Abstract
Background
The introduction of tumor necrosis factor (TNF) antagonists (adalimumab, infliximab, and etanercept) was a major advance and was highly important and beneficial in most rheumatoid arthritis (RA) patients. The adverse effects of this treatment are infrequent, but include opportunistic intracellular infection (especially the reactivation of latent Mycobacterium tuberculosis); exacerbation of demyelinating disorders; and the production of various types of antibodies such as antinuclear antibodies (ANA) or double-stranded DNA autoantibodies (dsDNA) and antiphospholipid antibodies (aPL) such as anti-cardiolipin antibodies (aCL) and anti-B2GP-I antibodies (B2GP-I). The aim of the study was to determine the prevalence of aCL and B2GP-I in IgM and IgG classes, using ELISA tests, during 6 months of follow-up in patients with refractory RA successfully treated with infliximab.
Material/Methods
We determined the prevalence of aCL and B2GP-I in IgM and IgG classes, using ELISA tests, during 6 months of follow-up in patients with refractory RA successfully treated with infliximab.
Results
We observed a statistically important increase only in the group of B2GP-I IgM (p<0.05). There are contradictory results concerning the ability of infliximab to induce aPL, but most authors confirm this phenomenon.
Conclusions
Further investigations are needed to determine if the new aPL appears in patients with β2-GPI gene polymorphisms such as leucine-to-valine substitution at position 247, which can lead to a conformational changes in β2-GPI protein, leading to aPL synthesis. The role of aPL in pathogenesis of APS is still unclear, but we should remember the immunogenic aspect of TNF antagonist treatment. Therefore, we recommend early detection of aPL and observation of the patient, paying special attention to signs and symptoms of thromboembolism.
MeSH Keywords: Antibodies, Antiphospholipid, Infliximab, Arthritis, Rheumatoid
Background
The introduction of the tumor necrosis factor (TNF) antagonists – adalimumab, infliximab, and etanercept – was a major advance and was highly important and beneficial in treating most rheumatoid arthritis (RA) patients who are refractory to a classic treatment with disease-modifying anti-rheumatic drugs (DMARDs) [1–5].
The adverse effects of this treatment are infrequent, opportunistic, intracellular infections, especially the reactivation of latent Mycobacterium tuberculosis, an exacerbation of demyelinating disorders. Moreover, the induction of severe neutropenia and thrombocytopenia can occur [6–8]. It is also possible to induce the production of various types of antibodies, such as antinuclear antibodies (ANA) or double-stranded DNA autoantibodies (dsDNA). Treatment with biological agents like infliximab can additionally induce synthesis of anti-drug antibodies, such as the human anti-mouse antibodies (HAMA) or human anti-chimera antibodies (HACA) [9].
The pathogenetic mechanism that changes the humoral response leading to development of autoimmunity during anti-TNF inhibitors therapy is unknown. A possible mechanism leads through the binding of infliximab to the transmembrane and soluble TNF, rapidly lowering TNF level and enhancing apoptotic cell death, which triggers the development of autoantibodies [10,11]. The other possible mechanisms that may result in autoantibodies production are: a) TNF-alpha inhibition that causes B-cell activation and production of autoantibodies through the upregulation of interleukin-10 [12], b) an increase in Th2 activity [13], and c) an increase in bacterial infections, which leads to the production of antibodies through molecular mimicry [6,14–17].
Only limited data have been published about the induction of antiphospholipid antibodies (aPL) during treatment using TNF inhibitors [18–20]. The stimulation mechanisms of its synthesis and role still remain unclear.
Antiphospholipid antibodies target phospholipid-binding proteins, and may cause a prolongation of phospholipid-dependent coagulation assays, although patients are at risk for thromboembolic rather than bleeding complications. The most often recognized antibodies from this group are now anti-cardiolipin antibodies (aCL) and the recently recognized antiphospholipid syndrome (APS) criteria anti-B2GP-I antibodies (B2GP-I). The aCL that are detected in patients with RA and other autoimmune diseases are directed against negatively charged phospholipids associated with B2-glycoprotein, whereas aCL are associated with infection are directed against negatively charged phospholipids alone [21,22].
