Abstract
Background
Thyroidectomized patients need variable doses of levothyroxine (LT4) to obtain target thyroid-stimulating hormone (TSH) levels. Individual feedback set-points have been hypothesized and the influence of several genes in the regulation of the pituitary-thyroid axis has been demonstrated.
Objectives
We hypothesized that genetic variants of the TRHR gene could be associated with a different hypothalamo-pituitary sensitivity to thyroid hormone feedback.
Methods
We retrospectively analyzed 84 thyroidectomized patients with no residual thyroid function and undetectable thyroglobulin levels. Patients were evaluated under LT4 resulting in TSH levels detectable but <0.5 μIU/ml. The two SNPs rs3134105 and rs3110040 were identified as informative markers of the TRHR gene. Genotyping was performed using high-resolution melting technology. Genotype distribution was compared between the patients and 99 euthyroid controls.
Results
The selected SNPs were in linkage disequilibrium and only rs3134105 was further considered. A significant difference between the three possible genotypes for rs3134105 was found for TSH (p = 0.04) and free thyroxine (fT4)/TSH ratio (p = 0.02). Moreover, despite similar serum concentrations of free triiodothyronine (fT3) and fT4, carriers of at least one A allele of rs3134105 had significantly lower serum TSH levels (p = 0.01) as well as higher fT3/TSH (p = 0.01) and fT4/TSH ratios (p < 0.01).
Conclusions
We demonstrated an association between serum TSH levels and discrete alleles of the TRHR gene in totally thyroidectomized patients under LT4 therapy. Therefore, the TRHR gene seems to be a determinant of hypothalamo-pituitary sensitivity to LT4.
Key Words: TRHR, Hypothalamo-pituitary sensitivity, TSH suppression
Introduction
Clinical experience suggests that the replacement dose of levothyroxine (LT4) in thyroidectomized patients is widely variable [1] and defined, in clinical practice, considering the levels of thyroid-stimulating hormone (TSH) and free thyroxine (fT4) [2].
Many factors are involved in thyroid hormone requirement [3], such as age [4], body weight [5], residual thyroid function [6], timing of LT4 administration [7], food and beverages [8], concomitant medications [9], high-fiber intake [10], gastrointestinal diseases [11] and, finally, patient compliance [12]. The mostly used approach to define appropriate LT4 dose, especially in athyreotic subjects, is to calculate the starting dosage based on body weight [13] and then to refine according to biochemical controls: each patient needs different doses of LT4 to obtain TSH suppression [14].
Besides these factors influencing directly levels of free triiodothyronine (fT3) and fT4, a predetermined ‘thyroid function set-point’ has been hypothesized so that the individual genetic background influences the regulation of the hypothalamo-pituitary-thyroid (HPT) axis [15]. Because of the absence of residual thyroid function, thyroidectomized patients represent a good model to study determinants of hypothalamo-pituitary sensitivity (HPs) to LT4. Individual differences in LT4 requirements could be justified by the presence of genetic variants, such as single nucleotide polymorphisms (SNPs), in the genes involved in the determination of thyroid hormone levels. Several genes of the HPT axis have been analyzed in relation to the thyroid function set-point: thyroid-stimulating hormone receptor [16], iodothyronine deiodinase 1 [17,18], iodothyronine deiodinase 2 [17,18,19,20,21], iodothyronine deiodinase 3 [22], monocarboxylate transporter 8 [23], monocarboxylate transporter 10 [24], several members of the organic anion transporting polypeptide family [24], thyroxine-binding globulin [25] and thyroid hormone receptors α and β [22,26].
In this study, we selected the TRHR gene, which was neglected so far in association studies, in spite of its biological function in HPT axis regulation. Thyroid-releasing hormone (TRH) produced in the hypothalamus stimulates biosynthesis and secretion of TSH from the pituitary [27], which in turn stimulates biosynthesis of thyroid hormones [28]. The maintenance of euthyroidism is dependent on TRH regulation of TSH synthesis and secretion [27] as well as on feedback regulation by thyroid hormones, suppressing preproTRH expression and TSH secretion [29].
We hypothesized that genetic variants of TRHR could be associated with the HPs to the thyroid hormone-negative feedback. To investigate this, we assessed selected SNPs in the genomic region encompassing the TRHR gene, using thyroidectomized patients treated with LT4 as a model. We selected two informative markers according to the current recommendations for genetic case-control association studies [30]: this approach allows to test the association between the chosen gene (TRHR) and the clinical parameter (HPs to thyroid hormones) without assuming any causal role for the selected SNPs.
