Abstract
Psoriasis is a common chronic inflammatory skin disease with a spectrum of clinical phenotypes and results from the interplay of genetic, environmental, and immunological factors. Four decades of clinical and basic research on psoriasis have elucidated many of the pathogenic mechanisms underlying disease and paved the way to effective targeted therapies. Here, we review this progress and identify future directions of study that are supported by a more integrative research approach and aim at further improving the patients' life.
Currently, there is no definitive cure for psoriasis, which affects 2%–4% of the Western population. Understanding its complex genetic, environmental, and immunological bases is critical for continued development of therapeutic options.
This skin is me, I can’t get out.
—John Updike5
The Journal of a Leper
The skin is the most exposed boundary with the outside world, thus, setting cutaneous conditions apart from those affecting internal organs. Skin diseases are often obvious and visible to others. Those who suffer from them have to cope with both their disease and the negative reaction of others because of the stigma traditionally associated with these types of conditions. Conversely, the unique accessibility of skin for tissue biopsy allows the study of the cellular and molecular determinants of cutaneous diseases in greater detail compared with other disorders, hence, facilitating the development of effective targeted therapies.
The chronic inflammatory skin disease psoriasis is one such condition, whose past and more recent history reflects both scenarios depicted above. Known since ancient time, various biblical references to “leprosy” more likely represent psoriasis; consequently, psoriasis patients have been cast out from society in biblical and medieval times because of fear, ignorance, and prejudice. Recognized as a distinct entity by Robert Willan in the early 19th century and named by Ferdinand Hebra in 1841, psoriasis’ impact on quality of life is still far-reaching and profound in modern times, even in the absence of stigmatization. On the other hand, being one of the most common skin conditions, psoriasis has received a great deal of attention from clinicians and basic scientists alike, becoming a model to study chronic inflammation. This joint effort has resulted in the elucidation of many underlying pathogenic mechanisms and, more importantly, has been translated in novel therapeutic strategies that have dramatically improved patient care. Here, we describe recent advances in understanding the complex genetic, environmental, and immunological basis of psoriasis, how some of the new findings have already resulted in novel targeted therapies, and why the integrative research approach currently being taken holds the promise of further enhancing our knowledge about psoriasis and ultimately improve patients’ lives.
EPIDEMIOLOGY
Psoriasis affects 2%–4% of the population in Western countries, with prevalence rates influenced by age, geographic location, and genetic background (Chandran and Raychaudhuri 2010).
A recent systematic review of psoriasis epidemiology confirms that psoriasis is a common disease, based on 46 studies reporting on prevalence of psoriasis and seven studies related to the incidence of disease in the general population (Parisi et al. 2013). Prevalence is higher in adults (from 0.91% to 8.5%) as compared with children (from 0% to 2.1%) with a dual peak of incidence: ∼30–39 years and ∼60 years of age. Disease prevalence is different across countries, with a geographical pattern suggesting less prevalence in those closer to the equator as compared with the more distant ones, in line with the beneficial effects of UV radiation exposure and clinical amelioration of psoriasis (Hart et al. 2011). Prevalence in Europe varies from 0.73% to 2.9%, similar to the Unites States (0.7%–2.6%) and higher than Latin America, Africa, and Asia (from 0 to <0.5%). Psoriasis has traditionally been considered to affect both genders equally; however, recent data about age stratification within gender shows a higher incidence in females <18 years old, and conversely a higher incidence in males ≥18 years old (Icen et al. 2009; Tollefson et al. 2010).
DISEASE CLASSIFICATION AND CLINICAL AND HISTOLOGICAL FEATURES
The term psoriasis (from the Greek psora, to itch) encompasses a number of distinct clinical phenotypes (van de Kerkhof and Nestle 2012), sometimes representing a dynamic, anatomical, or qualitative spectrum of the same disease (e.g., large and small plaque psoriasis), whereas, in other cases, most likely corresponds to a quite different entity (e.g., generalized pustular psoriasis [GPP]).
Historically, disease classification has been based on clinical appearance, mainly differentiating according to localization and morphology. Here, we follow the recent classification proposed by the International Psoriasis Council, which identifies four main forms of psoriasis: plaque-type, guttate, GPP, and erythroderma, and several further subphenotypes according to distribution (localized vs. widespread), anatomical localization (flexural, scalp, palms/soles/nail), size (large vs. small) and thickness (thick vs. thin) of plaques, onset (early vs. late), and disease activity (active vs. stable) (Griffiths et al. 2007).
Plaque-Type Psoriasis
Plaque-type psoriasis, occurring in 85%–90% of affected patients, is the most common form of psoriasis and is characterized by oval or irregularly shaped, red, sharply demarcated, raised plaques covered by silvery scales (Fig. 1A–C) (van de Kerkhof and Nestle 2012). Plaques occur mainly on the extensor surface of elbows and knees, on the scalp, and in the lower back, but can affect every area of the body, often with a symmetrical distribution. Size of the lesions can vary, from pinpoint to larger individual lesions or confluent areas leading to two clinical subphenotypes. The term large (>3 cm) plaque psoriasis describes thick (>0.75 cm), well-demarcated, red plaques with silvery scales. Small (<3 cm) plaque psoriasis presents with numerous lesions; the plaques are thinner (<0.75 cm), pinkish in color with a fine scale, and can be well-defined or merge with surrounding skin. A further classification takes into account the age of onset. Type I psoriasis has early onset (<40 yr), is often associated with familiar disease history and shows high association with the human leukocyte antigen (HLA)-Cw0602 allele, whereas type II psoriasis develops after the age of 40 (Henseler and Christophers 1985).
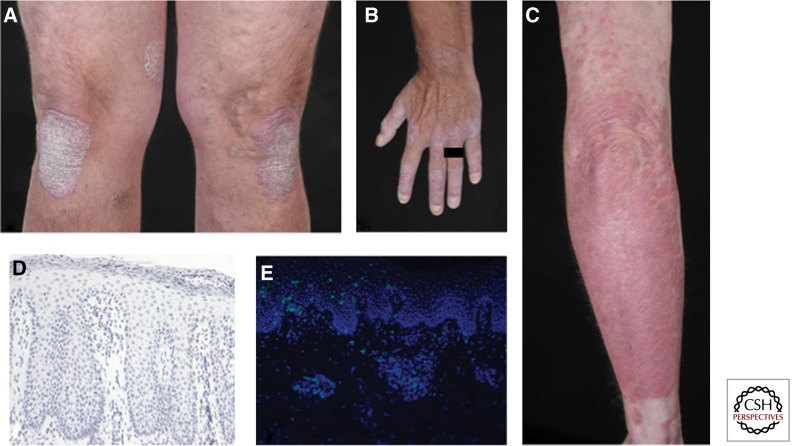
Figure 1.
Clinical and histopathological features of psoriasis. (A–C) Clinical pictures of chronic plaque psoriasis. Note nail involvement in B. (D) Hematoxylin-stained section from a chronic psoriatic plaque. Typical histological features are visible: acanthosis, papillomatosis, parakeratosis, as well as Munro abscess in the stratum corneum. (E) Immunofluorescence staining of chronic psoriatic plaque showing skin infiltrating CD3+ T cells in green.
Psoriasis is a dynamic disease; morphological changes accompany the evolution of a newly formed lesion into an advanced plaque that can slowly enlarge (active lesions, sharing most of the histological features of newly formed lesions) or remain static (stable lesions, retaining the morphology of the advanced stage) (Griffiths et al. 2007). In the early stages of a newly developing plaque, the first changes occur in the uppermost layer of the dermis, the papillary dermis. Blood vessels become dilated and tortuous, with lymphocytes and neutrophils emerging from their lumen (“squirting” papilla) and reaching for the epidermis, which looks still quite normal at this stage. Shortly after, however, aberrant keratinocyte (KC) proliferation and migration begin, resulting in epidermal thickening, incomplete terminal differentiation with initial loss of the “stratum granulosum,” and the appearance of foci of parakeratosis, that is, the retention of the nucleus by corneocytes. In the advanced stage, fully flagged psoriasis hyperplasia is present with acanthosis, which is the thickening of the “stratum spinosum,” and papillomatosis, the elongation of the rete ridges extending downward between dermal papillae. Parakeratosis becomes confluent, the stratum granulosum is absent, lymphocytes, mainly CD8+ T cells, are interspersed between KCs, and neutrophils accumulate into the parakeratotic scales, forming Munro microabscesses (Fig. 1D,E). The dilated blood vessels extend high into papillae, accounting for pinpoint bleeding when a scale is removed, known as Auspitz sign. The dermis is heavily infiltrated by T cells and dendritic cells (DC). Lesions can spontaneously resolve, although rarely. Resolving lesions after therapy can be encased by a distinctive rim of blanching (Woronoff’s ring), predictive of clearing and histologically characterized by orthokeratosis, that is thickening of the stratum corneum without parakeratosis and restoration of the stratum granulosum.
Guttate Psoriasis
Guttate psoriasis, from the Latin “gutta” for tear drop, is characterized by multiple small scaly plaques usually occurring around the trunk and upper arms and thighs. The rash has often sudden onset, usually within 2–4 wk after a bacterial infection of the upper ways, notably streptococcal pharyngitis in children and young adults, and is therefore associated with type I psoriasis (Griffiths et al. 2007). Guttate psoriasis can either completely clear spontaneously or following topical treatment, become chronic, or worsen into the plaque type.
Generalized Pustular Psoriasis
GPP, also known as von Zumbush psoriasis, is a rare but potentially life-threatening disease characterized by episodic, widespread skin and systemic inflammation. Typical histological feature of GPP is the presence of prominent aggregates of neutrophils infiltrating the stratum spinosum (spongiform pustules of Kogoj) and giving rise to sterile cutaneous pustules (van de Kerkhof and Nestle 2012). The skin manifestations are associated with marked systemic features: high fever, fatigue, and neutrophils leukocytosis. Acute attacks often occur during pregnancy and may be triggered by infection, exposure to or withdrawal from drugs. GPP can be frequently associated with plaque-type psoriasis and/or palmoplantar pustular psoriasis. Although still classified as a variant of psoriasis, the striking clinical and histological features of GPP have long suggested that it is a disease of distinct etiology. Recent genetic data lend further support to this hypothesis with the identification of some cases of familial GPP in which the disease is inherited as an autosomal recessive trait with mutations in the IL36RN gene encoding the anti-inflammatory IL-36-receptor antagonist, IL-36Ra (Marrakchi et al. 2011; Onoufriadis et al. 2011). IL-36Ra blocks the proinflammatory cytokines IL-36α/β/γ; when IL36RN is mutated, IL-36 signaling is uncontrolled with enhanced production of further proinflammatory cytokines (Onoufriadis et al. 2011). However, IL36RN mutations only occur in a minority of patients (Setta-Kaffetzi et al. 2013), thus, more genes are likely involved. Interestingly, a de novo mutation in the epidermal NF-κB activator CARD14 (Jordan et al. 2012b) has been described to underlay a sporadic case of severe GPP, suggesting that KCs dysfunction is likely to play a predominant role in this disease phenotype.
Erythrodermic Psoriasis
Erythrodermic psoriasis, one of the rarest forms of psoriasis (1%–2.25% of patients with psoriasis), represents the most severe phenotype; it carries substantial morbidity and can be potentially life threatening (Boyd and Menter 1989). It is characterized by diffuse erythema, with or without scaling, involving >75% of the skin surface. If present, scales are only superficial and differ from the adherent scales of plaque psoriasis. Systemic manifestations such as hypothermia and limb edema might occur because of the generalized vasodilation underlying the erythema, as well as myalgia, fatigue, and fever. GPP may revert to erythrodermic psoriasis when pustule formation stops. Both administration and abrupt withdrawals of systemic corticosteroids or methotrexate, sunburn, and emotional stress have been suggested as possible triggering factors (Ayala 2007).
PSORIATIC ARTHRITIS
About 20%–30% of psoriasis patients develop a seronegative, chronic inflammatory muscoskeletal disorder named psoriatic arthritis (PsA), which occurs, in most cases, about a decade after the appearance of psoriasis (Gladman et al. 2005).
PsA has a complex aetiology mirrored in a wide spectrum of clinical disease presentation, expression, and clinical course (Anandarajah and Ritchlin 2009).