In normal populations (healthy blood donors), aCL are found in 2–6% of people, and in an aging population are found in up to 12% and have been associated with the symptoms of APS such as recurrent thromboembolism and fetal loss [23,24]. In RA patients, the incidence of aCL may be even higher [25]. Their clinical significance in RA is uncertain and their presence has been considered to be a non-specific marker of activation of the immune system [26].
Material and Methods
We enrolled 32 infliximab-treated patients with refractory RA (28 females and 4 males, medium age 45.4 years, range 19–60 years). All of them were RF-positive and 25/32 (78%) were aCCP-positive. Patients were treated at the Department of Rheumatology and Connective Tissue Diseases, Medical University of Lublin, Poland.
All patients had a history of failed treatment with at least 1 DMARD. The patients were allowed to continue DMARDs, steroids, and non-steroid anti-inflammatory drugs before and during infliximab treatment. No patient had an infectious disease, active or latent tuberculosis, neoplastic disease, heart failure, cytopenia, or a demyelinating disorder.
The patients received 3 mg/kg infliximab intravenously at weeks 0, 2, and 6, and every 8 weeks thereafter. Methotrexate was given in a dose of 10 to 20 mg weekly. In addition to methotrexate, chloroquine (250 mg daily) and steroids (maximum daily dose 10 mg of oral prednisone or equivalent) were also permitted.
Written informed consent was obtained from all patients and the study was approved by the Bioethics Committee of the Medical University of Lublin.
Blood serum samples were collected from all patients at baseline and after 3 and 6 months of anti-TNF treatment. The sera were stored at −70°C until further analysis.
The patients were examined clinically at baseline and after 3 and 6 months of the study by the same physician during each visit for infliximab infusion.
The aCL and B2GP-I antibodies (IgG and IgM classes) were tested using a commercially available enzyme-linked immunoabsorbent assay (ELISA) (Euroimmun, Germany). All the serum samples of RA patients were analyzed in a single session according to the manufacturer’s instructions. The antibodies levels were measured in arbitrary units per milliliter and were considered to be positive at a cut off value of ≥20 U/ml. In further analysis, the sample was described as positive or negative.
In the present study, we investigate the prevalence of such autoantibodies during 6 months of follow-up in patients with RA successfully treated with infliximab. aCL and B2GP-I autoantibodies were evaluated at baseline and at 3 and 6 months after the beginning of infliximab treatment.
Statistical analysis
Statistical analysis was performed using Statistica 7.0 PL software. Differences between groups were analyzed using Mann-Whitney U test. A p value less than 0.05 was considered to be statistically significant.
Results
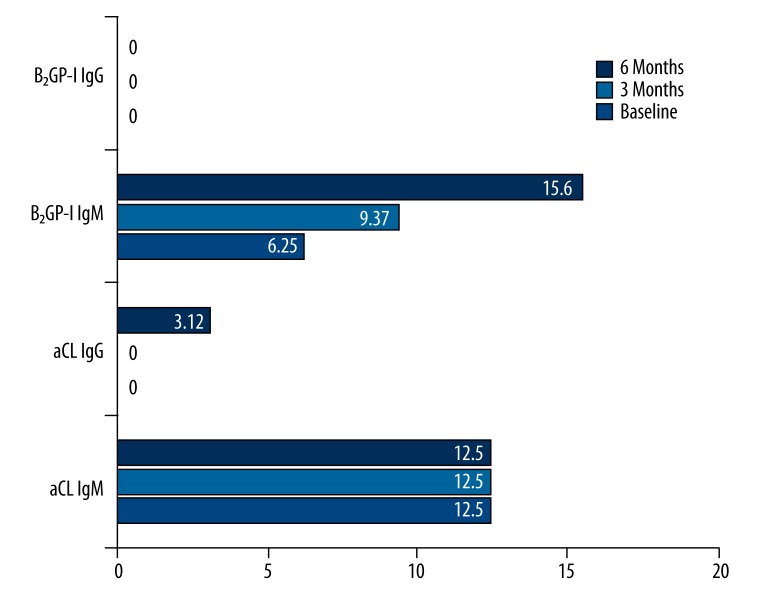
We observed 4 aCL IgM-positive (12.5%) patients before the beginning of infliximab treatment. In these cases, we found no changes after 3 and 6 months of observation. There were no aCL IgG-positive patients at the beginning and after 3 months, but 1 case (3.12%) of seroconversion was observed after 6 months in aCL IgG class. During the whole study, we did not observe any B2GP-I IgG-positive patients. Otherwise, we noticed a statistically important increase in the group of B2GP-I IgM-positive patients (p<0.05) (Figure 1). There were 2 (6.25%) seropositive patients before the start of the treatment, 1 (3.33%) after 3 months, and the next 3 (10.3%) after 6 months. Totally, we observed 4 (13.3%) new B2GP-I IgM-positive patients, which was statistically significant.