Materials and Methods
Ethics Statement
Written informed consent was obtained from all participants and the study protocol was approved by the local Ethics Committee of Modena (No. 122/08). No minors/children participants were involved in the study.
Subjects
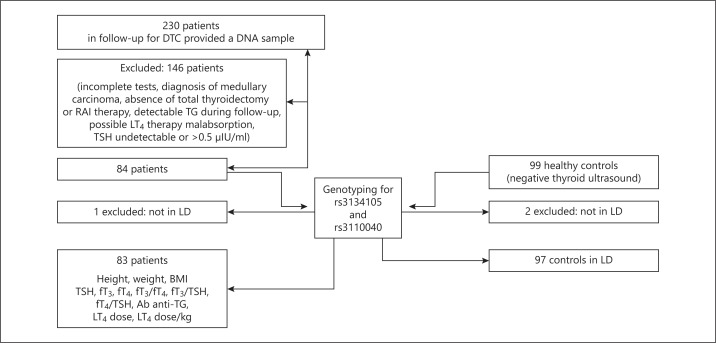
With a retrospective study design (fig. 1), we selected 230 patients (161 females, 69 males) recruited among outpatients attending the endocrinological oncology unit, who agreed to donate a DNA sample for retrospective association studies on differentiated thyroid cancer (DTC). 99 volunteers (61 females, 38 males) served as controls in order to exclude the association between genotype and occurrence of thyroid cancer.
Fig. 1.
Study design of 230 patients recruited attending the endocrinological oncology unit.
Clinical data of the patients were collected using the institutional database Endobase, built and modified from Androbase [31], using an open-source technology which allows the extraction of experimental data ready to be analyzed.
Inclusion criteria were: Caucasian ethnicity, total thyroidectomy for thyroid carcinoma followed by radioactive iodine (RAI) therapy, undetectable thyroglobulin (TG) during follow-up. TSH values were detectable but <0.5 μIU/ml, according to the target suggested for patients considered at low risk of DTC recurrence or at high risk but with a negative follow-up for at least 5 years [32,33].
Exclusion criteria were: gastrointestinal diseases, medications interfering with LT4 absorption/metabolism and medullary carcinoma.
According to the inclusion criteria, the selected patients should have no residual thyroid function and their thyroid hormones levels should be considered secondary to LT4 exogenous therapy. 84 patients (55 females, 29 males) were considered eligible.
All patients underwent physical examination (height, weight and body mass index (BMI)) and blood sampling at 08:00 a.m., before LT4 tablet ingestion, in order to measure TSH, fT3, fT4, TG and anti-TG antibodies (Ab anti-TG). They were genotyped for rs3134105 and rs3110040 of the TRHR. One patient was excluded because the two SNPs were not in linkage disequilibrium (LD): the final patient group consisted of 83 subjects.
Controls were selected among Caucasian volunteers recruited as a control group for a case-control study on thyroid cancer. None of the participants had a personal history of thyroid disease and had ever been exposed to ionizing radiations. They presented normal thyroid ultrasounds and no signs of dysthyroidism. Thyroid hormone levels were not measured in this group. They underwent genotyping for rs3134105 and rs3110040: 2 subjects were excluded because the two SNPs were not in LD. The final control group consisted of 97 subjects.
