Abstract
BACKGROUND. Patients with mutations that inactivate kisspeptin signaling are infertile. Kisspeptin-54, the major circulating isoform of kisspeptin in humans, potently stimulates reproductive hormone secretion in humans. Animal studies suggest that kisspeptin is involved in generation of the luteinizing hormone surge, which is required for ovulation; therefore, we hypothesized that kisspeptin-54 could be used to trigger egg maturation in women undergoing in vitro fertilization therapy.
METHODS. Following superovulation with recombinant follicle-stimulating hormone and administration of gonadotropin-releasing hormone antagonist to prevent premature ovulation, 53 women were administered a single subcutaneous injection of kisspeptin-54 (1.6 nmol/kg, n = 2; 3.2 nmol/kg, n = 3; 6.4 nmol/kg, n = 24; 12.8 nmol/kg, n = 24) to induce a luteinizing hormone surge and egg maturation. Eggs were retrieved transvaginally 36 hours after kisspeptin injection, assessed for maturation (primary outcome), and fertilized by intracytoplasmic sperm injection with subsequent transfer of one or two embryos.
RESULTS. Egg maturation was observed in response to each tested dose of kisspeptin-54, and the mean number of mature eggs per patient generally increased in a dose-dependent manner. Fertilization of eggs and transfer of embryos to the uterus occurred in 92% (49/53) of kisspeptin-54–treated patients. Biochemical and clinical pregnancy rates were 40% (21/53) and 23% (12/53), respectively.
CONCLUSION. This study demonstrates that a single injection of kisspeptin-54 can induce egg maturation in women with subfertility undergoing in vitro fertilization therapy. Subsequent fertilization of eggs matured following kisspeptin-54 administration and transfer of resulting embryos can lead to successful human pregnancy.
TRIAL REGISTRATION. ClinicalTrials.gov NCT01667406.
FUNDING. Medical Research Council, Wellcome Trust, and National Institute for Health Research.
Introduction
Kisspeptins are a group of recently identified, naturally occurring hypothalamic peptides that are essential for normal human fertility (1–3). Patients with genetic inactivation of the kisspeptin signaling pathway fail to undergo puberty and are infertile (4–6). Conversely patients with activating mutations undergo precocious (early) puberty (7). Within the hypothalamus, kisspeptin is coreleased from kisspeptin-neurokinin B-dynorphin (KNDy) neurons with other neuropeptides implicated in the regulation of reproduction, such as neurokinin B and dynorphin (8). KNDy neurons directly project to gonadotropin-releasing hormone (GnRH) neurons, which activate the secretion of gonadotropins (luteinizing hormone [LH]; follicle stimulating hormone [FSH]) from the pituitary gland (9, 10). Exogenous kisspeptin stimulates the firing of GnRH neurons in vitro (11). Furthermore, peripheral injection of kisspeptin has been shown to potently stimulate gonadotropin secretion in many nonhuman mammalian species including rats (9, 12), mice (13, 14), monkeys (15), sheep (16), and humans (17–24); these effects are abolished by preadministration of a GnRH antagonist (9, 13, 15). Kisspeptin therefore stimulates gonadotropin secretion through a GnRH-dependent mechanism. Administration of a pharmacological antagonist of the kisspeptin receptor inhibits reproductive hormone secretion in rodents, sheep, and monkeys (25). Collectively, these data suggest that endogenous kisspeptin is needed for the activation of the reproductive system in humans and other mammals. Recent evidence from animals suggests that kisspeptin may activate the LH surge required for egg maturation and ovulation. In rodents, the proestrus LH surge is abolished by a specific monoclonal antibody that blocks the actions of hypothalamic kisspeptin (26), and the LH surge has been observed to be absent in transgenic mice null for kisspeptin or its receptor (27). Conversely, peripheral administration of kisspeptin triggers egg maturation in female rats primed with gonadotropins (28). In addition, exogenous administration of kisspeptin induces ovulation in the musk shrew (26), rats (28), and sheep (29). In keeping with this, human studies demonstrate that kisspeptin stimulates LH release most potently during the preovulatory phase of the menstrual cycle (i.e., the time of the cycle just prior to ovulation) in females with regular, normal menstrual cycles (18, 21, 30).
Based on these data, we hypothesized that kisspeptin administration could be used as a novel method of triggering ovulation in humans. To test this hypothesis, we performed a single-center, proof-of-concept study to determine the effects of kisspeptin-54 administration on egg maturation (an objective marker of ovulation) in women undergoing in vitro fertilization (IVF) therapy.
Results
Women with subfertility requiring IVF therapy underwent superovulation with recombinant FSH and GnRH antagonist administration to prevent premature ovulation. A single subcutaneous injection of kisspeptin-54 was used to trigger egg maturation (Figure 1). The number of patients assessed for eligibility, study enrollment, and dose allocation are shown in Figure 2.
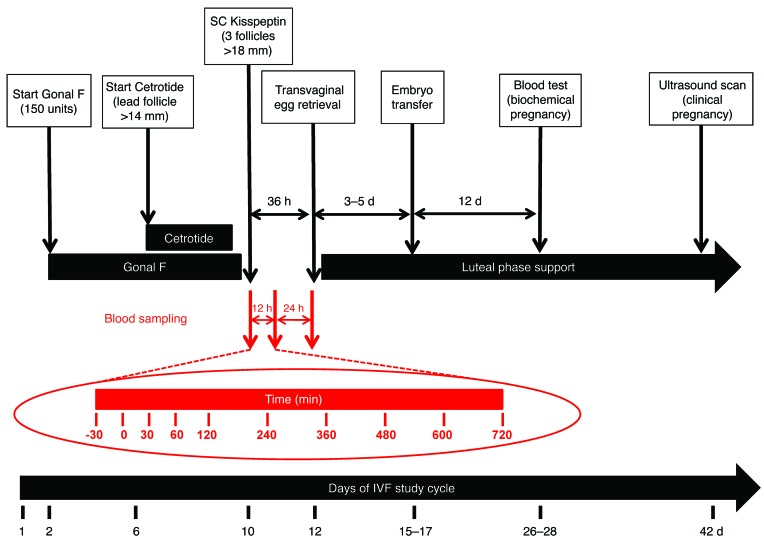
Figure 1. Superovulation protocol using subcutaneous kisspeptin-54 as a trigger to induce egg maturation.
The time line shows the day of menstrual cycle for a typical patient. On day two of the menstrual cycle, daily subcutaneous recombinant FSH (Gonal F, 150 IU) was commenced. Daily GnRH antagonist injections (Cetrotide, 0.25 mg) were commenced when the lead follicle was greater than 14 mm in diameter on an ultrasound scan. When at least three ovarian follicles of 18 mm or greater in diameter were visible on ultrasound, a subcutaneous bolus injection dose of kisspeptin-54 (1.6, 3.2, 6.4, or 12.8 nmol/kg) was administered to trigger egg maturation (between 8:30 pm and 9:30 pm). Injections of GnRH antagonist and FSH were stopped 24 and 12 hours, respectively, prior to administration of kisspeptin-54. Transvaginal ultrasound–directed egg retrieval was carried out 36 hours following kisspeptin-54 injection, and intracytoplasmic sperm injection was performed using sperm from the male partner. One or two embryos were transferred to the uterine cavity three to five days following egg collection. Progesterone (400 mg twice daily suppository/pessary) and estradiol valerate (2 mg orally 3 times daily) for luteal phase support were started after egg collection and continued until 12 weeks gestation. Biochemical pregnancy (βHCG > 10 miU/ml) was assessed 12 days following embryo transfer, and clinical pregnancy was assessed at six weeks gestation. Reproductive hormone secretion during the 12 hours following kisspeptin injection is presented in red: a subgroup of women receiving the two highest doses of kisspeptin-54 (6.4 or 12.8 nmol/kg, n = 10/dose) underwent overnight measurements of serum LH, FSH, estradiol and progesterone, and plasma kisspeptin IR just prior to and during the 12 hours following kisspeptin-54 injection.