PsA can affect different tissues (synovium, cartilage, bone, entheses, tendons); it presents common involvement of distal joints, asymmetric articular distribution, erythema over-affected joints, spinal involvement, and enthesitis (Gladman et al. 2005) and eventually leads to erosion and loss of function of the affected areas.
Because ∼80% of the patients develop PsA following psoriasis (Ellinghaus et al. 2012b), PsA is sometime considered as a disease within a disease (Eder et al. 2011). In keeping with this, several PsA susceptibility genes, such as HLA-C, IL-12B, IL-23R, TNIP1 overlap with psoriasis (Liu et al. 2008; Huffmeier et al. 2010; Ellinghaus et al. 2012b). On the other hand, differences in the genetic background of the two conditions do exist and unique genetic determinants have been identified, although not at genome-wide significance (Liu et al. 2008). Nevertheless, PsA shares several key cellular and molecular mediators with psoriasis, such as lymphocytes infiltrating the inflamed skin or joint (Pitzalis et al. 1996; Shen et al. 2006) and critical cytokines such as tumor necrosis factor (TNF), IL-23, and IL-17 (Gullick et al. 2010). Genetic association with class I HLA molecules and clinical evidence supports an important role of CD8 T cells in PsA, with the presence of oligoclonally expanded CD8 T cells in the joint fluids of individuals with active PsA (Costello et al. 2001). TNF is a critical disease player as it is in psoriasis and ∼70% of patients successfully respond to anti-TNF therapy in terms of signs and symptoms improvement, and, in some cases, also by radiographic progression (Anandarajah and Ritchlin 2009).
COMORBIDITIES
The association of psoriasis with physical and psychosocial comorbidities has been increasingly appreciated, and the synergistic contribution of psoriasis and its comorbidities to the establishment of systemic inflammation has been named “psoriatic march” (Griffiths and Barker 2007). The fact that psoriasis is more than skin deep is supported by the elevated levels of unspecific inflammation markers (such as C-reactive protein), as well as proinflammatory cytokines (such as TNF and interferon γ [IFN-γ]) and immune cells (such as T helper type 1 [Th1] and Th17) in the circulation of psoriasis patients as compared with healthy controls (Arican et al. 2005; Kagami et al. 2010). Most of these inflammatory markers are also increased in the skin lesions, indicating the blood is mirroring, at least in part, the inflammatory process taking place in the skin (Suarez-Farinas et al. 2012).
The systemic manifestation of the disease results in comorbidities including, but not limited to, metabolic syndrome, cardiovascular disease (CVD), diabetes, depression, and cancer (Griffiths and Barker 2007). Despite the importance of understanding the causal relationship between psoriasis and its comorbidities, the studies available so far are, generally, either scarce or heterogeneous because of different data-collection methods, limited control of confounding factors, and heterogeneous outcomes. Nevertheless, recent observations indicate a high prevalence of metabolic syndrome among psoriasis patients, with the odds ratio (OR), which measures the association between the exposure (psoriasis) and outcome (metabolic syndrome), increasing more than twofold as compared with matched healthy controls (Armstrong et al. 2013a). Metabolic syndrome increases the risk of developing CVD and diabetes, which are also associated with psoriasis. Interestingly, the association of type 2 diabetes with psoriasis is stronger in patients with severe disease (OR = 1.97) as compared with those with mild disease (OR = 1.53) (Armstrong et al. 2013b). Data about the relationship between psoriasis and CVD are still controversial (Ahlehoff et al. 2011; Dowlatshahi et al. 2013), but a recent meta-analysis showed increased risk of CVD in patients with severe disease, with OR relative to the general population of 1.37 for CVD mortality, 3.04 for myocardial infarction (MI), and 1.59 for stroke (Samarasekera et al. 2013). Interestingly, the relative risk (RR) for MI in psoriasis patients varies by age, with the greatest RR found in young patients with severe psoriasis (Gelfand et al. 2006). This association is clinically relevant and psoriasis has been included as an independent risk factor for CVD in recent guidelines for CVD prevention (Perk et al. 2012). The traditional risk factors for CVD such as smoking, excessive alcohol intake, hypertension, hyperlipidemia, obesity, and insulin resistance are also reported to be higher is psoriasis patients, therefore, making it difficult to account for the extent of CVD risk directly attributable to psoriasis. Indeed, psoriasis patients have high levels of lipids and lipid peroxidation, altered adipokine function (Kaur et al. 2008; Shibata et al. 2009), as well as abnormal coagulation profile (Marongiu et al. 1994; Karabudak et al. 2008).
Despite a definitive biological link between comorbidities and psoriasis, which has yet to be identified, it is reasonable to infer that the systemic inflammation and dyslipidaemia present in patients may predispose to impaired glucose tolerance and cardiovascular damage (Davidovici et al. 2010). It has been suggested that the proinflammatory molecules produced by the skin could be released into the systemic circulation and, in fact, several genes differentially regulated in psoriasis are linked to functional pathways associated with metabolic diseases/diabetes and cardiovascular risk (Suarez-Farinas et al. 2012). Among them, renin, an enzyme involved in the renin-angiotensin pathway ultimately regulating blood pressure, was overexpressed both at transcriptional and posttranscriptional level in psoriatic skin, showing a functional link between expression profile at skin level and peripheral functions. Further support to the close relationship between psoriasis and its comorbidities is the observation that IL-17A/IL-17F and CD4+ cells expressing IL-17 and IFN-γ are also found in atherosclerotic lesions (Eid et al. 2009; de Boer et al. 2010), and certain common risk alleles are shared between psoriasis and its metabolic and cardiovascular comorbidities (Lu et al. 2013). The increased risk of CVD is shared also with other inflammatory diseases such as rheumatoid arthritis (Wolfe et al. 2003; Maradit-Kremers et al. 2005) and inflammatory bowel diseases (IBDs) (Yarur et al. 2011), the latter themselves associated with psoriasis (Hsu and Armstrong 2012; Li et al. 2013). Remarkably, psoriasis and IBDs share several connections (Najarian and Gottlieb 2003), including genetic determinants (Wolf et al. 2008; Ellinghaus et al. 2012a).
Finally, psoriasis carries a severe psychosocial burden with anxiety, depression, and perceived stress appearing at a higher rate in psoriasis patients (O’Leary et al. 2004).
Psoriasis patients find it hard to adapt to the chronic, yet variable, and unpredictable nature of the disease. Another major component of psychological distress is the anticipated negative reactions of others, which can be shame or stigmatization. Coping mechanisms include avoidance and seclusion, which, in turn, affect the patients’ quality of life. Depression, which is one of the stronger predictors of suicidal ideation, is observed in more than 60% of patients (Esposito et al. 2006), and higher prevalence of suicidal ideation has been detected in psoriasis patients as compared with healthy controls and other skin-disease patients (Kurd et al. 2010; Picardi et al. 2013).
More studies are required to answer open questions regarding the effect of systemic therapy for psoriasis on CVD and diabetes (Solomon et al. 2011; Samarasekera et al. 2013) and whether an association exists between specific subtypes of psoriasis and comorbidities.
ETIOPATHOGENESIS
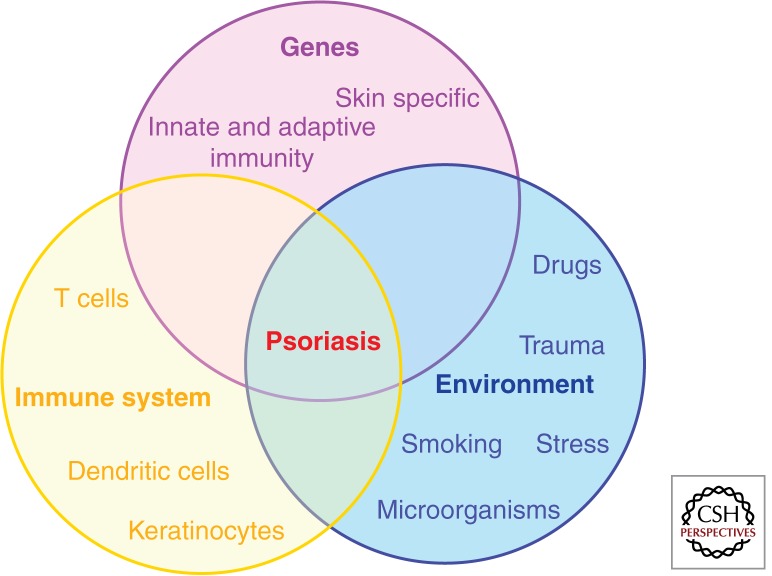
Disease initiation of complex diseases, such as psoriasis, takes place in genetically predisposed individuals in which a dysregulated immune response occurs following exposure to certain environmental triggers. Although mechanistic associations linking distinct environmental factors with specific genetic determinants and dysregulated immune processes are still scarce, critical determinants of this pathogenic interplay have been identified (Fig. 2).
Figure 2.
Psoriasis etiopathogenesis. Disease takes place in genetically predisposed individuals, carrying one or more psoriasis susceptibility genes (either skin specific or of immunological function) in which a dysregulated immune response (involving DC, T cells, and KCs) occurs, following exposure to certain environmental triggers.
GENETICS
Population and family studies support the existence of a genetic predisposition to psoriasis with higher incidences of psoriasis in relatives, compared with the general population (Lomholt 1963), and higher pairwise concordance rates in monozygotic twins (MZ) compared with dizygotic twins (DZ) (from 20% to 73% in MZ vs. 9% to 20% in DZ, depending on the population studied) (Farber et al. 1974; Brandrup et al. 1978; Duffy et al. 1993; Lonnberg et al. 2013). Lack of complete concordance between MZ and familial recurrence of disease not following a clear inheritance pattern, support the definition of psoriasis as a complex genetic trait, resulting from gene–gene and gene–environment interactions (Elder et al. 2010; Capon et al. 2012) and raising the question about the influence of epigenetics on disease. Large efforts to understand the genetic architecture of psoriasis have been undertaken using different approaches and technologies, resulting in the identification of a number of psoriasis genetic determinants (Table 1).
Table 1.