Criteria for SNPs Selection
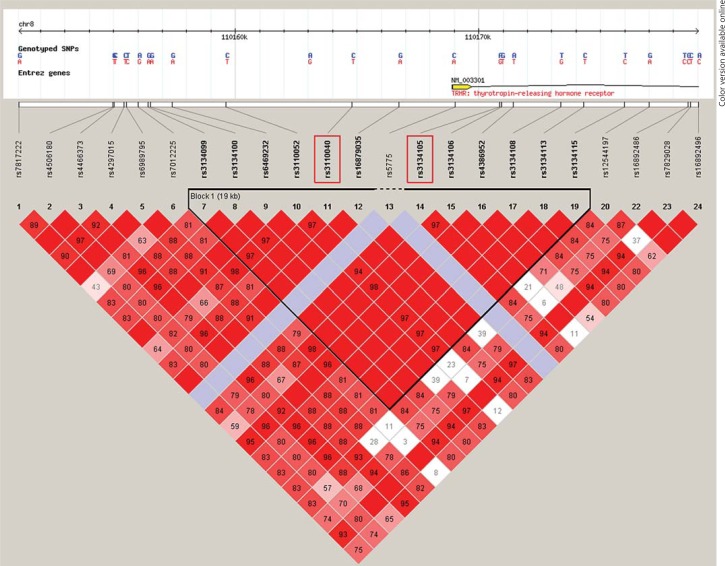
The genomic region corresponding to the TRHR gene was analyzed using the online database ‘HapMap’ (http://hapmap.ncbi.nlm.nih.gov/) to select the SNPs to be used as markers. We identified a genomic region of ∼20 kb encompassing the promoter, the first two exons and part of intron 2 of TRHR, which was contained in one large LD block (fig. 2). We then performed a frequency analysis of the 13 mapped SNPs, searching those with frequency close to 50% and not located in a region of low complexity. Using the Haploview software (Harvard University and Massachusetts Institute of Technology, Mass., USA), we performed a linkage analysis to verify that target SNPs were within a LD block [30,34]. After calculation of the minor allele frequency (MAF) and degree of allelic correlation between LD blocks [30], we finally selected rs3134105 and rs3110040 as the haplotype markers.
Fig. 2.
The chosen LD block in the genomic region encompassing a TRHR-relevant portion. This figure shows rs3134105 and rs3110040 (red-rimmed) and their location. The framed triangle defines the LD block identified by Haploview. Red squares represent strong LD (100%). Red tone and LD percentage (numbers in the squares) decrease with reduction in LD strength.
The TRHR gene is located at chromosome 8q23.1, spanning 35 kb. The first selected SNP, rs3134105, is located at about +2,000 bp in the second intron (between the second and third exon), while rs3110040 is located at about −4,000 bp upstream of the transcriptional start site. Moreover, rs3134105 presents a MAF = 39% for C allele, while rs3110040 presents a MAF = 40% for A allele. Given these characteristics the two SNPs were expected to be informative tags of two frequent alleles (≅40%) within our study population.
Laboratory Analyses
The hormonal data were collected from patients referred from the province and coming to the doctor's office with analyses performed in different laboratories. Moreover, different assay methods were used over the years. Considering the retrospective study design, it is not possible to identify with certainty the different assay methods used by the laboratories over the years. However, in all laboratories, the second-generation TSH assays (functional sensitivity: 0.1-0.2 μIU/ml) were abandoned in 2005, in favor of the third-generation assays (functional sensitivity: 0.01-0.02 μIU/ml). In this study, only 8 patients out of 83 obtained a TSH measurement before 2005, i.e. by second-generation assay.
Genomic DNA was purified from total peripheral blood (monocytes and leukocytes) with a Nucleon BACC1 kit (GE Healthcare, Milan, Italy). DNA concentration was quantified with a UV spectrophotometer UV-1601 (Shimadzu, Milan, Italy). Genotyping for the SNPs was performed using high-resolution melting (HRM) technology on a CFX96 real-time PCR detection system (Bio-Rad, Laboratories, Hercules, Calif., USA).
All raw data (preliminary melting curves) of HRM were analyzed with two specific softwares, CFX Manager (Bio-Rad) and Precision Melt Analysis (Bio-Rad). Uncertain results were verified by conventional sequencing using a 4-capillary AB-Prism 3130 Genetic Analyzer (Applied Biosystems, Foster City, Calif., USA).
Statistical Analysis
Non-parametric tests were used for comparisons since most of the variables were not normally distributed (D'Agostino and Pearson normality test). Statistical analysis was performed by a non-parametric Kruskal-Wallis test and Dunn's post-test for multiple comparison or the Mann-Whitney U test where appropriate. Deviation from the Hardy-Weinberg equilibrium was analyzed using χ2 test. p was considered significant when <0.05.
Results
The mean age of patients was 55 ± 14 years (min 18, max 83) and the mean age of controls was 44 ± 14 (min 28, max 80, p < 0.05 vs. patients). Given the perfect LD between the two SNPs, all subsequent analyses refer to rs3134105 only.
83 thyroidectomized patients (55 females and 28 males) were compared to 97 controls to evaluate frequency distribution of the TRHR alleles in the two groups. Patients and controls did not differ for allele frequency of rs3134105 (p = 0.18).