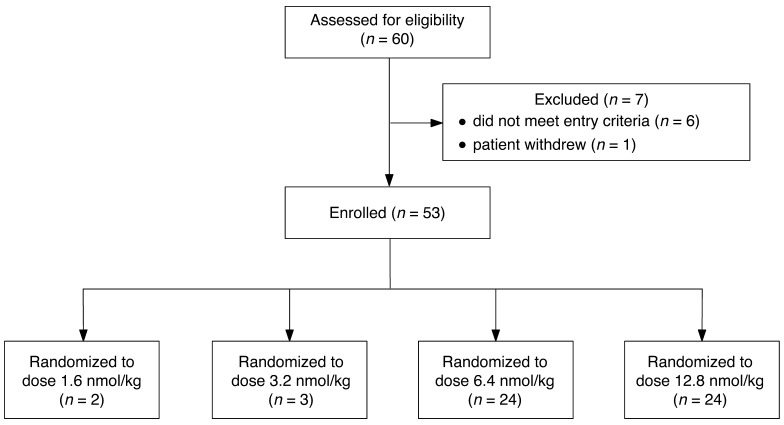
Figure 2. Enrollment of patients administered kisspeptin-54 to induce egg maturation during IVF therapy.
The first two patients were randomized to receive kisspeptin-54 at the dose of 1.6 nmol/kg during IVF therapy. Clinically difficult egg collection was reported 36 hours following kisspeptin-54 injection in both patients. The subsequent nine patients were therefore randomized 1:1:1 to 3.2, 6.4, or 12.8 nmol/kg. Clinically difficult egg collection was reported 36 hours following kisspeptin-54 injection in all patients in the 3.2 nmol/kg cohort. Subsequent patients were therefore randomized 1:1 to 6.4 or 12.8 nmol/kg kisspeptin-54 (n = 24 per group). No patients were lost to follow-up, and no patients discontinued the intervention. The outcome data for all patients who were randomized were included in the final data analysis.
Kisspeptin dose allocation and baseline characteristics.
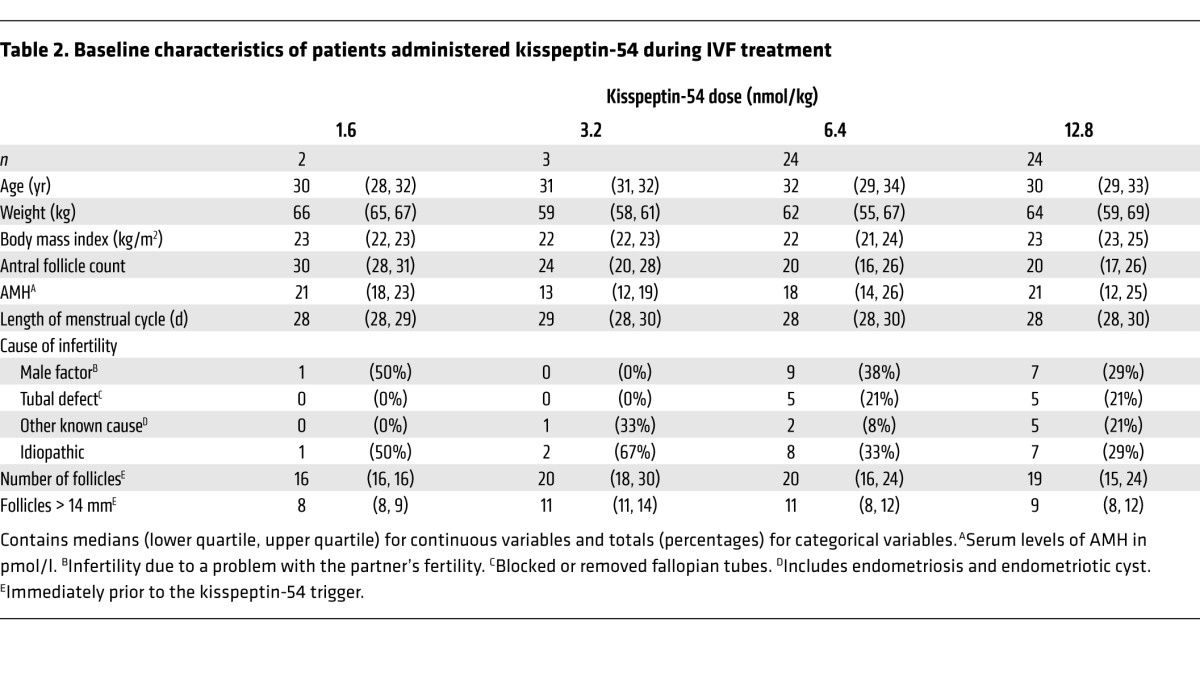
The first two patients were randomized to 1.6 nmol/kg of kisspeptin-54; in these patients, egg collection was substantially more difficult when compared with a typical treatment cycle using human chorionic gonadotropin (hCG) to induce egg maturation (Table 1). It was therefore decided to proceed to the second phase doses of 3.2, 6.4, and 12.8 nmol/kg. Clinically difficult egg collection was reported in all patients at the kisspeptin-54 dose, 3.2 nmol/kg. Subsequent patients were therefore randomized 1:1 between 6.4 and 12.8 nmol/kg kisspeptin (n = 24 per group). The dose allocation was approved by two clinical trialists who were independent of the study. No imbalances in baseline characteristics were observed between the dose groups (Table 2).
Table 1.
Clinical ease of egg collection 36 hours following kisspeptin-54 injection in patients undergoing IVF therapy
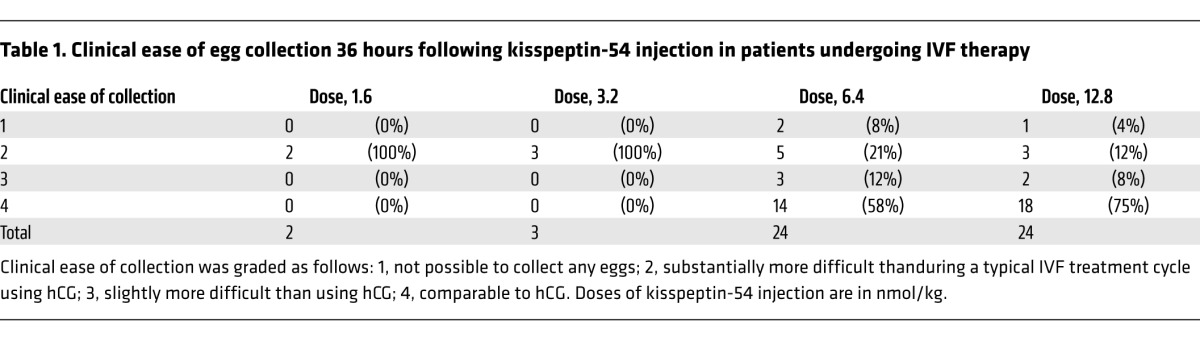
Table 2.
Baseline characteristics of patients administered kisspeptin-54 during IVF treatment
Primary outcome
Ability of kisspeptin to induce egg maturation in women undergoing IVF therapy.
This study was designed to determine whether egg maturation was achievable in women during IVF treatment following kisspeptin-54 administration. Egg maturation was observed following each dose of kisspeptin-54 (Table 3 and Figure 3). Similar rates of egg maturation (proportion of recovered eggs that were mature), between 75% and 85%, were observed following all doses of kisspeptin-54. However, oocyte yield (the proportion of eggs recovered when compared with the expected number, i.e., number of follicles > 14 mm diameter) and the absolute number of mature eggs appeared to increase with increasing doses of kisspeptin (Table 3).
Table 3.
