Abstract
During the course of many chronic viral infections, the antiviral T cell response becomes attenuated through a process that is regulated in part by the host. While elevated expression of the immunosuppressive cytokine IL-10 is involved in the suppression of viral-specific T cell responses, the relevant cellular sources of IL-10, as well as the pathways responsible for IL-10 induction, remain unclear. In this study, we traced IL-10 production over the course of chronic lymphocytic choriomeningitis virus (LCMV) infection in an IL-10 reporter mouse line. Using this model, we demonstrated that virus-specific T cells with reduced inflammatory function, particularly Th1 cells, display elevated and sustained IL-10 expression during chronic LCMV infection. Furthermore, ablation of IL-10 from the T cell compartment partially restored T cell function and reduced viral loads in LCMV-infected animals. We found that viral persistence is needed for sustained IL-10 production by Th1 cells and that the transcription factor BLIMP-1 is required for IL-10 expression by Th1 cells. Restimulation of Th1 cells from LCMV-infected mice promoted BLIMP-1 and subsequent IL-10 expression, suggesting that constant antigen exposure likely induces the BLIMP-1/IL-10 pathway during chronic viral infection. Together, these data indicate that effector T cells self-limit their responsiveness during persistent viral infection via an IL-10–dependent negative feedback loop.
Introduction
Chronic viral infections such as HIV, HCV, and HBV are a major burden on human health due to both their high rates of morbidity and mortality as well as to the lack of effective therapies. While viral evasion of the immune response can directly contribute to viral persistence, recent findings indicate that impaired viral clearance is also facilitated by host-regulated immunosuppression. In particular, both the CD4+ and CD8+ T cell response to chronic viral infection is impaired, with some antiviral T cells failing to survive (termed “deletion”) and others persisting in a dysfunctional or “exhausted” state characterized by diminished effector function (1, 2). In particular, exhausted antiviral T cells lose effector cytokine production capacity to varying degrees depending on exhaustion severity, with cells first losing IL-2 production, followed by TNF-α and finally IFN-γ. This process is regulated by T cell gene expression changes, including inhibitory receptor induction (3, 4), and by soluble factors such as IL-10 and TGF-β (5–7). Importantly, blockade of these pathways restores T cell numbers and function and triggers a reduction in viral loads (3–7), validating immunomodulation as a viable therapy for chronic viral infections.
Despite our increasing knowledge of the molecules involved in immunoregulation during chronic viral infection, the signals that induce inhibitory molecule expression remain unclear. In order to address this question, we focused on regulation of the cytokine IL-10. IL-10 expression is elevated during mouse infection with the chronic clone 13 (Cl.13) lymphocytic choriomeningitis virus (LCMV) strain relative to infection with acute LCMV Armstrong (Arm) (5, 6). In addition, Cl.13-infected Il10–/– mice display enhanced T cell function and augmented viral clearance (5, 6). Elevated IL-10 expression has also been implicated in immunoregulation during human HIV and HCV infection (8–11), suggesting that it is part of an evolutionarily conserved response to chronic viral infection with clinical relevance. To determine the factors controlling IL-10 induction during chronic viral infection, it is first necessary to determine the physiologically relevant cellular IL-10 sources. Hematopoietic cells are the primary source of IL-10 (12), however, while a large array of cell types, including DCs, NK cells, monocytes, B cells, and T cells, produce IL-10 during chronic viral infection (1, 5, 6, 8–15), the physiological relevance of these different IL-10 sources in vivo is controversial.
To better understand IL-10 regulation during chronic viral infection, we wished to first definitively trace the cellular sources of IL-10 during mouse LCMV-Cl.13 infection, then identify those cellular IL-10 sources that have an impact on viral clearance, and finally identify the factors responsible for IL-10 induction within these cells. We reasoned that cell types that produce more IL-10 in chronic versus acute LCMV infection (“overproducers”) would represent the most physiologically relevant sources of IL-10. Using an IL-10 reporter mouse, we identified virus-specific T cells, particularly CD4+ T cells, as one of the few cell types that overproduced IL-10 over the course of chronic LCMV infection and demonstrated that T cell–derived IL-10 was physiologically relevant. IL-10 expression was restricted to Th1 cells within the virus-specific CD4+ T cell population and was BLIMP-1 dependent. Strikingly, IL-10 production appeared enriched within Th1 cells with diminished inflammatory function. BLIMP-1 expression was higher in Th1 cells in chronic versus acute infection, and antigen engagement during acute infection was sufficient to cause BLIMP-1 upregulation and the rapid formation of IL-10–producing Th1 cells. Finally, the signaling requirements for antigen-induced IL-10 expression compared with antigen-induced inflammatory cytokine production appear distinct. Collectively, these data identify Th1 cells as a physiologically relevant IL-10 source and implicate antigen-induced BLIMP-1 expression within Th1 cells as a key factor that leads to sustained IL-10 production and dampened T cell responses during chronic viral infection.
Results
Multiple cell types produce IL-10 during chronic LCMV-Cl.13 infection.
To identify cell types that overproduce IL-10 during chronic versus acute LCMV infection, 10BiT reporter mice were infected with LCMV (16). 10BiT reporter mice possess a bacterial artificial chromosome transgene containing the Il10 gene locus, but with the Thy1.1 cDNA (containing a stop codon) inserted upstream of the Il10 locus. As a result, Thy1.1 expression is controlled by the Il10 gene regulatory elements in these mice, such that cells transcribing Il10 express Thy1.1 on their cell surface.
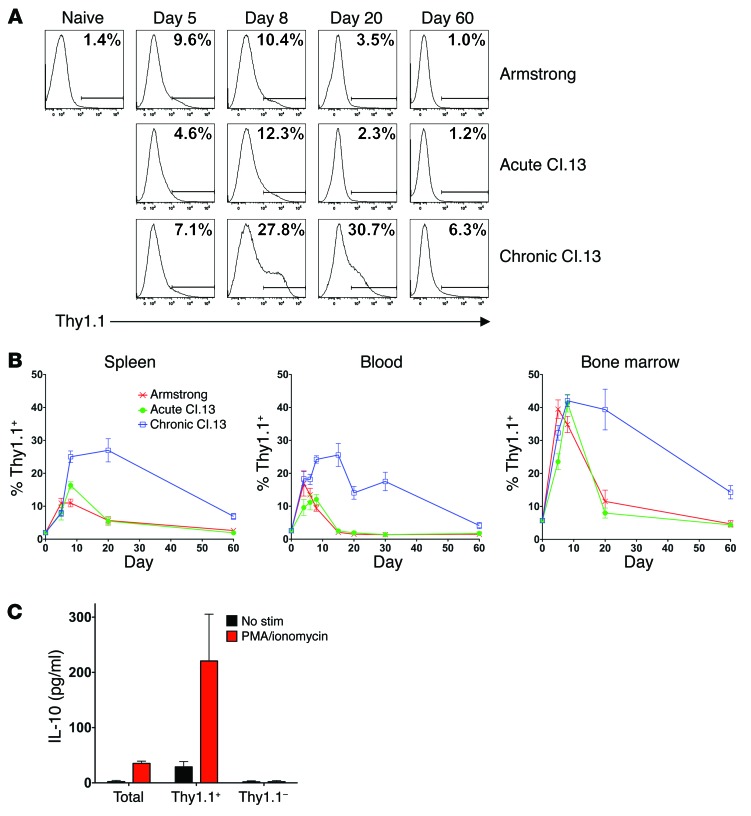
To validate that 10BiT mice respond normally to LCMV-Cl.13 infection, we first determined whether the T cell response kinetics and viral persistence were similar in C57BL/6 (B6) versus 10BiT mice. T cell responses in 10BiT mice were comparable to those seen in B6 mice, although viral clearance was slightly delayed in the reporter mice (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI66108DS1). To begin profiling IL-10 production kinetics, 10BiT mice were either infected with a high LCMV-Cl.13 dose (2 × 106 PFU) to establish chronic infection or 2 × 105 PFU LCMV-Arm to establish acute infection. To further confirm that any differences we observed in IL-10 production were due to viral persistence rather than to viral tropism differences between the LCMV-Arm and Cl.13 strains, we also infected mice with a low LCMV-Cl.13 dose (1 × 102 PFU) to generate an acute Cl.13 infection. When the IL-10 reporter (Thy1.1) expression kinetics were examined within all splenocytes, we observed a similar percentage of IL-10–expressing cells between acute and chronic LCMV infection at early time points (day 5 post infection [p.i.]), but we found a higher frequency of IL-10–expressing cells in chronic LCMV infection from day 8 p.i. onward (Figure 1, A and B). Specifically, 25%–30% of splenocytes produced IL-10 during peak viremia, with the IL-10+ cell proportion declining in parallel with the drop in viral titers from day 20 of infection onward. These kinetics are similar to published data on IL-10 protein and transcript expression (5, 6, 17, 18), validating the accuracy of reporter expression. IL-10 expression was additionally tracked in blood and BM cells, and similar kinetics were observed (Figure 1B), although the IL-10+ cell proportions varied between these tissues.
Figure 1. IL-10 reporter expression kinetics during chronic versus acute LCMV infection.
(A and B) 10BiT reporter mice were infected with either 2 × 105 PFU LCMV-Arm or 1 × 102 PFU LCMV-Cl.13 (Acute Cl.13) to establish an acute infection or 2 × 106 PFU LCMV-Cl.13 (Chronic Cl.13) to establish a chronic infection. The Thy1.1 IL-10 reporter–positive cell percentage was measured over time in either spleen (A and B), blood (B), or BM (B). Gates used to determine the Thy1.1+ percentage were set using transgene-negative control animals. (A) Representative Thy1.1 staining in splenocytes and (B) data compiled from 2 to 5 independent experiments (n = 4–16 mice/time point). (C) 10BiT reporter mice were infected with 2 × 106 PFU LCMV-Cl.13, and on day 8 p.i., FACS-purified Thy1.1+ and Thy1.1– splenocytes were left unstimulated or were stimulated for 6 hours with PMA and ionomycin. Supernatant IL-10 concentrations were then measured by ELISA. Bar graphs show the pooled data of the IL-10 amount produced per 1 × 106 cells from 3 independent experiments.