Subjects may be homo- or heterozygous for each allele with the expected frequency: possible genotypes for rs3134105 are AA, AG and GG. The MAF resulted in being 39.39% in patients and 45.92% in controls (p = 0.91). Genotype distribution was not statistically different in patients and controls (p = 0.38), suggesting that allelic variants of the TRHR gene are not involved in DTC pathogenesis. The genotypes of both patients and controls were in Hardy-Weinberg equilibrium (p > 0.05).
The distribution of genotypes (AA+AG vs. GG) between patients tested before (n = 8) and after 2005 (n = 75), year of transition from the second- to the third-generation TSH assay, did not differ (χ2, p = 0.75). The same results were found in relation to gender (χ2, p = 0.27) and age, comparing subjects older and younger than the calculated median age (56 years) (χ2, p = 0.97).
TG was undetectable for all patients. Dividing the patients into three groups according to genotype (table 1), no differences were observed in age (p = 0.84) and age at diagnosis of DTC (p = 0.99). Regarding weight and BMI, there were no differences among the three groups (respectively p = 0.71; p = 0.34). No significant differences were found in serum levels of fT3 (p = 0.59), fT4 (p = 0.16), Ab anti-TG (p = 0.48), fT3/fT4 ratio (p = 0.76) and fT3/TSH ratio (p = 0.05) (fig. 3a). Conversely, a significant difference was found for fT4/TSH ratio (p = 0.02) and TSH (p = 0.04) (fig. 3b, c).
Table 1.
Subjects’ characteristics and distribution according to the genotype of rs3134105
| NR | AA (n = 12) | AG (n = 39) | GG (n = 32) | p value | |
|---|---|---|---|---|---|
| Age at diagnosis, years | NA | 49 (21–72) | 48 (14–72) | 48 (21–75) | 0.9916 |
| Age, years | NA | 60 (32–83) | 54 (18–79) | 55.5 (29–81) | 0.8412 |
| Histology, NA | NA | 11 P; 1 F | 39 P | 26 P, 4 F; 2 H | NA |
| Weight, kg | NA | 78.5 (42–104) | 71 (51.5–135.5) | 69.5 (52–115.5) | 0.7084 |
| BMI, kg/m2 | 18–25 | 25.56 (17.71–40.12) | 25.81 (18.21–42.77) | 25.02 (20.45–33.38) | 0.3368 |
| TSH, μIU/ml | 0.35–4.94 | 0.10 (0.01–0.18) | 0.08 (0.01–0.29) | 0.12 (0.02–0.28) | 0.0411 |
| fT3, pmol/l | 2.6–5.7 | 4.54 (3.23–5.38) | 4.46 (3.38–5.38) | 4.31 (3.38–5.54) | 0.5858 |
| fT4, pmol/l | 9–19.2 | 16.79 (14.74–20) | 16.67 (14.23–22.56) | 15.64 (12.31–24.74) | 0.1604 |
| Ab anti-TG, IU/ml | 0–60 | 19.85 (1–46.90) | 28.40 (1–48.20) | 23.90 (14–29) | 0.4832 |
| LT4 dose, μg/day | NA | 110.71 (64.28–175) | 132.14 (85.71–200) | 103.57 (78.57–225) | 0.1348 |
| LT dose/kg, μg/kg/day | NA | 1.42 (1.12–2.64) | 1.62 (0.93–2.95) | 1.53 (1.22–2.44) | 0.4233 |
| fT3/fT4, NA | NA | 0.28 (0.19–0.32) | 0.27 (0.20–0.34) | 0.27 (0.15–0.38) | 0.7627 |
| fT3/TSH, NA | NA | 57.44 (24.85–369.23) | 55.77 (13.79–523.01) | 34.96 (12.53–200) | 0.0511 |
| fT4/TSH, NA | NA | 200 (81.91–1,525.64) | 224.18 (51.74–1,794.87) | 122.54 (57.20–839.74) | 0.0211 |
Data were analyzed using the Kruskal-Wallis test and Dunn's post-test for multiple comparison. p was considered significant when <0.05. Results are expressed as median (min–max). NR = Normal range; NA = not applicable; AA = homozygotes adenine-adenine; AG = heterozygotes adenine-guanine; GG = homozygotes guanine-guanine; P = papillary; F = follicular; H = Hurtle cells.
Fig. 3.