Psoriasis susceptibility genes identified by genome-wide association studies (GWASs)
| Class | Gene(s) | Pathway | Protein function | OR | Disease overlap | GWAS references |
|---|---|---|---|---|---|---|
| Skin specific | LCE3B/3C/3D | Skin barrier formation | KC structural protein | 1.26 | De cid et al. 2009; Zhang et al. 2009; Ellinghaus et al. 2010 | |
| KLF4 | Skin barrier formation, 17 signaling | Transcription factor | 1.12 | Tsoi et al. 2012 | ||
| ETS1 | Unknown | Transcription factor | 1.12 | Tsoi et al. 2012 | ||
| Innate immunity | IL-28RA | IFN signaling | IL-29 receptor subunit | 1.21 | Strange et al. 2010 | |
| IFIH1 | IFN signaling | Innate antiviral receptor | 1.27 | Strange et al. 2010 | ||
| RNF114 | IFN signaling | E3 ubiquitin ligase | 1.16 | Capon et al. 2008, Nair et al. 2009; Strange et al. 2010; Stuart et al. 2010 | ||
| ELMO1 | IFN signaling | Involved in TLR-mediated IFN-α signaling | 1.11 | Tsoi et al. 2012 | ||
| DDX58 | IFN signaling | Innate antiviral receptor | 1.11 | Tsoi et al. 2012 | ||
| NOS2 | Inflammation | Induced nitric oxide synthase | 1.22 | Stuart et al. 2010 | ||
| REL | NF-κB signaling | NF-κB subunit | 1.17 | RA | Strange et al. 2010 | |
| TNIP1 | NF-κB signaling | Inhibitor of TNF-induced NF-κB activation | 1.59 | Nair et al. 2009; Strange et al. 2010; Sun et al. 2010 | ||
| TNFAIP3 | NF-κB signaling | Inhibitor of TNF-induced NF-κB activation | 1.23 | Nair et al. 2009; Strange et al. 2010 | ||
| NFKBIA | NF-κB signaling | Inhibitor of NF-κB activation | 1.16 | Strange et al. 2010; Stuart et al. 2010 | ||
| FBXL19 | NF-κB signaling | Putative inhibitor of NF-κB activation | 1.16 | Stuart et al. 2010 | ||
| CARD14 | NF-κB signaling | Activator of NF-κB pathway | 1.11 | Tsoi et al. 2012 | ||
| CARM1a | NF-κB signaling | Transcriptional coactivator of NF-κB | 1.17 | Tsoi et al. 2012 | ||
| UBE2L3a | NF-κB signaling | Ubiquitin-conjugating enzyme | 1.13 | CeD, RA, CD | Ellinghaus et al. 2012a; Tsoi et al. 2012 | |
| At the interface between innate and adaptive immunity | TRAF3IP3 | IL-23/IL-17 axis NF-κB signaling | Adaptor molecule mediating IL-17-induced NF-kb activation | 1.52 | Ellinghaus et al. 2010; Strange et al. 2010 | |
| IL-12B | IL-23/IL-17 axis | Shared subunit of IL-12/IL-23 | 1.58 | Cargill et al. 2007; Capon et al. 2008; Nair et al. 2009; Zhang et al. 2009; Ellinghaus et al. 2010; Tsoi et al. 2012 | ||
| IL-23A | IL-23/IL-17 axis | Unique subunit of IL-23 | 1.39 | Nair et al. 2009; Strange et al. 2010 | ||
| TYK2 | IL-23/IL-17 axis IFN signaling | Tyrosine kinase associated with cytokines receptors | 1.88 | Strange et al. 2010 | ||
| HLA-C | Antigen presentation | MHC class I antigen | 4.32 | Capon et al. 2008; Nair et al. 2009; Zhang et al. 2009; Ellinghaus et al. 2010, Strange et al. 2010; Tsoi et al. 2012 | ||
| ERAP1 | Antigen presentation | Enzyme procesing MHC class I ligands | 1.2 | AS | Strange et al. 2010; Sun et al. 2010 | |
| Adaptive immunity | IL-23R | IL-23/IL-17 axis | Unique subunit of IL-23 receptor complex | 1.52 | AS, UC, CD | Cargill et al. 2007; Capon et al. 2008; Nair et al. 2009; Ellinghaus et al. 2010; Strange et al. 2010; Tsoi et al. 2012 |
| STAT3a | IL-23/IL-17 axis | Transcription factor | 1.15 | Tsoi et al. 2012 | ||
| IRF4a | IL-17 signaling | Transcription factor | 1.12 | Tsoi et al. 2012 | ||
| RUNX3 | Tbet pathway | Transcription factor | 1.13 | AS, CeD | ||
| IL-4/IL-13 | IL-4/IL-13 signaling | IL-4 and IL-13 cytokines | 1.18 | Nair et al. 2009 | ||
| TNFRSF9a | T-cell differentiation | Adaptor molecule | 1.13 | Ellinghaus et al. 2012a; Tsoi et al. 2012 | ||
| TAGAP | T-cell activation | RhoGTPase-activating protein | 1.12 | RA | Tsoi et al. 2012 | |
| ZMIZ1 | TGF-β signaling | Protein inhibitor of activated STAT(PIAS) family of proteins | 1.1 | MS | Ellinghaus et al. 2012a; Tsoi et al. 2012 | |
| SOCS1 | Type II IFN signaling | Suppressor of cytokine signaling | 1.13 | Tsoi et al. 2012 | ||
| Other | PRDX5 | Intracellular redox signaling | Antioxidant enzyme | 1.09 | Ellinghaus et al. 2012a; Tsoi et al. 2012 | |
| B3GNT2 | Carbohydrate metabolism | Enzyme | 1.12 | AS | Tsoi et al. 2012 | |
| MBD2a | Unknown | Transcriptional repressor | 1.12 | Tsoi et al. 2012 | ||
| ZC3H12C | Unknown | Zinc finger protein with putative RNase function | 1.14 | Tsoi et al. 2012 |
OR, odds ratio; KC, keratinocyte; TLR, toll-like receptor; GWAS, genome-wide association studies; TNF, tumor necrosis factor; IFN, interferon; IL, interleukin; HLA, human leukocyte antigen; LCE, late cornified envelope; MHC, major histocompatibility complex; RA, rheumatoid arthritis; CeD, celiac disease; CD, Crohn’s disease; AS, ankylosing spondylitis; MS, multiple sclerosis; UC, ulcerative colitis.
aMost notable gene mapped by the identified SNP.
PSORS1
The quest for psoriasis susceptibility genes using linkage analysis led to the identification of the major genetic determinant of psoriasis known as “psoriasis susceptibility 1” or PSORS1, a region spanning ∼250 kb within the major histocompatibility complex (MHC), on chromosome 6p21.3 (Nair et al. 1997, 2000; Trembath et al. 1997; Veal et al. 2002). A potential psoriasis locus in the MHC had been long suspected, based on serologic typing identifying an association between psoriasis and the HLA-Cw*060 allele of the MHC class I molecule HLA-C (Russell et al. 1972; Tiilikainen et al. 1980). However, the identification of the actual causative PSORS1 gene has proven to be challenging because of the extensive linkage disequilibrium (LD)—the tendency for particular alleles at two or more loci to be inherited together more often than would be predicted by chance—present within the MHC. Nevertheless, sequence and haplotype analysis (Nair et al. 2006), genome-wide association studies (GWASs) (Nair et al. 2009; Strange et al. 2010; Tsoi et al. 2012), and analysis of high-density single-nucleotide polymorphism (SNP) data, further refining PSORS1 interval to a 179-kb region encompassing HLA-C (Clop et al. 2013), have consistently indicated HLA-C as the most likely PSORS1 gene. SNPs within the minimal promoter region of HLA-C result in differential expression of various alleles, including HLA-Cw*0602 (Hundhausen et al. 2012). Moreover, regulatory variants in the putative HLA-C enhancer element are also likely to contribute to psoriasis susceptibility by affecting HLA-C expression (Clop et al. 2013). The expression pattern of MHC class I molecule on all nucleated cells makes HLA-C capable to regulate both innate and adaptive response (Mak et al. 2009). Nevertheless, despite the strong genetic evidence and the obvious immunological function of HLA-C, functional studies addressing the precise mechanism by which -Cw*0602 alleles predispose to psoriasis are still missing and no Cw*0602-specific antigen or interacting protein has been identified to date.
Psoriasis Susceptibility Genes Identified by GWASs
Advances in high-throughput genotyping technologies and the completion of the genome-wide database of common genetic sequence variation (HapMap project) have paved the way to the identification of a number of psoriasis susceptibility genes by means of GWAS (Capon et al. 2008; de Cid et al. 2009; Nair et al. 2009; Zhang et al. 2009; Ellinghaus et al. 2010; Huffmeier et al. 2010; Strange et al. 2010; Stuart et al. 2010) and subsequent meta-analysis (Ellinghaus et al. 2012a; Tsoi et al. 2012). Collectively, these studies identified 36 independent psoriasis-associated regions within individuals of European ancestry (Tsoi et al. 2012), plus five more uniquely associated in the Chinese population (Sun et al. 2010).
The psoriasis genetic landscape (Table 1) emerging from these studies includes skin-specific genes and immune-related genes, with the latter belonging to either the innate or the adaptive immunity, as well as being at the interface between the two arms of the immune system. SNPs and copy number variation (de Cid et al. 2009; Tsoi et al. 2012) in genes of the late cornified envelope (LCE) family within the epidermal cell differentiation complex, a cluster of genes involved in skin barrier formation, support a critical role for skin-specific genes in psoriasis susceptibility. Moreover, there is genetic interaction (epistasis) between LCE deletions and HLA-C (Tsoi et al. 2012). A closer look at the immune genes reveals the critical contribution to disease susceptibility of four fundamental immunological processes and pathways: antigen presentation, NF-κB signaling, IL-23/IL-17 axis, and type I IFN pathway. The fundamental importance of antigen presentation in psoriasis is highlighted by several observations. First, the HLA-C association has the greatest statistical significance observed in GWAS studies (Tsoi et al. 2012). Second, HLA-C accounts for about 6% of the total genetic variance (Chen et al. 2011), which is at least 10-fold more than that explained by any other known psoriasis susceptibility locus (Tsoi et al. 2012). Third, epistasis is present between HLA-C and ERAP1 (Strange et al. 2010), which encodes for an aminopeptidase involved in the trimming of peptide antigens. Among genes of the innate immunity, more than half belong to the NF-κB pathway, which has a pivotal role in amplifying and sustaining chronic inflammation. Of particular interest are genes encoding for molecules regulating NF-κB activation downstream from TNF (TNFAIP3 and TNIP1) or downstream from IL-17 (TRAF3IP2). Two recent studies have identified and evaluated the functional consequences of rare and common gene variants (Jordan et al. 2012a; Tsoi et al. 2012) and missense mutations (Jordan et al. 2012b) in CARD14, an activator of NF-kB primarily expressed in skin epidermis, providing further evidence that intrinsic defects in KCs can promote psoriasis. Genes belonging to the type I IFN pathway (e.g., IL-28RA and RNF114) support clinical and experimental findings, indicating an important role for innate antiviral responses in psoriasis (Nestle et al. 2005). Finally, a sizeable number of genes, including IL-23, IL-12B, and IL-23R, belong to the IL-23/IL-17 pathway, whose critical involvement in psoriasis pathogenesis has been well documented by a wealth of studies showing a pivotal role for IL-23-induced and IL-17-mediated responses in psoriasis (Di Cesare et al. 2009). Moreover, the genetic association with IL-23R is one of the very few supported by functional evidence with reduced IL-17 responses in carriers of the protective Arg381Gln IL-23R allele (Di Meglio et al. 2011a, 2013).
Interestingly, some genes from the above pathways influence multiple phenotypic traits, in particular, other immune-mediated conditions such as Crohn’s disease (CD), celiac disease, and ankylosing spondylitis (Table 1) (Tsoi et al. 2012), thus confirming the presence of a shared genetic basis among immune-mediated inflammatory diseases. It has been noted that pleiotropism is more pronounced in genes of the evolutionary more recent adaptive immunity (e.g., IL-23R), whereas genes uniquely associated with psoriasis are mainly involved in innate immune responses (e.g., CARD14), in keeping with the diverse and well-conserved set of innate immune mechanisms exploited by the skin to maintain homeostasis. The signals identified in the European population collectively account for ∼20% of estimated psoriasis heritability (Tsoi et al. 2012); therefore, the search for psoriasis genes is yet to be completed. Moreover, the gene variants identified have modest-effect size and it has been hypothesized that rare variants with bigger effect may explain this “missing heritability” (Manolio et al. 2009). However, a recent study, which has resequenced 25 genes from 20 GWAS-identified risk loci overlapping between six common autoimmune disease including psoriasis, has shown that rare variants at known loci have a negligible role in common immune-mediated disease susceptibility, suggesting that the missing heritability likely results from the coexistence of many common variants of weak effect (Hunt et al. 2013).
ENVIRONMENTAL TRIGGERS
In contrast to the fast-growing list of psoriasis susceptibility genes, the environmental factors concurring in initiating the disease are still ill-defined. Among known environmental triggers of psoriasis, there are drugs, infections, physical trauma, smoking, alcohol, and stress.
Drugs, such as the antiviral and antiproliferative agent imiquimod, antidepressants (lithium), antihypertensives (beta blockers), IFNs, and also anticytokine therapies used in the treatment of psoriasis (anti-TNF antibodies) have all been clinically associated with initiation, exacerbation, and worsening of the disease (Kim and Del Rosso 2010). Imiquimod, a toll-like receptor (TLR)7/8 agonist used to treat genital warts and nonmelanoma skin tumours, represents one of the best investigated examples of psoriasis trigger so far and activates the type I IFN-signaling pathway. The important role of the type I IFN pathway and main producers of type I IFN (plasmacytoid dendritic cells [pDC]) has been shown in preclinical models and verified in transcriptomic studies of psoriasis (Nestle et al. 2005; Yao et al. 2008). The initial clinical observation of a case of psoriasis exacerbated by topical treatment with imiquimod (Gilliet et al. 2004) has been translated into the clinically relevant imiquimod-induced psoriasiform skin inflammation mouse model (van der Fits et al. 2009), which faithfully reproduces most of the features of the human disease and has quickly become one of the most widely used experimental models to study psoriasis (Flutter and Nestle 2013).