To further validate the accuracy of Thy1.1 reporter expression, IL-10 production was examined on day 8 of chronic LCMV-Cl.13 infection in 10BiT mice. Since we were unable to reliably detect IL-10 protein by intracellular staining above the background seen in infection-matched Il10–/– control animals (data not shown), we used an ELISA approach to validate the reporter. On day 8 p.i., 10BiT splenocytes were either left unsorted or were sorted into IL-10 reporter–positive (Thy1.1-positive) and –negative subsets. To measure IL-10 production, cells were cultured for a short time (6 hours) with or without stimulation with the mitogens PMA and ionomycin, and IL-10 levels were measured in the cell supernatants by ELISA. This assay definitively demonstrated that all basal and inducible IL-10 production observed within the total splenocyte population was solely contained within the Thy1.1+ cells (Figure 1C). Thus, the Thy1.1 reporter accurately marks cells with the greatest basal and inducible capacity to produce IL-10 during chronic LCMV-Cl.13 infection.
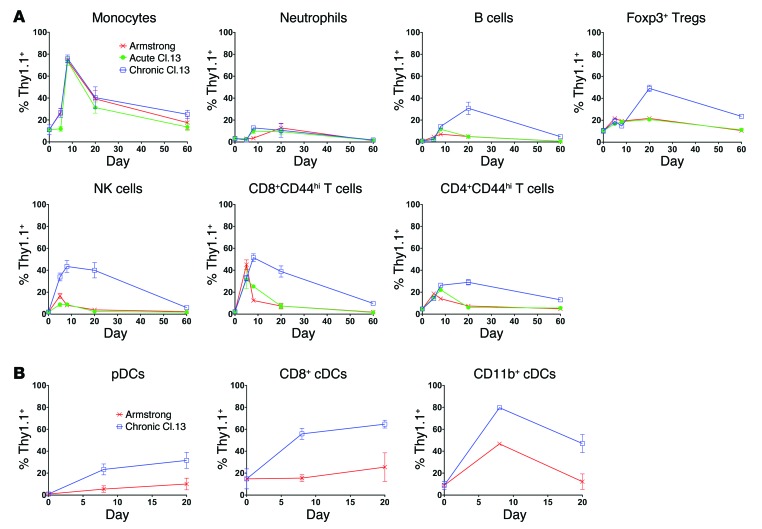
We next examined IL-10 expression levels within different cell populations over the course of chronic and acute LCMV infection. We were unable to consistently detect appreciable Thy1.1 expression within neutrophils, but all other cell types showed some degree of IL-10 upregulation during LCMV infection (Figure 2, A and B). Notably, B cells, FOXP3+ Tregs, NK cells, plasmacytoid DCs (pDCs), CD8+ and CD11b+ conventional DCs (cDCs), and activated (CD44hi) CD8+ and CD4+ T cells showed increased IL-10 production over the course of chronic versus acute LCMV infection (Figure 2, A and B). In contrast, while monocytes displayed increased IL-10 production during LCMV infection, we observed no difference in the IL-10 production kinetics between chronic and acute LCMV infection (Figure 2A). Collectively, these data suggest that many cell types contribute to the IL-10 pool during chronic LCMV-Cl.13 infection.
Figure 2. IL-10 production by different cell types across the course of chronic and acute LCMV infection.
(A and B) 10BiT reporter mice were infected with either LCMV-Arm, acute Cl.13, or chronic Cl.13, as in Figure 1. The Thy1.1 IL-10 reporter–positive cell percentage was measured in the spleen at various times p.i. IL-10 phenotyping in DCs was performed in a different set of experiments (B), in which the spleen was digested with collagenase and DNase followed by magnetic cell–sorting (MACS) enrichment of CD11c+ cells prior to measurement of Thy1.1 expression. Pooled data from 2 to 5 independent experiments are shown (n = 4–16 mice/time point).
Virus-specific T cells overproduce IL-10 over the course of chronic LCMV-Cl.13 infection.
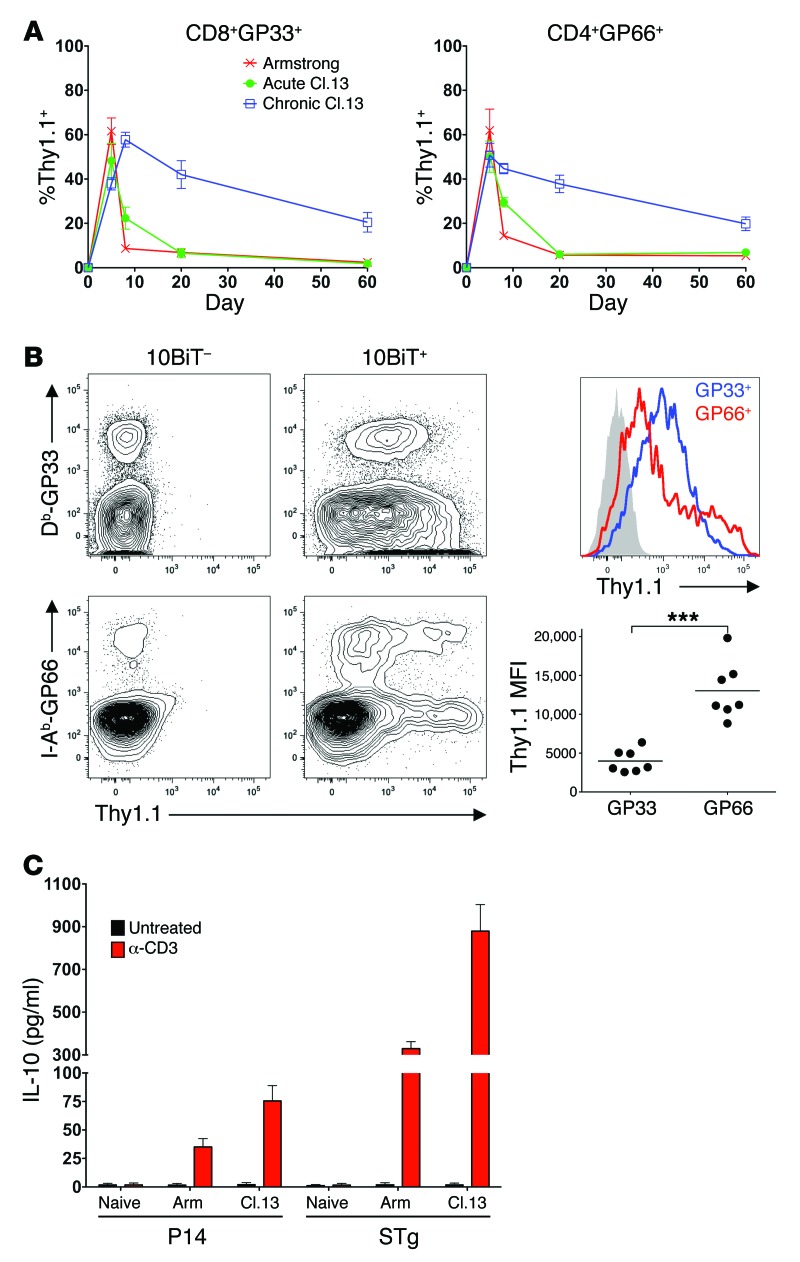
We decided to further investigate the IL-10 overproduction observed within the activated CD4+ and CD8+ T cell compartments, as it implied that virus-specific T cells might be self-regulating during LCMV-Cl.13 infection. To directly examine whether virus-specific T cells produce IL-10, we stained 10BiT splenocytes with either the LCMV GP33–41–specific MHC class I tetramer to identify CD8+GP33+ T cells or the LCMV GP66–77–specific MHC class II tetramer to identify CD4+GP66+ T cells and examined Thy1.1 expression. IL-10 production was evident within the virus-specific T cell compartment over the entire course of chronic LCMV-Cl.13 infection (Figure 3A and Supplemental Figure 2). While CD8+GP33+ and CD4+GP66+ cells expressed comparable levels of IL-10 between chronic and acute LCMV on day 5 p.i., elevated IL-10 production was evident during chronic LCMV from day 8 p.i. onward. The higher level of IL-10 reporter expression on day 8 p.i. was not restricted to CD8+GP33+ cells, as CD8+ cells specific for LCMV NP396–404 and GP276–284 also displayed elevated reporter expression in chronic versus acute LCMV infection at this time point (Supplemental Figure 3). Interestingly, while a larger CD8+GP33+ cell percentage expressed Thy1.1 than did CD4+GP66+ cells, the Thy1.1+ CD4+GP66+ cells had a 3-fold higher Thy1.1 MFI than did Thy1.1+ CD8+GP33+ cells, suggesting that the virus-specific CD4+ T cells produced more IL-10 on a per-cell basis than did CD8+ T cells (Figure 3B).
Figure 3. Virus-specific T cells overproduce IL-10 over the course of chronic LCMV infection.
(A) 10BiT mice were infected with either LCMV-Arm, acute Cl.13, or chronic Cl.13, as in Figure 1. Thy1.1 expression was examined in tetramer+ splenic CD8+GP33+ or CD4+GP66+ T cells. Data were pooled from 3 to 6 independent experiments (n = 7–16 mice/time point). (B) Thy1.1 levels were compared between CD8+GP33+ (GP33) and CD4+GP66+ (GP66) cells. Left panels show Thy1.1 staining in Db-GP33 tetramer–stained CD8+ cells (top panels, gated on CD8+ cells) or I-Ab-GP66 tetramer–stained CD4+ cells (bottom panels, gated on CD4+ cells) from 10Bit transgene–positive (right) or –negative (left) mice. Representative histogram (far right, top) shows CD4+GP66+ (red) and CD8+GP33+ (blue) cell Thy1.1 staining, with background staining from 10BiT– cells shown in gray. Graph (far right, bottom) depicts Thy1.1 MFI of Thy1.1+ cells. Data from 3 independent experiments are shown. ***P < 0.001. (C) Congenically marked Ly5.1+ P14 and STg cells were transferred into B6 mice that were subsequently infected with either 2 × 105 PFU LCMV-Arm or 2 × 106 PFU LCMV-Cl.13. On day 8 p.i., splenic P14 and STg cells were isolated by FACS and, along with FACS-purified naive P14 and STg cells, were cultured in the presence or absence of plate-bound α-CD3 for 12 hours. Supernatant IL-10 concentrations were measured by ELISA. Data show IL-10 production per 1.5 × 105 cells, with values pooled from 2 independent experiments.