Summary of egg maturation following administration of kisspeptin-54
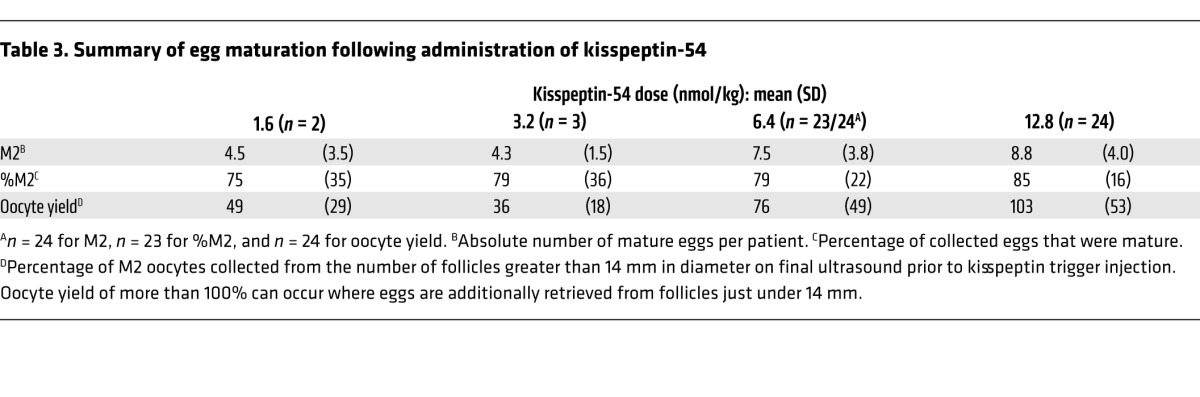
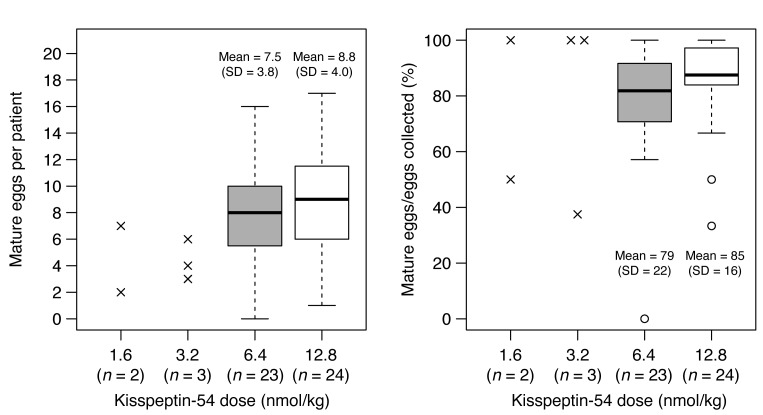
Figure 3. Egg maturation in patients following administration of kisspeptin-54.
Individual data are shown by x’s for the 1.6 and 3.2 nmol/kg doses. Box plots are shown for the 6.4 and 12.8 nmol/kg doses: line, median; box, interquartile range; whiskers extend to the extremes of the data (1.5× the interquartile range); open circles, very extreme outliers. The difference in absolute number of mature eggs between the 12.8 and 6.4 nmol/kg doses was 1.2 (95% CI, –1.0 to 3.5). For the percentage of eggs collected that were mature, this difference was 6% (95% CI, –5% to 17%).
Secondary outcomes
A descriptive analysis of secondary outcomes is presented.
Circulating reproductive hormones following kisspeptin administration.
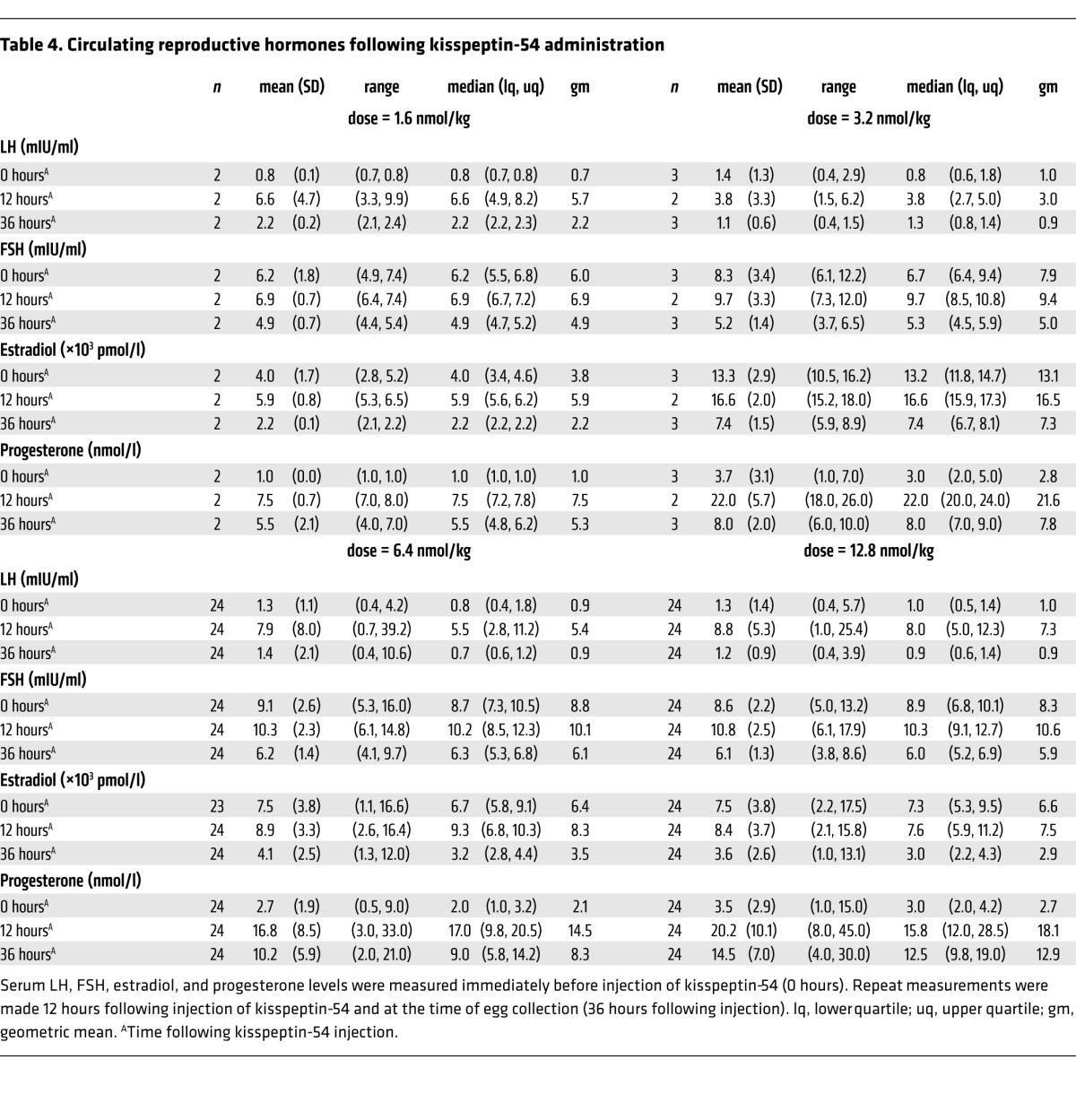
Circulating reproductive hormones were measured immediately before and 12 and 36 hours following kisspeptin-54 injection in all patients (except for one patient who missed her 12-hour blood test). Serum LH, FSH, and progesterone levels were observed to be elevated 12 hours following kisspeptin-54 injection (Table 4).
Table 4.
Circulating reproductive hormones following kisspeptin-54 administration
A surge in reproductive hormonal secretion was observed during the 12 hours following kisspeptin injection.