Figure 1.
The percentage of aCL and B2GP-I in IgM and IgG classes at the beginning and after 3 and 6 months of rheumatoid arthritis treatment with infliximab.
Discussion
Elliott et al. found aCL in 1 out of 20 patients with RA treated with anti-TNF (cA2) in the first 8-week open trial on humans [18]. These preliminary findings were confirmed by subsequent observations [14,17,27,28]. Rankin et al. measured the serological effects of repeated doses of the humanized anti-TNF antibody CDP 571 in patients with RA and found that some patients develop positive aCL (IgG) [29]. Ferraccioli et al. showed variations in aCL titers over time in etanercept-treated patients with concomitant bacterial infection, where lowering of titers was seen after the treatment with antibiotics [17].
Many studies have confirmed induction of ANA and anti-dsDNA in patients treated with infliximab [10,30–33]. Anti-dsDNA antibodies are usually IgM isotype [10] or IgM and IgA isotypes together [32].
Our results showed statistically significant changes only in B2GP-I IgM class. However, the period of our observation was short and subsequent seroconversions are possible in the near future. We were not able to find an association between new B2GP-I IgM and thromboembolism risk. A similar observation was presented by Jonsdottir et al., who noted a statistically significant increase in aCL IgM positivity after 3 and 6 months of infliximab treatment. This increase was seen in both aCL IgG and IgM classes. Another important observation was the worse clinical presentation in aCL-positive patients [34].
If aPL are not involved in APS, their role is difficult to explain. They may be nonspecific markers of the immune system activation and may vanish without a trace [25,26]. There are some reports that RA is associated with an increased frequency of aCL positivity [13,35,36]; the de novo production of aPL, especially aCL IgM, is associated with APS signs, such as thrombosis [37] or vasculitis [38].
According to Jonsdottir et al., a statistically significant increase in frequency of aCL IgM and IgG was induced in patients with RA at 3 months of treatment with infliximab [34].
The ASPIRE trial reported no statistically significant differences in aCL IgG and IgM classes between infliximab + MTX and MTX + placebo groups before or after treatment. Fewer than 3% of patients who received infliximab plus MTX and who had negative findings for IgG aCL at baseline were found to have positive results at week 30 or 54. For IgM aCL, a slightly higher proportion of patients who received infliximab plus MTX and had negative findings at baseline were found to have positive results at week 30 or week 54 (11.6%), as compared with the proportion of patients who received placebo plus MTX (7.6%) [39].
In the Ferraro-Peyret et al. study, the researchers found 21% of new aCL IgM antibodies [14].
Some authors suggest that the induction of aPL appears usually at the beginning of treatment, but Morris et al. demonstrated that it is important to check the aCL appearance even between the 30th and 54th weeks of observation [28]. Similarly, Visvanathan et al. emphasize that it is important to obtain serial samples over at least 1 year to ascertain a patient’s aPL status [40].