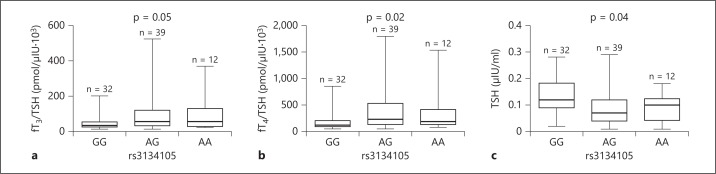
TSH and thyroid hormones/TSH ratios in patient groups subdivided according to the rs3134105 genotype. a fT3/TSH ratio. b fT4/TSH ratio. c TSH. Data were analyzed using the Kruskal-Wallis test and Dunn's post-test for multiple comparison. p was considered significant when <0.05. AA = Homozygotes adenine-adenine; AG = heterozygotes adenine-guanine; GG = homozygotes guanine-guanine.
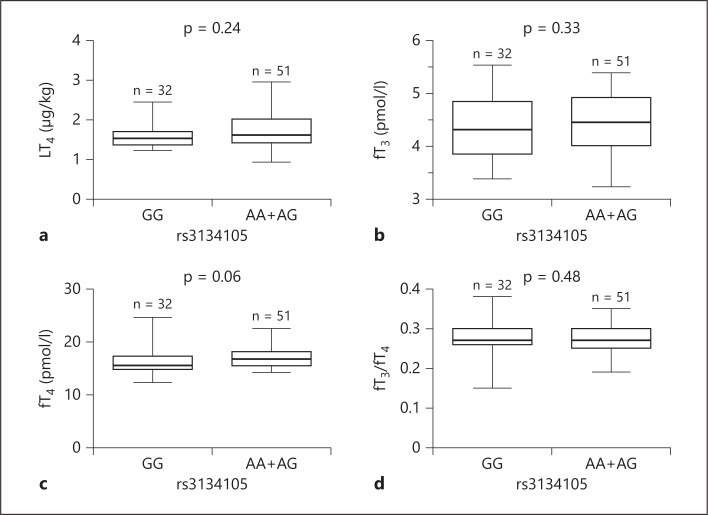
Considering that both fT3/TSH and fT4/TSH were higher in carriers of at least one A allele, we then subdivided patients into two groups, A carriers (genotypes AA/AG) versus non-carriers (genotype GG), reflecting a dominant genetic model. No significant difference was found in LT4 dose (p = 0.24) (fig. 4a), fT3 (p = 0.33) (fig. 4b), fT4 (p = 0.06) (fig. 4c) and in fT3/fT4 (p = 0.48) (fig. 4d). In contrast, A carriers demonstrated significantly lower TSH levels (p = 0.01) (fig. 5a) as well as higher fT3/TSH (p = 0.01) (fig. 5b) and fT4/TSH (p < 0.01) (fig. 5c).
Fig. 4.
Thyroid hormones and LT4 dose/body weight in patient groups subdivided according to the rs3134105 genotype. Subjects are divided into homozygotes GG, homozygotes AA and heterozygotes AG. a LT4 dose. b fT3. c fT4. d fT3/fT4 ratio. Data were analyzed using the Mann-Whitney U test. p was considered significant when <0.05.
Fig. 5.
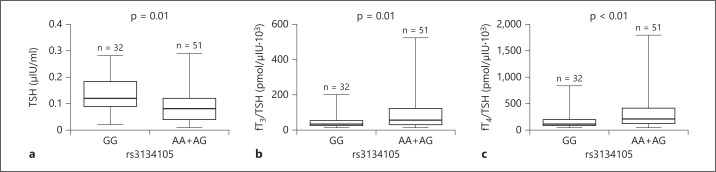
TSH and thyroid hormones/TSH ratios in patient groups subdivided according to the rs3134105 genotype. Subjects are divided into homozygotes GG, homozygotes AA and heterozygotes AG. a TSH. b fT3/TSH ratio. c fT4/TSH ratio. Data were analyzed using the Mann-Whitney U test. p was considered significant when <0.05.
These results suggest that the carriers of at least one A allele of the rs3134105 SNP have significantly lower TSH levels in the presence of the same concentrations of fT3 and fT4, obtained by similar doses of exogenous LT4.
Discussion
This study demonstrates an association between TSH levels and discrete alleles of the TRHR gene, which represents a determinant of HPs to LT4. In order to increase the definition of discrete alleles and to analyze the association with the entire LD block and not only with specific SNPs, we chose two SNPs in LD, considered as informative markers of a wider genome region. Thus, the selected SNPs are not expected to be directly involved in the HPs to thyroid hormones.