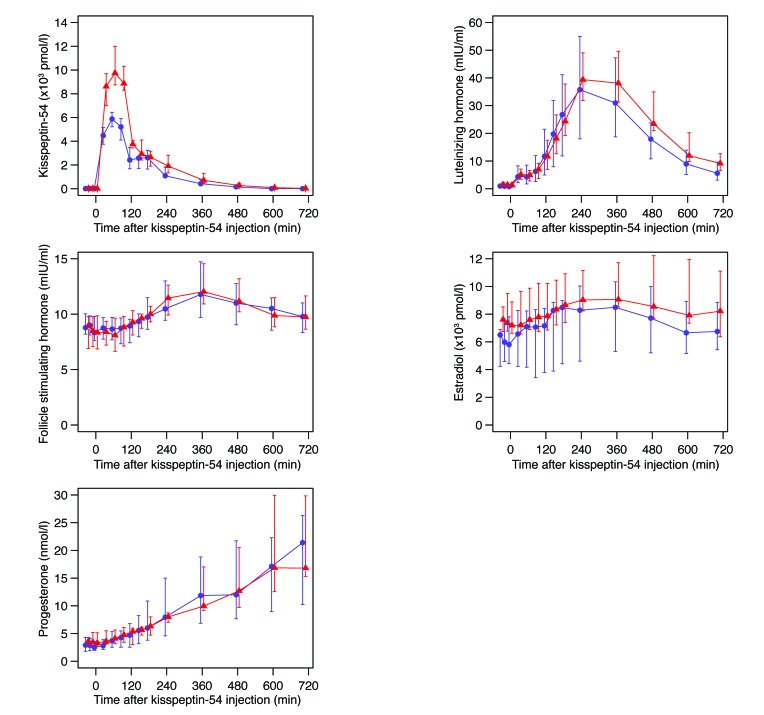
Overnight blood sampling was performed to determine the time profile of hormone release in 20 of the subjects treated with the two highest doses of kisspeptin-54 (6.4 or 12.8 nmol/kg; n = 10/dose). Peak levels of plasma kisspeptin were observed approximately one hour after injection and then fell to preinjection levels by 12 hours following injection (Figure 4). Serum LH levels peaked four to six hours following kisspeptin-54 injection and decreased thereafter. Similar but less prominent patterns of elevation were observed in FSH and estradiol secretion following kisspeptin-54 injection. In contrast, progesterone levels rose continually during the 12 hours following kisspeptin-54 injection (Figure 4).
Figure 4. Detailed overnight blood sampling following kisspeptin-54 injection reveals a surge in reproductive hormonal secretion in patients undergoing IVF therapy.
A subgroup of women receiving the two highest doses of kisspeptin-54 (6.4 or 12.8 nmol/kg; n = 10 per group) at t = 0 minutes underwent overnight measurements of circulating kisspeptin-54, LH, FSH, estradiol, and progesterone at t = –30, –15, 0, 30, 60, 90, 120, 150, 180, 240, 360, 480, 600, and 705 minutes. Blue circles show median for 6.4 nmol/kg kisspeptin-54; red triangles show median for 12.8 nmol/kg kisspeptin-54. Vertical lines indicate the interquartile ranges. Conversion factor for serum estradiol: 1 pmol/l = 0.27 pg/ml; conversion factor for serum progesterone: 1 nmol/l = 0.31 ng/ml.
Fertilization of embryos and pregnancy.
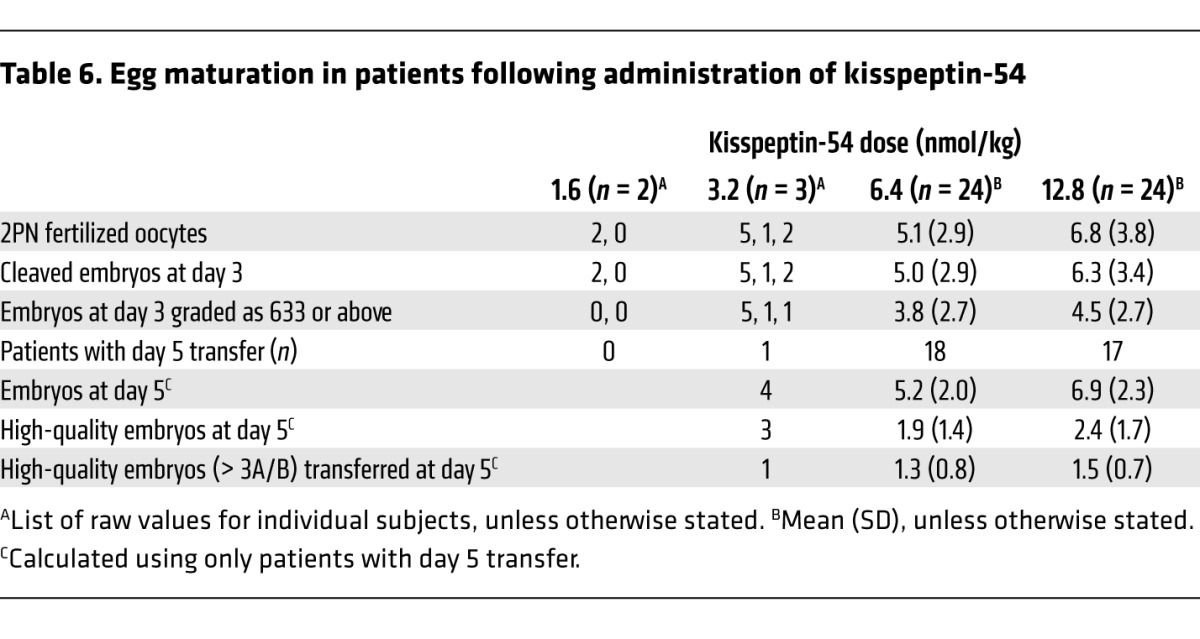
Although this study was primarily designed to investigate whether egg maturation could be achieved in women administered kisspeptin-54 during IVF treatment, we also recorded the rates of egg fertilization, biochemical pregnancy (at 12 days following embryo transfer), and clinical pregnancy (six weeks gestation). Results are shown in Table 5. Overall, fertilization occurred in 92% (defined as at least one fertilized egg), rate of embryo transfer was 92%, and high-quality embryo transfer occurred in 58%. The embryo quality results are shown in Table 6. In addition, 36 of 49 patients who had an embryo transfer did so on day five following egg retrieval. Furthermore, the blastocyst formation rate (defined as the total number of blastocysts divided by the total number of 2 pronuclei [2PN] zygotes formed) in these patients was 49.4% (129 blastocysts from 261 2PN zygotes), and 31 of 36 patients who had a day-five embryo transfer had at least one high-quality embryo transferred. The biochemical pregnancy rate was 40%, and the clinical pregnancy rate was 23% (12 of all 53 treated patients). The following outcomes were observed in the 12 women achieving clinical pregnancies: ten women each gave birth to healthy babies (eight women had singleton pregnancies and two women had twin pregnancies); two further women had miscarriages (one at nine weeks and the other at 12 weeks gestation).
Table 5.
