a further 15 minutes at room temperature. It was precipitated and washed with ice-cold methanol and recovered by centrifugation, as before. The pellet was redissolved in ammonium bicarbonate solution (50 mM, 30 μl) and assayed for protein content. The remainder of the solution (protein concentration, 3.2 mg/ml) was digested with trypsin (5 μg) at 37°C overnight. The digests were desalted using 0.6-μl bed C18 Zip-Tip pipette tips (Millipore, Billerica, MA), eluted with acetonitrile-0.1% trifluoroacetic acid (1:1, v/v; 10 μl), and dried by centrifugation under vacuum (SpeedVac; Eppendorf UK Ltd, Cambridge, UK) before LC-MS/MS analysis.
Fig. 2.
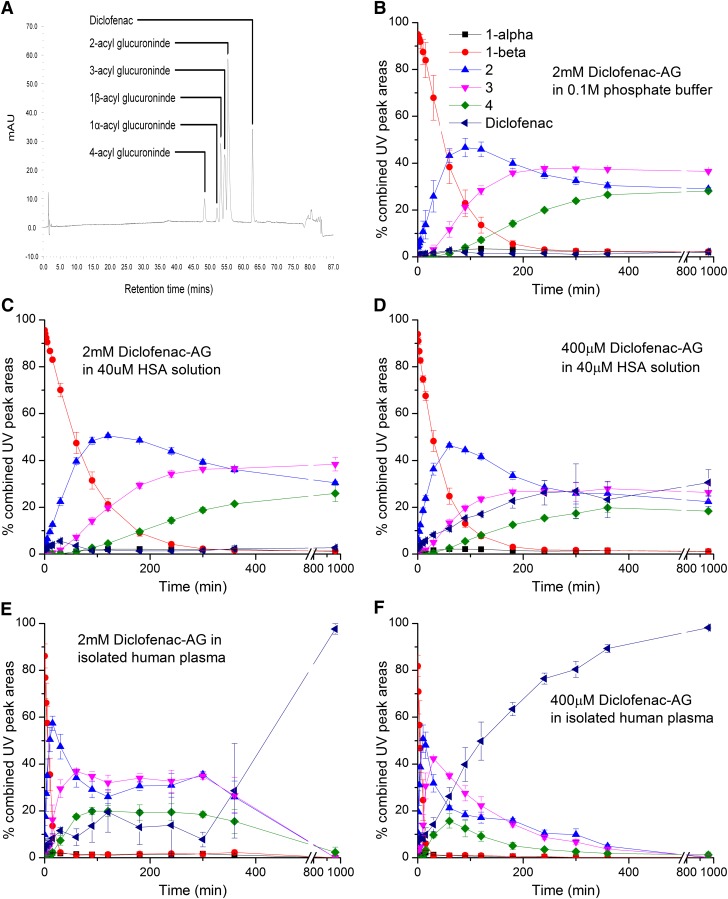
Acyl migration, anomerization, and hydrolysis of synthetic diclofenac 1-β AG in vitro at 37°C. (A) LC-UV chromatogram (λ = 254 nm) of diclofenac 1-β AG; its anomer; and C-2, C-3, and C-4 positional isomers and liberated diclofenac after a 90-minute incubation of the 1-β AG (2 mM) in 0.1 M potassium phosphate, pH 7.4. (B) 1-β AG (2 mM) in 0.1 M potassium phosphate buffer, pH 7.4. (C) 1-β AG (2 mM) in HSA solution (40 μM; 2.66 mg/ml) buffered with 0.1 M potassium phosphate buffer, pH 7.4. (D) 1-β AG (400 μM) in HSA solution (40 μM) buffered with 0.1 M potassium phosphate, pH 7.4. (E) 1-β AG (2 mM) in pooled human plasma. (F) 1-β AG (400 μM) in pooled human plasma. The relative proportions (peak areas) of the AG isomers and diclofenac were determined by LC-UV. Analytes were identified fundamentally by LC-MS. The AG positional isomers were assigned from their chronological order of appearance. Only the anomers of the 1-isomer were seen to be resolved. The 1-α anomer was assigned by chromatographic comparisons with fully characterized 1-O-acyl anomers of other AG. Data shown represent means ± S.D. as error bars (n = 3 separate experiments).
Isolation of HSA from Diclofenac Patients.
HSA was isolated by affinity chromatography from stored unacidified plasma samples (60 μl; equivalent to approximately 2.4 mg HSA) of six diclofenac patients (Table 1) and control plasma, stored under the same conditions. The samples were processed immediately after they were thawed. HSA from patients N01, N02, N03, and N08, and also HSA from the control plasma samples, was captured at room temperature on a 2-ml (4.6 × 50 mm) POROS anti-HSA affinity cartridge installed in a PerSeptive BioSystems Vision Workstation (Applied Biosystems, Foster City, CA) and was eluted with 12 mM HCl as described previously (Greenough et al., 2004; Jenkins et al., 2009). This cartridge expired before any more plasma samples were processed and could not be replaced because the manufacturer had discontinued production. Therefore, HSA from patients N09 and N10 was captured using an Affinity Removal System column (HSA only, 4.6 × 50 mm; Agilent Technologies, Santa Clara, CA) according to the same general procedure and eluted with the proprietary acidic elution buffer. In all cases, the eluted protein fractions were immediately neutralized with 0.1 M Tris-HCl buffer, pH 9. Protein was precipitated with ice-cold methanol, processed, and digested with trypsin overnight as described for HSA modified in vitro. The digest was subjected to ion exchange chromatography on a PolySULFOETHYL A strong cation-exchange column (200 × 4.6 mm, 5 μm, 300 Å; PolyLC, Columbia, MD), a procedure that enhances substantially the sensitivity of the peptide analyses by LC-MS/MS (Jenkins et al., 2009). Peptides were eluted with a linear gradient (0–50% over 75 minutes) of 10 mM KH2PO4 containing 1 M KCl and acetonitrile (3:1, v/v), pH < 3, against 10 mM KH2PO4 and acetonitrile (3:1,v/v), pH < 3, at a flow rate of 1 ml/min. The eluate was monitored at 214 nm. Approximately 15 peptide-containing fractions (2 ml) were collected per elution. They were dried by centrifugation under vacuum, reconstituted in 0.1% (v/v) trifluoroacetic acid, desalted using a Macroporous Reversed-Phase C18 High-Recovery column (4.6 × 50 mm; Agilent Technologies) installed on a Vision Workstation, and finally dried under vacuum for LC-MS/MS analysis.
Mass Spectrometric Characterization of Adducted Tryptic Peptides of HSA.
Adducted tryptic peptides in the Zip-Tip eluates (HSA modified in vitro) and the desalted ion-exchange fractions (HSA modified in vivo) were analyzed on a 5500 QTRAP hybrid triple-quadrupole/linear ion trap instrument fitted with a Nanospray II source (AB Sciex, Foster City, CA). From earlier mass spectrometric and spectrophotometric studies on adductions of HSA by NSAID AG in vitro (Ding et al., 1993, 1995; Qiu et al., 1998), ε-amino groups of lysine residues were known to be the principal identified sites of modification by these AG even in the absence of imine-adduct stabilizing reagents. Although Cys34 of HSA is also recognized as a nucleophilic residue, standard thiol-masking agents have failed to block covalent modification of the protein by NSAID AGs (Smith et al., 1990). None of the previous studies that characterized adducts of NSAID AGs on HSA reported a thioester adduct (Ding et al., 1993, 1995; Qiu et al., 1998). The established chemistry of thioesters certainly suggested stable S-acylation of Cys34 by diclofenac AG was an improbable expectation under the experimental conditions employed. Nonetheless, attempts were made using LC-MS/MS to identify an adduct at Cys34 in the tryptic digest but they revealed no evidence for covalent modification by the AG in vitro (data not shown). Therefore, multiple reaction monitoring (MRM) transitions specific for peptides containing N-acylated or glycated lysines were selected as follows: the mass values of all HSA peptides with a missed trypsin cleavage at a modified lysine residue (Jenkins et al., 2009; Meng et al., 2011; Whitaker et al., 2011) and mass additions of either 277 amu (acylated peptide) or 453 amu (acyl-glucuronide glycated peptide) for the [35Cl2]diclofenac-derived residues were calculated and these were paired with the m/z values of the dominant fragment ions of diclofenac, namely m/z 250 and m/z 215, to complete the MRM transitions. The amino acid sequence of UniProtKB/Swiss-Prot entry P02768 for HSA (monoisotopic mass, 66,429; 585 residues) was used. This sequence omits the 24 N-terminal residues of preproalbumin. The choice of mass spectrometric method was also influenced pragmatically by the reasonable assumption that only highly selective peptide survey scans would be sufficiently sensitive to detect any protein modifications in diclofenac patients. This choice was reinforced somewhat by failures of tryptic peptide analyses to detect covalently modified serum albumin in rats administered single large intravenous doses (60 mg/kg) of either diclofenac or diclofenac AG (unpublished data), plausible indications that only very low levels of modified serum albumin might be expected in patients. Sample aliquots (2.4–5.0 pmol) were delivered into the mass spectrometer by an Ultimate 3000 high-performance liquid chromatography system through a 5-mm C18 nano-precolumn, a C18 PepMap column (75 µm × 15 cm; Dionex, Sunnyvale, CA), and a 10-µm i.d. PicoTip ionspray emitter (New Objective, Woburn, MA). The ionspray potential was set to 2200–3500 V, the nebulizer gas to level 19, and the interface heater to 150°C. A gradient from 2% acetonitrile/0.1% formic acid (v/v) to 50% acetonitrile/0.1% formic acid (v/v) over 60 minutes was applied at a flow rate of 300 nl/min. MRM transitions were acquired at unit resolution in both Q1 and Q3 to maximize specificity. The collision energy was optimized for each MRM transition, and the dwell time was 20 milliseconds. MRM survey scans were set to trigger up to three enhanced product-ion scans of modified peptides according to the MIDAS technique (Unwin et al., 2005), with Q1 set to unit resolution and with dynamic fill of the ion trap. These product-ion spectra were used variously to confirm the peptide sequence, the site of adduction, and the identity of the lysyl adduct (acylation or glycation). The relative MRM peak heights of the modified peptides were computed using MultiQuant software version 2.0 (AB Sciex) to produce an "epitope profile" for each type of adducting species, i.e., acylating and glycating. However, the peak-height ratios are regarded as approximations of molar ratios in the absence of knowledge of the peptides’ relative ionization and transmission efficiencies. The total ion count for each digest sample was normalized to that of the HSA adduct produced in vitro at a molar ratio of diclofenac 1-β AG to protein of 50:1 over 16 hours, thereby allowing the magnitudes of the MRM signals to be adjusted for differences between on-column sample loading (Meng et al., 2011). Modified tryptic peptides of HSA isolated from diclofenac patients were also analyzed on an AB Sciex Triple TOF 5600. The higher resolution and broader mass range of this instrument allowed for more confident assignments of some peptide sequences and adduct structures. Peptide aliquots (2.4–5.0 pmol) were delivered into the mass spectrometer via a 10-µm i.d. PicoTip (New Objective) by a direct-flow nano-LC system (Eksigent, Dublin, CA). The system was comprised of a NanoLC-Ultra chromatograph linked to a cHiPLC-Nanoflex docking station, ChromXP C18 trap column (200 μm × 0.5 mm), and ChromXP C18 column (75 µm × 15 cm). The ionspray potential was set to 2200–3500 V, the nebulizer gas to level 5, and the interface heater to 150°C. A gradient from 2% acetonitrile/0.1% formic acid (v/v) to 50% acetonitrile/0.1% formic acid (v/v) in 90 minutes was applied at a flow rate of 300 nl/min. Data were acquired at 25 MS/MS spectra per cycle with an accumulation time of 100 ms each, sorted in PeakView (AB Sciex) to highlight spectra containing fragment ions at m/z 215 and m/z 250, and then interpreted manually.