Further investigations are needed to determine if the new aPL appears in patients with β2-GPI gene polymorphisms, such as leucine-to-valine substitution at position 247, which can lead to conformational changes in β2-GPI protein, leading to aPL synthesis [41,42].
Conclusions
The role of aPL in the pathogenesis of APS is still unclear but we should remember the immunogenic aspect of TNF antagonist treatment. Therefore, we recommend early detection of aPL and observation of the patient, paying special attention to signs and symptoms of thromboembolism. A possible preventive treatment should be discussed in certain patients.
Footnotes
Source of support: Departmental sources
References
- 1.Bathon J, Martin R, Fleischmann R, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343(22):1586–93. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 2.Elliott M, Maini R, Feldmann M, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344(8930):1105–10. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 3.Illei G, Lipsky P. Novel, non-antigen-specific therapeutic approaches to autoimmune/inflammatory diseases. Curr Opin Immunol. 2000;12(6):712–18. doi: 10.1016/s0952-7915(00)00167-9. [DOI] [PubMed] [Google Scholar]
- 4.Lipsky PE, van der Heijde DM, StClair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343(22):1594–602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 5.Furst DE, Breedveld FC, Kalden JR, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2006. Ann Rheum Dis. 2006;65(Suppl 3):iii 2–15. doi: 10.1136/ard.2006.061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoni C, Braun J. Side effects of anti-TNF therapy: current knowledge. Clin Exp Rheumatol. 2002;20(6 Suppl 28):S152–57. [PubMed] [Google Scholar]
- 7.Vidal F, Fontova R, Richart C. Severe neutropenia and thrombocytopenia associated with infliximab. Ann Intern Med. 2003;139(3):W–W63. doi: 10.7326/0003-4819-139-3-200308050-00021-w4. [DOI] [PubMed] [Google Scholar]
- 8.Day R. Adverse reactions to TNF-alpha inhibitors in rheumatoid arthritis. Lancet. 2002;359(9306):540–41. doi: 10.1016/S0140-6736(02)07718-8. [DOI] [PubMed] [Google Scholar]
- 9.Mirick GR, Bradt BM, Denardo SJ, Denardo GL. A review of human anti-globulin antibody (HAGA, HAMA, HACA, HAHA) responses to monoclonal antibodies. Not four letter words. Q J Nucl Med Mol Imaging. 2004;48(4):251–57. [PubMed] [Google Scholar]
- 10.Charles PJ, Smeenk RJT, De Jong J, et al. Assessment of antibodies to double stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor: Findings in open label and randomized placebo controlled trials. Arthritis Rheum. 2000;43(11):2383–90. doi: 10.1002/1529-0131(200011)43:11<2383::AID-ANR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Aringer M, Steiner G, Graninger WB, et al. Effects of short term infliximab therapy on autoantibodies in systemic lupus erythematosus. Arthritis Rheum. 2007;56(1):274–79. doi: 10.1002/art.22327. [DOI] [PubMed] [Google Scholar]
- 12.Isomäki P, Punnonen J. Pro-and anti-inflammatory cytokines in rheumatoid arthritis. Ann Med. 1997;29(6):499–507. doi: 10.3109/07853899709007474. [DOI] [PubMed] [Google Scholar]
- 13.Seriolo B, Cutolo M, Fasciolo D, et al. Anticardiolipin antibodies in rheumatoid arthritis. Ann Rheum Dis. 1992;51(9):1100. [PubMed] [Google Scholar]