Summary of response to treatment and progression of pregnancy following IVF treatment
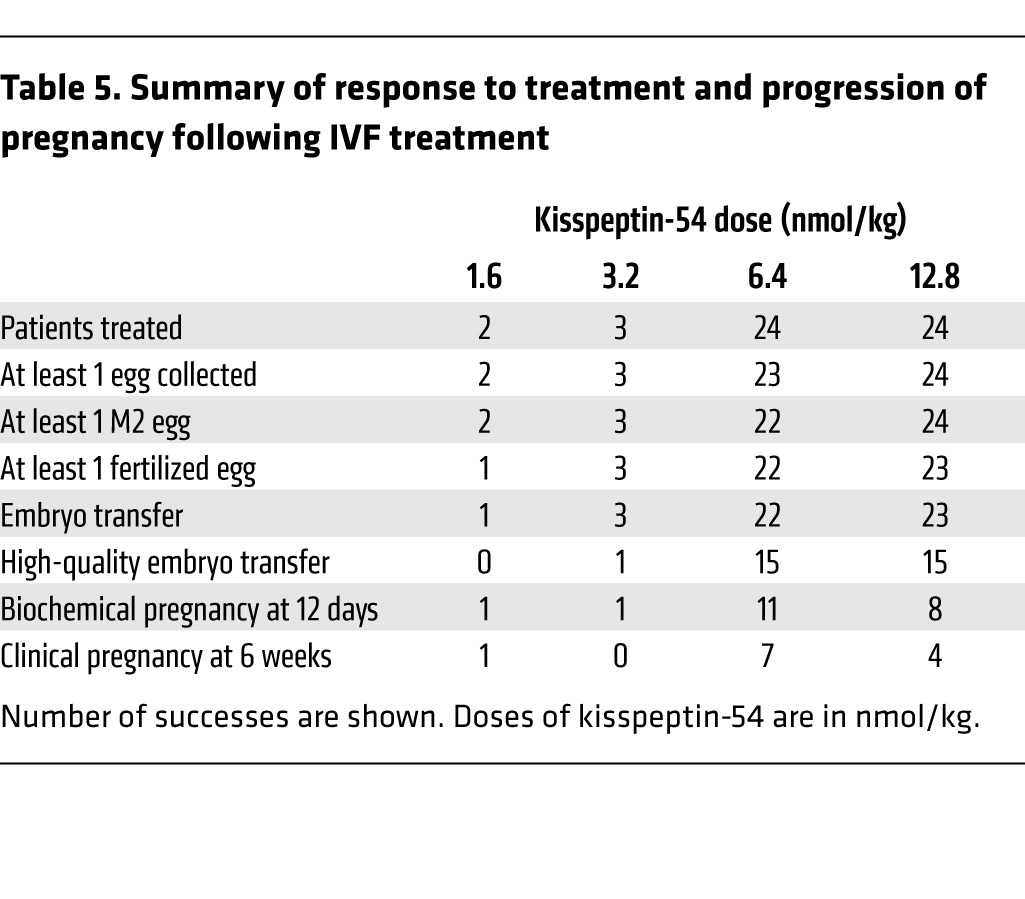
Table 6.
Egg maturation in patients following administration of kisspeptin-54
Adverse events.
Kisspeptin-54 administration was well tolerated in all 53 subfertile patients in this study (in keeping with the safety reported in previous studies that have administered kisspeptin in men and women) (17–24). Five adverse events occurred that are established complications of IVF treatment and pregnancy (two patients experienced an ectopic pregnancy, one had a heterotopic pregnancy, and two had miscarriages).
Discussion
Kisspeptin signaling is obligatory for mammalian fertility (4–6), and exogenous kisspeptin administration potently stimulates endogenous reproductive hormones in all mammalian species studied to date (9, 11–24, 28). Kisspeptin therefore offers a potentially novel method for treating patients with infertility. Animal studies suggest that kisspeptin may play a physiological role in generating the endogenous LH surge, which is required for ovulation. We therefore investigated whether a single injection of kisspeptin-54 could be used as a method to trigger egg maturation (an objective marker of ovulation) in patients during IVF therapy. Herein, we demonstrate for the first time, to our knowledge, that a single injection of kisspeptin-54 triggers egg maturation sufficient to result in fertilization, embryo implantation, and successful live birth in women with subfertility undergoing IVF therapy.
The major objective of this study was to investigate whether egg maturation was achievable in women administered kisspeptin-54 during IVF therapy. Our primary end point was the most definitive marker of oocyte maturation, which is the presence of an intact first polar body and a translucent, homogeneously colored cytoplasm without inclusions graded as “metaphase 2” (31). However, there is no conventional scoring system to determine the ease of egg collection. We therefore devised a scoring system to allow some objectivity for the dose escalation as a secondary end point. The difficulty of egg collection as determined using our scoring system correlated well with oocyte yield. The oocyte yield was 13%, 34%, 96%, and 111% when the clinical ease of collection was scored as 1, 2, 3, or 4 respectively. This suggests that the clinical ease of collection score made a reasonable assessment of the difficulty of egg retrieval as subjectively encountered by the assisted reproductive technology (ART) doctor. We also prospectively tracked the progress of patients following treatment with kisspeptin-54. Embryogenesis was observed in almost all patients who had egg maturation following administration of kisspeptin-54 at doses of 6.4 or 12.8 nmol/kg. It is of interest to consider how the blastocyst formation rate using kisspeptin as a trigger for oocyte maturation compares with that found following other standard triggers of oocyte maturation. Kisspeptin was associated with a blastocyst formation rate of 49.4%; this is comparable to the blastocyst formation rate of 51.2% observed in our department for patients aged less than 35 years following intracytoplasmic sperm injection (ICSI) using established triggers of oocyte maturation. Furthermore, 12 clinical pregnancies were observed during the study. The number of patients in each treatment group was very small, and numerous factors can affect pregnancy outcome following egg maturation; the observed pregnancy rate at each dose of kisspeptin-54 should therefore be interpreted with caution. However, our observations importantly demonstrate that successful human pregnancy can result from kisspeptin-54 treatment in women undergoing IVF therapy.
There were important ethical issues to consider in the design of this study of patients with subfertility undergoing IVF treatment. First, it is known that omitting the trigger for egg maturation results in failed egg maturation (termed “empty follicle syndrome”); therefore, a placebo trigger was not considered (32). Second, a preliminary dose-finding study without attempted fertilization was considered unethical, since it would have exposed vulnerable patients to a medically invasive procedure and substantial psychological stress and resulted in any mature eggs generated being discarded. Instead, the independent ethics committee approved a step-wise strategy by which oocyte maturation was assessed as the primary end point by an embryologist independent of the study team; any retrieved eggs not meeting the criteria for egg maturation were not injected with sperm and were discarded. Only if egg maturation was achieved were eggs injected with sperm, and thereafter only if fertilization was achieved did patients proceed to embryo transfer. As a result of this ethically approved strategy designed with patient safety in mind, 4 of 53 patients during the study did not proceed to embryo transfer.
It is also important to consider the safety of kisspeptin in IVF treatment. Kisspeptin is a naturally occurring peptide hormone that plays a physiological role in triggering ovulation (25–29). Reassuringly, circulating levels of kisspeptin-54 rise dramatically, up to 7,000-fold, during normal human pregnancy and remain elevated for the duration of a normal nine-month pregnancy (33). Using kisspeptin as a trigger for egg maturation exposes the patient to only a very short duration of kisspeptin, since the half-life of exogenously administered kisspeptin is 28 minutes (17). Furthermore, a number of clinical studies have demonstrated that kisspeptin can be safely administered to human subjects without any observed adverse effects (17–24). In the present study, 12 healthy babies have been born without any abnormalities. However, valid concerns have been raised regarding the potential association of IVF treatment with alterations in epigenetic markers that regulate gene expression (34). To date, there is no published data investigating whether kisspeptin alters epigenetic markers in any experimental model. Further studies are therefore needed to determine the full risk and safety profile using kisspeptin in IVF treatment. Since kisspeptin had not previously been administered to treat patients with subfertility undergoing IVF treatment, the effectiveness of kisspeptin to trigger egg maturation was not known. To minimize the number of patients exposed to clinically ineffective kisspeptin doses (i.e., doses not resulting in egg maturation), we chose a study design that allowed for rapid dose escalation. Therefore, although we have identified two doses of kisspeptin-54 that effectively induce egg maturation in subfertile patients undergoing IVF therapy, further research is required to ascertain the optimal dose of kisspeptin and verify that lower doses are insufficient to induce egg maturation.
Three of the patients had tubal pregnancies during the current study. It is therefore important to consider the possible reasons for this. Ectopic pregnancies are more common during ART when compared with spontaneous pregnancies. This may be partly explained by the selection of women with tubal infertility and also by alterations in tubal contractility as a consequence of the hormonal milieu during IVF cycles (35). In unselected IVF cycles, ectopic pregnancy is thought to occur in 2% to 8.6% of pregnancies (35). Furthermore, ectopic pregnancy has been reported to occur in up to 11% of IVF pregnancies in women with tubal infertility (35–37). In our current study, 10 of the 53 women had a diagnosis of tubal infertility as the indication for ART, which may have therefore contributed to our results. The overall number of patients in this study is small, so the rate of ectopic pregnancy may have occurred due to statistical artifact. Furthermore, the half-life of exogenously administered kisspeptin is short (28 minutes); we would therefore not expect a single injection of kisspeptin to have an effect that was long enough to alter susceptibility to tubal pregnancy (17). However, it is important to recognize that a higher than expected rate of tubal pregnancy was observed, based on the total number of patients in this study and the published literature. It is therefore possible that kisspeptin could have resulted in an increased rate of tubal pregnancy by a currently unidentified mechanism; for example, some dysfunctionality in the triggering of egg maturation following kisspeptin may have led to dyskinetic alterations in uterine contractility that may have resulted in an increased tubal pregnancy rate. Further and larger studies are therefore needed to evaluate the outcomes of IVF treatment using kisspeptin as a trigger for oocyte maturation.