Results
Isomerization and Hydrolysis of Diclofenac 1-β AG In Vitro.
Synthetic diclofenac 1-β AG in phosphate buffer, pH 7.4, at 37°C (Fig. 2B) and also in buffered HSA solution at a high molar ratio (50:1; Fig. 2C) underwent rapid rearrangement through acyl migration (Fig. 1) but very little hydrolysis to parent carboxylate. In the HSA solution, the quantities of C-2, C-3, and C-4 positional isomers exceeded that of the 1-β AG after approximately 1, 2, and 3 hours, respectively. Similar isomer successions occurred in the phosphate buffer. The relative proportions of the isomers (C-3 > C-2 > C-4) stabilized from about 6 hours in the presence and absence of HSA and remained essentially unchanged for the subsequent 10 hours. Reducing the molar ratio AG:HSA from 50:1 to 10:1 produced a dramatic acceleration of conjugate hydrolysis without greatly affecting the relative proportions of the positional isomers (≤10% variance of isomer exposure over 16 hours): the quantity of deconjugated diclofenac equaled or exceeded the individual quantities of the AG isomers after approximately 4 hours (Fig. 2D). However, as in the buffer and other HSA incubations, the mixture of degradation products stabilized from about 6 hours. In the human plasma incubations (Fig. 2, E and F), although some acceleration of acyl migration was also apparent, hydrolysis of 1-β AG and all the regioisomers was the dominant pathway, outstandingly at the lower concentration of 1-β AG (400 μM) when the quantity of diclofenac exceeded the quantities of all the positional isomers after 1.5 hours and only minor residues of the isomers (≤ 5%) remained by 6 hours (Fig. 2F). These observations of faster migration and hydrolysis reactions in plasma conform with physicochemical measurements made on other NSAID AG (Karlsson et al., 2010). In all cases, the 1-α anomer was only formed in trace amounts (Fig. 1). The half-lives of diclofenac 1-β AG in phosphate buffer, HSA solution (2 mM and 400 μM), and human plasma (2 mM and 400 μM), estimated by nonlinear regression analysis of the first-order rates of decay of the conjugate (Supplemental Table 4), were 46.5, 56.8, 31.9, 7.0, and 5.4 minutes, respectively.
Covalent Binding of Diclofenac Residues to HSA Incubated with 1-β AG In Vitro.
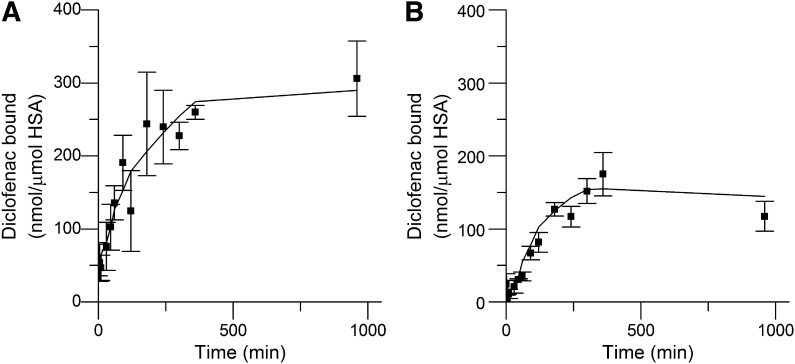
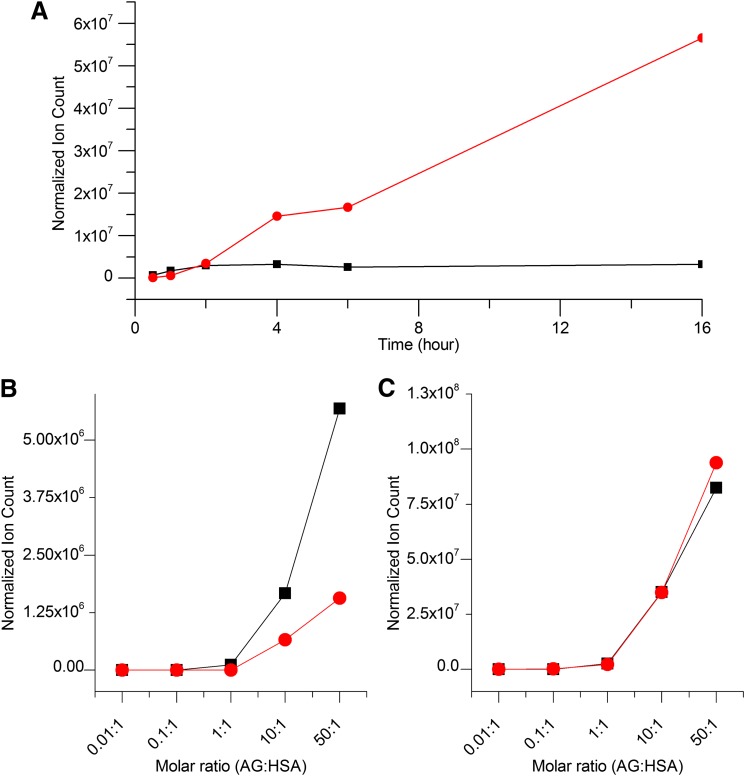
Incubation of diclofenac 1-β AG with HSA at pH 7.4 under the conditions favoring acyl-group migration over hydrolysis, i.e., a 50:1 molar ratio of AG to HSA (Fig. 2C), resulted in rapid protein modification, which was assessed from the quantity of diclofenac liberated by strong alkaline hydrolysis (Fig. 3A). This method does not differentiate between glycation structures and acyl residues on protein side chains (Fig. 1). Covalent binding was measurable from 5 minutes onward and reached an essentially stable maximum at about 6 hours. The covalent binding at the lower molar ratio of AG to HSA (10:1), when conjugate hydrolysis was substantial (Fig. 2D), followed a similar time course (Fig. 3B). The maximum measured protein adduction was 0.62 ± 0.10 and 1.78 ± 0.28% molar equivalents of diclofenac (mean ± S.D., n = 3) at the lower and higher ratio, respectively. The corresponding area under the liberated diclofenac time curve, which represents a measure of protein modification over the 16-hour incubations (AUC0–16), was 238.37 and 124.13 ng⋅h/ml, respectively.
Fig. 3.
Covalent binding of diclofenac residues to HSA (40 μM) incubated with synthetic diclofenac 1-β AG (400 μM and 2 mM) in 0.1 M potassium phosphate, pH 7.4, at 37°C for 16 hours. (A) Binding at 50:1 molar ratio of AG:HSA. (B) Binding at 10:1 molar ratio of AG:HSA. Covalent binding was estimated using a LC-MS/MS assay of diclofenac liberated from the protein adducts by alkaline hydrolytic deconjugation. Data shown represent means ± S.D. as error bars (n = 3 separate experiments).
Structural Characterization of Diclofenac AG–Derived Adducts Formed on HSA In Vitro.