To confirm that the elevated reporter expression within virus-specific CD4+ and CD8+ T cells leads to higher IL-10 protein levels, we assessed IL-10 production by virus-specific T cells by ELISA. Naive, congenically marked CD8+GP33+ TCR transgenic (P14) and CD4+GP66+ TCR transgenic (Smarta, or STg) cells were transferred into B6 mice that were then infected with either LCMV-Arm or Cl.13. On day 8 p.i., the splenic STg and P14 cells were isolated and their IL-10 production assessed following α-CD3 stimulation. Naive P14 and STg cells were unable to produce IL-10, but cells isolated from either LCMV-Arm– or LCMV-Cl.13–infected mice produced IL-10 upon TCR ligation (Figure 3C). Importantly, both P14 and STg cells from LCMV-Cl.13–infected mice produced more IL-10 than did their LCMV-Arm–derived counterparts (2- and 3-fold more IL-10, respectively) (Figure 3C). Furthermore, consistent with the higher Thy1.1 reporter MFI within CD4+GP66+ cells, STg cells generated approximately 10-fold more IL-10 than did P14 cells during LCMV-Cl.13 infection. Thus, virus-specific T cells produce more IL-10 protein in chronic versus acute infection, with CD4+ T cells producing considerably more IL-10 than do CD8+ T cells on a per-cell basis.
Virus-specific T cell–derived IL-10 is required to fully suppress T cell responses during LCMV-Cl.13 infection.
To assess the in vivo relevance of T cell–derived IL-10 in viral persistence and T cell dysfunction, a series of BM chimeras were generated. As a positive control for the IL-10 KO phenotype, 1 group of mice was lethally irradiated and reconstituted with Il10–/– BM to generate a mouse in which all hematopoietic cells were IL-10 deficient (Total KO group). Next, we created mixed BM chimeras with 75% Tcra–/– BM mixed with 25% Il10–/– BM (T cell KO group) to generate mice with an IL-10 deficiency selectively within the T cell compartment. In these T cell KO chimeras, all T cells will be IL-10 deficient, while the majority (~75%) of the non–T cells will be IL-10 sufficient. As a control for WT mice, we also generated mixed BM chimeras containing 75% Tcra–/– BM and 25% Il10+/+ B6 BM (T cell WT group). To examine the role of IL-10 production by CD4+ and CD8+ T cells individually, we also generated chimeras containing 75% Cd4–/– BM mixed with 25% Il10–/– BM (CD4 T cell KO group) or 75% Cd8a–/– BM mixed with 25% Il10–/– BM (CD8 T cell KO group), along with their corresponding WT control groups.
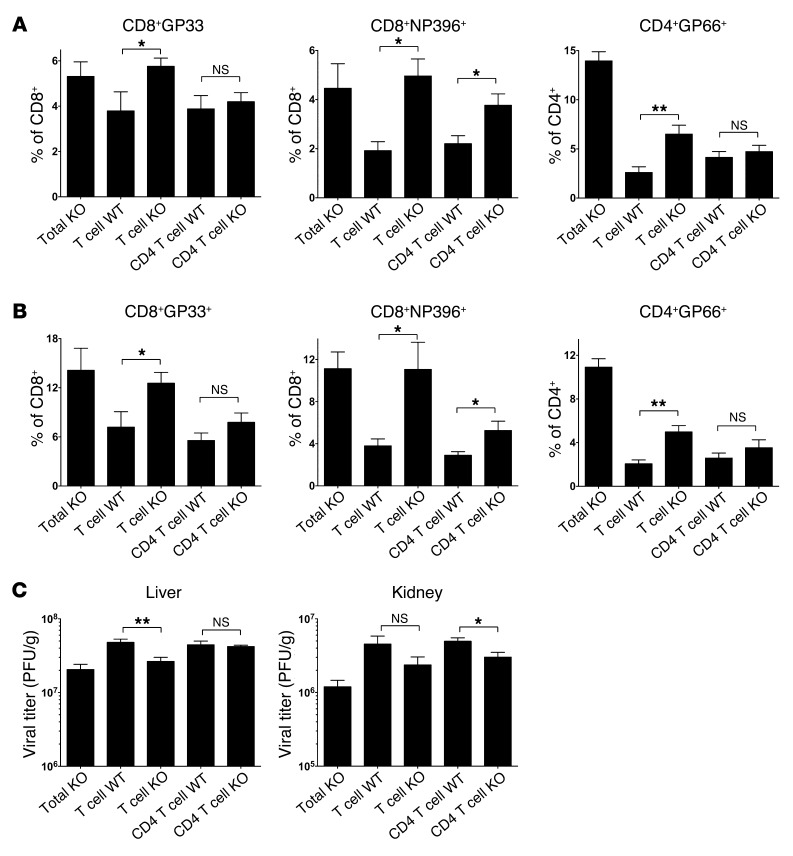
The BM chimeras were infected with LCMV-Cl.13, and on day 8 p.i., we examined both the antiviral T cell response and viral titers. As some sickness and mortality was observed within the BM chimeras from ~day 10 of infection onward, we restricted our analysis to day 8 p.i. Consistent with previously published data on IL-10 KO mice (5, 6), there was a considerable increase in the magnitude of both the CD8+GP33+ and CD4+GP66+ responses in total KO chimeras compared with those of the WT control groups (Figure 4, A and B, and Supplemental Figure 4A). Furthermore, IL-10 deficiency rescued CD8+ T cells specific for the NP396–404 epitope (CD8+NP396+) from deletion, as was previously reported (5). We observed a corresponding decrease in organ viral titers (Figure 4C and Supplemental Figure 4B), suggesting that the augmented T cell response provides better viral control. We observed no consistent decline in serum viral titers within total KO chimeras relative to those in WT controls (data not shown), and the magnitude of organ viral clearance within total KO chimeras was weaker than originally published, likely because BM chimeras have lower overall T cell numbers than do untreated mice due to incomplete reconstitution and altered lymphoid architecture. Nevertheless, a clear boost in the antiviral immune response was observed in total KO chimeras that could be used as a reference for the other BM chimera groups.
Figure 4. T cell–derived IL-10 suppresses virus-specific T cell responses and viral clearance during chronic LCMV infection.
Irradiated BM chimeras were generated with either 100% Il10–/– BM (Total KO), 75% Tcra–/– plus 25% B6 BM (T cell WT), 75% Tcra–/– plus 25% Il10–/– BM (T cell KO), 75% Cd4–/– plus 25% B6 BM (CD4 T cell WT), or 75% Cd4–/– plus 25% Il10–/– BM (CD4 T cell KO). BM chimeras were infected with 2 × 106 PFU LCMV-Cl.13, and the T cell response and viral load were measured on day 8 p.i. (A and B) Bar graphs show the percentage of either tetramer+ cells specific for the indicated epitopes (A) or IFN-γ–producing (B) CD8+ T cells in response to either GP33 (CD8+GP33+) or NP396 (CD8+NP396+) peptide stimulation (left 2 panels) or the percentage of IFN-γ–producing CD4+ T cells in response to GP66 peptide stimulation (CD4+GP66+, far right panel). (C) Viral titers in the liver or kidney. *P < 0.05; **P < 0.01; NS, indicates P > 0.05. Data were pooled from 2 independent experiments (n = 4–8 mice/group).
When we examined the magnitude of the CD8+GP33+ and CD8+NP396+ T cell responses in T cell KO BM chimeras, the responses were of a similarly elevated magnitude to those in total KO chimeras (Figure 4A, B). IL-10 loss from the CD4 compartment only partially phenocopied the total KO CD8+GP33+ and CD8+NP396+ responses, while IL-10 loss from the CD8 compartment had no effect on the CD8+ T cell response (Figure 4, A and B, and Supplemental Figure 4A). IL-10 ablation from the total T cell compartment only partially boosted the CD4+GP66+ T cell response to the levels seen in the total KO chimera controls. Again, the response was weaker in CD4 T cell KO chimeras and unchanged in CD8 T cell KO mice.
Importantly, IL-10 ablation from the total T cell compartment, and to a lesser extent the CD4 T cell compartment, also contributed to better viral control (Figure 4C). Consistent with the unchanged T cell response in CD8 T cell KO mice, these mice exhibited no change in viral control (Supplemental Figure 4B). However, viral titers in the total T cell KO mice (and in the CD4 T cell KO mice) were still slightly higher than titers in mice lacking IL-10 in all immune cells (Total KO), indicating that IL-10 produced by non–T cells also regulates viral control during chronic LCMV infection (Figure 4C). Importantly, it is unlikely that Tregs are an important IL-10 source, as Treg depletion was unable to phenocopy the Il10–/– phenotype (Supplemental Figure 5). Collectively, these data indicate that T cell–derived IL-10 is physiologically relevant and contributes to both viral persistence and suppression of virus-specific T cells during chronic viral infection. Furthermore, while combined IL-10 production by both CD4+ and CD8+ T cells has the greatest effect on the immune response, CD4+ T cell–derived IL-10 (likely from FOXP3– effector cells) has a bigger impact than CD8+ T cell–derived IL-10.
IL-10 is produced by virus-specific Th1 cells with diminished inflammatory function, not by Tfh cells.