An association between preceding streptococcal throat infection and psoriasis (Gudjonsson et al. 2003) has been reported, mainly with guttate psoriasis (Prinz 2001), and homologous T-cell clones have been found in both the tonsils and skin lesions of plaque-type psoriasis patients (Diluvio et al. 2006).
Tattoos and surgical incisions give rise to the Koebner phenomenon with psoriasis plaques appearing at the site of the trauma (Weiss et al. 2002).
The association of modifiable behavioral risk factors, such as smoking and alcohol consumption, as well as comorbidites such as stress, is traditionally more difficult to investigate. Although a number of studies have offered evidence linking stress and smoking with psoriasis (Naldi et al. 2005; Jin et al. 2009; Ozden et al. 2011), there is no consensus on whether these factors do actually cause or aggravate psoriasis (Dellavalle and Johnson 2005).
IMMUNOPATHOGENESIS
The contribution of the immune system to psoriasis is not less complex than the overall disease pathogenesis, with a variety of innate and adaptive immune cells and proinflammatory mediators involved, possibly at different stages of the disease. Moreover, the question of whether psoriasis is primarily an epithelial- or immune-mediated disease has recurred for several decades in the scientific community, with researchers torn between the prominent changes in the skin and increasingly recognized importance of immune-mediated mechanisms. If, why, and how the immune system instructs KCs to deviate from their differentiation program and how KCs, in turn, contribute to the aberrant immune response have been crucial questions for almost four decades of psoriasis research.
Not surprisingly, given the macroscopic alterations occurring in psoriatic skin, the focus has initially been on KCs and the aberrant terminal differentiation program they undertake in psoriasis. In normal skin, basal KCs differentiate through spinal and granular layers of the epidermis to become dead, enucleated corneocytes, which constitute the protective physical barrier (Watt 1989). Cells of the stratum spinosum withdraw from the cell cycle and enter the terminal differentiation program consisting of the synthesis of several proteins encoded by the epidermal differentiation complex, expression of specific keratins, synthesis and release of extracellular lipids, and, ultimately, the creation of a cornified envelope. Normally, the overall process takes ∼28 d. In psoriatic lesions, KCs have higher proliferation rate and shortened (5–8 d) differentiation process, resulting in nuclei retention in the corneocytes, reduced lipid secretion, loss of the stratum granulosum, and retention of partially differentiated KCs leading to the typical scales accumulating on the skin. Following the unexpected efficacy of serendipitously administered immunosuppressive agents, innate and adaptive immune cells have attracted attention and been the focus of clinical and experimental studies. These efforts have elucidated many of the pathogenic immune mechanisms and highlighted the chief contribution of tissue resident T cells and TNF, leading to effective anti-T-cell and anticytokine targeted therapies. Nevertheless, KCs, equipped with innate immune receptors and actively taking part in the inflammatory skin response with ancillary immune functions, have recently gained the status of skin sentinel cells (Nestle et al. 2009), and their contribution to disease pathogenesis is being reevaluated. Thus, the current view of psoriasis pathogenesis implies that the aberrant immune and epidermal response seen in psoriasis is sustained by a pathogenic cross talk between epithelial and immune cells (Di Meglio et al. 2011b; Lowes et al. 2013). This interplay is primarily driven by the critical proinflammatory molecules, TNF, IL-23, and IL-17, whose direct therapeutic targeting has proven to be clinically effective, with other mediators such as IFN-α, IFN-γ, and IL-22 also contributing to the initiation, amplification, and maintenance of the disease.
In the following section, we discuss the distinct roles and contribution of the different cell types and proinflammatory molecules to disease initiation and established disease by integrating findings drawn from studies of clinical samples, xenotransplant models in which human skin is transplanted onto immuno-compromised hosts, and experimental mouse model of psoriasis-like skin inflammation. Although no mouse model can fully recapitulate the development and features of a disease only occurring in humans (Gudjonsson et al. 2007), lessons from a number of animal models showing most of the crucial clinical traits and molecular signatures of psoriasis (Swindell et al. 2011) should not be discarded as they can provide valuable insights to dissect pathogenic mechanisms.
Disease Initiation
One of the best-characterized initiation mechanisms, leading to dysregulated skin immune responses, involves KCs releasing the cationic antimicrobial peptide (AMP) LL-37 following physical trauma (Koebner phenomenon) or infection. LL-37 binds to self-DNA/RNA fragments (Lande et al. 2007; Ganguly et al. 2009) also released by damaged skin cells (Ganguly et al. 2009; Lin et al. 2011), forming LL-37/self-DNA/RNA complexes found in psoriasis lesions. These complexes activate TLR7/9-bearing pDC, a subset of circulatory DC normally absent in human healthy skin, but highly infiltrating developing psoriasis lesions (Nestle et al. 2005), to release type I IFN, which is thought to play important roles in the early phases of psoriasis development. In the AGR129 xenotransplantation model in which human nonlesional psoriatic skin is transplanted onto AGR129 mice lacking T/B cells and having severely impaired NK activity, type I IFN triggers activation and expansion of autoimmune T cells present in the transplant, leading to fully fledged psoriasis plaque formation (Nestle et al. 2005). Moreover, type I IFN, as well as proinflammatory cytokines released by activated KCs (IL-1β, IL-6, and TNF), can also mature and activate myeloid DC. Psoriatic skin lesions have a 30-fold increase in myeloid dermal dendritic cells (DDC) (Zaba et al. 2007) and, in addition to “classical” CD11c+CD1c+ DDC found in healthy skin, it harbors a distinct population of “inflammatory” CD1c– DC (Zaba et al. 2009b) producing TNF, iNOS, IL-20, and IL-23 (Lee et al. 2004; Wang et al. 2006; Zaba et al. 2009a). The clinical benefit of anti-TNF agents strongly shows that elevated TNF levels in the skin are critical for the pathogenic interaction between DDC and T cells with TNF blockade-impairing DDC maturation and IL-23 production (Zaba et al. 2007). In keeping with their role as antigen presenting cells, mature DDC are thought to migrate to skin-draining lymph nodes to present an as-yet elusive antigen to naïve T cells. The presence of oligoclonal T cells in psoriatic lesions, identification of conserved clonal T-cell receptor (TCR) rearrangements in different patients, and presence of the same T-cell clone over time (Chang et al. 1994; Menssen et al. 1995; Prinz et al. 1999; Vollmer et al. 2001) imply the presence of a common antigen triggering the disease. LL-37/self-RNA complexes have been shown to activate TLR8-bearing myeloid DC and, thus, they could fulfill this role. In line with the association between psoriasis and streptococcal infection, molecular mimicry (Valdimarsson et al. 2009), with several streptococcal antigens such as M protein (which shares high similarity structure with certain human keratins), peptidoglycan, and CpG DNA, has been suggested as possible mechanism for specific T-cell activation (Cai et al. 2012), but conclusive evidence identifying psoriasis (auto)antigen(s) has yet to be provided.
Nevertheless, T cells are critical players in the initiation phase of disease and particularly important are those residing in the skin as tissue-resident memory T (TRM) cells. TRM are memory cells that do not circulate, but are strategically positioned as the first-line of defense in the tissue (Boyman et al. 2007; Clark 2010) and have been implicated in long-term peripheral immunity (Gebhardt et al. 2009; Jiang et al. 2012). Early evidence of their existence came from the AGR129 xenotransplantation model of human nonlesional psoriatic skin onto immunodeficient mice. In this model, development of fully fledged psoriatic lesions occurs in the absence of T-cell recruitment from blood (Boyman et al. 2004) and depends on the ability of locally activated skin TRM cells, present in the initial graft, to migrate into the epidermis (Conrad et al. 2007).
Established Disease
DDC activation and their interaction with T cells is central to plaque progression as it creates an IL-23/IL-17 inflammatory environment in which DC and macrophage-derived IL-23 promotes type 17 helper (Th17) and cytotoxic (Tc17) cell effector functions. The initial definition of psoriasis as Th1 and IFN-γ-driven disease, based on a strong type II IFN transcriptomic signature and the high frequency of Th1 and Tc1 cells in both psoriasis plaques and peripheral blood (Schlaak et al. 1994; Austin et al. 1999; Friedrich et al. 2000), has been challenged by the discovery of IL-23 and Th17 cells and a wealth of genetic, clinical, and experimental findings indicating a key role for the IL-23/IL-17 axis in psoriasis (Di Cesare et al. 2009).
IL-23 is a heterodimeric cytokine consisting of a unique IL-23p19 subunit coupled with a common IL-12p40 subunit, which is shared with IL-12. Increased levels of IL-23p19, IL-12p40 (Lee et al. 2004), and IL-23R (Wilson et al. 2007; Tonel et al. 2010), but not IL-12p35, are detected in psoriatic skin. IL-23 induces and sustains psoriasis-like skin inflammation in murine models (Chan et al. 2006; Zheng et al. 2007) and selective targeting of IL-23p19 is effective in the AGR129 xenotransplant model (Tonel et al. 2010). Ustekinumab, a monoclonal antibody directed against the common IL-12/23p40 subunit is highly effective in psoriasis (Griffiths et al. 2010) and investigational antibodies targeting IL-23p19, IL-17A, and IL-17R, discussed later in the text, also show promising efficacy and safety profiles. Th17 cells, abundantly infiltrating psoriatic skin dermis (Lowes et al. 2008), are increased in the blood of psoriasis patients (Kagami et al. 2010), and together with Tc17 and γδ-T cells are an important source of IL-17A, IL-17F, and IL-22 (Ortega et al. 2009; Cai et al. 2011). IL-17A/IFN-γ or IL-17/IL-22 double-producing cells have also been described in psoriasis patients (Kryczek et al. 2008; Lowes et al. 2008; Eyerich et al. 2009; Kagami et al. 2010). IL-17A and IL-17F share high structural and functional homology and function primarily by activating KCs to produce neutrophil- (CXCL1, CXCL2, CXCL5, CXCL8) and T-cell- (CCL20) recruiting chemokines and AMP, including LL37 and S100 family members (S100A7/8/9/15) (Wilson et al. 2007) that also have leukocytes’ chemoattractant properties (Wolf et al. 2010). Thus, IL-17 is central in a pathogenic loop linking T cells and KCs (Chiricozzi et al. 2011). Moreover, another IL-17 family member, IL-17C, induces an autocrine proinflamamtory loop in KCs (Ramirez-Carrozzi et al. 2011; Johnston et al. 2013). Finally, IL-22 mediates most of the epidermal hyperplasia by impairing KC differentiation (Zheng et al. 2007; Ma et al. 2008).
Besides Th17 cells, cytotoxic CD8 T cells increased in the epidermis of lesional psoriatic skin (Hammar et al. 1984) have been recently recognized as a previously unappreciated source of disease-relevant cytokines IL-17A, TNF, IFN-γ, and IL-22 in the epidermis (Austin et al. 1999; Kryczek et al. 2008; Ortega et al. 2009; Hijnen et al. 2013). In the AGR129 xenotransplant model, there is a strong positive correlation between disease progression and epidermal T-cell expansion, and disease development is prevented by blocking CD8 T-cell dermal–epidermal transition (Conrad et al. 2007). In a transgenic mouse model of psoriasiform inflammation overexpressing RAS in KCs, CD8 T cells initiate skin inflammation and KC proliferation via cytokine production (Gunderson et al. 2013).
γδ-T cells, an innate-like T-cell population involved in surveillance of epithelial surfaces, are the major pathogenic source of IL-17 in psoriasiform skin inflammation in mouse models, which strongly rely on their prominent presence in naïve mouse skin as compared with αβ-T cell, and their ablation associates with amelioration of pathology (Cai et al. 2011; Pantelyushin et al. 2012). IL-17-producing γδ-T cells have been identified also in psoriasis (Laggner et al. 2011) and the Vγ9Vδ2 T-cell subset, producing also IFN-γ, TNF, and Th1-recruiting chemokines, has been shown to be increased in the skin and simultaneously decreased in the peripheral blood of psoriasis patients as compared with healthy controls. Although representing a small subset of skin-infiltrating T cells, the clinical relevance of this subset is indicated by the significant correlation between increased disease severity and lower numbers of Vγ9Vδ2 T cells in the circulation, as well as their peripheral increase following successful antipsoriatic therapy (Laggner et al. 2011).