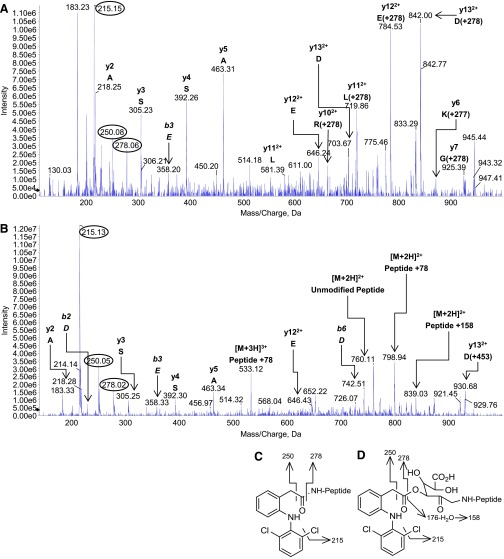
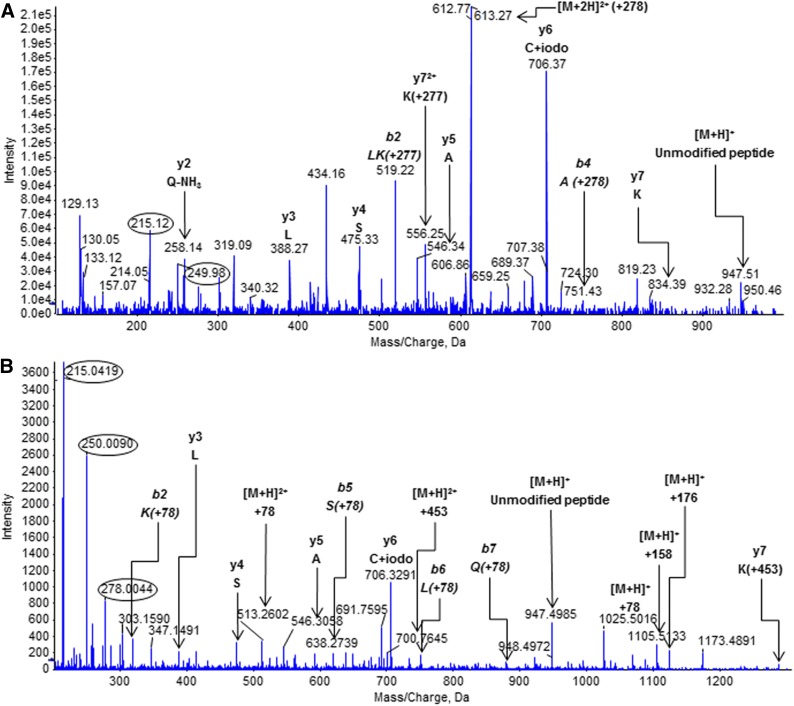
Incubation of diclofenac 1-β AG with HSA (50:1 molar ratio) for 16 hours under the conditions conducive to rapid and selective acyl migration (Fig. 2C) produced two predicted adduct types (Fig. 1) that were identified by LC-MS/MS analysis of tryptic digests: lysine residues that were either N-acylated or glycated by the complete AG (diclofenac carboxyl and glucuronyl residues). The use of a high reactant:HSA ratio was known from earlier studies on HSA adduction by β-lactams (Meng et al., 2011) to provide a secure starting point for the considerably more challenging in vivo analyses. It enables identification under controlled conditions of (almost) all the selected HSA adducts formed detectably in patients and thereby delivers a working inventory of adducts for the in vivo study. Because trypsin is unable to cleave the protein at adducted amino acids (Jenkins et al., 2009; Meng et al., 2011; Whitaker et al., 2011), all of the peptides with a modified lysine residue will have either an N-terminal lysine—as when the residue is one of a pair—or a single subterminal lysine (Table 2). A priori, any tryptic peptide detected by the specific MRM survey-scan method used here will be acylated or glycated with diclofenac AG–derived residues (diclofenac AG– and/or thioester-derived residues in vivo) at the lysine that is the site of the missed enzymic cleavage. Eight of the 59 lysine residues, which constitute 1.4% of the entire sequence and 13.6% of the lysines, were modified consistently. Seven of the residues were adducted when the AG:HSA molar ratio was 1:1, and all eight when the ratio was ≥10:1 notwithstanding the extensive AG hydrolysis at 10:1 (Fig. 2D). All except one of those eight modified residues underwent both types of modification consistently when the ratio was ≥10:1. Exceptionally, Lys351 was not always found to be acylated (Table 2). Similarly, the glycation of Lys162 and the acylation and glycation of Lys436 were not observed invariably. Two of the modified residues, i.e., Lys137 and Lys525, were paired lysines—HSA has four lysine pairs—but neither of the adjacent residues (Lys136 and Lys524) was adducted detectably. The marked localized selectivity of lysine modification was also exemplified by the adduction of only one of the five residues (Lys541) between Lys534 and Lys545, inclusive. Nevertheless, derivatization within short amino acid sequences was highly variable: all three of the lysine residues between Lys190 and Lys199, inclusive, were adducted. Typical product-ion spectra of the N-ε-acylated and N-ε-glycated forms of a modified miscleaved tryptic peptide, namely those of 182LDELRDEGKASSAK195 modified at Lys190, are shown in Fig. 4, A and B, respectively. Fragment ions at m/z 250 (benzyl moiety) and m/z 215 (m/z 250-Cl·) were diagnostic of modifications that included the diclofenac residue, namely N-acylations and complete glycations, whereas the ion at m/z 278 (the diclofenac acyl residue) was observed less frequently, but all the fragmentations (Fig. 4, C and D) could be rationalized in terms of the two predicted adduct structures (Fig. 1) and are known from the product-ion spectrum of diclofenac (Galmier et al., 2005). The glycated peptide alone yielded an ion at m/z 839, corresponding to [M + 2H]2+ for the unmodified miscleaved peptide plus 158 amu. This ion was assigned to a fragment of the glycated peptide that retained only the dehydrated residue of the dehydroglucuronic acid moiety (Fig. 4D). A glucuronate species is apparently more susceptible to this dehydration if it is derived from non–1-O-β-AG structures (Karlsson et al., 2010), as it must be in this case. The peptide + 78 amu ion seen in spectra of glycated peptides (Fig. 4B; Supplemental Fig. 2B) was assigned by analogy to a pyrylium species ([C5H4O] = NR) produced by triple loss of water and elimination of CO2 from the deacylated glucuronic acid residue (Jerić et al., 2002; Karlsson et al., 2010). It is putatively diagnostic of a C-N glycation linkage. The AG-derived structures were evidently more labile in the MS collision cell than the peptide bonds, leading to multiple overlapping and weak spectra within the same analytical space and difficulties with interpreting amino acid sequences. Fragment ions corresponding to the full-length peptide minus the modification were also visible in most spectra, as were full-length peptides with partial adduct structures and peptide fragments with either no or partial adducts. Nonetheless, it was possible to design MRM transitions that detected the AG-modified peptides and to interpret the MRM-triggered MS/MS spectra.
TABLE 2.
Modified tryptic peptides of HSA reacted with diclofenac 1β-AG in vitro
HSA was incubated with synthetic diclofenac 1β-AG in phosphate buffer, pH 7.4, at 37°C for 16 hours. The peptides were characterized by LC-MS/MS (AB Sciex 5500 QTRAP). Glycated Lys162 was not detected consistently and was only ever seen at low signal strength (acylated Lys162 was never detected). Acylated Lys351 was not detected consistently and was only found at the 50:1 molar ratio. Acylated and glycated Lys436 were not detected consistently. Inconsistently observed adducts are shown as bold letters.
| Modified Lysinea | Tryptic Peptideb | Molar Ratio Diclofenac 1β-AG: HSA |
||||
|---|---|---|---|---|---|---|
| 0.01:1 | 0.1:1 | 1:1 | 10:1 | 50:1 | ||
| 137 | K*YLEIAR | — | — | — | G+T | G+T |
| 162 | YK*AAFTECCQAADK | — | — | — | G | G |
| 190 | LDELRDEGK*ASSAK | G | G | G+T | G+T | G+T |
| 195 | ASSAK*QR | — | — | G+T | G+T | G+T |
| 199 | LK*CASLQK | — | G | G+T | G+T | G+T |
| 351 | LAK*TYETTLEK | — | — | G | G | G+T |
| 432 | NLGK*VGSK | — | G | G | G+T | G+T |
| 436 | VGSK*CCK | — | — | — | G+T | G+T |
| 525 | K*QTALVELVK | — | — | G | G+T | G+T |
| 541 | ATK*EQLK | — | — | G+T | G+T | G+T |
G, complete glycation structure (diclofenac carboxyl and glucuronyl residues); T, diclofenac transacylation adduct.
Benoxaprofen AG modified Lys159 and Lys199 in vitro without reductive stabilization (Qiu et al., 1998). Tolmetin AG modified Lys195, 199, 525, and 541 in vitro without reductive stabilization (Ding et al., 1995). Lys159 and Lys199, respectively, were the principal adduction sites.
Asterisk indicates lysine modification site on the miscleaved peptide. The methodology of adduct identification is described under “Mass Spectrometric Characterization of Adducted Tryptic Peptides of HSA” in Materials and Methods. Cysteine residues of the recovered and reduced protein were carboxyamidomethylated before trypsin digestion.
Fig. 4.
Product-ion spectra of modified HSA peptide 182LDELRDEGKASSAK195 acquired during LC-MS/MS analysis of a tryptic digest of protein reacted in vitro with synthetic diclofenac 1β-AG (50:1 molar ratio of AG:HSA). (A) The diclofenac-acylated peptide (peptide + 277 amu; parent ion, [M + 3H]3+ at m/z 599.2). (B) The glycated peptide (peptide + 453 amu; incorporating glucuronyl and drug carboxyl residues; parent ion, [M + 3H]3+ at m/z 658.6). The MRM survey scans were set up to search for acylated and glycated HSA peptides with missed trypsin cleavages, i.e., covalent modifications, at lysine residues. The m/z values of the modified peptides and fragments correspond to the 35Cl2 isobars. The y6 ion of the acylated peptide (m/z 868) was adducted (+277 amu, the diclofenac acyl residue) at Lys190. The glycated peptide did not yield any observable y or b ions bearing the complete glycation structure. The multiply charged peptide + 158 amu ion was assigned to a whole-peptide species that retained the dehydrated residue of the dehydroglucuronic acid moiety. The acylated peptide’s parent ion also yielded peptide fragments that had undergone collision-induced elimination of the entire adduct residue. Fragment ions of the adducts are circled. The principal adduct-derived fragment ions of the modified peptide were rationalized as shown. (C) Fragment ions of the acyl adduct. (D) Fragment ions of the glycation adduct.
Epitope Profiles of HSA Adducted by Diclofenac AG In Vitro.