A significant difference between the three genotype groups was found with regard to TSH and fT4/TSH, suggesting a different HPs. In particular, fT4/TSH and fT3/TSH in GG subjects were significantly lower than in AG subjects. This finding indicates that these individuals may have a decreased HPs to thyroid hormone-negative feedback.
Given the limited number of subjects in each group, we then considered a recessive model: a highly significant association of A allele with TSH, fT3/TSH and fT4/TSH was found. Homozygotes GG produce and secrete more TSH, despite comparable doses of LT4 and levels of free thyroid hormones. Indeed, no difference was found with regard to fT3, fT4 and LT4 dose, reinforcing the conclusion of a different pituitary response to comparable levels of peripheral thyroid hormones.
We selected a population with the lowest possible number of factors affecting LT4 dose requirement: all patients underwent total thyroidectomy and RAI therapy, with undetectable TG at follow-up. Thus, we considered that they no longer had any residual thyroid function, the main confounding factor in studies on this topic [6]. Our strict exclusion criteria (especially gastrointestinal disease and medications interfering with LT4 absorption or metabolism) allowed to further reduce the influence of external confounding factors. Moreover, when subdivided into genotype groups, the selected patients resulted in being similar in weight, BMI and age, factors to be considered in the study of HPT axis regulation [3].
No differences in LT4 dose and fT4 levels were found between genotypes, suggesting indirectly a correct therapy intake and no differences in absorption and metabolism of LT4. Moreover, no significant differences were found considering fT3, fT4 and fT3/fT4, suggesting a comparable activity of the other genes involved in the thyroid function set-point [16,17,18,19,20,21,22,23,24,25,26].
The retrospective design represents a limit of our study, especially concerning the different assay methods that have occurred over the years. In any case, the distribution of genotypes before and after the year of transition from the second- to third-generation assays did not differ, indicating that TSH variations in relation to the genotype should not depend on the assay used. Moreover the distribution of genotypes did not differ in relation to gender and age. Together, these data suggest that all these factors should not influence the outcome of our study.
The association of A allele with lower TSH levels might reflect a higher HPs to the negative feedback by thyroid hormones due to possible differences in gene transcription and/or activity of TRHR. This could be due to differences in gene transcription mediated by changes in the promoter activity or in the activity of intronic response elements.
The role of TRHR SNPs in HPT axis regulation is still a matter of debate. Although several genes may be involved in thyroid hormone feedback, the different levels of TSH found in our population may be the result of changes in TRHR activity and in its stimulation of TSH production/secretion. It could depend on different numbers of expressed receptors or ligand-receptor affinity or, finally, signal transduction by the activated receptor.
At the genomic level, the TRHR consists of three exons and two introns [35]. Our selected SNPs are markers of a LD block including the promoter, the first two exons, first intron and part of the second intron of the TRHR gene, containing several regulatory elements. Therefore, this LD region includes genomic elements capable of regulating the transcriptional activity of the TRHR gene [35], thereby influencing the expression of TRHR at the pituitary level and the response to TRH. However, the lack of in vitro experiments confirming this hypothesis needs to be addressed.
While the results of the present study are not expected to modify the therapeutical approach in thyroidectomized patients and need to be strengthened by future replication in an independent cohort, the TRHR gene was identified as a novel determinant of the HPs to LT4.
Disclosure Statement
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Acknowledgements
This research was partly supported by grant IDEAS RBID08777T from the Italian Ministry of University and Research and by the Fondazione Cassa di Risparmio di Modena.