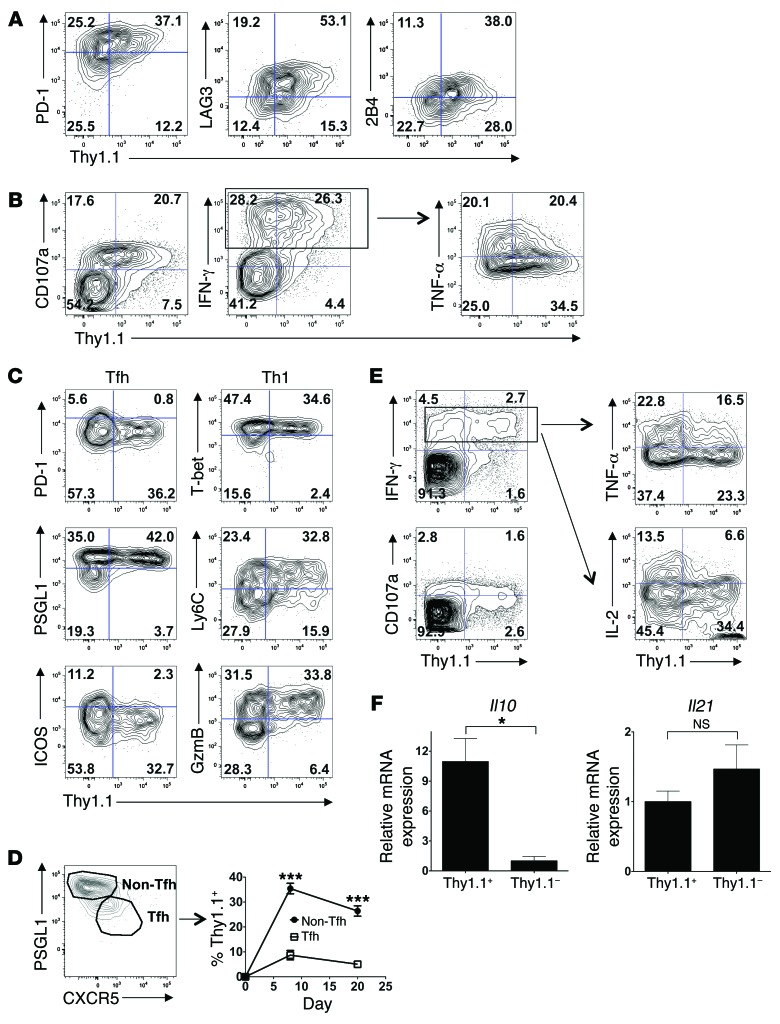
We next characterized the phenotype of the IL-10–producing, virus-specific CD8+ and CD4+ T cells. Consistent with previously published findings (13, 19), the IL-10–producing CD8+ T cells expressed greater amounts of the inhibitory receptors PD-1, LAG3, and 2B4 (Figure 5A and Supplemental Figure 6A). We observed that the IL-10+ CD8+ T cells could degranulate (surface CD107a+) and produce IFN-γ, however, the IL-10+ cells were enriched in cells with lower TNF-α production, a key signature of T cell exhaustion (Figure 5B and Supplemental Figure 6B). As almost no IL-2 production was detected within the stimulated CD8+ T cells during LCMV-Cl.13 infection, the correlation between IL-10 and IL-2 could not be assessed. Thus, IL-10 production appears to be restricted to exhausted CD8+ effector T cells with less inflammatory function and higher inhibitory receptor expression.
Figure 5. IL-10 production is restricted to exhausted CD8+ T cells and Th1 cells with diminished inflammatory function.
(A) Splenic Thy1.1+ CD8+GP33+ tetramer+ cell phenotypes in LCMV-Cl.13–infected (2 × 106 PFU) 10BiT mice on day 8 p.i. Data are representative of 2 independent experiments (n = 5 mice). (B) Correlation between cytokine or CD107a expression and Thy1.1 levels within α-CD3–stimulated CD8+ T cells on day 8 p.i. with Cl.13. The TNF-α plot was gated on CD8+IFN-γ+ cells. Data are representative of 2 independent experiments (n = 5 mice). Numbers represent the quadrant frequency. (C) The Tfh and Th1 phenotype of splenic Thy1.1+ CD4+GP66+ tetramer+ 10BiT cells on day 8 p.i. with Cl.13. Data are representative of 3 independent experiments (n = 8–10 mice). (D) The Thy1.1+ 10BiT cell proportion within the non-Tfh (PSGL1hiCXCR5lo) and Tfh (PSGL1loCXCR5hi) cell populations on days 8 and 20 p.i. with Cl.13. Data were pooled from 2 to 4 independent experiments (n = 11–13/time point). ***P < 0.001. (E) Plots showing IFN-γ and CD107a production within α-CD3–stimulated CD4+ T cells or TNF-α and IL-2 production within CD4+IFN-γ+ cells on day 8 p.i. with Cl.13. Data are representative of 2 to 3 independent experiments (n = 5–8 mice total). Numbers represent the quadrant frequency. (F) Congenically marked Ly5.1+ STg 10BiT cells were transferred into B6 mice subsequently infected with 2 × 106 PFU LCMV-Cl.13. Donor Thy1.1+ and Thy1.1– STg 10BiT cells were sorted on day 8 p.i., and Il10 and Il21 mRNA levels were assessed by quantitative RT-PCR. *P < 0.05; NS, indicates P > 0.05. Data were pooled from 3 independent experiments.
To examine the phenotype of the IL-10–expressing virus-specific CD4+ T cells, we first characterized the Th effector subsets that form on day 8 of LCMV-Cl.13 infection. Similar to LCMV-Arm infection (20) and consistent with other LCMV-Cl.13 phenotyping studies (1), we found little evidence of Th17, Th2, or induced Treg (iTreg) cell formation within virus-specific CD4+GP66+ T cells on day 8 p.i. (data not shown). Instead, as during LCMV-Arm infection (20), we found primarily Th1 cells and T follicular helper (Tfh) CD4+GP66+ cells on day 8 p.i. (Figure 5C), as has been shown previously (1).
Tfh cells typically express high levels of PD-1, ICOS, and CXCR5, while expressing low levels of PSGL-1 (20–23). IL-10 reporter (Thy1.1) expression within the CD4+GP66+ cell population on day 8 following LCMV-Cl.13 infection generally negatively correlated with these Tfh cell surface markers (Figure 5C, left, and Supplemental Figure 7A). To further confirm that IL-10 production was associated with non-Tfh cells, we examined IL-10 production by PSGL1hiCXCR5lo (non-Tfh) and PSGL1loCXCR5hi (Tfh) cells. IL-10 production was heavily enriched in the non–Tfh cell population on both days 8 and 20 p.i. (Figure 5D). In contrast, IL-10 reporter expression positively correlated with Th1 cell markers (Figure 5C, right, and Supplemental Figure 7B). Strikingly, IL-10 expression was restricted to T cells with higher expression of the Th1 master transcriptional regulator T-bet, Th1 cell surface molecule Ly6C, and Th1-restricted intracellular cytolytic molecule granzyme B (GZMB) (20, 22). We observed similar results in CD4+ T cells during acute LCMV infection (Supplemental Figure 8, A and B). Overall, these data suggest that IL-10 is predominantly produced by Th1 cells, not by Tfh cells.
To examine whether IL-10 expression is associated with IFN-γ–producing CD4+ T cells, CD4+ T cells from LCMV-Cl.13–infected 10BiT mice were polyclonally stimulated with α-CD3 and stained for IFN-γ and IL-10 reporter expression. Strikingly, we found that IL-10 production was almost exclusively limited to IFN-γ–producing CD4+ T cells that could degranulate (CD107a+) (Figure 5E, left, and Supplemental Figure 7C). However, similar to effector CD8+ T cells, there was a negative correlation between IL-10 expression and TNF-α and IL-2 production within the IFN-γ+ CD4+ Th1 cell population. Specifically, relative to IL-10– Th1 cells, IL-10+ Th1 cells had a lower TNF-α MFI and produced little IL-2 (Figure 5E, right, and Supplemental Figure 7C). Interestingly, although the few IL-10–producing Th1 cells that formed in acute LCMV infection exhibited normal TNF-α production, we found that they produced less IL-2 (Supplemental Figure 8, C and D). Collectively, these data argue that the Th1 cell subset with the most impaired inflammatory function is the predominant IL-10 source.
Because IL-2 helps sustain the antiviral CD8+ T cell response during LCMV-Cl.13 infection (24), the inverse relationship between IL-2 and IL-10 expression suggested a separation between effector CD4+ T cell helper and suppressive functions. IL-21 is another key cytokine that helps maintain antiviral CD8+ T cell function and survival during LCMV-Cl.13 infection (25–27), so we examined its expression in virus-specific CD4+ T cells. Il10 expression correlated tightly with Thy1.1 expression, as expected (Figure 5F, left), but while there was some evidence of elevated Il21 expression within Thy1.1– cells, this difference was not statistically significant (Figure 5F, right). Thus, the separation of CD8+ T cell helper and regulatory functions within the virus-specific CD4+ T cells is evident in terms of IL-2, but not IL-21, production.
IL-10 induction within Th1 cells is NFIL3 and NOTCH independent.
We next investigated the transcriptional pathways responsible for IL-10 induction within virus-specific T cells. We chose to focus on IL-10 regulation within virus-specific CD4+ T cells during chronic infection, as these cells appeared to be a more abundant and physiologically relevant IL-10 source than were virus-specific CD8+ T cells. Transcription factors such as AhR, c-MAF, NFIL3, and Ikaros (IKZF) induce IL-10 expression within CD4+ T cells, and increased expression of these factors is typically required for IL-10 induction. However, we failed to observe elevated expression of most of these factors in STg cells during chronic versus acute LCMV infection, and these factors were not enriched within the IL-10+ STg cell population (Supplemental Figure 9A, and data not shown). While Nfil3 expression was elevated in STg cells during LCMV-Cl.13, retroviral Nfil3 knockdown using an shRNA vector had no impact on STg IL-10 production (Supplemental Figure 9, A–C). NOTCH can cause IL-10 induction in Th1 cells in cooperation with the transcription factor RBP-Jκ (28), however RBP-Jκ knockdown also had no effect on IL-10 production (Supplemental Figure 9, B and C). Thus, IL-10 induction within Th1 cells during LCMV-Cl.13 infection is NOTCH and NFIL3 independent.
Elevated BLIMP-1 expression sustains Th1 IL-10 production during chronic viral infection.