Computation of the relative MS/MS ion count for each modified HSA peptide in a tryptic digest provides an "epitope profile" that is characteristic of the adducting drug or drug metabolite (Jenkins et al., 2009; Meng et al., 2011; Whitaker et al., 2011). These profiles can be used to visualize the time and concentration dependency of covalent modifications. The acylation and glycation structures derived from diclofenac 1-β AG were located on the subset of eight lysine residues described previously (Fig. 5; Table 2). All except two of the 16 observed modifications—acylation of Lys199 and glycation of Lys351—were detected after 0.5 hour of incubation at the highest AG:HSA molar ratio, namely 50:1. Acylation of Lys199 and glycation of Lys351 was first detected after 2 and 1 hour, respectively (data not shown). In general, the glycated adducts were identified at lower AG:HSA molar ratios (Table 2). The proportions of the normalized ion counts for the two adduct types varied considerably between adducted residues and with time at certain lysines. Thus the lowest AG:HSA molar ratio at which a glycation adduct was found was 0.01:1, whereas the corresponding ratio for the acyl adducts was 1:1. The levels of both modifications were dependent on the concentration of AG (Fig. 6, B and C). However, because the individual proportionalities of ion counts and peptide abundances will have been influenced by unknown efficiencies of ionization and ion transmission in the mass spectrometer, it cannot be assumed the measured counts equate with relative molar abundances of the peptides. The time courses of totalized ion counts showed that the acyl adducts collectively were formed early, but no greater acylation occurred after about 2 hours (Fig. 6A). The glycation structures appeared no more rapidly than the acyl adducts, but the collective level of this modification increased continuously to the end of the 16-hour incubation. A representative selection of ion-count time courses for individual modified peptides is shown in Supplemental Fig. 1. The representative relationship between AG concentration and adduction of the Lys195 peptide is shown in Supplemental Fig. 1C. Although the totalized ion counts for glycation products were progressively greater than those for acylation products after about 3 hours, the glycated tryptic peptides, for the analytical reasons mentioned previously, were not necessarily more abundant in total.
Fig. 5.
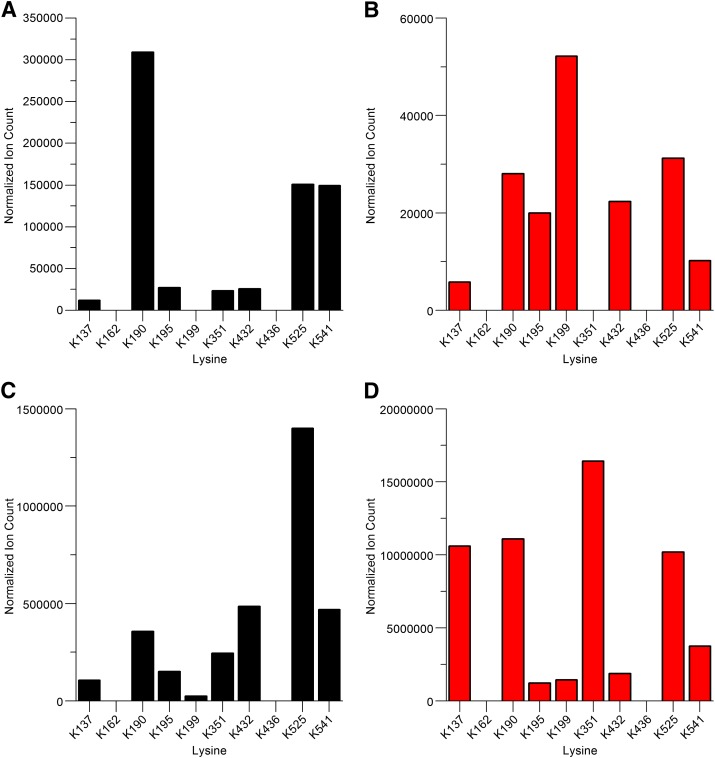
Ion-count epitope profiles of HSA modified at lysine residues by reactions with diclofenac 1-β AG in vitro. (A) Acylation adducts at 0.5 hour. (B) Glycation adducts at 0.5 hour. (C) Acylation adducts at 16 hours. (D) Glycation adducts at 16 hours. Data represent relative ion intensities of lysine-modified peptides detected during LC-MS/MS analyses of tryptic digests. Each relative ion intensity was derived from the area under the curve for the relevant extracted ion chromatogram by normalization to the total ion count of the sample. Synthetic AG was incubated with HSA (AG:HSA molar ratio, 50:1) at pH 7.4 and 37°C. Acylated Lys351 was detected in these analyses, but this modification of Lys351 and also modifications of Lys162 and Lys436 were not detected consistently (Table 2). Lys436 was modified detectably in patient N08.
Fig. 6.
Acylation and glycation of HSA by synthetic diclofenac 1β-AG and preformed diclofenac AG positional isomers in 0.1 M potassium phosphate, pH 7.4, at 37°C over 16 hours demonstrating the transacylation reactions were effected principally by the 1-β isomer. (A) Time-dependent acylation (black line) and glycation (red line) of HSA (40 μM) by 1β-AG (2 mM). (B) Concentration-dependent acylation of HSA (40 μM; final AG:HSA molar ratio, 0.01–50:1) by freshly prepared solutions of 1β-AG (black line) and a preformed mixture of positional isomers (red line). (C) Concentration-dependent glycation of HSA (40 μM; final AG:HSA molar ratio, 0.01–50:1) by freshly prepared solutions of 1β-AG (black line) and a preformed mixture of positional isomers (red line). The extent of each generic modification of HSA (acylation or glycation) in an incubation sample was estimated by summing the normalized ion counts for all the acylated or glycated peptides detected during a LC-MS/MS analysis of a tryptic digest. The mixture of positional isomers was produced by preincubating the 1β-AG in phosphate buffer, pH 7.4, at 37°C for 3 hours (see Fig. 2B). The data are representative of at least two separate experiments.
Time- and Isomer-Dependent Adduction of HSA by Diclofenac AG In Vitro.
A closer examination of the dynamics of the two types of adduction in vitro provided an important insight into the mechanism of protein modification by diclofenac AG. The rapidity of the isomerization of diclofenac 1-β AG in protein solutions, even when the glucuronide underwent somewhat extensive hydrolysis (Fig. 2, D and E), suggested an explanation for early completion of the acylation reactions in HSA incubations (Fig. 6A), namely that they were affected principally by the 1-β isomer. In fact, from reaction mechanism theory and all other considerations being equal, the anomeric acyl group will always be a better acylating agent than its regioisomeric forms. This proposition was tested by reacting HSA with a preformed mixture of positional AG isomers (∼8:7:3 of the C-2, C-3, and C-4 isomers, respectively, estimated from LC-UV analysis; Fig. 2B) generated by incubating 1-β AG in phosphate buffer, pH 7.4, at 37°C for 3 hours, by which time only approximately 5% of the parent isomer remained. Parallel incubations of HSA with degraded and undegraded glucuronide at pH 7.4 for 16 hours (AG:HSA molar ratio, 0.01–50:1) revealed that the total level of protein acylation in solutions containing rearranged AG, as represented by summated ion counts of modified peptide ions, was approximately 30% of that observed in solutions containing only 1-β AG at the outset (Fig. 6B). In contrast, the total level of glycated peptide (peptide + 453 amu) ions, as dictated by the established mechanism of protein glycation (Fig. 1), was unaffected by extensive preincubation depletion of parent AG (Fig. 6C). Therefore, it was deduced that the 1-β AG was at least the principal source of lysine acylation adducts collectively on HSA in vitro. A detailed representation of the differential adductions of individual peptides in these experiments is shown in Supplemental Fig. 1. Collective and individual contributions of the positional isomers to acylation reactions appear to be minor. The data do not reveal which of the three positional isomers is principally responsible for the glycations, although the timescales of isomer formation (Fig. 2, C and D) and protein glycation (Fig. 6A) in HSA solutions suggest all three isomers might contribute to this modification after the fourth hour. Glycations at the anomeric carbon of the C-2 regioisomer are likely to be less durable than those of the other migration products, however, because their structure prevents a stabilizing Amadori tautomeric rearrangement.
Diclofenac and Diclofenac AG in Clinical Plasma Samples.
All of the acid-stabilized single plasma samples taken from the six patients 1–3 hours after their last tablet contained detectable concentrations of diclofenac, although in three cases the concentration was below the lower limit of LC-MS/MS quantification (Table 3). Tmax values for the narrow peaks of diclofenac can display considerable variability, although Crook et al. (1982) calculated 2.0 ± 0.50 hours (mean ± S.D.) for a 50-mg oral dose in rheumatoid patients. Only four of the samples contained detectable concentrations of diclofenac AG, which was assayed without chromatographic resolution of the positional isomers, and in only one patient (N08) was the conjugate quantifiable. The latter sample was taken 3 hours after the last tablet. Notably, the concentration of parent drug in that spot sample was by far the highest concentration of diclofenac found in any of the patients. Nevertheless, it was substantially lower than the Cmax of 3.3±1.4 μM reported for a single 50-mg dose (Crook et al., 1982). The molar ratio of AG to drug in N08 was 0.21. Two of the other plasma samples that contained detectable concentrations of diclofenac AG also contained quantifiable amounts of diclofenac. The relatively low concentrations of diclofenac in the patients’ plasma have no clear explanation. Certainly neither the clinical conditions of the patients nor the comedications (Supplemental Table 3) suggest an explanation. Most of the patients took a delayed release formulation, but delaying blood sampling from 1 to 2.5/3 hours had no marked or consistent effect on drug concentrations, notwithstanding the sampling times were within the range of published Tmax for enteric-coated diclofenac (Willis et al., 1981; Crook et al., 1982).
TABLE 3.
Concentrations of diclofenac and diclofenac AG in plasma of patients
See Table 1 for individual patient data.
| Patient ID Number | Time of Blood Samplinga | Diclofenac AGb | Diclofenacb |
|---|---|---|---|
| hrs | nM | ||
| N01 | 1 | N.D. | N.Q. |
| N02 | 1 | N.D. | N.Q. |
| N03 | 1 | N.Q. | 166.7 |
| N08 | 3 | 90.8 | 423.3 |
| N09 | 2.5 | N.Q. | N.Q. |
| N10 | 2.5 | N.Q. | 63.91 |
N.D., not detected (MRM signal-to-noise ratio <3 at confirmed Rt of the analyte); N.Q., not quantified (analyte concentration >lower limit of detection, <lower limit of quantification).
Single blood samples were removed by venipuncture at the indicated time after the last diclofenac tablet had been taken.
Diclofenac and diclofenac AG were assayed by LC-MS/MS.