Innate lymphoid cells (ILC) (Spits and Cupedo 2012; Spits et al. 2013), bearing lymphoid morphology, but no immune cell lineage markers, has been recently identified as a key source of IL-17 and IL-22 in psoriasiform skin inflammation (Pantelyushin et al. 2012) and clinical samples of IBD (Geremia et al. 2011), as well as psoriasis patients (Villanova et al. 2013b). Both γδ-T cells and ILC constituitively express IL-23R, thus, representing an immediate target for IL-23-mediated IL-17 and IL-22 production. Finally, mast cells and neutophils might represent other innate sources of IL-17 (Lin et al. 2011), although more definitive evidences are awaited.
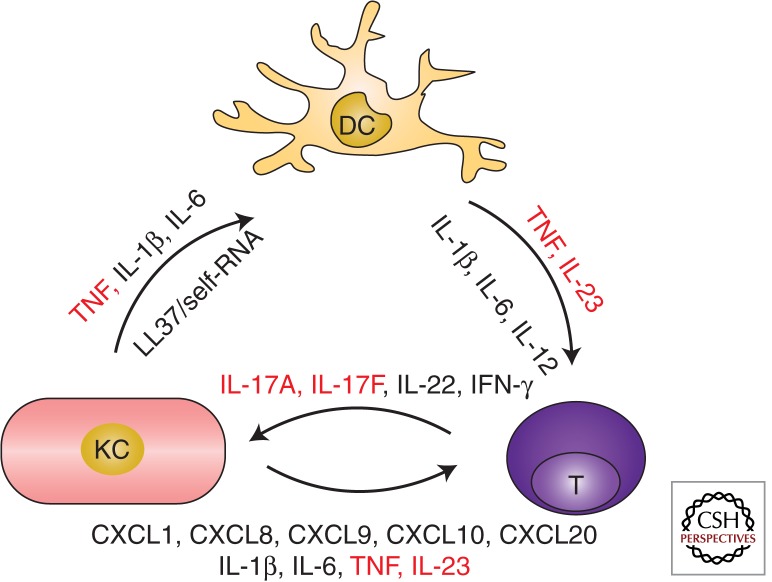
Taken together, a pathogenic cross talk between KCs, DC, and T cells sustained by TNF, IL-23, and IL-17, and possibly supported by other immune cell players and further proinflammatory molecules, underlay the dysregulated immune response observed in psoriasis (Fig. 3).
Figure 3.
Psoriasis immunopathogenesis. A pathogenic cross talk between innate and adaptive immune cells, sustained by proinflammatory mediators, underlies the dysregulated immune response seen in psoriasis. The three main cellular players and their products are depicted in this diagram. KCs produce key cytokines (TNF, interleukin-[IL] 1β, and IL-6), as well as the AMP LL37-binding self-RNA activating myeloid DC in the dermis. Activated DC present, yet not identified, antigens and secrete mediators such as TNF, IL-23, IL-1β, IL-6 leading to the differentiation and activation of IL-17-producing T cells (T, here representing both αβ and γδ TCR T cells). T cells, in turn, secrete cytokines (IL-17A, IL-17F, IL-22, IFN-γ) that activate KC aberrant differentiation program and induce the production of further proinflammatory mediators, especially chemokines (CXCL1, CXCL8,) recruiting neutrophils (not shown) or other immune cells (CXCL9, CXCL10, CCL20), as well as other antimicrobial peptides (not shown). Critical proinflammatory molecules, effectively targeted by biologic drugs, are shown in red.
THERAPY
In common with other immune-mediated complex diseases, there is no definitive cure for psoriasis, and available treatment is only to decrease disease activity and improve symptoms.
Therapies are administered according to disease severity and assessed by the Psoriasis Area and Severity Index (PASI, ranging from 0 to 72), which takes into account appearance and extension of the lesions.
Classic therapies span from topical treatments (emollients, topical corticosteroids, vitamin D analogs) used in mild-to-moderate psoriasis, to UVA/UVB phototherapy or systemic therapies reserved to moderate-to-severe cases. Among systemic therapies, which include retinoids, methotrexate, and cyclosporine, the folic acid antagonist methotrexate, which has immunosuppressive, cytostatic, and anti-inflammatory activity and is rather inexpensive, is often used as first line of treatment. However, classic therapy has not completely met patients’ needs, especially in the most severe cases. In the past decade, a better understanding of disease immunopathogenesis has been successfully translated into new drugs, known as “biologics,” targeting key inflammatory mediators and currently representing an effective third-line therapy in moderate-to-severe psoriasis patients, unresponsive to nonbiologic systemic agents.
An overview of the newest therapeutic options, both in clinical use and under clinical investigation, is shown in Table 2.
Table 2.
Targeted therapies in psoriasis, either approved or under investigation as of July 2013
| Type | Mechanism of action | Name | Molecular target | Phase | Formulation | Administration route | Company | Reference |
|---|---|---|---|---|---|---|---|---|
| Biologic | Anti-T cells | Alefacept | CD2 | Approved 2003 (U.S.) | Human LFA-3/IgG1 fusion protein | IM or IV | Biogen | Krueger et al. 2002; Lebwohl et al. 2003a |
| Efalizumab | CD11a | Approved 2003, withdrawn 2009 | Humanized IgG1 monoclonal antibody | SC | Genentech | Gordon et al. 2003; Lebwohl et al. 2003b; Leonardi et al. 2005; Menter et al. 2005; Gottlieb et al. 2006; Papp et al. 2006 | ||
| Abatacept (BMS188667) | CTLA-4 | Phase II | Human CTLA4–Ig-IgG1 fusion protein | SC or IV | Bristol-Myers Squibb | Bristol-Myers Squibb 2013 | ||
| Anticytokine | Etanercept | TNF | Approved 2004 (U.S. and E.U.) | Human TNF-R (p75)-lgG1 fusion protein | SC | Amgen | Leonardi et al. 2003; Papp et al. 2005; Tyring et al. 2006 | |
| Infliximab | TNF | Approved 2006 (U.S. and E.U.) | Mouse-human IgG1 chimeric monoclonal antibody | IV | Janssen Biotech | Gottlieb et al. 2004; Reich et al. 2005; Menter et al. 2007 | ||
| Adalimumab | TNF | Approved 2007 (E.U.) 2008 (U.S.) | Human IgG1 monoclonal antibody | SC | Abbott | Gordon et al. 2006 ; Menter et al. 2008; Saurat et al. 2008; | ||
| Ustekinumab | IL-12p40 (IL-2, IL-23) | Approved 2009 (U.S. and E.U.) | Human IgG1 monoclonal antibody | SC | Janssen Biotech | Papp et al. 2008; Leonardi et al. 2008b | ||
| Briakinumab (ABT-874) | IL-12p40 (IL-12, IL-23) | Phase III, then discontinued 2011 | Human IgG1 monoclonal antibody | SC | Abbott | Gottlieb et al. 2011; Strober et al. 2011 | ||
| MK-3222 (SCH900222) | IL-23p19 | Phase III | Humanized IgG1 monoclonal antibody | SC | Merck | Merck 2013a,b | ||
| Guselkumab (CNTO 1959) | IL-23p19 | Phase II | Human IgG1 monoclonal antibody | SC | Janssen Biotech | Janssen 2013 | ||
| Brodalumab (AMG 827) | IL-17R | Phase III | Human IgG2 monoclonal antibody | SC | Amgen | Papp et al. 2012b; Amgen 2013a,b,c | ||
| Ixekizumab (LY2439821) | IL-17 | Phase III | Humanized IgG4 monoclonal antibody | SC | Eli Lilly | Leonardi et al. 2012 ; Eli Lilly 2013a,c,d,e,f | ||
| Secukinumab (AIN457) | IL-17 | Phase III | Human IgG1 monoclonal antibody | SC or IV | Novartis | Novartis 2013b; Papp et al. 2013b; Rich et al. 2013 | ||
| Fezakinumab (ILV-094) | IL-22 | Phase I | Human IgG1 monoclonal antibody | SC or IV | Pfizer | Pfizer 2013 | ||
| Small molecule | PDE4 inhibitor | Apremilast (CC-10004) | PDE4 | Phase III | NA | Oral | Celgene | Papp et al. 2012a; Celgene 2013a,b,c |
| AN2728 | PDE4 | Phase II | NA | Topical | Anacor | Anacor 2013a,b,c,d | ||
| JAK inhibitor | Tofacitinib (CP-690, 550) | JAK1 and JAK3 | Phase II | NA | Oral | Pfizer | Boy et al. 2009; Papp et al. 2012c | |
| Tofacitinib (CP-690, 550) | JAK1 and JAK3 | Phase II | NA | Topical | Pfizer | Ports et al. 2013 | ||
| INCB01824 | JAK1 and JAK2 | Phase II | NA | Topical | Incyte | Punwani et al. 2012 | ||
| INCB039110 | JAK1 | Phase II | NA | Oral | Incyte | Incyte 2013 | ||
| Baricitinib (INCB028050) | JAK1 and JAK2 | Phase II | NA | Oral | Eli Lilly | Eli Lilly 2013b | ||
| PKC inhibitor | AEB071 | PKC | Phase II | NA | Oral | Novartis | Novartis 2013a | |
| A3AR agonist | CF101 | A3AR | Phase II/III | NA | Oral | Can-Fite BioPharma | David et al. 2012; Can-Fite 2013 |
As of mid-2013, there are five biologics approved for the treatment of psoriasis, targeting either T cells or cytokines such as TNF or IL-12/IL-23 (Perera et al. 2012).
LFA, lymphocyte function-associated antigen; IM, intramuscular; IV, intravenous, SC, subcutaneous; A3AR = A3 adenosine receptor; PKC, protein kinase C; PDE4, phosphodiesterase 4; CTLA4, cytotoxic T-lymphocyte antigen 4; NA, not applicable; U.S., United States; E.U., European Union.
Anti-T-Cell Therapies
The first biologic to be approved was Alefacept in 2003, a lymphocyte function-associated antigen (LFA)-3/IgG1 fusion protein binding CD2 on T cells and, thus, selectively inducing apoptosis of CD2+ human memory-effector T cells in vivo (da Silva et al. 2002), although further studies suggested a broader immunomodulatory effect (Chamian et al. 2005; Haider et al. 2007). Clinical efficacy was shown in phase III studies with 40% of patients achieving a PASI75 response (75% reduction of the PASI) (Krueger et al. 2002) and more that 50% achieving PASI 50 (50% reduction of the PASI) (Lebwohl et al. 2003a).
A second anti-T-cell strategy was approved in 2003 and consisted of a humanized antibody (Efalizumab) binding and blocking CD11a, a key molecule for T-cell activation and migration through the circulation into the skin (Jullien et al. 2004).
Despite a good efficacy profile, efalizumab (Gordon et al. 2003; Lebwohl et al. 2003b; Leonardi et al. 2005, 2008a; Menter et al. 2005; Gottlieb et al. 2006; Papp et al. 2006) has been withdrawn from the market in 2009 because of three cases of progressive multifocal leukoencephalopathy (Tan and Koralnik 2010), highlighting the importance of carefully monitoring the long-term safety of immunomodulatory therapies.
Anticytokine Therapies
An alternative strategy to anti-T-cell targeting aims at interfering with the psoriasis cytokine network (Nickoloff 1991) by using anticytokine biologic drugs.
TNF blockade, using etanercept, a human p75 TNF receptor fusion protein (approved in 2004), infliximab, a humanized chimeric anti-TNF monoclonal antibody (approved in 2006), or adalimumab, a fully human monoclonal antibody (approved in 2008) is another effective therapeutic strategy. Etanercept efficacy have been shown in three phase III trials with about 50% of patients achieving PASI75 at week 12 in the high-dose group (Leonardi et al. 2003; Papp et al. 2005, 2011d; Tyring et al. 2006). TNF neutralization causes early down-modulation of myeloid cell-related genes, with decrease of Th17 cell products and downstream molecules in just 2 wk after commencing therapy (Gottlieb et al. 2005; Zaba et al. 2007; Johansen et al. 2010). Interestingly, only patients who downregulate the expression of Th17 pathway genes successfully respond to etanercept treatment (Zaba et al. 2007; Zaba et al. 2009c).