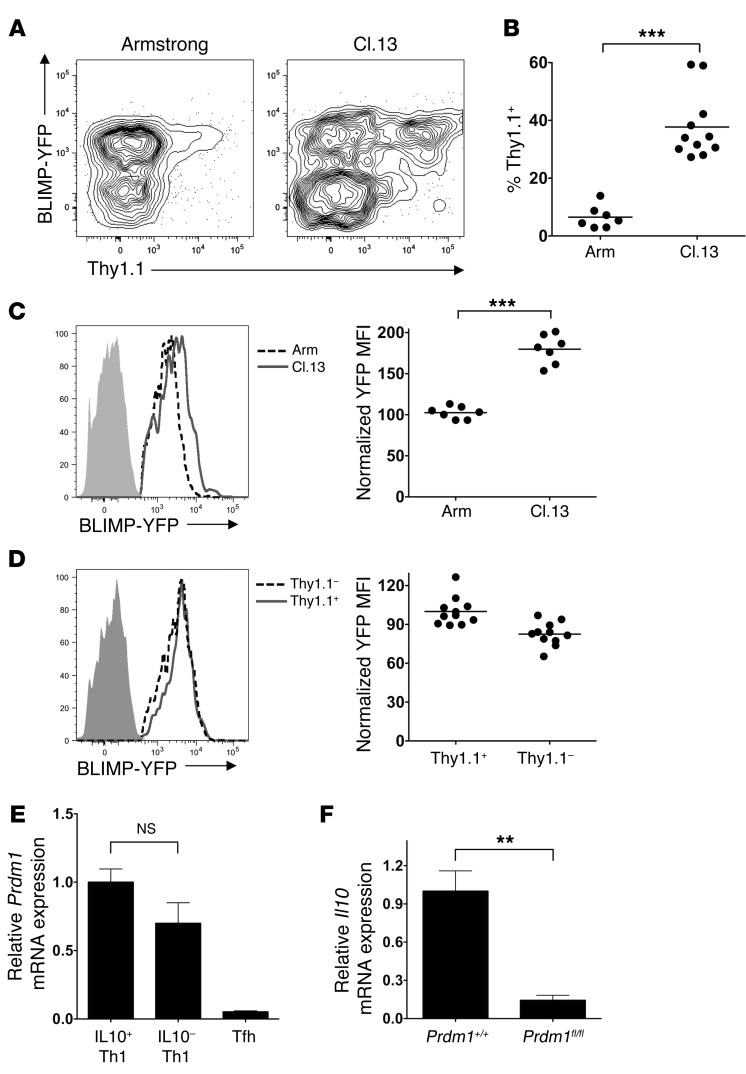
The transcription factor BLIMP-1 (encoded by the Prdm1 gene) was recently demonstrated to regulate IL-10 expression in CD8+ T cells and FOXP3+ Tregs (29, 30), so we next examined whether BLIMP-1 was responsible for IL-10 induction in Th1 CD4+ T cells during chronic LCMV infection. To first assess BLIMP-1 expression during LCMV infection, 10BiT mice were crossed with a BLIMP-YFP reporter mouse (31). The resulting 10BiT BLIMP-YFP mice were infected with LCMV-Cl.13 or Arm, and YFP expression was assessed within CD4+GP66+ cells on day 8 of infection. We observed a bimodal distribution of BLIMP-1 expression in both LCMV-Arm and Cl.13 infection, with YFPhi and YFPlo cell populations evident (Figure 6A). Based on surface marker expression and consistent with recent observations (32), the YFPhi cell population represented Th1 cells, while the YFPlo cell population represented Tfh cells (Supplemental Figure 10A). Consistent with recent findings, we also observed an increased proportion of YFPlo Tfh cells in chronic versus acute LCMV infection (Supplemental Figure 10B). In line with our earlier phenotyping data, IL-10 production was largely confined to the YFPhi Th1 cell population, with IL-10 production elevated within YFPhi Th1 cells in chronic versus acute infection (Figure 6, A and B). To examine whether BLIMP-1 expression was higher in Th1 cells from chronic versus acute LCMV infection, we determined the YFP MFI within the YFP+-gated (Th1) cells from mice infected with LCMV-Arm or Cl.13. BLIMP-YFP reporter expression was almost 2-fold higher in Th1 cells derived from LCMV-Cl.13 compared with those derived from Arm infection (Figure 6C), suggesting that BLIMP-1 plays a role in IL-10 regulation. However, BLIMP-YFP expression analysis and quantitative RT-PCR failed to demonstrate a statistically significant increase in BLIMP-1 expression in IL-10+ versus IL-10– CD4+GP66+ Th1 cells during Cl.13 infection (Figure 6, D and E). To examine the role of BLIMP-1 in IL-10 expression within virus-specific CD4+ T cells, Il10 transcript levels were measured in CD4+GP66+ cells sorted from Cd4-Cre Prdm1+/+ or Cd4-Cre Prdm1fl/fl mice. BLIMP-1 is a negative regulator of Tfh formation (33), so to ensure that any observed differences in IL-10 expression were not due to the lower Th1 cell proportion within the CD4+GP66+ cell population (Supplemental Figure 11A), we isolated PSGL1hiLy6Chi Th1 cells from both BLIMP-1–sufficient and –deficient CD4+GP66+ cells. We observed a striking loss of IL-10 expression in CD4+GP66+ Th1 cells deficient in BLIMP-1 expression (Figure 6F).
Figure 6. BLIMP-1 regulates IL-10 production by CD4+GP66+ cells during LCMV-Cl.13 infection.
(A–D) 10BiT BLIMP-YFP mice were infected with LCMV-Arm or LCMV-Cl.13 and BLIMP-YFP reporter, and Thy1.1 IL-10 reporter expression was examined within CD4+GP66+ tetramer+ cells on day 8 p.i. Representative profiles in A show YFP and Thy1.1 expression in total CD4+GP66+ cells in LCMV-Arm and Cl.13 infection, while B quantitates the Thy1.1+ cell percentage within BLIMP-YFP+ cells. The BLIMP-YFP MFI within YFP+-gated CD4+GP66+ Th1 cells was compared between LCMV-Arm versus Cl.13 infection (C) or between Thy1.1+ versus Thy1.1– cells during LCMV-Cl.13 infection (D). Shaded histograms illustrate background YFP in BLIMP-YFP transgene–negative mice. The BLIMP-YFP MFI was normalized to the average MFI of cells either from the LCMV-Arm (C) or the Thy1.1+ cells (D). Representative and pooled data are from 2 (A–C) or 4 (D) independent experiments. (E) Ly5.1+ STg 10BiT cells were transferred into B6 mice subsequently infected with 2 × 106 PFU LCMV-Cl.13. On day 8 p.i., the Thy1.1+PSGL1hi (IL-10+ Th1), Thy1.1–PSGL1hi (IL10- Th1), and (Thy1.1–) PSGL1lo (Tfh) STg 10BiT cells were isolated by cell sorting, and Prdm1 mRNA was measured by quantitative RT-PCR. Data were pooled from 3 independent experiments. (F) Cd4-Cre+ Prdm1+/+ or Cd4-Cre+ Prdm1fl/fl mice were infected with 2 × 106 PFU LCMV-Cl.13. At day 8 p.i., the CD4+GP66+ tetramer–stained PSGL1hiLy6Chi Th1 cells were isolated by FACS, and Il10 mRNA was measured by quantitative RT-PCR. Data were compiled from 3 independent experiments. ***P < 0.001; **P < 0.01; NS, indicates P > 0.05.
Importantly, although Th1 proportions were diminished in Cd4-Cre Prdm1fl/fl mice, the Th1 cells that did form appeared functionally normal with regard to IFN-γ, TNF-α, and T-bet expression (Supplemental Figure 11, A–C), suggesting that IL-10 loss upon BLIMP-1 ablation was not due to abnormal Th1 differentiation. Despite the IL-10 reduction in BLIMP-1–deficient T cells in Cd4-Cre Prdm1fl/fl mice, we did not observe augmented viral clearance (data not shown). However, this is likely because the additional defects in effector CD8+ T cell function that occur in the absence of BLIMP-1 (34) override any advantage of IL-10 reduction. These data indicate that chronic viral infection elevates BLIMP-1 expression within antiviral Th1 cells, which leads to immunoregulatory IL-10 production.
Antigen engagement induces BLIMP-1 and IL-10 in Th1 cells.
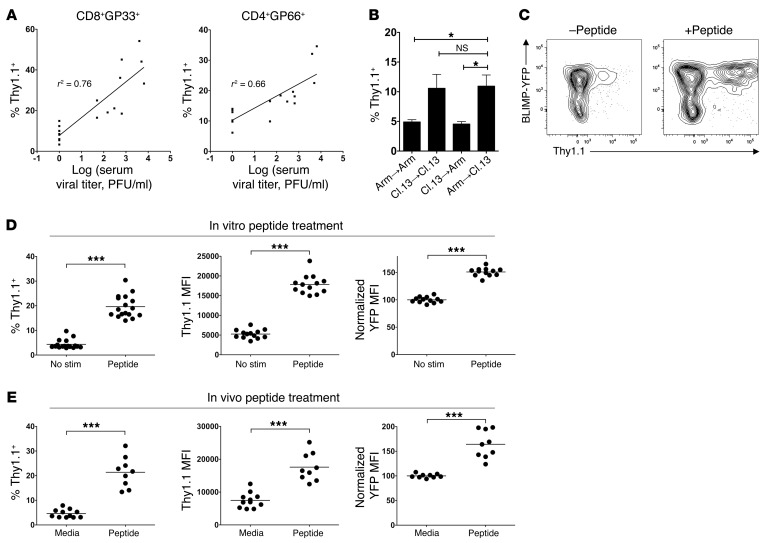
A key environmental signal that T cells are exposed to in chronic, but not acute, viral infection is persistent antigen encounter. We thus speculated that the elevated BLIMP-1 and IL-10 expression in virus-specific T cells during LCMV-Cl.13 versus Arm infection was mediated by prolonged TCR engagement. On day 60 p.i. with Cl.13, we observed considerable variability between mice in IL-10 expression by virus-specific T cells. Consistent with the idea that persistent virus encounter sustains IL-10 production by virus-specific T cells, we observed a direct positive correlation between serum viral titers on day 60 p.i. and Thy1.1 expression of both CD8+GP33+ and CD4+GP66+ cells (Figure 7A). Importantly, both serum viral titers and CD4+GP66+ cell IL-10 production also positively correlated with CD8+GP33+ exhaustion (as assessed by PD-1 expression) (Supplemental Figure 12). To more directly test whether viral persistence could sustain IL-10 production, we performed adoptive transfer experiments in which polyclonal effector (CD44hi) CD4+ T cells were isolated from either LCMV-Arm– or Cl.13-infected 10BiT mice on day 8 p.i. and transferred into LCMV-Arm– or Cl.13 infection–matched, congenically marked recipients. Seven days after transfer (day 15 p.i.), we assessed the IL-10–producing (Thy1.1+) cell percentage within the transferred effector cells. Strikingly, we found that effector cell transfer from either the LCMV-Arm– or the Cl.13-infected donors into Arm-infected recipients led to loss of IL-10 expression (Figure 7B). In direct contrast, effector cell transfer from either LCMV-Arm– or Cl.13-infected donors into Cl.13-infected recipients led to sustained IL-10 expression (Figure 7B). Collectively, these data demonstrate that virus persistence is required for sustained IL-10 production and argue that Th1 cells from both chronic and acute infection can sustain IL-10 expression when exposed to persistent virus.