Characterization of Drug-Derived Adducts Formed on HSA in Diclofenac Patients.
Plasma samples from the six diclofenac patients were processed by HSA concentration (affinity chromatography) and tryptic peptide fractionation (cation exchange chromatography) before peptide analysis by nano–LC-MS/MS. These steps were essential to achieving the high sensitivity required for characterization of circulating adducts. Although two of the eight lysines modified consistently in vitro were not modified detectably in vivo (Lys137 and Lys351), a collective total of seven adducted residues and ten modifications were identified (Table 4). The detection of adducts on 75% of the HSA residues modified consistently in vitro exceeded expectations. The lower limit of adduct detection in vitro after a 16-hour incubation was an AG:HSA molar ratio of 0.01:1 (Table 2)—and reaction at that ratio yielded only one modification. Contrastingly, the molar ratio in the one patient (N08) with a measurable plasma AG concentration (90.8 nM; Table 3) was estimated to be only 0.0001:1 3 hours after the final drug dose, taking an HSA concentration of 45 mg/ml for the calculation (Veering et al., 1990). This disparity is attributed to an accumulation of modified protein (Zia-Amirhosseini et al., 1994) and cautions against any tendency to discount adduct detectability in vivo based on chemical analysis of adduction. Acylation of one, three, four, or five lysine residues was observed on HSA isolated from the patients. The acylated residues detected in each person were variously one to five from a total of six of the seven lysines that underwent this adduction reaction reproducibly in vitro (Table 2). One of the two residues adducted invariably in vitro although not modified detectably by either acylation or glycation in any of the subjects, Lys137, was only modified in vitro when the AG:HSA molar ratio was ≥10:1. Acylation of the other residue, Lys351, was detected inconsistently in vitro, i.e., near the limit of mass spectrometric identification, although its glycation was detected reproducibly at an AG:HSA ratio of ≥1:1. Acylated Lys436 and Lys525 were identified exclusively in patient N08. Unusually, Lys436 was not adducted consistently in vitro. Three of the lysines (Lys195, 199, and 541) were acylated in four patients. Two of these residues and two of the other four acylated lysines were modified in vitro when the AG:HSA molar ratio was 1:1. A typical product-ion spectrum of an acylated peptide is shown in Fig. 7A. Glycation adducts were found on one or three lysines (Lys195 or Lys199 and Lys195, 199, and 432, respectively) but in only three of the patients (N01, N08, and N09). All of these residues were among the eight lysines that were glycated in vitro. Modified peptide 198LKCASLQK205 was detected most readily (Fig. 7B), notwithstanding in-source fragmentation that resulted in this peptide being found additionally at m/z 691.9 after loss of water. Two of the residues glycated in vivo were glycated detectably by synthetic diclofenac 1-β AG when the AG:HSA molar ratio was 0.1:1 and all of them when the ratio was ≥1:1 (Table 2). Product-ion spectra of other glycated peptides are shown in Supplemental Fig. 2, A and B. Two of the three glycated lysines (Lys195 and Lys199) were also acylated in the same patients. The three residues were among the seven lysines acylated and glycated in vitro. Supplemental Table 5 itemizes the nine modified HSA tryptic peptides obtained from N08, the greatest number obtained from any of the patients. Exceptionally, peptide 525KQTALVELVK534 from HSA of patient N08 (Table 1) was detected with an addition of 176 amu at Lys525 (Supplemental Fig. 3). Although the biochemical context of the mass increment immediately suggested conjugation with glucuronic acid alone (Fig. 1), the LC-MS/MS analysis was not able to differentiate between a glucuronyl and other, isomeric, hexuronyl modifications. Lys525 was also acylated in N08 and in vitro and was glycated in vitro (Table 2) but was not modified in any of the other patients. The 176-amu modified peptide was recovered in a later cation exchange fraction than the corresponding acylated peptide (Supplemental Table 5). On the basis of the retention times of other modified peptides, a corresponding AG-glycated peptide would have eluted earlier still from the cation exchange column, suggesting that detection of the unique glucuronylated species was not a result of in-source fragmentation of an AG-glycated precursor ion. This seemingly novel adduct is attributed tentatively to a somewhat rare, nonenzymic, post-translational modification occurring in vivo by the slow reaction of HSA with glucuronic acid in plasma (Mazzuchin et al., 1971; Smith et al., 1990). In a separate study using similar analytical methods, we found glucosylated Lys525 (Barnaby et al., 2011) in vivo (data not shown). Neither glucuronylated Lys525 nor any of the N-acylations and glycations associable with either diclofenac AG or reactive thioester metabolites of diclofenac were found on HSA isolated from the three control subjects. Finally, neither the detection nor the chemical characteristics of the drug-protein adducts were associated consistently with measured concentrations of drug or AG in plasma (Tables 3 and 4). N08 had the greatest number of HSA adducts and the highest concentrations of diclofenac and AG but there was not a consistent alignment of the two plasma concentrations and the number of adducts in the six patients. Thus, N01 and N02 had nonquantifiable drug concentrations and yet they each yielded multiple adducts. The spot drug and AG concentrations are unlikely to have any relevance to the detection or composition of the HSA adducts found in these patients. Those phenomena will have been determined by protracted accumulation and chemical evolution of the adducts during the extended pharmacotherapy preceding the study.
TABLE 4.
Modified tryptic peptides of HSA isolated from diclofenac patients
HSA was isolated by affinity chromatography from plasma of patients who had taken diclofenac (100–150 mg/day) as two or three times daily doses for at least 1 year. The peptides were characterized by LC-MS/MS (AB Sciex 5500 QTRAP and TripleTOF 5600). See Table 1 for individual patient data.
| Patient ID Number | Modified Lysine | Tryptic Peptidea | Modificationb |
|---|---|---|---|
| N01 | 190 | LDELRDEGK*ASSAK | T |
| 195 | ASSAK*QR | T | |
| 199 | LK*CASLQK | T+G | |
| 432 | NLGK*VGSK | T | |
| 541 | ATK*EQLK | T | |
| N02 | 190 | LDELRDEGK*ASSAK | T |
| 195 | ASSAK*QR | T | |
| 199 | LK*CASLQK | T | |
| 432 | NLGK*VGSK | T | |
| 541 | ATK*EQLK | T | |
| N03 | 195 | ASSAK*QR | T |
| 199 | LK*CASLQK | T | |
| 541 | ATK*EQLK | T | |
| N08 | 195 | ASSAK*QR | T+G |
| 199 | LK*CASLQK | T+G | |
| 432 | NLGK*VGSK | G | |
| 436 | VGSK*CCK | T | |
| 525 | K*QTALVELVK | T+Glucuronylationb | |
| 541 | ATK*EQLK | T | |
| N09 | 195 | ASSAK*QR | T+G |
| N10 | 195 | ASSAK*QR | T |
G, complete glycation structure (incorporating the diclofenac carboxyl and glucuronic acid residues); T, diclofenac transacylation adduct.
Asterisk indicates lysine modification site on the miscleaved peptide. Cysteine residues of isolated and reduced HSA were carboxyamidomethylated before trypsin digestion.
Modified by acylation and glucuronylation/hexuronylation.
Fig. 7.
Product-ion spectra of modified HSA peptide 198LKCASLQK205 acquired during a LC-MS/MS analysis of a tryptic digest of the protein isolated from diclofenac patient N08. HSA was isolated from a 60-μl plasma sample by affinity chromatography. (A) The diclofenac-acylated peptide (peptide + 277 amu; parent ion, [M + 2H]2+ at m/z 612.8). (B) The glycated peptide (peptide + 453 amu; incorporating glucuronyl and drug carboxyl residues; parent ion, [M + 2H]2+ at m/z 700.8). The MRM survey scans were set up to search for acylated and glycated HSA peptides with missed trypsin cleavages, i.e., covalent modifications, at lysine residues. The m/z values of the modified peptides and fragments (b and y ions) correspond to the 35Cl2 isobars. The b2 (LK) ion (m/z 519.22) of the acylated peptide and the y7 ion (m/z 556.25) were both adducted (+277 amu, the diclofenac acyl residue) at Lys199. The y7 ion of the glycated peptide was adducted (+453 amu) but the peptide did not yield any observable b ions bearing the complete glycation structure. The peptide + 158 amu ion was assigned to a whole-peptide species that retained the dehydrated residue of the dehydroglucuronic acid moiety. The peptide + 176 amu ion retained the complete dehydroglucuronic acid moiety. The parent ions also yielded peptide fragments that had evidently undergone collision-induced elimination of the entire adduct residue. Fragment ions of the modifications are circled. The principal adduct-derived fragment ions of the modified peptide were rationalized as shown in Fig. 4. Cysteine residues of isolated and reduced HSA were carboxyamidomethylated before trypsin digestion. The presence of an alkylated cysteine in a peptide fragment is indicated by the annotation C + iodo.
Discussion
Mass spectrometric analyses of HSA from diclofenac patients have proven definitively for the first time that an AG metabolite can modify a protein covalently in vivo. This was achieved through the identification of signature lysyl glycations retaining glucuronyl and carboxyl residues (Fig. 1). It was also shown that a bioactivated carboxylate drug can modify multiple lysines via N-acylation and glycation. Ten adductions to seven lysines in highly variable combinations were characterized. Each patient had a unique combination, and the number of modifications in a subject varied almost continuously between one and eight. Only one modification was common to all the patients. Apart from an N-acylation, found in one subject, all of these adductions were predicted from reactions of diclofenac AG with HSA in vitro.