References
- 1.Verhaert N, Vander Poorten V, Delaere P, Bex M, Debruyne F. Levothyroxine replacement therapy after thyroid surgery. B-ENT. 2006;2:129–133. [PubMed] [Google Scholar]
- 2.Wiersinga WM. Thyroid hormone replacement therapy. Horm Res. 2001;56(suppl 1):74–81. doi: 10.1159/000048140. [DOI] [PubMed] [Google Scholar]
- 3.Jonklaas J. Sex and age differences in levothyroxine dosage requirement. Endocr Pract. 2010;16:71–79. doi: 10.4158/EP09257.OR. [DOI] [PubMed] [Google Scholar]
- 4.Sawin CT, Herman T, Molitch ME, London MH, Kramer SM. Aging and the thyroid. Decreased requirement for thyroid hormone in older hypothyroid patients. Am J Med. 1983;75:206–209. doi: 10.1016/0002-9343(83)91192-0. [DOI] [PubMed] [Google Scholar]
- 5.Olubowale O, Chadwick DR. Optimization of thyroxine replacement therapy after total or near-total thyroidectomy for benign thyroid disease. Br J Surg. 2006;93:57–60. doi: 10.1002/bjs.5157. [DOI] [PubMed] [Google Scholar]
- 6.Gordon MB, Gordon MS. Variations in adequate levothyroxine replacement therapy in patients with different causes of hypothyroidism. Endocr Pract. 1999;5:233–238. doi: 10.4158/EP.5.5.233. [DOI] [PubMed] [Google Scholar]
- 7.Bach-Huynh T-G, Nayak B, Loh J, Soldin S, Jonklaas J. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab. 2009;94:3905–3912. doi: 10.1210/jc.2009-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benvenga S, Bartolone L, Pappalardo MA, Pappalardo MA, Russo A, Lapa D, Giorgianni G, Saraceno G, Trimarchi F. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid. 2008;18:293–301. doi: 10.1089/thy.2007.0222. [DOI] [PubMed] [Google Scholar]
- 9.Singh N, Singh PN, Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA. 2000;283:2822–2825. doi: 10.1001/jama.283.21.2822. [DOI] [PubMed] [Google Scholar]
- 10.Liel Y, Harman-Boehm I, Shany S. Evidence for a clinically important adverse effect of fiber-enriched diet on the bioavailability of levothyroxine in adult hypothyroid patients. J Clin Endocrinol Metab. 1996;81:857–859. doi: 10.1210/jcem.81.2.8636317. [DOI] [PubMed] [Google Scholar]
- 11.Centanni M, Gargano L, Canettieri G, Viceconti N, Franchi A, Delle Fave G, Annibale B. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787–1795. doi: 10.1056/NEJMoa043903. [DOI] [PubMed] [Google Scholar]
- 12.Ain KB, Refetoff S, Fein HG, Weintraub BD. Pseudomalabsorption of levothyroxine. JAMA. 1991;266:2118–2120. [PubMed] [Google Scholar]
- 13.Mandel SJ, Brent GA, Larsen PR. Levothyroxine therapy in patients with thyroid disease. Ann Intern Med. 1993;119:492–502. doi: 10.7326/0003-4819-119-6-199309150-00009. [DOI] [PubMed] [Google Scholar]
- 14.Biondi B, Filetti S, Schlumberger M. Thyroid-hormone therapy and thyroid cancer: a reassessment. Nat Clin Pract Endocrinol Metab. 2005;1:32–40. doi: 10.1038/ncpendmet0020. [DOI] [PubMed] [Google Scholar]
- 15.Hansen PS, Brix TH, Sørensen TIA, Kyvik KO, Hegedüs L. Major genetic influence on the regulation of the pituitary-thyroid axis: a study of healthy Danish twins. J Clin Endocrinol Metab. 2004;89:1181–1187. doi: 10.1210/jc.2003-031641. [DOI] [PubMed] [Google Scholar]
- 16.Yin X, Latif R, Bahn R, Tomer Y, Davies TF. Influence of the TSH receptor gene on susceptibility to Graves' disease and Graves' ophthalmopathy. Thyroid. 2008;18:1201–1206. doi: 10.1089/thy.2008.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Jong FJ, Peeters RP, den Heijer T, van der Deure WM, Hofman A, Uitterlinden AG, Visser TJ, Breteler MM. The association of polymorphisms in the type 1 and 2 deiodinase genes with circulating thyroid hormone parameters and atrophy of the medial temporal lobe. J Clin Endocrinol Metab. 2007;92:636–640. doi: 10.1210/jc.2006-1331. [DOI] [PubMed] [Google Scholar]