The latest biologic to be approved for psoriasis in 2009, ustekinumab, is a monoclonal antibody simultaneously blocking the heterodimeric proteins IL-12 and IL- 23 via its biding to the shared subunit p40. Its efficacy is quite high, with 67% of patients achieving PASI75 at 12 wk of treatment (Leonardi et al. 2008b; Papp et al. 2008).
Some serious adverse events, such as opportunistic infections and reactivation of latent tubercolosis, have been reported for these monoclonal antibodies (Sivamani et al. 2013). Long-term safety data (up to 4 and 5 yr treatment) are now available for etanercept and ustekinumab (Leonardi et al. 2003; Papp et al. 2005, 2012d, 2013a; Tyring et al. 2006), suggesting a safe use of these drugs.
A number of other biologic drugs is currently (mid-2013) being investigated in clinical trials. In line with the prominent role of IL-23/IL-17 axis in psoriasis, two antibodies (guselkumab [formerly CNTO1959] and MK-3222) targeting the specific IL-23p19 subunit are in phase II and III, respectively (Janssen 2013; Merck 2013a,b).
Monoclonal antibodies blocking either IL-17A (ixekizumab and secukinumab) (Leonardi et al. 2012; Papp et al. 2013b; Rich et al. 2013) or IL-17R brodalumab (Papp et al. 2012b) have shown striking efficacy in phase II clinical trials with >70% of patients achieving PASI75 and more than half receiving a remarkable PASI90. Although phase III clinical trials are currently ongoing (Amgen 2013a,b,c; Eli Lilly 2013a,c,d,e,f; Novartis 2013b), initial molecular data showed that the effect of IL-17 blockade on expression of genes synergistically regulated by IL-17 and TNF-α is greater than in previous studies with anti-TNF therapy (Krueger et al. 2012).
Notwithstanding the efficacy of the biologic drug currently available in the clinic, at least one third of patients do not respond to biologic therapy (Gudjonsson et al. 2012) or lose initial responsiveness because of the development of antidrug antibodies (ADA), which causes decreased drug efficacy and/or induction of adverse events (Sathish et al. 2013; Vincent et al. 2013). Moreover, biologic drugs pose a considerable economical burden because of their costs (Poulin et al. 2009; Liu et al. 2012). Finally, a sizable number of patients with mild-to-moderate psoriasis still rely on traditional topical treatments.
Small Molecule Drugs
The aforementioned limitations of biologic drugs has led to other therapeutic options being explored, such as small molecule, that is, low molecular weight, organic compounds targeting key molecules involved in cellular signaling (Garcia-Perez et al. 2013) aimed at minimizing general immunosuppression.
Within this category, tofacitinib and apremilast are currently the molecules most advanced in the clinical development. Tofacitinib is an inhibitor of Janus kinases (JAK)1 and JAK3, key intracellular enzymes transducing cytokine-initiated signals, which showed efficacy both in the oral and topical formulation (Boy et al. 2009; Ports et al. 2013), with 66.7% of patients reaching PASI75 at 12 wk in a phase IIb trial (Papp et al. 2012c). These encouraging results are in agreement with the efficacy displayed by tofacitinib in phase III clinical trials in rheumatoid arthritis (RA) (Fleischmann et al. 2012; van Vollenhoven et al. 2012), which have led to its approval in the United States of America for the treatment of patients with moderate-to-severe RA who inadequately responded to metothrexate.
Apremilast is an inhibitor of phosphodiesterase 4, an enzyme involved in the breakdown of cAMP. Apremilast inhibitory activity increases cAMP levels, thus inhibiting the production on proinflammatory cytokines. Phase IIb data on efficacy (41% of patients achieving PASI75 at week 16), safety, and tolerability of apremilast in the treatment of psoriasis have been recently published, supporting the transition of this drug to the next level of clinical development (Papp et al. 2012a).
Even if their efficacy is, in some cases, far from that of biologic drugs, these molecules have a cheaper manufacturing process, can be administered orally or topically, and have shown a good safety profile so far, although long-term safety data are needed to confirm the initial studies. Thus, they could find their therapeutic niche in the treatment of less severe forms of psoriasis.
BIOMARKER DISCOVERY IN PSORIASIS: STATE OF THE ART AND INNOVATIVE DISCOVERY APPROACHES
The identification of disease-specific molecular patterns or, more precisely, biomarkers, is of invaluable usefulness in the clinic for disease prognosis, therapy response prediction, and patient stratification. Biomarkers can also be used to identify disease risk factors, guide further investigation, and, overall, contribute to better elucidate disease etiopathogenesis. Using a variety of hypothesis-driven experimental approaches, candidate biomarkers have been described for psoriasis, although none of them has so far met the sensitivity, specificity, and accuracy criteria that would allow their translation into clinical use (Villanova et al. 2013a).
Traditional investigation of circulating biomarkers assessing psoriasis blood and serum have shown increased levels of unspecific inflammation markers (Rocha-Pereira et al. 2004; Garbaraviciene et al. 2010; Gisondi et al. 2010), several pro inflammatory cells (Kagami et al. 2010) and their mediators (Arican et al. 2005), as well as altered lipid and coagulation profiles (Marongiu et al. 1994; Rashmi et al. 2009) compared with healthy individuals, in keeping with the described cardiovascular comorbidities. Specific cell subsets present in the periphery, correlate well not only with disease status, but also with therapy response. Both pathogenic Th cell subsets (Th1, Th17, and Th22) and circulating endothelial cells, which can be used as an indirect measure of vascular injury, decrease after successful anti-TNF therapy, suggesting simultaneous improvement of disease activity and cardiovascular health during efficacious treatment (Kagami et al. 2010; De Simone et al. 2013). Interestingly, cutaneous lymphocyte-associated antigen expression on lymphocytes has been shown to negatively correlate to PASI score during anti-TNF therapy, allowing to predict responders and nonresponders within the first six weeks of treatment (Jokai et al. 2013). Good response to biologics can be affected by their immunogenicity and consequent development of ADA, which can interefere with pharmacokinetics, pharmacodynamics, efficacy, and safety of the therapeutic. Therefore, biomarkers to assess drug levels and test ADA would be helpful to optimize long-term management of biologic therapies (Vincent et al. 2013).
An overall increase in proinflammatory mediators is detectable in skin lesions, in particular, prominence for molecules of the IL-23/IL-17 axis (Lee et al. 2004; Wilson et al. 2007; Tonel et al. 2010). Nonetheless, the biggest changes at tissue levels are observed in KC-related molecules, in keeping with their altered proliferation and differentiation and increased production of antimicrobial peptides.
Finally, based on the growing list of psoriasis susceptibility genes identified by GWASs, genetic biomarkers are being investigated. SNPs in genes involved in drug transport and metabolism (ABCC1 and ABCG2), as well as in the regulation of TNF-induced pathways (TNFAIP3), are associated with improved metotrexathe or anti-TNF treatment response (Hebert et al. 2012; Tejasvi et al. 2012), calling for further pharmacogenetic studies.
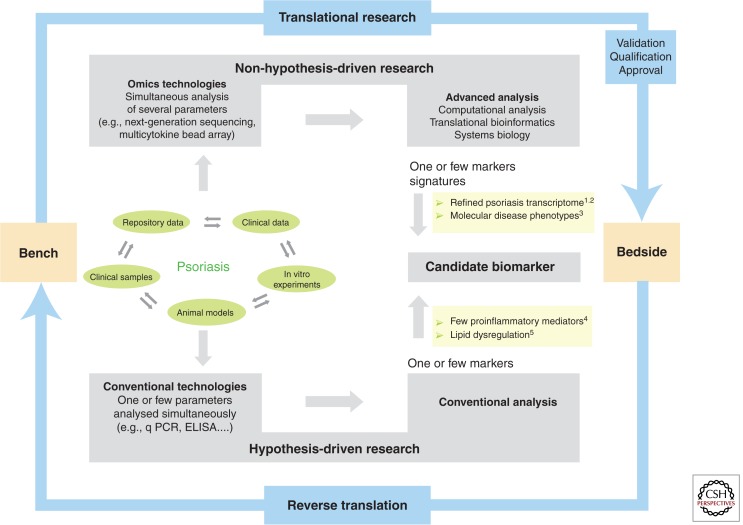
Recent advances in technology, improved cost effectiveness, and the development of powerful computational tools have led to the implementation of high-throughput platforms, which can be exploited in both research and clinical settings for a de novo discovery approach and the successful identification of translational biomarkers (Fig. 4).
Figure 4.
Psoriasis research for disease-specific biomarker discovery. Psoriasis research progresses via reciprocal inputs from the bench to the bedside (translational research) and vice versa (reverse translation). Clinical samples and data, repository data, in vitro, and in vivo animal models can be assessed and analyzed using conventional technologies measuring one or few parameters according to a preformed hypothesis, or innovative high-throughput platforms, which simultaneously measure many parameters according to a non–a priori hypothesis approach. Both strategies can lead to the identification of a candidate biomarker, which coincides with one, several, or multiple markers leading to molecular signatures in the case of omics data. Whatever the discovery approach, the clinical translation of a biomarker will then require the successful achievement of the validation, qualification, and approval process. Examples in the figure are from: 1Suarez-Farinas et al. 2012; 2Tian et al. 2012; 3Ainali et al. 2012; 4Arican et al. 2005; 5Gupta et al. 2011.
Technologies, such as gene expression microarray and next-generation DNA and RNA sequencing for the rapid sequencing of entire genomes, transcriptomes, and epigenomes have been successfully applied to psoriasis, resulting, for instance, in the identification of the IL36RN (Onoufriadis et al. 2011) and CARD14 mutations (Jordan et al. 2012b), refinement of the psoriasis “transcriptome” (Jabbari et al. 2012; Tian et al. 2012), and description of the global changes of methylation in lesional versus nonlesional psoriatic skin (Roberson et al. 2012). Multiparameter technologies such as multiplex cytokine bead arrays and flow cytometry have provided an additional layer of complexity, fine tuning the molecular and cellular immune signature of psoriatic serum (Suarez-Farinas et al. 2012), blood, and skin (Sjogren et al. 2012) using little starting material.
As these technologies produce a vast amount of data (“omics”), the collection of genetic variations, gene expression, proteomics, and clinical information call for integrative computational methods, or “translational bioinformatics,” to develop and improve methods for storing, retrieving, organizing, and analyzing biological data.
The derivation of molecular signatures using omics data is increasingly expanding and can be further implemented by mining publically available data sets.
For instance, a large amount of gene expression data from psoriasis skin is now available and a meta-analytic approach has recently been used to combine the results of five microarray data sets obtaining the meta-analysis-derived psoriasis transcriptome (Tian et al. 2012). The overrepresentation of atherosclerosis signaling and fatty acid metabolism pathways in lesional skin supports the close relationship between psoriasis and systemic manifestations (Tian et al. 2012) This study has also identified a set of 20 “classifier” genes clearly separating lesional from nonlesional psoriasis skin, thus representing possible biomarkers. This set contained many genes that were part of the residual disease genomic profile, or “molecular scar,” still present in psoriasis skin after successful treatment (Suarez-Farinas et al. 2011), and also genes with differential methylation status (Roberson et al. 2012). Moreover, an innovative pipeline for patient stratification has been developed through an integrated analysis of the psoriasis transcriptome using decision-tree predictors (Ainali et al. 2012). Psoriatic samples were clustered on the basis of distinct gene expression patterns identifying two molecular disease subtypes: pathways particularly enriched in one of the two subgroups included transforming growth factor β and ErbB, thus, suggesting putative therapeutic targets for these patients. A network-based methodology has also been used for the integrative analysis of proteomics and transcriptomics data sets identifying similarities and differences between the two levels of cellular organization (Piruzian et al. 2010)
Moreover, systems biology is used to analyze and visualize the complex connections and multiple levels of biological hierarchies of subcellular processes, such as gene regulatory networks and signal transduction pathways. A systems biology approach has been used to model and quantify immune cell interactions contributing to skin inflammation via cytokine signaling (Valeyev et al. 2010). The relationship between genetic variants and small alterations in cytokine production can modify the feedback loop between immune cells and lead to pathological inflammation.
CONCLUSIONS AND FUTURE PERSPECTIVES
Four decades of clinical and basic research have greatly advanced our understanding of the complex architecture of psoriasis and, more importantly, increased the number of effective therapeutic options available to patients.