Hitherto the identification of protein adducts of diclofenac in vivo rested on radiotracing, which showed relatively low binding in rat liver and plasma (Masubuchi et al., 2007), and immunovisualization in rodent (Hargus et al., 1995; Wade et al., 1997) and human (Aithal et al., 2004) liver. Although some targeted hepatic proteins were assigned, and glucuronyltransferase catalyzed diclofenac binding to rat liver homogenate (Hargus et al., 1994), the structures and metabolic derivations of modifications were not defined precisely. The method used often to characterize carboxylate-derived adducts of plasma proteins, namely alkalinolysis (Zia-Amirhosseini et al., 1994; Sallustio et al., 1997; Hermening et al., 2000), has not been applied to diclofenac patients. Correlations of plasma protein adduction with NSAID AG exposure in humans (Castillo et al., 1995), and especially the higher correlation with exposure to regioisomers (Hyneck et al., 1988), implicated covalent combination with AG and possibly intravascular reactions. However, protracted alkalinolysis releases carboxylic acid unspecifically from amide and ester side-chain linkages and ester bonds of isomeric glycations (Smith et al., 1990). Without identification of AG-definitive glycations it cannot be assumed any of these circulating adducts are derived from AG rather than reactive thioester metabolites (Skonberg et al., 2008). The strongest evidence of protein modification by diclofenac AG within biologic systems was the observation that glucuronidation inhibitors reduced [14C]diclofenac binding to rat hepatocytes (Kretz-Rommel and Boelsterli, 1993).
Diclofenac AG incubated with HSA under conditions favoring acyl-group migration produced the dominant adduct types, N-acyl and glycation, obtained when tolmetin AG (Ding et al., 1993; Ding et al., 1995) and benoxaprofen AG (Qiu et al., 1998) were reacted similarly with HSA. The half-lives of tolmetin and R/S-benoxaprofen AG in phosphate buffer are 0.26 and 2.0/4.1 hours, respectively (Stachulski et al., 2006). The values of the half-life of diclofenac AG reported here, and by Ebner et al. (1999) and Sawamura et al. (2010), of 0.78, 0.51, and 0.7 hour, respectively, all rate the conjugate as highly reactive. Several of the modified residues are common targets of electrophilic compounds and, thereby, potentially frequent locations of antigen formation. Lys195, adducted in every diclofenac patient, also reacts with β-lactams (Jenkins et al., 2009; Meng et al., 2011; Whitaker et al., 2011), a cyclic imide (Meng et al., 2007), and tolmetin AG (Ding et al., 1995), although not benoxaprofen AG without reductive imine-adduct stabilization (Qiu et al., 1998). AG of diclofenac, benoxaprofen, and tolmetin modify, respectively, three, two, and four lysines detectably without stabilization (Table 2). Lys195 and Lys199 (adducted in four patients) lie at the wide and flexible entrance to HSA drug site 1, a predominantly apolar ligand binding pocket (Ghuman et al., 2005) where the regioisomers of diflunisal AG react selectively (Williams and Dickinson, 1994). They and Lys432 were the only residues glycated detectably in patients. Lys414, in HSA site 2, diclofenac’s high-affinity binding site (Chamouard et al., 1985), is modified by the cyclic imide but not by diclofenac AG or benzylpenicillin. Lys199 and Lys414, but neither Lys195 nor Lys432, are glycated spontaneously by glucose (Barnaby et al., 2011) via the same pathway of reversible imine formation and slow, cumulative, Amadori rearrangement. Diclofenac AG glycations were identified notwithstanding incubations did not contain cyanoborohydride, which reduces imines and enhances binding of AG, including diclofenac AG (Kenny et al., 2004), to HSA (Smith et al., 1990). HSA adducts of diclofenac AG are unstable at pH 7.4 (Ebner et al., 1999). Stabilizing additives were excluded to avoid compromising prediction of HSA’s glycations in patients. Contrarily, even without additives, five of the eight consistent glycations were not detected in patients. Detection of six from seven reproducible N-acylations suggests, hypothetically, a relatively low abundance of AG isomers or greater instability of glycation adducts in vivo. Although C-3 and C-4 lysyl aldimines of AG might stabilize via rearrangements (Fig. 1), and this has been proposed (Smith et al., 1990; Ding et al., 1993), no experimental confirmation is available. Hydroxyimine and ketoamine peptide adducts are in equilibrium (Acharya and Sussman, 1984) and therefore probably indistinguishable by LC-MS/MS methods. Because progressive acyl migrations, cyclization, hydrolysis, and even back migrations (Johnson et al., 2008), might occur after adduction, the glycation adducts are prospectively a complex, dynamic, set of regioisomeric structures.
The N-acyl adducts could have derived additionally and independently from the minor glutathione and CoA thioesters of diclofenac formed in human hepatocytes (Grillo et al., 2003), other NSAID thioesters acylate HSA in vitro (Skonberg et al., 2008). Consequently acylations by multiple metabolites as well as glycations might occur before and after HSA is transported to the blood. However, unlike AG, highly reactive S-acyl metabolites are improbable plasma constituents. The known susceptibility of a thioester prodrug to biochemical hydrolysis implies any diclofenac thioester entering the circulation will be degraded in the same way (Bentley et al., 2012). In fact, in rat liver homogenate, diclofenac does not undergo acyl-CoA–dependent covalent binding to protein (Hargus et al., 1994).
The adduction pathways of AG are now better characterized, but relative extents of acylation versus glycation and of glycation by individual regioisomers remain uncertain. Oxaprozin AG modifies HSA only through transacylation (Ruelius et al., 1986). Five NSAID AG modify insulin only through glycation (Liu et al., 1998). Whereas HSA transacylation was effected principally by the diclofenac 1-β AG, the rank order of binding of diflunisal AGs to HSA is C-4 > >C-3 > C-2 > C-1 (Dickinson and King, 1991). High reactivity of diclofenac C-4 AG would, however, be counterbalanced by the isomer’s lower abundance. In contrast, (S)-naproxen C-2 AG modifies HSA slower than the 1β-conjugate (Iwaki et al., 1999). Apparently, the balance of acylation versus glycation differs substantially between AG and proteins. Further analyses might expand the diclofenac adduct inventory by finding structures derived from hydroxydiclofenac AG (Kumar et al., 2002) and cytochrome P450–generated metabolites (Boerma et al., 2012).
Although HSA was chosen for adduct analysis because of abundance, experience suggests it will be the principal target in vivo. Several undefined rat plasma proteins are adducted by zomepirac and diflunisal, but the major target is albumin (Bailey and Dickinson, 1996). Ketoprofen AG reacts selectively with HSA in vitro (Dubois et al., 1993), without detectable adduction of fibrinogen and γ-globulins and only low-level binding to α- and β-globulins. HSA adducts were found in every patient notwithstanding diclofenac AG underwent rapid hydrolysis in plasma ex vivo and even when the glucuronide was undetectable in plasma. Five of eight lysines modified reproducibly in vitro at high AG:HSA molar ratios were also modified in two or more patients, one in all of them. Because the patients received diclofenac daily for ≥12 months, adduct levels will probably have stabilized; t1/2 of plasma protein adducts of carboxylate drugs in humans are approximately 5–13.5 days (Zia-Amirhosseini et al., 1994).
We have obtained direct evidence of multiple, variable, drug-derived protein modifications in diclofenac patients without adverse reactions. However, studies on β-lactams have established that albumin adduction per se is insufficient to induce drug hypersensitivity (Jenkins et al., 2009; Meng et al., 2011; Whitaker et al., 2011). Induction of an immune response is a function of the chemistry of the drug and the biology of the patient. Moreover, multiple pathways have been proposed for initiation of hypersensitivity, including haptenation, pharmacological interaction, and altered self-peptide presentation (Louis-Dit-Sully and Schamel, 2014). Therefore, to determine the clinical consequences of AG-protein adduct formation, it will be necessary to test T cells and antibodies from hypersensitive and nonhypersensitive patients for recognition of the epitopes defined in this study. Such studies are essential to confirm or dispel the notion that AG are metabolite alerts for idiosyncratic toxicity and should be considered a liability when identified during drug discovery.
Supplementary Material
Acknowledgments
The authors thank Marie-Josephe Pradere, Research Nurse, Department of Rheumatology, Nottingham University Hospitals NHS Trust, for identifying eligible patients, drawing blood samples, and undertaking numerous administrative duties associated with clinic aspects of the study; Rawinder Banwait and Melanie Lingaya, Research Technicians, NIHR Nottingham Digestive Diseases-Biomedical Research Unit, Nottingham University Hospitals NHS Trust and University of Nottingham, for additional handling of the plasma samples from diclofenac patients; Emma J. Williams, Steve Swallow, and the Flexi Chemistry Team, Alderley Park, AstraZeneca U.K., for contributing to the synthesis of diclofenac acyl glucuronide; Claire Prince, Research Nurse, The Wolfson Centre for Personalised Medicine, Department of Molecular and Clinical Pharmacology, University of Liverpool, for blood collections from healthy volunteers; and Laura Dickinson, Department of Molecular and Clinical Pharmacology, for advice on WinNonlin analyses. The authors thank Prof. Ann K. Daly, Institute of Cellular Medicine, Newcastle University, Newcastle upon Tyne, UK; James E. Sidaway, AstraZeneca U.K., Safety Assessment; and Ian D. Wilson, Drug Metabolism and Pharmacokinetics IM, Alderley Park, AstraZeneca U.K., for their advice and assistance. The authors are indebted to Pfizer Global Research and Development, U.K., and AstraZeneca for gifts of mass spectrometric (API 2000) and chromatographic equipment, respectively.
Abbreviations
- AG
acyl glucuronide
- HSA
human serum albumin
- LC
liquid chromatography
- MRM
multiple reaction monitoring
- MS
mass spectrometry
- NSAIDs
nonsteroidal anti-inflammatory drugs
Authorship Contributions
Participated in research design: Hammond, Meng, Jenkins, Maggs, Aithal, Pande, Kenna, Stachulski, Park, Williams.
Conducted experiments: Hammond, Meng, Jenkins, Maggs, Santoyo Castelazo, Regan, Earnshaw.
Contributed new reagents or analytic tools: Meng, Jenkins, Bennett.
Performed data analysis: Hammond, Meng, Jenkins, Maggs, Pande.
Wrote or contributed to the writing of the manuscript: Hammond, Meng, Jenkins, Maggs, Bennett, Aithal, Pande, Kenna, Stachulski, Park, Williams.