- 18.Panicker V, Cluett C, Shields B, Murray A, Parnell KS, Perry JR, Weedon MN, Singleton A, Hernandez D, Evans J, Durant C, Ferrucci L, Melzer D, Saravanan P, Visser TJ, Ceresini G, Hattersley AT, Vaidya B, Dayan CM, Frayling TM. A common variation in deiodinase 1 gene DIO1 is associated with the relative levels of free thyroxine and triiodothyronine. J Clin Endocrinol Metab. 2008;93:3075–3081. doi: 10.1210/jc.2008-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peeters RP, van den Beld AW, Attalki H, Toor H, de Rijke YB, Kuiper GG, Lamberts SW, Janssen JA, Uitterlinden AG, Visser TJ. A new polymorphism in the type II deiodinase gene is associated with circulating thyroid hormone parameters. Am J Physiol Endocrinol Metab. 2005;289:E75–E81. doi: 10.1152/ajpendo.00571.2004. [DOI] [PubMed] [Google Scholar]
- 20.Torlontano M, Durante C, Torrente I, Crocetti U, Augello G, Ronga G, Montesano T, Travascio L, Verrienti A, Bruno R, Santini S, D'Arcangelo P, Dallapiccola B, Filetti S, Trischitta V. Type 2 deiodinase polymorphism (threonine 92 alanine) predicts L-thyroxine dose to achieve target thyrotropin levels in thyroidectomized patients. J Clin Endocrinol Metab. 2008;93:910–913. doi: 10.1210/jc.2007-1067. [DOI] [PubMed] [Google Scholar]
- 21.Hoftijzer HC, Heemstra KA, Visser TJ, le Cessie S, Peeters RP, Corssmit EP, Smit JW. The type 2 deiodinase ORFa-Gly3Asp polymorphism (rs12885300) influences the set point of the hypothalamus-pituitary-thyroid axis in patients treated for differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2011;96:E1527–E1533. doi: 10.1210/jc.2011-0235. [DOI] [PubMed] [Google Scholar]
- 22.Peeters RP, van Toor H, Klootwijk W, de Rijke YB, Kuiper GG, Uitterlinden AG, Visser TJ. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab. 2003;88:2880–2888. doi: 10.1210/jc.2002-021592. [DOI] [PubMed] [Google Scholar]
- 23.Lago-Lestón R, Iglesias MJ, San-José E, Areal C, Eiras A, Araújo-Vilar D, Lado-Abeal J, Domínguez-Gerpe L. Prevalence and functional analysis of the S107P polymorphism (rs6647476) of the monocarboxylate transporter 8 (SLC16A2) gene in the male population of north-west Spain (Galicia) Clin Endocrinol (Oxf) 2009;70:636–643. doi: 10.1111/j.1365-2265.2008.03377.x. [DOI] [PubMed] [Google Scholar]
- 24.Van der Deure WM, Peeters RP, Visser TJ. Molecular aspects of thyroid hormone transporters, including MCT8, MCT10, and OATPs, and the effects of genetic variation in these transporters. J Mol Endocrinol. 2010;44:1–11. doi: 10.1677/JME-09-0042. [DOI] [PubMed] [Google Scholar]
- 25.Refetoff S, Murata Y, Mori Y, Janssen OE, Takeda K, Hayashi Y. Thyroxine-binding globulin: organization of the gene and variants. Horm Res. 1996;45:128–138. doi: 10.1159/000184775. [DOI] [PubMed] [Google Scholar]
- 26.Sørensen HG, van der Deure WM, Hansen PS, Peeters RP, Breteler MM, Kyvik KO, Sørensen TI, Hegedüs L, Visser TJ. Identification and consequences of polymorphisms in the thyroid hormone receptor α and β genes. Thyroid. 2008;18:1087–1094. doi: 10.1089/thy.2008.0236. [DOI] [PubMed] [Google Scholar]
- 27.Hall R, Amos J, Garry R, Buxton RL. Thyroid-stimulating hormone response to synthetic thyrotropin-releasing hormone in man. Br Med J. 1970;2:274–277. doi: 10.1136/bmj.2.5704.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morley JE. Extrahypothalamic thyrotropin-releasing hormone (TRH) – its distribution and its functions. Life Sci. 1979;25:1539–1550. doi: 10.1016/0024-3205(79)90435-1. [DOI] [PubMed] [Google Scholar]
- 30.Pettersson FH, Anderson CA, Clarke GM, Barrett JC, Cardon LR, Morris AP, Zondervan KT. Marker selection for genetic case-control association studies. Nat Protoc. 2009;4:743–752. doi: 10.1038/nprot.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tüttelmann F, Luetjens CM, Nieschlag E. Optimising workflow in andrology: a new electronic patient record and database. Asian J Androl. 2006;8:235–241. doi: 10.1111/j.1745-7262.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- 32.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 33.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 34.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 35.Matre V, Høvring PI, Orstavik S, Frengen E, Rian E, Velickovic Z, Murray-McIntosh RP, Gautvik KM. Structural and functional organization of the gene encoding the human thyrotropin-releasing hormone receptor. J Neurochem. 1999;72:40–50. doi: 10.1046/j.1471-4159.1999.0720040.x. [DOI] [PubMed] [Google Scholar]