Figure 7. TCR engagement converts CD4+GP66+ Th1 cells from acute viral infection into BLIMPhi IL-10 producers.
(A) 10BiT mice were infected with 2 × 106 PFU LCMV-Cl.13, and serum viral titers and the Thy1.1+ CD4+GP66+ and CD8+GP33+ cell frequency were correlated on day 60 p.i. for each mouse. Correlation coefficients (r2) are indicated, and data were pooled from 2 independent experiments. (B) 10BiT splenocytes were isolated on day 8 p.i. with either LCMV-Arm or LCMV-Cl.13 and transferred into infection-matched LCMV-Arm or LCMV-Cl.13 mice. Seven days later (day 15 p.i.), the Thy1.1+ cell percentage was measured within the activated (CD44hi) CD4+ T cells. Data were pooled from 3 independent experiments (n = 6–8 mice). (C and D) Ly5.1+ STg 10BiT BLIMP-YFP cells were transferred into B6 mice subsequently infected with LCMV-Arm. On day 8 p.i., splenocytes were incubated with GP66–77 peptide (Peptide) or left untreated (No stim) for 8 hours. (C) Thy1.1 versus BLIMP-YFP expression in STg cells with or without peptide treatment. (D) Pooled data. Left graph shows the percentage of Thy1.1+ cells; center graph shows Thy1.1 MFI of Thy1.1+ cells; right graph shows normalized YFPhi (Th1) cell YFP MFI, with BLIMP-YFP MFI normalized to the “No stim” group. Data were pooled from 3 to 5 independent experiments. (E) 10BiT BLIMP-YFP mice were infected with LCMV-Arm, and were injected 7 days p.i. with either media or GP66–77 peptide. Twelve hours later, splenic CD4+GP66+ tetramer–stained cells were analyzed for Thy1.1 and YFP expression as in C. Data were pooled from 3 independent experiments. ***P < 0.001; *P < 0.05.
To more directly test whether antigen alone was sufficient to induce IL-10 expression, we examined whether antigen reexposure could convert IL-10– STg cells from acute LCMV-Arm infection into IL-10 producers. B6 mice given STg cells that express the 10BiT and BLIMP-YFP reporters were infected with LCMV-Arm, and 8 days p.i., the cells were incubated in vitro for 8 hours in the presence or absence of GP66–77 peptide. We observed a marked IL-10 (Thy1.1) induction in peptide-treated cells relative to untreated controls, with a substantial increase in both Thy1.1+ cell percentage and Thy1.1 MFI (Figure 7, C and D). The IL-10–producing cells were restricted to the Th1 population, because IL-10 expression segregated with PSGL1hi Ly6Chi IFN-γhi and BLIMP-YFPhi cells (Figure 7C and Supplemental Figure 13A). Peptide stimulation did not alter the BLIMP-YFPhi cell percentage, but it did cause an increase in BLIMP-YFP reporter expression within the YFPhi Th1 cell population (Figure 7D, right graph, and data not shown). IL-10 and BLIMP-1 induction in these cultures appeared ICOS and IL-2 independent, since antibody blockade of these factors had little impact on IL-10 and BLIMP-1 reporter expression (Supplemental Figure 13B). Importantly, restimulation-induced IL-10 expression was BLIMP-1 dependent, since BLIMP-1–deficient Th1 cells from LCMV-Arm infection were severely deficient in IL-10 expression upon restimulation (Supplemental Figure 14). Antigen recognition in vivo was also able to induce IL-10 and BLIMP-1 expression in LCMV-specific Th1 cells. Intravenous GP66–77 peptide administration to LCMV-Arm–infected 10BiT BLIMP-YFP mice caused a significant increase in both IL-10 and BLIMP-1 reporter expression in CD4+GP66+ Th1 cells (Figure 7E). Again, the IL-10–producing cells were exclusively within the PSGL1hi BLIMP-1-YFPhi Th1 cell population (Supplemental Figure 13C). Together, these data show that antigen recognition in vitro and in vivo can rapidly induce IL-10 and BLIMP-1 expression selectively within LCMV-specific Th1 cells. These data therefore suggest that TCR signaling is the primary driver of sustained IL-10 production in virus-specific T cells during chronic infection and that antigen-induced, BLIMP-1–dependent IL-10 expression is a common feature of Th1 cells in both chronic and acute infection. Collectively, these results support a negative feedback loop model during chronic viral infection, in which persisting viral antigens sustain IL-10 production in virus-specific T cells, thereby promoting CTL exhaustion and viral persistence.
Differential requirements for ERK signaling in IL-10 versus inflammatory cytokine induction.
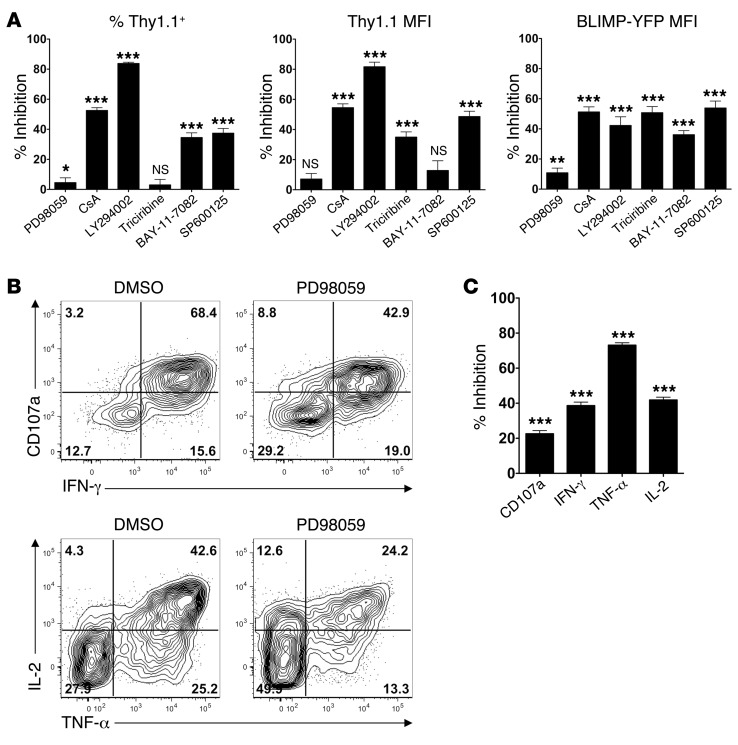
We next examined the signaling pathways responsible for IL-10 induction downstream of TCR engagement. The in vitro antigen–induced IL-10 expression experiments outlined in Figure 7, C and D, were repeated in the presence of a panel of inhibitors that interfere with various TCR signaling pathways. From this analysis, blockade of PI3 kinase signaling (LY294002), as well as many of the pathways downstream of PI3 kinase (Akt [triciribine], JNK [SP600125], and NF-κB [BAY-11-7082]), inhibited both BLIMP-1 and IL-10 induction (Figure 8A). Additionally, blockade of calcineurin/NFAT signaling (CsA) also significantly impaired BLIMP-1 and IL-10 induction (Figure 8A). In contrast, ERK blockade (PD98059) had little or no effect on IL-10 and BLIMP-1 induction.
Figure 8. Differential ERK dependency for TCR-induced IL-10 versus inflammatory cytokine production.
(A) Ly5.1+ STg 10BiT BLIMP-YFP cells were transferred into B6 mice that were subsequently infected with LCMV-Arm. Splenocytes were isolated on day 8 p.i. and incubated with GP66–77 peptide for 8 hours along with the indicated inhibitors. The percentage of inhibition with each inhibitor relative to the appropriate vehicle control–treated cells is shown for the percentage of Thy1.1+ cells, Thy1.1 MFI (of Thy1.1+ cells), and BLIMP-YFP MFI (of BLIMP-YFPhi Th1 cells). Data were pooled from 2 to 7 independent experiments (7–21 mice/group). (B and C) Ly5.1+ STg cells were transferred into B6 mice that were subsequently infected with LCMV-Arm. Splenocytes were isolated on day 8 p.i. and incubated with GP66–77 peptide for 8 hours as in A in the presence of either vehicle (DMSO) or PD98059. CD107a, IFN-γ, TNF-α, and IL-2 production was examined (B), and the percentage of inhibition of total MFI induction relative to DMSO control was calculated (C). Representative (B) or pooled (C) data from 3 independent experiments are shown (15 mice/group). ***P < 0.001; **P < 0.01; *P < 0.05; NS, indicates P > 0.05. P values in A and C measure the significance of treated versus control cells for each condition.
The failure of ERK blockade to interfere with IL-10 induction was surprising, given previous findings that TCR-induced IL-10 expression within Th1 cells is ERK dependent (35). Furthermore, as ERK is thought to be important in inflammatory cytokine induction, this potentially implied that there are distinct signaling requirements for IL-10 versus inflammatory cytokine induction. To test this idea, we examined whether TCR-induced expression of the inflammatory cytokines IFN-γ, TNF-α, and IL-2, as well as degranulation (CD107a expression), within Th1 cells was ERK dependent. While ERK inhibition only slightly inhibited degranulation (CD107a expression), it moderately inhibited IFN-γ and IL-2 induction and substantially impaired TNF-α expression (Figure 8, B and C). Thus, the signaling requirements for TCR-induced inflammatory cytokine versus antiinflammatory IL-10 induction are somewhat distinct.