Footnotes
This work was undertaken principally through a CASE studentship awarded to T.G.H. that was funded by the BBSRC (Integrative Mammalian Biology award) and Safety Assessment U.K., AstraZeneca U.K. Ltd, as part of the Centre for Drug Safety Science supported by the Medical Research Council [Gant G0700654]. X.M. was funded by the NIHR Biomedical Research Centre in Microbial Diseases; S.L.R. by Safety Assessment U.K., AstraZeneca U.K. Ltd; and C.J.E. by the MRC Centre for Drug Safety Science.
Some of the data in this article were published previously in the form of conference abstracts: Hammond T, Regan S, Meng X, Jenkins R, Kenna G, Sathish J, and Williams D (2009) An investigation into the in vitro and in vivo fate of acyl glucuronides. British Pharmacology Society Winter Meeting; 2009 Dec 15–17; London, UK. British Pharmacology Society; http://www.pA2online.org/abstracts/Vol7Issue4abst074P.pdf; Hammond TG, Regan SL, Meng X, Maggs JL, Jenkins RE, Kenna GL, Sathish JG, Williams DP, and Park BK (2010) Protein binding and pharmacokinetics of reactive acyl glucuronide drug metabolites, in Toxicology; 2010 28–31 Mar; Edinburgh, Scotland. Vol 278, pp 357–358, British Toxicology Society Annual Congress; Hammond T, Regan S, Meng X, Berry N, Maggs J, Jenkins R, Kenna G, Sathish J, Williams D, and Park K (2011) In vitro assessment of the interactions between acyl glucuronide drug metabolites and human serum albumin. British Pharmacology Society Winter Meeting; 2010 14–16 Dec; London, UK. British Pharmacology Society; http://www.pA2online.org/abstracts/Vol8Issue1abst019P.pdf; Hammond TG, Regan SL, Meng X, Maggs JL, Jenkins RE, Kenna JG, Sathish JG, Williams DP, and Park BK (2011) In vitro assessment of the interactions between diclofenac and tolmetin acyl glucuronide drug metabolites and human serum albumin, in Toxicology; 2011 27–30 Mar; Durham, UK. Vol 290, pp 40–41. British Toxicology Society Annual Congress.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Acharya AS, Sussman LG. (1984) The reversibility of the ketoamine linkages of aldoses with proteins. J Biol Chem 259:4372–4378 [PubMed] [Google Scholar]
- Aithal GP, Ramsay L, Daly AK, Sonchit N, Leathart JBS, Alexander G, Kenna JG, Caldwell J, Day CP. (2004) Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology 39:1430–1440 [DOI] [PubMed] [Google Scholar]
- Bailey MJ, Dickinson RG. (1996) Chemical and immunochemical comparison of protein adduct formation of four carboxylate drugs in rat liver and plasma. Chem Res Toxicol 9:659–666 [DOI] [PubMed] [Google Scholar]
- Barnaby OS, Cerny RL, Clarke W, Hage DS. (2011) Comparison of modification sites formed on human serum albumin at various stages of glycation. Clin Chim Acta 412:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet LZ, Spahn-Langguth H, Iwakawa S, Volland C, Mizuma T, Mayer S, Mutschler E, Lin ET. (1993) Predictability of the covalent binding of acidic drugs in man. Life Sci 53:PL141–PL146 [DOI] [PubMed] [Google Scholar]
- Bentley D, Young AM, Rowell L, Gross G, Tardio J, Carlile D. (2012) Evidence of a drug-drug interaction linked to inhibition of ester hydrolysis by orlistat. J Cardiovasc Pharmacol 60:390–396 [DOI] [PubMed] [Google Scholar]
- Boerma JS, Dragovic S, Vermeulen NPE, Commandeur JNM. (2012) Mass spectrometric characterization of protein adducts of multiple P450-dependent reactive intermediates of diclofenac to human glutathione-S-transferase P1-1. Chem Res Toxicol 25:2532–2541 [DOI] [PubMed] [Google Scholar]
- Bowkett ER, Harding JR, Maggs JL, Park BK, Perrie JA, Stachulski AV. (2007) Efficient synthesis of 1β-O-acyl glucuronides via selective acylation of allyl or benzyl D-glucuronate. Tetrahedron 63:7596–7605 [Google Scholar]
- Castillo M, Lam YWF, Dooley MA, Stahl E, Smith PC. (1995) Disposition and covalent binding of ibuprofen and its acyl glucuronide in the elderly. Clin Pharmacol Ther 57:636–644 [DOI] [PubMed] [Google Scholar]
- Chamouard JM, Barre J, Urien S, Houin G, Tillement JP. (1985) Diclofenac binding to albumin and lipoproteins in human serum. Biochem Pharmacol 34:1695–1700 [DOI] [PubMed] [Google Scholar]
- Crook PR, Willis JV, Kendall MJ, Jack DB, Fowler PD. (1982) The pharmacokinetics of diclofenac sodium in patients with active rheumatoid disease. Eur J Clin Pharmacol 21:331–334 [DOI] [PubMed] [Google Scholar]
- Dickinson RG, King AR. (1991) Studies on the reactivity of acyl glucuronides—II. Interaction of diflunisal acyl glucuronide and its isomers with human serum albumin in vitro. Biochem Pharmacol 42:2301–2306 [DOI] [PubMed] [Google Scholar]
- Ding A, Ojingwa JC, McDonagh AF, Burlingame AL, Benet LZ. (1993) Evidence for covalent binding of acyl glucuronides to serum albumin via an imine mechanism as revealed by tandem mass spectrometry. Proc Natl Acad Sci USA 90:3797–3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A, Zia-Amirhosseini P, McDonagh AF, Burlingame AL, Benet LZ. (1995) Reactivity of tolmetin glucuronide with human serum albumin. Identification of binding sites and mechanisms of reaction by tandem mass spectrometry. Drug Metab Dispos 23:369–376 [PubMed] [Google Scholar]
- Dockens RC, Santone KS, Mitroka JG, Morrison RA, Jemal M, Greene DS, Barbhaiya RH. (2000) Disposition of radiolabeled ifetroban in rats, dogs, monkeys, and humans. Drug Metab Dispos 28:973–980 [PubMed] [Google Scholar]
- Dubois N, Lapicque F, Maurice MH, Pritchard M, Fournel-Gigleux S, Magdalou J, Abiteboul M, Siest G, Netter P. (1993) In vitro irreversible binding of ketoprofen glucuronide to plasma proteins. Drug Metab Dispos 21:617–623 [PubMed] [Google Scholar]
- Ebner T, Heinzel G, Prox A, Beschke K, Wachsmuth H. (1999) Disposition and chemical stability of telmisartan 1-O-acylglucuronide. Drug Metab Dispos 27:1143–1149 [PubMed] [Google Scholar]
- Galmier MJ, Bouchon B, Madelmont JC, Mercier F, Pilotaz F, Lartigue C. (2005) Identification of degradation products of diclofenac by electrospray ion trap mass spectrometry. J Pharm Biomed Anal 38:790–796 [DOI] [PubMed] [Google Scholar]
- Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S. (2005) Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol 353:38–52 [DOI] [PubMed] [Google Scholar]
- Greenough C, Jenkins RE, Kitteringham NR, Pirmohamed M, Park BK, Pennington SR. (2004) A method for the rapid depletion of albumin and immunoglobulin from human plasma. Proteomics 4:3107–3111 [DOI] [PubMed] [Google Scholar]
- Grillo MP, Hua F, Knutson CG, Ware JA, Li C. (2003) Mechanistic studies on the bioactivation of diclofenac: identification of diclofenac-S-acyl-glutathione in vitro in incubations with rat and human hepatocytes. Chem Res Toxicol 16:1410–1417 [DOI] [PubMed] [Google Scholar]
- Hargus SJ, Amouzedeh HR, Pumford NR, Myers TG, McCoy SC, Pohl LR. (1994) Metabolic activation and immunochemical localization of liver protein adducts of the nonsteroidal anti-inflammatory drug diclofenac. Chem Res Toxicol 7:575–582 [DOI] [PubMed] [Google Scholar]
- Hargus SJ, Martin BM, George JW, Pohl LR. (1995) Covalent modification of rat liver dipeptidyl peptidase IV (CD26) by the nonsteroidal anti-inflammatory drug diclofenac. Chem Res Toxicol 8:993–996 [DOI] [PubMed] [Google Scholar]
- Hermening A, Gräfe AK, Baktir G, Mutschler E, Spahn-Langguth H. (2000) Gemfibrozil and its oxidative metabolites: quantification of aglycones, acyl glucuronides, and covalent adducts in samples from preclinical and clinical kinetic studies. J Chromatogr B Biomed Sci Appl 741:129–144 [DOI] [PubMed] [Google Scholar]
- Hyneck ML, Smith PC, Munafo A, McDonagh AF, Benet LZ. (1988) Disposition and irreversible plasma protein binding of tolmetin in humans. Clin Pharmacol Ther 44:107–114 [DOI] [PubMed] [Google Scholar]
- Iwaki M, Ogiso T, Inagawa S, Kakehi K. (1999) In vitro regioselective stability of β-1-O- and 2-O-acyl glucuronides of naproxen and their covalent binding to human serum albumin. J Pharm Sci 88:52–57 [DOI] [PubMed] [Google Scholar]
- Jenkins RE, Meng X, Elliott VL, Kitteringham NR, Pirmohamed M, Park BK. (2009) Characterisation of flucloxacillin and 5-hydroxymethyl flucloxacillin haptenated HSA in vitro and in vivo. Proteomics Clin Appl 3:720–729 [DOI] [PubMed] [Google Scholar]
- Jerić I, Versluis C, Horvat S, Heck AJR. (2002) Tracing glycoprotein structures: electrospray ionization tandem mass spectrometric analysis of sugar-peptide adducts. J Mass Spectrom 37:803–811 [DOI] [PubMed] [Google Scholar]
- Johnson CH, Athersuch TJ, Wilson ID, Iddon L, Meng X, Stachulski AV, Lindon JC, Nicholson JK. (2008) Kinetic and J-resolved statistical total correlation NMR spectroscopy approaches to structural information recovery in complex reacting mixtures: application to acyl glucuronide intramolecular transacylation reactions. Anal Chem 80:4886–4895 [DOI] [PubMed] [Google Scholar]