Future directions call for a refinement of the current knowledge through a more integrative approach. Psoriasis susceptibility genes identified so far clearly point toward critical pathogenic pathways warranting further studies and more genetic studies to identify the so-called “missing heritability.” Although challenging, functional investigation of genetic determinants is needed to better exploit them in a clinical setting, for example, in pharmacogenetic studies assessing possible response-to-treatment predictive roles. Investigation of mechanistic associations linking distinct environmental triggers with certain genetic determinants and specific dysregulated immune responses would also increase the possibility to implement a much-needed personalized medicine approach in the near future. The integration of different types of large datasets, obtained through high-throughput platforms and powerful analytical tools, supports the discovery of disease biomarkers with multiple applications in patients' stratification, treatment of comorbidites, and new drug discovery. Taken together, these efforts hold the promise to further benefit psoriasis patients.
ACKNOWLEDGMENTS
We are indebted to psoriasis patients and healthy volunteers for their courage, trust, and generosity in donating clinical specimens to make psoriasis research possible. We thank F.O.N. laboratory members for their contribution over the years to the work cited in this review. We thank Dr. Catherine Smith, Thomas Walters, Hemawtee Sreeneebus, Luca Napolitano, and Lucia Dunajova for their help with Figure 1. We acknowledge support by the following grant bodies: Wellcome Trust Programme GR078173MA (F.O.N.) and National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St. Thomas' National Health Service (NHS) Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, NIHR, or Department of Health. F.O.N. has been a consultant for companies producing targeted therapies for treatment of patients with psoriasis. The other authors state no conflict of interest.
Footnotes
John Updike (1932–2009), was a Pulitzer Prize-winning American writer who had psoriasis. His description of his lifelong personal journey with psoriasis is both touching and inspiring for whoever is engaged in psoriasis research (Updike 1976, 1985, 1989).
Editors: Anthony E. Oro and Fiona M. Watt
Additional Perspectives on The Skin and Its Diseases available at www.perspectivesinmedicine.org
REFERENCES
- Ahlehoff O, Gislason GH, Charlot M, Jorgensen CH, Lindhardsen J, Olesen JB, Abildstrom SZ, Skov L, Torp-Pedersen C, Hansen PR 2011. Psoriasis is associated with clinically significant cardiovascular risk: A Danish nationwide cohort study. J Intern Med 270: 147–157 [DOI] [PubMed] [Google Scholar]
- Ainali C, Valeyev N, Perera G, Williams A, Gudjonsson JE, Ouzounis CA, Nestle FO, Tsoka S 2012. Transcriptome classification reveals molecular subtypes in psoriasis. BMC Genomics 13: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amgen. 2013a. Study of efficacy and safety of brodalumab compared with placebo and ustekinumab in moderate to severe plaque psoriasis subjects (AMAGINE-2). clinicaltrials.gov/ct2/show/NCT01708603.
- Amgen. 2013b. Study of efficacy and safety of brodalumab compared with placebo and ustekinumab in moderate to severe plaque psoriasis subjects (AMAGINE-3). clinicaltrials.gov/ct2/show/NCT01708629.
- Amgen. 2013c. Study of efficacy, safety, and withdrawal and retreatment with brodalumab in moderate to severe plaque psoriasis subjects (AMAGINE-1). http://clinicaltrials.gov/ct2/show/NCT01708590.
- Anacor. 2013a. AN2728 Topical ointment to treat mild-to-moderate plaque-type psoriasis. clinicaltrials.gov/ct2/show/NCT01300052?term=01300052&rank=1.
- Anacor. 2013b. Safety and efficacy study of a novel ointment to treat plaque type psoriasis. clinicaltrials.gov/ct2/show/NCT01029405?term=01029405&rank=1.
- Anacor. 2013c. Safety and efficacy study of a novel ointment to treat plaque type psoriasis. clinicaltrials.gov/ct2/results?term=00759161.
- Anancor. 2013d. Safety and efficacy study of a novel ointment to treat plaque type psoriasis. clinicaltrials.gov/ct2/show/NCT00755196
- Anandarajah AP, Ritchlin CT 2009. The diagnosis and treatment of early psoriatic arthritis. Nat Rev Rheumatol 5: 634–641 [DOI] [PubMed] [Google Scholar]
- Arican O, Aral M, Sasmaz S, Ciragil P 2005. Serum levels of TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm 2005: 273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AW, Harskamp CT, Armstrong EJ 2013a. Psoriasis and metabolic syndrome: A systematic review and meta-analysis of observational studies. J Am Acad Dermatol 68: 654–662 [DOI] [PubMed] [Google Scholar]
- Armstrong AW, Harskamp CT, Armstrong EJ 2013b. Psoriasis and the risk of diabetes mellitus: A systematic review and meta-analysis. JAMA Dermatol 149: 84–91 [DOI] [PubMed] [Google Scholar]
- Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG 1999. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-γ, interleukin-2, and tumor necrosis factor-α, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: A type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol 113: 752–759 [DOI] [PubMed] [Google Scholar]
- Ayala F 2007. Clinical presentation of psoriasis. Reumatismo 59: 40–45 [PubMed] [Google Scholar]
- Boy MG, Wang C, Wilkinson BE, Chow VF, Clucas AT, Krueger JG, Gaweco AS, Zwillich SH, Changelian PS, Chan G 2009. Double-blind, placebo-controlled, dose-escalation study to evaluate the pharmacologic effect of CP-690,550 in patients with psoriasis. J Invest Dermatol 129: 2299–2302 [DOI] [PubMed] [Google Scholar]
- Boyd AS, Menter A 1989. Erythrodermic psoriasis. Precipitating factors, course, and prognosis in 50 patients. J Am Acad Dermatol 21: 985–991 [PubMed] [Google Scholar]
- Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO 2004. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J Exp Med 199: 731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O, Conrad C, Tonel G, Gilliet M, Nestle FO 2007. The pathogenic role of tissue-resident immune cells in psoriasis. Trends Immunol 28: 51–57 [DOI] [PubMed] [Google Scholar]
- Brandrup F, Hauge M, Henningsen K, Eriksen B 1978. Psoriasis in an unselected series of twins. Arch Dermatol 114: 874–878 [PubMed] [Google Scholar]
- Bristol-Myers Squibb. 2013. Phase II randomized, double-blind, placebo-controlled study of BMS-188667 (CTLA4Ig) in patients with psoriasis vulgaris. clinicaltrials.gov/ct2/show/NCT00287547?term=00287547&rank=1.
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, et al. 2011. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35: 596–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Fleming C, Yan J 2012. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol 9: 302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can-Fite. 2013. Trial of CF101 to treat patients with psoriasis. clinicaltrials.gov/ct2/show/NCT01265667?term=Cf101%2C+psoriasis&rank=2
- Capon F, Bijlmakers MJ, Wolf N, Quaranta M, Huffmeier U, Allen M, Timms K, Abkevich V, Gutin A, Smith R, et al. 2008. Identification of ZNF313/RNF114 as a novel psoriasis susceptibility gene. Hum Mol Genet 17: 1938–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon F, Burden AD, Trembath RC, Barker JN 2012. Psoriasis and other complex trait dermatoses: From Loci to functional pathways. J Invest Dermatol 132: 915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, Matsunami N, Ardlie KG, Civello D, Catanes JJ, et al. 2007. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet 80: 273–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celgene. 2013a. Phase 3b Safety and efficacy study of apremilast to treat moderate to severe plaque-plaque psoriasis. clinicaltrials.gov/ct2/show/NCT01690299.
- Celgene. 2013b. Study to evaluate safety and effectiveness of oral apremilast (CC-10004) in patients with moderate to severe plaque psoriasis (ESTEEM 1). clinicaltrials.gov/ct2/show/NCT01194219.
- Celgene. 2013c. Study to evaluate safety and effectiveness of oral apremilast (CC-10004) in patients with moderate to severe plaque psoriasis. (ESTEEM 2). clinicaltrials.gov/ct2/show/NCT01232283
- Chamian F, Lowes MA, Lin SL, Lee E, Kikuchi T, Gilleaudeau P, Sullivan-Whalen M, Cardinale I, Khatcherian A, Novitskaya I, et al. 2005. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc Natl Acad Sci 102: 2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB, et al. 2006. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med 203: 2577–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran V, Raychaudhuri SP 2010. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun 34: J314–J321 [DOI] [PubMed] [Google Scholar]
- Chang JC, Smith LR, Froning KJ, Schwabe BJ, Laxer JA, Caralli LL, Kurland HH, Karasek MA, Wilkinson DI, Carlo DJ, et al. 1994. CD8+ T cells in psoriatic lesions preferentially use T-cell receptor V β3 and/or V β13.1 genes. Proc Natl Acad Sci 91: 9282–9286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Poon A, Yeung C, Helms C, Pons J, Bowcock AM, Kwok PY, Liao W 2011. A genetic risk score combining ten psoriasis risk loci improves disease prediction. PloS ONE 6: e19454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, Nograles KE, Tian S, Cardinale I, Chimenti S, Krueger JG 2011. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol 131: 677–687 [DOI] [PubMed] [Google Scholar]
- Clark RA 2010. Skin-resident T cells: The ups and downs of on site immunity. J Invest Dermatol 130: 362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clop A, Bertoni A, Spain SL, Simpson MA, Pullabhatla V, Tonda R, Hundhausen C, Di Meglio P, De Jong P, Hayday AC, et al. 2013. An in-depth characterization of the major psoriasis susceptibility locus identifies candidate susceptibility alleles within an HLA-C enhancer element. PloS ONE 8: e71690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de Fougerolles A, Kotelianski V, Gardner H, Nestle FO 2007. α1β1 Integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med 13: 836–842 [DOI] [PubMed] [Google Scholar]
- Costello PJ, Winchester RJ, Curran SA, Peterson KS, Kane DJ, Bresnihan B, FitzGerald OM 2001. Psoriatic arthritis joint fluids are characterized by CD8 and CD4 T cell clonal expansions appear antigen driven. J Immunol 166: 2878–2886 [DOI] [PubMed] [Google Scholar]
- da Silva AJ, Brickelmaier M, Majeau GR, Li Z, Su L, Hsu YM, Hochman PS 2002. Alefacept, an immunomodulatory recombinant LFA-3/IgG1 fusion protein, induces CD16 signaling and CD2/CD16-dependent apoptosis of CD2+ cells. J Immunol 168: 4462–4471 [DOI] [PubMed] [Google Scholar]
- David M, Akerman L, Ziv M, Kadurina M, Gospodinov D, Pavlotsky F, Yankova R, Kouzeva V, Ramon M, Silverman MH, et al. 2012. Treatment of plaque-type psoriasis with oral CF101: Data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol 26: 361–367 [DOI] [PubMed] [Google Scholar]
- Davidovici BB, Sattar N, Prinz J, Puig L, Emery P, Barker JN, van de Kerkhof P, Stahle M, Nestle FO, Girolomoni G, et al. 2010. Psoriasis and systemic inflammatory diseases: Potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol 130: 1785–1796 [DOI] [PubMed] [Google Scholar]
- de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, Idu MM, van Maldegem F, Aten J, van der Wal AC 2010. Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J Pathol 220: 499–508 [DOI] [PubMed] [Google Scholar]
- de Cid R, Riveira-Munoz E, Zeeuwen PL, Robarge J, Liao W, Dannhauser EN, Giardina E, Stuart PE, Nair R, Helms C, et al. 2009. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat Genet 41: 211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle RP, Johnson KR 2005. Do smoking, obesity, and stress cause psoriasis? J Invest Dermatol 125: vi–vii [DOI] [PubMed] [Google Scholar]
- De Simone C, Caldarola G, Coco V, Palumbo S, Pocino K, Sgambato A, Maiorino A, Corbi M, Sandri MT, Vendittelli F, et al. 2013. Circulating endothelial cell levels in psoriatic patients and their modification after an anti-TNF-α (Etanercept) treatment. J Eur Acad Dermatol Venereol 10.1111/jdv.12140 [DOI] [PubMed] [Google Scholar]
- Di Cesare A, Di Meglio P, Nestle FO 2009. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 129: 1339–1350 [DOI] [PubMed] [Google Scholar]
- Diluvio L, Vollmer S, Besgen P, Ellwart JW, Chimenti S, Prinz JC 2006. Identical TCR β-chain rearrangements in streptococcal angina and skin lesions of patients with psoriasis vulgaris. J Immunol 176: 7104–7111 [DOI] [PubMed] [Google Scholar]
- Di Meglio P, Di Cesare A, Laggner U, Chu C-C, Napolitano L, Villanova F, Tosi I, Capon F, Trembath RC, Peris K, et al. 2011a. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans PLoS ONE 6: e17160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meglio P, Perera GK, Nestle FO 2011b. The multitasking organ: Recent insights into skin immune function. Immunity 35: 857–869 [DOI] [PubMed] [Google Scholar]
- Di Meglio P, Villanova F, Napolitano L, Tosi I, Terranova Barberio M, Mak RK, Nutland S, Smith CH, Barker JN, Todd JA, et al. 2013. The IL23R A/Gln381 allele promotes IL-23 unresponsiveness in human memory T-helper 17 cells and impairs Th17 responses in psoriasis patients. J Invest Dermatol 133: 2381–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlatshahi EA, Kavousi M, Nijsten T, Ikram MA, Hofman A, Franco OH, Wakkee M 2013. Psoriasis is not associated with atherosclerosis and incident cardiovascular events: The Rotterdam Study. J Invest Dermatol 133: 2347–2354 [DOI] [PubMed] [Google Scholar]
- Duffy DL, Spelman LS, Martin NG 1993. Psoriasis in Australian twins. J Am Acad Dermatol 29: 428–434 [DOI] [PubMed] [Google Scholar]
- Eder L, Chandran V, Pellett F, Pollock R, Shanmugarajah S, Rosen CF, Rahman P, Gladman DD 2011. IL13 gene polymorphism is a marker for psoriatic arthritis among psoriasis patients. Ann Rheum Dis 70: 1594–1598 [DOI] [PubMed] [Google Scholar]
- Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G 2009. Interleukin-17 and interferon-γ are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 119: 1424–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE, Tejasvi T, Voorhees JJ, Abecasis GR, Nair RP 2010. Molecular dissection of psoriasis: Integrating genetics and biology. J Invest Dermatol 130: 1213–1226 [DOI] [PubMed] [Google Scholar]
- Eli Lilly 2013a. Evaluation of ixekizumab using auto-injector or prefilled syringe in participants with moderate to severe plaque psoriasis (UNCOVER-A). clinicaltrials.gov/ct2/show/NCT01777191.