- 14.Ferraro-Peyret C, Coury F, Tebib JG, et al. Infliximab therapy in rheumatoid arthritis and ankylosing spondylitis-induced specific antinuclear and antiphospholipid autoantibodies without autoimmune clinical manifestations: a two-year prospective study. Arthritis Res Ther. 2004;6(6):R535–43. doi: 10.1186/ar1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Miguel S, Jover J, Vadillo C, et al. B cell activation in rheumatoid arthritis patients under infliximab treatment. Clin Exp Rheumatol. 2003;21(6):726–32. [PubMed] [Google Scholar]
- 16.Via CS, Shustov A, Rus V, et al. In Vivo Neutralization of TNFα Promotes Humoral Autoimmunity by Preventing the Induction of CTL 1. J Immunol. 2001;167(12):6821–26. doi: 10.4049/jimmunol.167.12.6821. [DOI] [PubMed] [Google Scholar]
- 17.Ferraccioli G, Mecchia F, Di Poi E, Fabris M. Anticardiolipin antibodies in rheumatoid patients treated with etanercept or conventional combination therapy: direct and indirect evidence for a possible association with infections. Br Med J. 2002;61(4):358–61. doi: 10.1136/ard.61.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott M, Maini R, Feldmann M, et al. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet. 1994;344(8930):1125–27. doi: 10.1016/s0140-6736(94)90632-7. [DOI] [PubMed] [Google Scholar]
- 19.Galli M, Barbui T. Prevalence of different anti-phospholipid antibodies in systemic lupus erythematosus and their relationship with the antiphospholipid syndrome. Clin Chem. 2001;47(6):985–87. [PubMed] [Google Scholar]
- 20.Sinico R, Bollini B, Sabadini E, et al. The use of laboratory tests in diagnosis and monitoring of systemic lupus erythematosus. J Nephrol. 2002;15(Suppl 6):S20–27. [PubMed] [Google Scholar]
- 21.Hunt J, McNeil H, Morgan G, et al. A Phospholipid-{beta} 2-Glycoprotein I Complex Is an Antigen for Anticardiolipin Antibodies Occurring in Autoimmune Disease But Not with Infection. Lupus. 1992;1(2):75–81. doi: 10.1177/096120339200100204. [DOI] [PubMed] [Google Scholar]
- 22.McNeil H, Chesterman C, Krilis S. Immunology and clinical importance of antiphospholipid antibodies. Adv Immunol. 1991;49:193–280. doi: 10.1016/s0065-2776(08)60777-4. [DOI] [PubMed] [Google Scholar]
- 23.Shi W, Krilis S, Chong B, et al. Prevalence of lupus anticoagulant and anticardiolipin antibodies in a healthy population. Aust N Z J Med. 1990;20(3):231–36. doi: 10.1111/j.1445-5994.1990.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 24.Fields R, Toubbeh H, Searles R, Bankhurst A. The prevalence of anticardiolipin antibodies in a healthy elderly population and its association with antinuclear antibodies. J Rheumatol. 1989;16(5):623–25. [PubMed] [Google Scholar]
- 25.Wolf P, Gretler J, Aglas F, et al. Anticardiolipin antibodies in rheumatoid arthritis: their relation to rheumatoid nodules and cutaneous vascular manifestations. Br J Dermatol. 1994;131(1):48–51. doi: 10.1111/j.1365-2133.1994.tb08456.x. [DOI] [PubMed] [Google Scholar]
- 26.Kapiotis S, Speiser W, Pabinger-Fasching I, et al. Anticardiolipin antibodies in patients with venous thrombosis. Haemostasis. 1991;21(1):19–24. doi: 10.1159/000216197. [DOI] [PubMed] [Google Scholar]
- 27.Jonsdottir T, Bratt J, Klareskog L, Van Vollenhoven R. Development of ACLA (anti-cardiolipin antibodies) in patients treated with infliximab (Remicade) Arthritis Rheum. 2001;44:373–75. [Google Scholar]
- 28.Morris A, Morris C, Hernandez C. Anticardiolipin antibodies developing during infliximab therapy. Arthritis Rheum. 2001;44:S373. [Google Scholar]