- Karlsson ES, Johnson CH, Sarda S, Iddon L, Iqbal M, Meng X, Harding JR, Stachulski AV, Nicholson JK, Wilson ID, et al. (2010) High-performance liquid chromatography/mass spectrometric and proton nuclear magnetic resonance spectroscopic studies of the transacylation and hydrolysis of the acyl glucuronides of a series of phenylacetic acids in buffer and human plasma. Rapid Commun Mass Spectrom 24:3043–3051 [DOI] [PubMed] [Google Scholar]
- Kenny JR, Maggs JL, Meng X, Sinnott D, Clarke SE, Park BK, Stachulski AV. (2004) Syntheses and characterization of the acyl glucuronide and hydroxy metabolites of diclofenac. J Med Chem 47:2816–2825 [DOI] [PubMed] [Google Scholar]
- Kretz-Rommel A, Boelsterli UA. (1993) Diclofenac covalent protein binding is dependent on acyl glucuronide formation and is inversely related to P450-mediated acute cell injury in cultured rat hepatocytes. Toxicol Appl Pharmacol 120:155–161 [DOI] [PubMed] [Google Scholar]
- Kretz-Rommel A, Boelsterli UA. (1994) Mechanism of covalent adduct formation of diclofenac to rat hepatic microsomal proteins. Retention of the glucuronic acid moiety in the adduct. Drug Metab Dispos 22:956–961 [PubMed] [Google Scholar]
- Kumar S, Samuel K, Subramanian R, Braun MP, Stearns RA, Chiu SHL, Evans DC, Baillie TA. (2002) Extrapolation of diclofenac clearance from in vitro microsomal metabolism data: role of acyl glucuronidation and sequential oxidative metabolism of the acyl glucuronide. J Pharmacol Exp Ther 303:969–978 [DOI] [PubMed] [Google Scholar]
- Liu JH, Marquez CD, Weintraub ST, Smith PC. (1998) Reaction of acyl glucuronides with insulin in vitro: identification of an imine mechanism by electrospray ionization mass spectrometry. Pharm Res 15:343–346 [DOI] [PubMed] [Google Scholar]
- Louis-Dit-Sully C, Schamel WWA. (2014) Activation of the TCR complex by small chemical compounds, in T Lymphocytes as Tools in Diagnostics and Immunotoxicology (Martin SF, ed) pp 25–39, Springer, Basel: [DOI] [PubMed] [Google Scholar]
- Masubuchi N, Makino C, Murayama N. (2007) Prediction of in vivo potential for metabolic activation of drugs into chemically reactive intermediate: correlation of in vitro and in vivo generation of reactive intermediates and in vitro glutathione conjugate formation in rats and humans. Chem Res Toxicol 20:455–464 [DOI] [PubMed] [Google Scholar]
- Matthews CZ, Woolf EJ. (2008) Determination of a novel gamma-secretase inhibitor in human plasma and cerebrospinal fluid using automated 96 well solid phase extraction and liquid chromatography/tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 863:36–45 [DOI] [PubMed] [Google Scholar]
- Mazzuchin A, Walton RJ, Thibert RJ. (1971) Determination of total and conjugated glucuronic acid in serum and urine employing a modified naphthoresorcinol reagent. Biochem Med 5:135–157 [DOI] [PubMed] [Google Scholar]
- Meng X, Jenkins RE, Berry NG, Maggs JL, Farrell J, Lane CS, Stachulski AV, French NS, Naisbitt DJ, Pirmohamed M, et al. (2011) Direct evidence for the formation of diastereoisomeric benzylpenicilloyl haptens from benzylpenicillin and benzylpenicillenic acid in patients. J Pharmacol Exp Ther 338:841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Maggs JL, Pryde DC, Planken S, Jenkins RE, Peakman TM, Beaumont K, Kohl C, Park BK, Stachulski AV. (2007) Cyclization of the acyl glucuronide metabolite of a neutral endopeptidase inhibitor to an electrophilic glutarimide: synthesis, reactivity, and mechanistic analysis. J Med Chem 50:6165–6176 [DOI] [PubMed] [Google Scholar]
- Nicholson JP, Wolmarans MR, Park GR. (2000) The role of albumin in critical illness. Br J Anaesth 85:599–610 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Burlingame AL, Benet LZ. (1998) Mechanisms for covalent binding of benoxaprofen glucuronide to human serum albumin. Studies By tandem mass spectrometry. Drug Metab Dispos 26:246–256 [PubMed] [Google Scholar]
- Regan SL, Maggs JL, Hammond TG, Lambert C, Williams DP, Park BK. (2010) Acyl glucuronides: the good, the bad and the ugly. Biopharm Drug Dispos 31:367–395 [DOI] [PubMed] [Google Scholar]
- Ruelius HW, Kirkman SK, Young EM, Janssen FW. (1986) Reactions of oxaprozin-1-O-acyl glucuronide in solutions of human plasma and albumin. Adv Exp Med Biol 197:431–441 [DOI] [PubMed] [Google Scholar]
- Sallustio BC, Fairchild BA, Pannall PR. (1997) Interaction of human serum albumin with the electrophilic metabolite 1-O-gemfibrozil-β-D-glucuronide. Drug Metab Dispos 25:55–60 [PubMed] [Google Scholar]
- Sawamura R, Okudaira N, Watanabe K, Murai T, Kobayashi Y, Tachibana M, Ohnuki T, Masuda K, Honma H, Kurihara A, et al. (2010) Predictability of idiosyncratic drug toxicity risk for carboxylic acid-containing drugs based on the chemical stability of acyl glucuronide. Drug Metab Dispos 38:1857–1864 [DOI] [PubMed] [Google Scholar]
- Schiffer R, Neis M, Höller D, Rodríguez F, Geier A, Gartung C, Lammert F, Dreuw A, Zwadlo-Klarwasser G, Merk H, et al. (2003) Active influx transport is mediated by members of the organic anion transporting polypeptide family in human epidermal keratinocytes. J Invest Dermatol 120:285–291 [DOI] [PubMed] [Google Scholar]
- Skonberg C, Olsen J, Madsen KG, Hansen SH, Grillo MP. (2008) Metabolic activation of carboxylic acids. Expert Opin Drug Metab Toxicol 4:425–438 [DOI] [PubMed] [Google Scholar]
- Smith PC, Benet LZ, McDonagh AF. (1990) Covalent binding of zomepirac glucuronide to proteins: evidence for a Schiff base mechanism. Drug Metab Dispos 18:639–644 [PubMed] [Google Scholar]
- Sparidans RW, Lagas JS, Schinkel AH, Schellens JHM, Beijnen JH. (2008) Liquid chromatography-tandem mass spectrometric assay for diclofenac and three primary metabolites in mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci 872:77–82 [DOI] [PubMed] [Google Scholar]
- Stachulski AV, Harding JR, Lindon JC, Maggs JL, Park BK, Wilson ID. (2006) Acyl glucuronides: biological activity, chemical reactivity, and chemical synthesis. J Med Chem 49:6931–6945 [DOI] [PubMed] [Google Scholar]
- Stepan AF, Walker DP, Bauman J, Price DA, Baillie TA, Kalgutkar AS, Aleo MD. (2011) Structural alert/reactive metabolite concept as applied in medicinal chemistry to mitigate the risk of idiosyncratic drug toxicity: a perspective based on the critical examination of trends in the top 200 drugs marketed in the United States. Chem Res Toxicol 24:1345–1410 [DOI] [PubMed] [Google Scholar]
- Stierlin H, Faigle JW. (1979) Biotransformation of diclofenac sodium (Voltaren) in animals and in man. II. Quantitative determination of the unchanged drug and principal phenolic metabolites, in urine and bile. Xenobiotica 9:611–621 [DOI] [PubMed] [Google Scholar]
- Unwin RD, Griffiths JR, Leverentz MK, Grallert A, Hagan IM, Whetton AD. (2005) Multiple reaction monitoring to identify sites of protein phosphorylation with high sensitivity. Mol Cell Proteomics 4:1134–1144 [DOI] [PubMed] [Google Scholar]
- Veering BT, Burm AGL, Souverijn JHM, Serree JMP, Spierdijk J. (1990) The effect of age on serum concentrations of albumin and α1-acid glycoprotein. Br J Clin Pharmacol 29:201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade LT, Kenna JG, Caldwell J. (1997) Immunochemical identification of mouse hepatic protein adducts derived from the nonsteroidal anti-inflammatory drugs diclofenac, sulindac, and ibuprofen. Chem Res Toxicol 10:546–555 [DOI] [PubMed] [Google Scholar]
- Whitaker P, Meng X, Lavergne SN, El-Ghaiesh S, Monshi M, Earnshaw C, Peckham D, Gooi J, Conway S, Pirmohamed M, et al. (2011) Mass spectrometric characterization of circulating and functional antigens derived from piperacillin in patients with cystic fibrosis. J Immunol 187:200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Dickinson RG. (1994) Studies on the reactivity of acyl glucuronides—VI. Modulation of reversible and covalent interaction of diflunisal acyl glucuronide and its isomers with human plasma protein in vitro. Biochem Pharmacol 47:457–467 [DOI] [PubMed] [Google Scholar]
- Willis JV, Kendall MJ, Jack DB. (1981) The influence of food on the absorption of diclofenac after single and multiple oral doses. Eur J Clin Pharmacol 19:33–37 [DOI] [PubMed] [Google Scholar]
- Zhang D, Raghavan N, Wang L, Xue Y, Obermeier M, Chen S, Tao S, Zhang H, Cheng PT, Li W, et al. (2011) Plasma stability-dependent circulation of acyl glucuronide metabolites in humans: how circulating metabolite profiles of muraglitazar and peliglitazar can lead to misleading risk assessment. Drug Metab Dispos 39:123–131 [DOI] [PubMed] [Google Scholar]
- Zia-Amirhosseini P, Ojingwa JC, Spahn-Langguth H, McDonagh AF, Benet LZ. (1994) Enhanced covalent binding of tolmetin to proteins in humans after multiple dosing. Clin Pharmacol Ther 55:21–27 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.