It is interesting to consider the mechanism of action of kisspeptin-54 in relation to other hormones currently used to trigger egg maturation during IVF therapy. hCG is the most commonly used hormonal trigger for egg maturation in clinical practice (31), stimulating the ovaries directly by binding to the LH receptor. hCG has LH-like activity that is far longer in duration than the endogenous physiological LH surge (38), and its effects are not subject to any negative feedback. As such, hCG can be associated with excessive ovarian stimulation that can lead to ovarian hyperstimulation syndrome (OHSS) (39). GnRH agonists stimulate the GnRH receptor on the pituitary gland to release LH. The LH profile following a GnRH agonist trigger has previously been assessed in 17 women who received 0.2 mg of triptorelin and 15 women who received 0.5 mg of leuprorelin following a recombinant FSH/GnRH antagonist protocol using the GnRH antagonist ganirelix (40). Serum LH levels rose from (mean ± SD) 0.9 ± 0.4 IU/l prior to trigger administration to peak levels of 130 ± 60 IU/l at four hours after receiving triptorelin and to 107 ± 55 IU/l four hours after receiving leuprorelin. Serum LH levels fell to approximately 55 IU/l at 12 hours following administration of either GnRH agonist and had returned to pretrigger baseline levels by 36 hours following administration (40). The use of GnRH agonists is associated with lower rates of OHSS, but can also be associated with lower pregnancy rates when compared with hCG (41, 42). The latter has been hypothesized to be due to luteal phase insufficiency (43), which may be a result of GnRH triggers causing excessive pharmacological stimulation and consequent desensitization of the GnRH receptor. In contrast with GnRH, kisspeptin-54 is implicated in generating the physiological endogenous LH surge by activating the hypothalamus to stimulate endogenous GnRH secretion, which then stimulates endogenous LH release (9–11, 13–15). As such, kisspeptin administration stimulates an individual’s endogenous GnRH reserve to stimulate LH release, whereas GnRH triggers cause direct pharmacological activation of the GnRH receptors on the pituitary gonadotrophs. In keeping with this, using kisspeptin as a trigger for egg maturation resulted in mean peak levels of serum LH (37.1 and 42.1 IU/l following 6.4 and 12.8 nmol/kg kisspeptin-54, respectively) that were lower than those previously observed using a GnRH agonist trigger (40).
The kisspeptin signaling pathway is therefore a mechanism that can be therapeutically exploited to trigger egg maturation in patients. Further research will be required to determine whether the therapeutic application of kisspeptin-54 to trigger egg maturation leads to better outcomes for patients undergoing IVF treatment than other hormones currently used for this purpose (such as hCG and GnRH agonists).
In summary, we provide proof of concept that a single injection of kisspeptin-54 induces egg maturation in women undergoing IVF therapy. Furthermore, eggs matured using kisspeptin-54 therapy can be fertilized in vitro, transferred to the uterus, and thus provide successful human pregnancy for couples with infertility. Further studies will determine the clinical utility of kisspeptin-54 during IVF therapy when compared with established pharmacological triggers of egg maturation.
Methods
Peptide
Kisspeptin-54 was synthesized and purified by Bachem (Bachem Holding AG). Sterile vials of kisspeptin-54 were produced by Bachem (Clinalfa; Bachem Distribution Services GmbH). Both products were prepared according to good manufacturing practice (GMP). Vials of freeze-dried kisspeptin-54 were stored at –20°C and reconstituted in 0.9% saline as described previously (17, 18).
Subjects
Sixty patients requiring IVF treatment at Hammersmith Hospital were screened for participation between the start (August 2012) and end of patient recruitment (September 2013). Eligible patients received a single treatment cycle; all treatment costs for this study cycle were funded through study participation, as the efficacy of kisspeptin to trigger egg maturation was unknown prior to starting the study. The inclusion criteria were as follows: age, 18–34 years; early follicular phase level of serum, FSH ≤ 12 mIU/ml; serum anti-Müllerian hormone (AMH), 10–40 pmol/l (1.4–5.6 ng/ml); both ovaries intact, regular menstrual cycles of 24 to 35 days duration; and body mass index 18–29 kg/m2. Exclusion criteria were as follows: moderate/severe endometriosis; poor response to, or more than one previous cycle of, IVF treatment; clinical or biochemical hyperandrogenemia; polycystic ovarian syndrome. The number of patients assessed for eligibility, study enrollment, and dose allocation are shown in Figure 1.
Study outcomes
The primary outcome was egg maturation during a single treatment cycle, which was assessed in two ways: first, the absolute number of mature eggs; second, the percentage of eggs collected that were mature. Eggs were classified as mature by the presence of the first polar body and round ooplasm (31).
Secondary outcomes were circulating levels of reproductive hormones following kisspeptin-54 injection, fertilization rate, rate of embryo transfer, embryo quality, biochemical pregnancy rate at 12 days following transfer, and clinical pregnancy rate at six weeks gestation. Fertilization rate was defined as the number of two pronuclear zygotes divided by the number of mature eggs injected (31). Biochemical pregnancy was defined by a plasma hCG concentration greater than 10 mIU/ml 12 days after embryo transfer. Clinical pregnancy was defined as an intrauterine gestational sac with heartbeat at six weeks gestation. Pregnancy outcomes and live births were documented in women with clinical pregnancy.
Dose allocation
Doses were to be allocated in two phases. During the first phase, the first nine women were to be randomized equally to the three lowest doses (0.4, 0.8, and 1.6 nmol/kg) of kisspeptin. If fewer than two women in each group experienced clinically effective egg maturation, then, during the second phase, the next nine women were to be randomized to the three higher doses (3.2, 6.4, and 12.8 nmol/kg) of kisspeptin. During the first phase of the study, randomization was performed using an online resource (www.random.org). The clinical ease of egg collection following kisspeptin-54 injection (compared with hCG, which is the trigger most commonly used in clinical practice to trigger oocyte maturation in IVF treatment) was also noted. The rationale for this was that a clinically effective dose of kisspeptin would need to result in maturation of eggs that could be collected with ease similar to that observed with triggers of egg maturation currently used in clinical practice. Hence, the clinical ease of collection was graded 1 to 4 by clinicians blinded to the dose allocation using the following as a guide: grade 1, not possible to collect any eggs; grade 2, substantially more difficult than during a typical IVF treatment cycle using hCG (excessive number of flushes were required in order to aspirate eggs from follicles and very scanty number of granulosa cells present in follicular aspirates); grade 3, slightly more difficult than using hCG (granulosa cells were present in the aspirates, although less abundant than following optimal triggering; cumulus cells were more densely packed than following optimal triggering); grade 4, comparable level of difficulty when compared to hCG. Flushing was performed where deemed clinically necessary by the ART doctor performing the egg collection. The presence of granulosa cells in the follicular fluid can provide an indication of follicular maturity (44). We did not explicitly measure the number of granulosa cells in follicular fluid during egg collection, since it is not universally regarded as a reliable marker of egg maturation (45, 46), as the concentration of granulosa cells can be heavily altered by flushing. However, the ART doctor performing the egg collection was able to take into account information regarding the estimated concentration of granulosa cells in the follicular fluid related by the embryologist during the procedure, in addition to his or her own experience, in scoring the ease of collection. This allowed us to assess whether the ART specialist encountered an abnormal level of difficulty in comparison with the ART doctors’ own experience of egg retrieval when hCG was used as a trigger for egg maturation. Once a dose of kisspeptin had been shown to induce clinically effective egg maturation in at least two women (per cohort of three), then further women were recruited to increase the sample size at that dose to a minimum of 15 patients. During the second phase of the study, randomization was performed using printed lists that were prepared by a statistician using NQuery (http://www.statsols.com/products/nquery-advisor-nterim/). Subjects were assigned to the next unassigned dose strictly in the order in which they received the trigger injection. The decision to escalate to higher dosing tiers of kisspeptin administration was determined by two clinical investigators with experience in running clinical trials, who were independent of the study. The clinicians who retrieved the eggs were blinded to the dose allocation of patients. Embryologists and patients were also blinded to the dose allocation.