Discussion
In this study, IL-10 expression kinetics were followed over the course of chronic LCMV-Cl.13 infection using an IL-10 reporter mouse. We found that virus-specific T cells, particularly virus-specific CD4+ T cells, overproduced IL-10 in chronic versus acute LCMV infection. Selective IL-10 loss from the T cell compartment had an impact on viral clearance that partially mimicked the effect of complete IL-10 loss, suggesting that T cell–derived IL-10 was biologically relevant. When IL-10 expression was examined within virus-specific CD4+ T cells, IL-10 production was restricted to the Th1 compartment, although it was only evident in those Th1 cells with the most diminished inflammatory function. IL-10 production was similarly observed within the most exhausted virus-specific CD8+ T cells, suggesting that IL-10 production is a conserved feature of T cell exhaustion during chronic viral infection. IL-10 induction within virus-specific Th1 cells appeared independent of many well-characterized factors linked to IL-10 induction, such as c-MAF, AhR, Ikaros, NFIL3, and NOTCH, with IL-10 expression instead dependent on elevated BLIMP-1 expression during chronic viral infection. Finally, TCR engagement selectively acts on the Th1 cell population, likely in an IL-2–independent manner, to convert virus-specific CD4+ T cells from an acute infection into BLIMP-1hi IL-10 producers. Thus, persistent TCR engagement elevates BLIMP-1 levels in Th1 cells during chronic LCMV infection, causing IL-10 production and negative feedback on the T cell response.
This study is one of the first to examine IL-10 production kinetics in chronic versus acute viral infection across many cell types. While the cell types producing IL-10 have been examined in human chronic viral infections (8–11, 13, 14), an equivalent acute viral infection was not available for comparison. Our data demonstrate that many cell types contribute to the total IL-10 pool, particularly at later time points of infection. However, only 3 cell types exhibited elevated IL-10 expression from day 8 of infection throughout the course of disease: NK cells, DCs (particularly cDCs), and virus-specific T cells. Cell types that overproduce IL-10 from day 8 of infection onward are likely to play a larger role in IL-10–mediated suppression, as IL-10 receptor blockade is more efficacious over the first 8 days of infection than at later time points (5, 6, 18). Thus, coupled with our finding that T cell–derived IL-10 is only partially responsible for IL-10–mediated immunosuppression, we speculate that these 3 cell types in combination are required for optimal IL-10–mediated immunosuppression. Together, IL-10 production from these sources could elevate IL-10 levels above a threshold level required for immunosuppression during the early time points of chronic viral infection. Nevertheless, a recent study demonstrated a minimal role for NK cell–derived (and B cell–derived) IL-10 in immunosuppression, but supported our conclusion that T cell–derived IL-10 is one of the most important IL-10 sources during chronic viral infection (36). The role of DC-derived IL-10 is still unclear, as one study suggested that IL-10 ablation from DCs has no effect on the immune response and viral control (36), while another study showed a substantial effect (37).
Our study also directly demonstrates sustained and elevated IL-10 expression by virus-specific T cells during chronic versus acute LCMV infection and identifies quantitative differences in IL-10 production between CD4+ and CD8+ T cells. T cells were originally dismissed as an important IL-10 source during LCMV-Cl.13 infection, because IL-10 expression was only modestly increased within the total CD4+ and CD8+ T cell populations in chronic versus acute LCMV infection (5). However, virus-specific T cells, which only represent a portion of the total T cell population, were not examined directly, hence the magnitude of IL-10 induction within T cells was likely underestimated. Indeed, DCs derived from Cl.13-infected mice preferentially prime IL-10–producing CD4+ T cells in vitro (6), and T cells produce high IL-10 levels during chronic HIV infection in humans (8, 13, 14). Importantly, we provide some of the first in vivo evidence that IL-10 elimination from either the total T cell compartment, or to a lesser extent from the CD4+ T cell compartment, has an effect on both the antiviral T cell response and viral clearance during chronic viral infection. This conclusion is supported by another recent study (36), although the relative contributions of CD4+ and CD8+ T cell–derived IL-10 were not assessed in this study. These data are in line with observations in other persistent nonviral infections, such as malaria, Leishmania, and Toxoplasma, in which effector T cell–derived IL-10 has an impact on disease outcome (38–40). While our BM chimera experiments also eliminated IL-10 from Tregs, it is unlikely that Treg-derived IL-10 contributed to our phenotype, as Treg depletion during days 0–8 of LCMV-Cl.13 infection had little impact on the T cell response (Supplemental Figure 5).
The physiological relevance and high per-cell production levels of virus-specific CD4+ T cell–derived IL-10 led us to study these cells in more detail. Consistent with other infectious models, IL-10 production was observed from FOXP3– virus–specific effector Th1 cells (38–41). Strikingly, IL-10 production was only evident within those Th1 cells that had diminished inflammatory function, an observation not made in previous studies. IL-10 production similarly segregated with the most exhausted virus-specific CD8+ T cells, consistent with previous findings (13, 19). Collectively, these data highlight that T cell exhaustion is not only associated with a loss of inflammatory function, but also with a gain of suppressive function. Importantly, IL-10+ Th1 cells specific for persistent pathogens have been isolated from human blood (42), indicating that these immunoregulatory Th1 cells are evolutionarily conserved, that they similarly form in response to persistent infection in humans, and are thus likely clinically relevant to the persistence of human infections.
Surprisingly, IL-10 production was excluded from the Tfh compartment. The reasons for this remain unclear, as IL-10 has been described as a Tfh cytokine in humans (43), but it could be due to differences between mouse and human Tfh cells. Alternatively, the signals required for Tfh IL-10 production are distinct from those that trigger Th1 IL-10 production, and LCMV-Cl.13 infection may not generate these signals. Consistent with this idea, Th1 IL-10 production during LCMV-Cl.13 infection was BLIMP-1 dependent, and BLIMP-1 is an inhibitor of Tfh differentiation that is typically not expressed within Tfh cells (32, 33).
Perhaps of most interest is the novel pathway responsible for IL-10+ Th1 cell formation during LCMV-Cl.13 infection. Many transcription factors, including c-MAF (44), AhR (45), NFIL3 (46), and RBP-Jκ (28), have been linked to IL-10 expression within FOXP3– CD4+ T cells. However, these factors were either not upregulated within virus-specific CD4+ T cells during LCMV-Cl.13 infection or were not required for IL-10 production. BLIMP-1 is a transcription factor recently reported to induce IL-10 expression both in virus-specific CD8+ T cells during influenza infection (30) and in Tregs (29), however it has not been implicated in IL-10 expression in effector CD4+ T cells. Thus, to our knowledge, our data represent the first demonstration that BLIMP-1 expression is elevated in Th1 cells during chronic viral infection and also the first report that BLIMP-1 regulates IL-10 in effector CD4+ T cells. Coupled with observations that BLIMP-1 negatively regulates CD8+ T cell function during LCMV-Cl.13 infection by inhibitory receptor induction (34), these data argue that BLIMP-1 plays diverse roles in inhibiting the T cell response during chronic viral infection.
Finally, we found that TCR engagement induced BLIMP-1 and IL-10 expression in Th1 cells from an acute infection, which implicates antigen as a key signal that sustains IL-10 production during LCMV-Cl.13 infection. In other systems, BLIMP-1 upregulation in T cells depends on IL-2 and STAT3-dependent cytokines (30, 47, 48). We currently have little evidence for a role of STAT3-dependent cytokines in IL-10 induction during LCMV-Cl.13 infection, as CD4+GP66+ cells isolated from Gzmb-Cre Stat3fl/fl mice, in which STAT3 is partially floxed in activated CD4+ T cells, have no defect in IL-10 expression during LCMV-Cl.13 infection (data not shown). IL-2 was recently linked to both BLIMP-1 and IL-10 induction within CD8+ T cells (30), but chronic infection is associated with diminished IL-2 production by virus-specific T cells and lower serum IL-2 levels (49). Furthermore, IL-2 administration promotes, rather than inhibits, viral clearance (50), meaning IL-2 is unlikely to mediate IL-10 induction. Consistent with this idea, IL-2 blockade had little impact on BLIMP-1 and IL-10 induction in vitro (Supplemental Figure 13B). Furthermore, in vivo IL-2 blockade during chronic LCMV infection did not affect BLIMP-1 or IL-10 expression in preliminary experiments (data not shown). Instead, IRF4 is a likely downstream candidate involved in TCR-induced BLIMP-1 and IL-10 expression, because IRF4 is a known activator of BLIMP-1 expression and can be upregulated by TCR stimulation (29, 48, 51). In support of this model, IRF4 expression is elevated in Th1 cells during chronic versus acute LCMV infection in vivo and was induced by in vitro peptide stimulation in parallel with BLIMP-1 and IL-10 (data not shown). Furthermore, inhibitors that prevented BLIMP-1 and IL-10 induction also interfered with IRF4 induction in Th1 cells upon in vitro antigen engagement (data not shown). Indeed, 2 of the pathways required for IL-10 production (NF-κB and NFAT) (Figure 8A) induce IRF4 expression downstream of TCR engagement (51). Therefore, we postulate that sustained TCR engagement triggers BLIMP-1 upregulation and IL-10 expression via IRF4 induction. This pathway may represent an alternative way to engage the IRF4/BLIMP-1/IL-10 axis in effector (and possibly regulatory) T cells.
While TCR engagement induces expression of many cytokines, our data additionally reveal that the signaling requirements for TCR-induced antiinflammatory IL-10 expression differ from those for inflammatory cytokine production. Our data indicate that IL-10 induction is ERK independent, a surprising finding, given that TCR-dependent IL-10 induction in in vitro–generated Th1 cells is ERK dependent (35). The reason for this discrepancy may relate to the c-MAF dependency of IL-10 production; while c-MAF did not appear to be involved in IL-10 expression by Th1 cells during Cl.13 infection, c-MAF was implicated in IL-10 production in the study by Saraiva et al. (35). The differential signaling requirements for IL-10 versus inflammatory cytokine production may have clinical implications, as it implies that in certain contexts, Th1 cell inflammatory function could be selectively and therapeutically blocked, while leaving their antiinflammatory functions intact. This approach may have efficacy in Th1-driven inflammatory diseases.
It remains unclear, though, why only a portion of Th1 cells produced IL-10 during LCMV-Cl.13 infection, given that there was no significant difference in BLIMP-1 (or IRF4) expression between IL-10+ and IL-10– Th1 cells (Figure 6, D and E, and data not shown). This observation suggests that factors other than BLIMP-1 and IRF4 may contribute to the formation of the IL-10+ Th1 subset. While we have not yet identified the molecular reason for this discrepancy, we have noted lower Bcl6 expression in IL-10+ versus IL-10– Th1 cells (Supplemental Figure 15). Since T-bet represses IL-10 production in T cells (52), and BCL-6 is a T-bet corepressor in Th1 cells (53), it is possible that lower BCL-6 expression in Th1 cells relieves the Il10 gene from T-bet–mediated repression, thereby allowing BLIMP-1–mediated transcriptional activation. Indeed, BCL-6 loss promotes IL-10 production by CD4+ T cells (54). Future work is needed to dissect the complex transcriptional regulation of IL-10 in antiviral CD4+ T cells during chronic infection, but our work identifying BLIMP-1 as an essential factor provides both an important first step in this direction and better insight into how the host regulates the balance between antiviral immunity and immunopathology during a chronic infection.