- 29.Rankin E, Choy E, Kassimos D, et al. The therapeutic effects of an engineered human anti-tumour necrosis factor alpha antibody (CDP571) in rheumatoid arthritis. Rheumatology (Oxford) 1995;34(4):334–42. doi: 10.1093/rheumatology/34.4.334. [DOI] [PubMed] [Google Scholar]
- 30.Bobbio-Pallavicini F, Alpini C, Caporali R, et al. Autoantibody profile in rheumatoid arthritis during long-term infliximab treatment. Arthritis Res Ther. 2004;6(3):R264–72. doi: 10.1186/ar1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Rycke L, Baeten D, Kruithof E, et al. Infliximab, but not etanercept, induces IgM anti-double-stranded DNA autoantibodies as main antinuclear reactivity: biologic and clinical implications in autoimmune arthritis. Arthritis Care Res. 2003;52(7):2192–201. doi: 10.1002/art.21190. [DOI] [PubMed] [Google Scholar]
- 32.De Rycke L, Kruithof E, Van Damme N, et al. Antinuclear antibodies following infliximab treatment in patients with rheumatoid arthritis or spondylarthropathy. Arthritis Rheum. 2003;48(4):1015–23. doi: 10.1002/art.10876. [DOI] [PubMed] [Google Scholar]
- 33.Comby E, Tanaff P, Mariotte D, et al. Evolution of antinuclear antibodies and clinical patterns in patients with active rheumatoid arthritis with longterm infliximab therapy. J Rheumatol. 2006;33(1):24–30. [PubMed] [Google Scholar]
- 34.Jonsdottir T, Forslid J, van Vollenhoven A, et al. Treatment with tumour necrosis factor alpha antagonists in patients with rheumatoid arthritis induces anticardiolipin antibodies. Ann Rheum Dis. 2004;63(9):1075–78. doi: 10.1136/ard.2003.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fort J, Gowchoc S, Abruzzo J. Anticardiolipin antibodies in patients with rheumatic disease. Arthritis Care Res. 1987;30(7):752–60. doi: 10.1002/art.1780300705. [DOI] [PubMed] [Google Scholar]
- 36.Merkel P, Chang Y, Pierangeli S, et al. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101(6):576–83. doi: 10.1016/s0002-9343(96)00335-x. [DOI] [PubMed] [Google Scholar]
- 37.Seriolo B, Accardo S, Garnero A, et al. Anticardiolipin antibodies, free protein S levels and thrombosis: a survey in a selected population of rheumatoid arthritis patients. Rheumatology (Oxford) 1999;38(7):675–78. doi: 10.1093/rheumatology/38.7.675. [DOI] [PubMed] [Google Scholar]
- 38.Vereckei E, Kriván G, Réti M, et al. Anti-TNF-alpha-induced anti-phospholipid syndrome manifested as necrotizing vasculitis. Scand J Rheumatol. 2010;39(1):175–80. doi: 10.3109/03009740902832753. [DOI] [PubMed] [Google Scholar]
- 39.St Clair EW, Van Der Heijde D, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: A randomized, controlled trial. Arthritis Rheum. 2004;50(11):3432–43. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 40.Visvanathan S, Wagner C, Smolen J, et al. IgG and IgM anticardiolipin antibodies following treatment with infliximab plus methotrexate in patients with early rheumatoid arthritis. Arthritis Rheum. 2006;54(9):2840–44. doi: 10.1002/art.22054. [DOI] [PubMed] [Google Scholar]
- 41.Pernambuco Climaco JM, Brochado MJF, Freitas MVC, et al. Val/Leu247 Polymorphism of 2 glycoprotein I in Brazilian Patients with Antiphospholipid Syndrome – A Genetic Risk Factor? Ann NY Acad Sci. 2009;1173(1):509–14. doi: 10.1111/j.1749-6632.2009.04655.x. [DOI] [PubMed] [Google Scholar]
- 42.von Scheven E, Elder M. Association between 2-glycoprotein I gene polymorphisms and pediatric SLE and antiphospholipid antibodies. Lupus. 2005;14(6):440–44. doi: 10.1191/0961203305lu2126oa. [DOI] [PubMed] [Google Scholar]