Protocols
All of the women underwent superovulation using a recombinant FSH and GnRH antagonist protocol. Kisspeptin-54 was used to trigger egg maturation.
Superovulation protocol.
On day two of the menstrual cycle, daily subcutaneous recombinant FSH injections (Gonal F 150 IU; Merck Serono), administered at 9:00 am daily, were commenced in all patients; pelvic ultrasound scan was performed five days after commencing injections to determine ovarian follicle development. Daily GnRH antagonist injections (Cetrotide 0.25 mg; Merck Serono), injected at 9:00 pm daily, were used to inhibit a premature LH surge and were commenced when the lead follicle was greater than 14 mm in diameter. Further ultrasound scans were performed according to follicular size and response to superovulation.
Kisspeptin-54 trigger of egg maturation.
When at least three ovarian follicles of 18 mm or greater diameter were visible on ultrasound, a subcutaneous bolus injection dose of kisspeptin-54 was administered by an investigator to trigger egg maturation. All doses of kisspeptin-54 were weight adjusted, and injection volumes ranged from a minimum of 330 μl to a maximum of 530 μl. This is comparable to the injection volume of hCG used to trigger oocyte maturation during IVF (500 μl mcl injection volume containing 250 mcg/6500 IU Ovitrelle; Merck Serono). Kisspeptin-54 was administered 36 hours prior to egg retrieval (between 8:30 pm and 9:30 pm). Injections of FSH were stopped 12 hours prior to kisspeptin-54 administration. In order to minimize any effect that the GnRH antagonist had on kisspeptin-54 response, the last injection of GnRH antagonist was administered 24 hours prior to kisspeptin-54 administration (47, 48). Serum LH, FSH, estradiol and progesterone were measured immediately before and 12 hours after kisspeptin-54 injection and at the time of egg retrieval in all women.
Reproductive hormone secretion during the 12 hours following kisspeptin injection.
A subgroup of women receiving the two highest doses of kisspeptin-54 (6.4 or 12.8 nmol/kg, n = 10/dose) volunteered to undergo overnight measurements of serum LH, FSH, estradiol and progesterone, and plasma kisspeptin immunoreactivity (IR) just prior to and after kisspeptin-54 injection (t = –30, –15, 0, 30, 60, 90, 120, 150, 180, 240, 360, 480, 600, and 705 minutes after injection). These hormonal measurements allowed us to determine the detailed time course of reproductive hormone release following kisspeptin-54 injection.
Egg retrieval, fertilization, and reimplantation of embryos.
Transvaginal ultrasound–directed egg retrieval was carried out 36 hours following kisspeptin-54 injection, and ICSI was performed in all study cycles using sperm from the male partner. The oocyte retrievals were carried out in line with the routine departmental policy of our IVF unit using other established triggers of oocyte maturation. Specifically, the embryologist routinely informs the clinician if an oocyte or granulosa cells are observed in the follicular aspirate before the clinician progresses to the next follicle. Eggs were collected using a single-lumen 16-gauge ovum aspiration needle (Cook Medical) for the majority of patients. However, a double-lumen 16-gauge ovum aspiration needle (Cook Medical), which allows for follicular flushing, was used if fewer than five follicles with a mean diameter greater than 14 mm were observed on the day of the kisspeptin trigger or if no oocyte was retrieved after aspiration of the first three follicles. All embryos were graded at day three by an independent embryologist who was not part of the study team using the British Fertility Society (BFS) and Association of Clinical Embryologists (ACE) embryo-grading scheme, which is used for cleavage embryos and describes embryos based on cell number, blastomere size, and fragmentation, giving a numerical value to each of these parameters (49). If on day three, at least two embryos had six or more cells, less than 20% difference in blastomere diameter, and less than 20% fragmentation, they were incubated until day five after egg retrieval so that the strongest embryos could be identified for transfer. At day five, embryos were graded by an independent embryologist for blastocyst expansion (grades 1 to 6), inner cell mass (grades A to E), and trophectoderm (grades A to C) (50). At this blastocyst stage, embryos that scored at least three for blastocyst expansion and A or B for inner cell mass and trophectoderm were classified as “high-quality embryos.” One or two embryos of highest quality during morphological assessment were transferred to the uterine cavity three to five days following egg collection. Progesterone (400 mg twice daily suppository/pessary) and estradiol valerate (2 mg orally 3 times daily) were started after egg collection and continued until 12 weeks gestation for luteal phase supplementation.
Hormonal assay methodology
Blood samples were collected as previously described (17, 18, 23, 24, 30). Serum LH, FSH, estradiol, and progesterone were measured using automated chemiluminescent immunoassays (Abbott Diagnostics). Interassay coefficients of variation were as follows: LH, 3.4%; FSH, 3.5%; estradiol, 3.4%; progesterone, 1.8%. Limits of detectability for each assay were as follows: LH, 0.07 mIU/ml; FSH, 0.05 mIU/ml; estradiol, 70 pmol/l (19 pg/ml); and progesterone 0.3 nmol/l (0.1 ng/ml).
AMH was measured using ELISA (Beckman Coulter Inc.). The reference range was 2.2–48.5 pmol/l (0.3–6.8 ng/ml). The lower limit of detection was 0.6 pmol/l (0.08 ng/ml). The interassay coefficient of variation was 4.6%; the intraassay coefficient of variation was 4.0%.
Measurement of plasma kisspeptin IR was performed using an established RIA (17, 18, 23, 24, 30). The antibody crossreacted 100% with human kisspeptin-54, kisspeptin-14, and kisspeptin-10 and less than 0.01% with other related arginine-phenylalanine amide proteins, including prolactin-releasing peptide, arginine-phenylalanine amide–related peptide 1, arginine-phenylalanine amide–related peptide 2, arginine-phenylalanine amide–related peptide 3, QRFP43, neuropeptide FF, and neuropeptide AF. The limit of detectability was 2 pmol/l, and the intra- and interassay coefficients of variation were 8.3% and 10.2%, respectively.
Statistics
The study data are summarized using standard descriptive methods. Data analysis was performed by A. Mason, J. Warwick, and D. Ashby, who had no involvement in patient management or data collection. Histograms and box plots were used to assess the distributional assumptions and check for possible outliers. Continuous variables following an approximately normal distribution are summarized using the mean and SD. Skewed continuous variables are summarized using the median and interquartile range. Categorical variables (binary, ordered, and multinomial) are presented in terms of frequencies and percentages. Where possible, outcomes are presented graphically, using scatter plots and box plots.
Study approval
The Hammersmith Hospital and Queen Charlotte’s and Chelsea Hospital Research Ethics Committee (London, United Kingdom) approved this study (reference: 10/H0707/2). The study was carried out in the IVF Unit at Hammersmith Hospital under a license from the United Kingdom Human Fertilization and Embryology Authority. Written informed consent was obtained from all subjects prior to inclusion in the study. Approval of this study as a clinical trial of an investigational medicinal product was granted by the Medicines and Healthcare Products Regulatory Agency (United Kingdom). The study was registered on the National Institutes of Health Clinical Trials database (NCT01667406). The study was performed in accordance with the Declaration of Helsinki.