Methods
Mice, infections, and plaque assays.
C57BL/6NCr and CD45.1 (Ly5.1) mice were purchased from the National Cancer Institute. B6.129S2 Tcratm1Mom/J (Tcra–/–), B6.129S6 Cd4tm1Knw/J (Cd4–/–), B6.129S2 Cd8atm1Mak/J (Cd8a–/–), and B6.129P2 Il10tm1Cgn/J (Il10–/–) mice were purchased from The Jackson Laboratory. Ly5.1+ P14 (55), Prdm1fl/fl Cd4-Cre (56), BLIMP-YFP (31), 10BiT (16), and STg (57) mice have been previously described. Mice were infected with 2 × 105 PFU LCMV-Arm (i.p.), 1 × 102 PFU LCMV-clone 13 (i.v.) for acute infections, or 2 × 106 PFU LCMV-clone 13 (i.v.) for chronic infections. Viral titers were measured by plaque assay (31). For details on adoptive transfers, see Supplemental Methods.
Antibodies for surface and intracellular staining.
Lymphocyte isolation, surface staining, tetramer staining, and intracellular staining were performed as described previously (20, 31). See Supplemental Methods for more details.
BM chimeras.
BM chimeras were generated as previously described (31). Thy1.1 mice were used as recipients, and injections with the Thy1.1-depleting antibody 19E12 were used to ensure depletion of any surviving host T cells.
Retroviral knockdown.
An LMP retroviral vector (Open Biosystems) with the puromycin resistance gene removed (32) was used for retroviral transductions and was a gift of Shane Crotty (La Jolla Institute for Allergy and Immunology, La Jolla, California, USA.). The sequences for knockdown were: NFIL3, CCCGCACAAGCTTCGGATTAAA, and RBP-Jκ, AAAGGAGAGGAGTTCACAGTTA from Open Biosystems, using shRNAmir flanking sequences. See Supplemental Methods for additional details.
Cell isolation and stimulation.
Cells were sorted using a FACSAria sorter (BD Biosciences). In some experiments, transferred Ly5.1+ STg cells were enriched using biotinylated Ly5.1 antibody and streptavidin microbeads (Miltenyi Biotec) prior to sorting. In experiments involving stimulation, cells were either stimulated in tissue culture plates precoated with 1 μg/ml α-CD3 (clone 17A2) or with 20 ng/ml PMA and 1 μM Ca2+ ionophore (ionomycin) for the indicated times (Figure 1, Figure 3, Figure 5, Supplemental Figure 8, and Supplemental Figure 14). For IL-10 ELISAs, the CBA Mouse IL-10 Flex Set was used (BD Biosciences). For more details, see Supplemental Methods.
RNA isolation and quantitative RT-PCR.
RNA was isolated from cells using an RNeasy Kit (QIAGEN), and cDNA was synthesized with Superscript II (Invitrogen). Real-time PCR was performed using the Brilliant III Ultra-Fast QPCR Master Mix (Agilent Technologies) on a Stratagene MX3000P real-time PCR machine (Agilent Technologies). Expression was normalized using the housekeeping gene Rpl9. The primers used for amplification are listed in Supplemental Table 1. AhR expression was measured using the Mm00478930-ml primer-probe mixture from Applied Biosystems.
Statistics.
All error bars represent SEM. A P value of less than 0.05 was considered statistically significant. For more details, see Supplemental Methods.
Study approval.
All animal experiments were performed with the approval of the IACUC of Yale University (New Haven, Connecticut, USA).
Supplementary Material
Acknowledgments
The authors would like to thank C. Dominguez, A. Chandele, B. Lu, K. Park, Y. Liu, other Kaech Laboratory members, J. Weinstein, and J. Craft for suggestions and technical help. We also wish to thank Shane Crotty for providing a modified LMP retroviral vector for gene knockdown. This work was supported by an Australian NHMRC Overseas Biomedical Postdoctoral Fellowship (to I.A. Parish); a Yale School of Medicine Brown-Coxe Postdoctoral Fellowship (to I.A. Parish); the Alexander von Humboldt Foundation (SKA2010, to P.A. Lang); a CIHR grant (to P.S. Ohashi); and by the Howard Hughes Medical Institute and NIH grant RO1AI074699 (to S.M. Kaech). P.S. Ohashi holds a Canada Research Chair in Autoimmunity and Tumor immunity.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2014;124(8):3455–3468. doi:10.1172/JCI66108.
Ian A. Parish’s present address is: Department of Immunology, The John Curtin School of Medical Research, The Australian National University, Canberra, Australia.
References
- 1.Crawford A, et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity. 2014;40(2):289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19(4):408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12(11):1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ejrnaes M, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203(111):2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. Cell-intrinsic transforming growth factor-β signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity. 2009;31(1):145–157. doi: 10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brockman MA, et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114(2):346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Maria A, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37(2):445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 10.Liu BS, Groothuismink ZM, Janssen HL, Boonstra A. Role for IL-10 in inducing functional impairment of monocytes upon TLR4 ligation in patients with chronic HCV infections. J Leukoc Biol. 2011;89(6):981–988. doi: 10.1189/jlb.1210680. [DOI] [PubMed] [Google Scholar]
- 11.Said EA, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16(4):452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson EB, et al. Emergence of distinct multiarmed immunoregulatory antigen-presenting cells during persistent viral infection. Cell Host Microbe. 2012;11(5):481–491. doi: 10.1016/j.chom.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elrefaei M, Baker CA, Jones NG, Bangsberg DR, Cao H. Presence of suppressor HIV-specific CD8+ T cells is associated with increased PD-1 expression on effector CD8+ T cells. J Immunol. 2008;180(11):7757–7763. doi: 10.4049/jimmunol.180.11.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graziosi C, et al. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science. 1994;265(5169):248–252. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 15.Ng CT, Oldstone MB. Infected CD8α-dendritic cells are the predominant source of IL-10 during establishment of persistent viral infection. Proc Natl Acad Sci U S A. 2012;109(35):14116–14121. doi: 10.1073/pnas.1211910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maynard CL, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3– precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8(9):931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 17.Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc Natl Acad Sci U S A. 2010;107(7):3018–3023. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maris CH, Chappell CP, Jacob J. Interleukin-10 plays an early role in generating virus-specific T cell anergy. BMC Immunol. 2007;8:8. doi: 10.1186/1471-2172-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin HT, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107(33):14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall HD, et al. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011;35(4):633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 22.Hale JS, et al. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38(4):805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odegard JM, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205(12):2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37(6):1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 25.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frohlich A, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324(5934):1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 27.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324(5934):1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kassner N, et al. Cutting edge: Plasmacytoid dendritic cells induce IL-10 production in T cells via the Delta-like-4/Notch axis. J Immunol. 2010;184(2):550–554. doi: 10.4049/jimmunol.0903152. [DOI] [PubMed] [Google Scholar]
- 29.Cretney E, et al. The transcription factors BLIMP-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12(4):304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 30.Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. CD4+ T cell help and innate-derived IL-27 induce BLIMP-1-dependent IL-10 production by antiviral CTLs. Nat Immunol. 2011;12(4):327–334. doi: 10.1038/ni.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutishauser RL, et al. Transcriptional repressor BLIMP-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31(2):296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi YS, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34(6):932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston RJ, et al. Bcl6 and BLIMP-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin H, et al. A role for the transcriptional repressor BLIMP-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31(2):309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O’Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31(2):209–219. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richter K, et al. Macrophage and T cell produced IL-10 promotes viral chronicity. PLoS Pathog. 2013;9(11):e1003735. doi: 10.1371/journal.ppat.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohyagi H, et al. Monocyte-derived dendritic cells perform hemophagocytosis to fine-tune excessive immune responses. Immunity. 2013;39(3):584–598. doi: 10.1016/j.immuni.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 38.Jankovic D, et al. Conventional T-bet(+)Foxp3(–) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204(2):273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nylen S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, Sacks D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med. 2007;204(4):805–817. doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freitas do Rosario AP, et al. IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol. 2012;188(3):1178–1190. doi: 10.4049/jimmunol.1102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15(3):277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haringer B, Lozza L, Steckel B, Geginat J. Identification and characterization of IL-10/IFN-γ-producing effector-like T cells with regulatory function in human blood. J Exp Med. 2009;206(5):1009–1017. doi: 10.1084/jem.20082238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma CS, et al. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest. 2005;115(4):1049–1059. doi: 10.1172/JCI23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pot C, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183(2):797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apetoh L, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11(9):854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motomura Y, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol. 2011;12(5):450–459. doi: 10.1038/ni.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong D, Malek TR. Cytokine-dependent BLIMP-1 expression in activated T cells inhibits IL-2 production. J Immunol. 2007;178(1):242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- 48.Kwon H, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31(6):941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Grevenynghe J, et al. Loss of memory B cells during chronic HIV infection is driven by Foxo3a- and TRAIL-mediated apoptosis. J Clin Invest. 2011;121(10):3877–3888. doi: 10.1172/JCI59211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9(5):540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 51.Biswas PS, Bhagat G, Pernis AB. IRF4 and its regulators: evolving insights into the pathogenesis of inflammatory arthritis? Immunol Rev. 2010;233(1):79–96. doi: 10.1111/j.0105-2896.2009.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100(26):15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oestreich KJ, Huang AC, Weinmann AS. The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J Exp Med. 2011;208(5):1001–1013. doi: 10.1084/jem.20102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hollister K, et al. Insights into the role of Bcl6 in follicular Th cells using a new conditional mutant mouse model. J Immunol. 2013;191(7):3705–3711. doi: 10.4049/jimmunol.1300378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 56.Cimmino L, et al. BLIMP-1 attenuates Th1 differentiation by repression of ifng, tbx21, and bcl6 gene expression. J Immunol. 2008;181(4):2338–2347. doi: 10.4049/jimmunol.181.4.2338. [DOI] [PubMed] [Google Scholar]
- 57.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28(1):390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.