Supplementary Material
Acknowledgments
This work was funded by a Medical Research Council (MRC) Developmental Clinical Studies grant. C.N. Jayasena and G.M.K. Nijher are supported by National Institute of Health Research (NIHR) Clinical Lectureships. A. Abbara is supported by a Wellcome Clinical Research Training Fellowship. A.N. Comninos is supported by a Wellcome Translational Medicine Research Fellowship. C. Izzi-Engbeaya is supported by an NIHR Academic Clinical Fellowship. W.S. Dhillo is supported by an NIHR Career Development Fellowship. We are grateful to the NIHR Imperial Biomedical Research Centre Funding Scheme and NIHR/Wellcome Trust Imperial Clinical Research Facility for providing infrastructure for this study.The study was designed, conducted, analyzed and reported entirely by the authors. The MRC, Wellcome Trust, and NIHR provided research funding to carry out the studies.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2014;124(8):3667–3677. doi:10.1172/JCI75730.
See the related Attending physician beginning on page 3277.
References
- 1.Ohtaki T, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411(6837):613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 2.Kotani M, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276(37):34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 3.Muir AI, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276(31):28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 4.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seminara SB, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 6.Topaloglu AK, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629–635. doi: 10.1056/NEJMoa1111184. [DOI] [PubMed] [Google Scholar]
- 7.Teles MG, et al. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358(7):709–715. doi: 10.1056/NEJMoa073443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman RL, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 9.Irwig MS, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 10.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han SK, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;7(25):11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro VM, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinising hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145(10):4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 13.Gottsch ML, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 14.Messager S, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102(5):1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102(6):2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caraty A, et al. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148(11):5258–5267. doi: 10.1210/en.2007-0554. [DOI] [PubMed] [Google Scholar]
- 17.Dhillo WS, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary-gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 18.Dhillo WS, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle. J Clin Endocrinol Metab. 2007;92(10):3958–3966. doi: 10.1210/jc.2007-1116. [DOI] [PubMed] [Google Scholar]
- 19.Chan YM, et al. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab. 2011;96(6):E908–E915. doi: 10.1210/jc.2010-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George JT, et al. Kisspeptin is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab. 2011;96(8):E1228–E1236. doi: 10.1210/jc.2011-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan YM, Butler JP, Sidhoum VF, Pinnell NE, Seminara SB. Kisspeptin administration to women: a window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. J Clin Endocrinol Metab. 2012;97(8):E1458–E1467. doi: 10.1210/jc.2012-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young J, et al. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology. 2013;97(2):193–202. doi: 10.1159/000336376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayasena CN, et al. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab. 2009;94(11):4315–4323. doi: 10.1210/jc.2009-0406. [DOI] [PubMed] [Google Scholar]
- 24.Jayasena CN, et al. Twice-daily subcutaneous injection of kisspeptin-54 does not abolish menstrual cyclicity in healthy female volunteers. J Clin Endocrinol Metab. 2013;98(11):4464–4474. doi: 10.1210/jc.2013-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roseweir AK, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue N, et al. Kisspeptin neurons mediate reflex ovulation in the musk shrew (Sunsus murinus). Proc Natl Acad Sci U S A. 2011;108(42):17527–17532. doi: 10.1073/pnas.1113035108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28(35):8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320(2):383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 29.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinising hormone surge. J Neurosci. 2006;26(25):6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayasena CN, et al. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;96(12):E1963–E1972. doi: 10.1210/jc.2011-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27(2):170–207. doi: 10.1210/er.2005-0015. [DOI] [PubMed] [Google Scholar]
- 32.Coulam CB, Bustillo M, Schulman JD. Empty follicle syndrome. Fertil Steril. 1986;46(6):1153–1155. doi: 10.1016/s0015-0282(16)49898-5. [DOI] [PubMed] [Google Scholar]
- 33.Horikoshi Y, et al. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88(2):914–919. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- 34.Shufaro Y, Laufer N. Epigenetic concerns in assisted reproduction: update and critical review of the current literature. Fertil Steril. 2013;99(3):605–606. doi: 10.1016/j.fertnstert.2013.01.126. [DOI] [PubMed] [Google Scholar]
- 35.Chang HJ, Suh CS. Ectopic pregnancy after assisted reproductive technology: what are the risk factors? Curr Opin Obstet Gynecol. 2010;22(3):202–207. doi: 10.1097/GCO.0b013e32833848fd. [DOI] [PubMed] [Google Scholar]
- 36.Malak M, Tawfeeq T, Holzer H, Tulandi T. Risk factors for ectopic pregnancy after in vitro fertilization treatment. J Obstet Gynaecol Can. 2011;33(6):617–619. doi: 10.1016/S1701-2163(16)34910-6. [DOI] [PubMed] [Google Scholar]
- 37.Dubuisson JB, Aubriot FX, Mathieu L, Foulot H, Mandelbrot L, de Jolière JB. Risk factors for ectopic pregnancy in 556 pregnancies after in vitro fertilization: implications for preventive management. Fertil Steril. 1991;56(4):686–690. doi: 10.1016/s0015-0282(16)54600-7. [DOI] [PubMed] [Google Scholar]
- 38.Damewood MD, Shen W, Zacur HA, Schlaff WD, Rock JA, Wallach EE. Disappearance of exogenously administered human chorionic gonadotropin. Fertil Steril. 1989;52(3):398–400. doi: 10.1016/s0015-0282(16)60906-8. [DOI] [PubMed] [Google Scholar]
- 39.Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv. 1989;44(6):430–440. doi: 10.1097/00006254-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Fauser BC, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87(2):709–715. doi: 10.1210/jcem.87.2.8197. [DOI] [PubMed] [Google Scholar]
- 41.Humaidan P, Kol S, Papanikolaou EG, Copenhagen GnRH Agonist Triggering Workshop Group GnRH agonist for triggering of final egg maturation: time for a change of practice? Hum Reprod Update. 2011;17(4):510–524. doi: 10.1093/humupd/dmr008. [DOI] [PubMed] [Google Scholar]
- 42.Humaidan P, et al. GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Hum Reprod. 2005;20(5):1213–1220. doi: 10.1093/humrep/deh765. [DOI] [PubMed] [Google Scholar]
- 43.Beckers NG, et al. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab. 2003;88(9):4186–4192. doi: 10.1210/jc.2002-021953. [DOI] [PubMed] [Google Scholar]
- 44.Enien WM, Chantler E, Seif MW, Elstein M. Human ovarian granulosa cells and follicular fluid indices: the relationship to oocyte maturity and fertilization in vitro. Hum Reprod. 1998;13(5):1303–1306. doi: 10.1093/humrep/13.5.1303. [DOI] [PubMed] [Google Scholar]
- 45.Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rattanachaiyanont M, Leader A, Léveillé MC. Lack of correlation between oocyte-corona-cumulus complex morphology and nuclear maturity of oocytes collected in stimulated cycles for intracytoplasmic sperm injection. Fertil Steril. 1999;71(5):937–940. doi: 10.1016/S0015-0282(99)00100-4. [DOI] [PubMed] [Google Scholar]
- 47.DiLuigi AJ, Nulsen JC. Effects of gonadotropin-releasing hormone agonists and antagonists on luteal function. Curr Opin Obstet Gynecol. 2007;19(3):258–265. doi: 10.1097/GCO.0b013e3281338874. [DOI] [PubMed] [Google Scholar]
- 48.Felberbaum RE, et al. Preserved pituitary response under ovarian stimulation with HMG and GnRH antagonists (Cetrorelix) in women with tubal infertility. Eur J Obstet Gynecol Reprod Biol. 1995;61(2):151–155. doi: 10.1016/0301-2115(95)02138-W. [DOI] [PubMed] [Google Scholar]
- 49.Cutting R, Morroll D, Roberts SA, Pickering S, Rutherford A, BFS ACE Elective single embryo transfer: guidelines for practice British Fertility Society and Association of Clinical Embryologists. Hum Fertil (Camb). 2008;11(3):131–146. doi: 10.1080/14647270802302629. [DOI] [PubMed] [Google Scholar]
- 50.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.