- Eli Lilly 2013b. A phase 2b study of LY3009104 in participants with moderate to severe psoriasis. clinicaltrials.gov/ct2/show/NCT01490632?term=01490632&rank=1.
- Eli Lilly 2013c. A phase 3 study in participants with moderate to severe psoriasis (UNCOVER-2). clinicaltrials.gov/ct2/show/NCT01597245.
- Eli Lilly 2013d. A study in Japanese participants with moderate-to-severe psoriasis. clinicaltrials.gov/ct2/show/NCT01624233.
- Eli Lilly 2013e. A study in participants with moderate to severe psoriasis. clinicaltrials.gov/ct2/show/NCT01708590.
- Eli Lilly 2013f. A study in participants with moderate to severe psoriasis (UNCOVER-3). clinicaltrials.gov/ct2/show/NCT01646177.
- Ellinghaus E, Ellinghaus D, Stuart PE, Nair RP, Debrus S, Raelson JV, Belouchi M, Fournier H, Reinhard C, Ding J, et al. 2010. Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat Genet 42: 991–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus D, Ellinghaus E, Nair RP, Stuart PE, Esko T, Metspalu A, Debrus S, Raelson JV, Tejasvi T, Belouchi M, et al. 2012a. Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am J Hum Genet 90: 636–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus E, Stuart PE, Ellinghaus D, Nair RP, Debrus S, Raelson JV, Belouchi M, Tejasvi T, Li Y, Tsoi LC, et al. 2012b. Genome-wide meta-analysis of psoriatic arthritis identifies susceptibility locus at REL. J Invest Dermatol 132: 1133–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M, Saraceno R, Giunta A, Maccarone M, Chimenti S 2006. An Italian study on psoriasis and depression. Dermatology 212: 123–127 [DOI] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, et al. 2009. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 119: 3573–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber EM, Nall ML, Watson W 1974. Natural history of psoriasis in 61 twin pairs. Arch Dermatol 109: 207–211 [PubMed] [Google Scholar]
- Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, Gruben D, Wallenstein GV, Zwillich SH, Kanik KS 2012. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 367: 495–507 [DOI] [PubMed] [Google Scholar]
- Flutter B, Nestle FO 2013. TLRs to cytokines: Mechanistic insights from the imiquimod mouse model of psoriasis. Eur J Immunol 43: 3138–3146 [DOI] [PubMed] [Google Scholar]
- Friedrich M, Krammig S, Henze M, Docke WD, Sterry W, Asadullah K 2000. Flow cytometric characterization of lesional T cells in psoriasis: Intracellular cytokine and surface antigen expression indicates an activated, memory/effector type 1 immunophenotype. Arch Dermatol Res 292: 519–521 [DOI] [PubMed] [Google Scholar]
- Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, Homey B, Barrat FJ, Zal T, Gilliet M 2009. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med 206: 1983–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbaraviciene J, Diehl S, Varwig D, Bylaite M, Ackermann H, Ludwig RJ, Boehncke WH 2010. Platelet P-selectin reflects a state of cutaneous inflammation: Possible application to monitor treatment efficacy in psoriasis. Exp Dermatol 19: 736–741 [DOI] [PubMed] [Google Scholar]
- Garcia-Perez ME, Stevanovic T, Poubelle PE 2013. New therapies under development for psoriasis treatment. Curr Opin Pediatr 25: 480–487 [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10: 524–530 [DOI] [PubMed] [Google Scholar]
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB 2006. Risk of myocardial infarction in patients with psoriasis. JAMA 296: 1735–1741 [DOI] [PubMed] [Google Scholar]
- Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F 2011. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med 208: 1127–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet M, Conrad C, Geiges M, Cozzio A, Thurlimann W, Burg G, Nestle FO, Dummer R 2004. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol 140: 1490–1495 [DOI] [PubMed] [Google Scholar]
- Gisondi P, Malerba M, Malara G, Puglisi Guerra A, Sala R, Radaeli A, Calzavara-Pinton P, Girolomoni G 2010. C-reactive protein and markers for thrombophilia in patients with chronic plaque psoriasis. Int J Immunopathol Pharmacol 23: 1195–1202 [DOI] [PubMed] [Google Scholar]
- Gladman DD, Antoni C, Mease P, Clegg DO, Nash P 2005. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann Rheum Dis 64: i14–i17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KB, Papp KA, Hamilton TK, Walicke PA, Dummer W, Li N, Bresnahan BW, Menter A 2003. Efalizumab for patients with moderate to severe plaque psoriasis: A randomized controlled trial. JAMA 290: 3073–3080 [DOI] [PubMed] [Google Scholar]
- Gordon KB, Langley RG, Leonardi C, Toth D, Menter MA, Kang S, Heffernan M, Miller B, Hamlin R, Lim L, et al. 2006. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: Double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol 55: 598–606 [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, Bala M, Marano CW, Menter A 2004. Infliximab induction therapy for patients with severe plaque-type psoriasis: A randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol 51: 534–542 [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Chamian F, Masud S, Cardinale I, Abello MV, Lowes MA, Chen F, Magliocco M, Krueger JG 2005. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol 175: 2721–2729 [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Hamilton T, Caro I, Kwon P, Compton PG, Leonardi CL 2006. Long-term continuous efalizumab therapy in patients with moderate to severe chronic plaque psoriasis: Updated results from an ongoing trial. J Am Acad Dermatol 54: S154–S163 [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Leonardi C, Kerdel F, Mehlis S, Olds M, Williams DA 2011. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol 165: 652–660 [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Barker JN 2007. Pathogenesis and clinical features of psoriasis. Lancet 370: 263–271 [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Christophers E, Barker JN, Chalmers RJ, Chimenti S, Krueger GG, Leonardi C, Menter A, Ortonne JP, Fry L 2007. A classification of psoriasis vulgaris according to phenotype. Br J Dermatol 156: 258–262 [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, Guzzo C, Xia Y, Zhou B, Li S, et al. 2010. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 362: 118–128 [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Karason A, Antonsdottir A, Runarsdottir EH, Hauksson VB, Upmanyu R, Gulcher J, Stefansson K, Valdimarsson H 2003. Psoriasis patients who are homozygous for the HLA-Cw*0602 allele have a 2.5-fold increased risk of developing psoriasis compared with Cw6 heterozygotes. Br J Dermatol 148: 233–235 [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT 2007. Mouse models of psoriasis. J Invest Dermatol 127: 1292–1308 [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Johnston A, Ellis CN 2012. Novel systemic drugs under investigation for the treatment of psoriasis. J Am Acad Dermatol 67: 139–147 [DOI] [PubMed] [Google Scholar]
- Gullick NJ, Evans HG, Church LD, Jayaraj DM, Filer A, Kirkham BW, Taams LS 2010. Linking power Doppler ultrasound to the presence of th17 cells in the rheumatoid arthritis joint. PloS ONE 5: pii: e12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson AJ, Mohammed J, Horvath FJ, Podolsky MA, Anderson CR, Glick AB 2013. CD8+ T cells mediate RAS-induced psoriasis-like skin inflammation through IFN-γ. J Invest Dermatol 133: 955–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta MCS, Borkar M, Chandankhede M 2011. Dyslipidemia and oxidative stress in patients of psoriasis. Biomed Res 22: 221–224 [Google Scholar]
- Haider AS, Lowes MA, Gardner H, Bandaru R, Darabi K, Chamian F, Kikuchi T, Gilleaudeau P, Whalen MS, Cardinale I, et al. 2007. Novel insight into the agonistic mechanism of alefacept in vivo: Differentially expressed genes may serve as biomarkers of response in psoriasis patients. J Immunol 178: 7442–7449 [DOI] [PubMed] [Google Scholar]
- Hammar H, Gu SQ, Johannesson A, Sundkvist KG, Biberfeld P 1984. Subpopulations of mononuclear cells in microscopic lesions of psoriatic patients. Selective accumulation of suppressor/cytotoxic T cells in epidermis during the evolution of the lesion. J Invest Dermatol 83: 416–420 [DOI] [PubMed] [Google Scholar]
- Hart PH, Gorman S, Finlay-Jones JJ 2011. Modulation of the immune system by UV radiation: More than just the effects of vitamin D? Nat Rev Immunol 11: 584–596 [DOI] [PubMed] [Google Scholar]
- Hebert HL, Ali FR, Bowes J, Griffiths CE, Barton A, Warren RB 2012. Genetic susceptibility to psoriasis and psoriatic arthritis: Implications for therapy. Br J Dermatol 166: 474–482 [DOI] [PubMed] [Google Scholar]
- Henseler T, Christophers E 1985. Psoriasis of early and late onset: Characterization of two types of psoriasis vulgaris. J Am Acad Dermatol 13: 450–456 [DOI] [PubMed] [Google Scholar]
- Hijnen D, Knol EF, Gent YY, Giovannone B, Beijn SJ, Kupper TS, Bruijnzeel-Koomen CA, Clark RA 2013. CD8+ T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-γ, IL-13, IL-17, and IL-22. J Invest Dermatol 133: 973–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LN, Armstrong AW 2012. Psoriasis and autoimmune disorders: A review of the literature. J Am Acad Dermatol 67: 1076–1079 [DOI] [PubMed] [Google Scholar]
- Huffmeier U, Uebe S, Ekici AB, Bowes J, Giardina E, Korendowych E, Juneblad K, Apel M, McManus R, Ho P, et al. 2010. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat Genet 42: 996–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundhausen C, Bertoni A, Mak RK, Botti E, Di Meglio P, Clop A, Laggner U, Chimenti S, Hayday AC, Barker JN, et al. 2012. Allele-specific cytokine responses at the HLA-C locus: Implications for psoriasis. J Invest Dermatol 132: 635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KA, Mistry V, Bockett NA, Ahmad T, Ban M, Barker JN, Barrett JC, Blackburn H, Brand O, Burren O, et al. 2013. Negligible impact of rare autoimmune-locus coding-region variants on missing heritability. Nature 498: 232–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Maradit Kremers H 2009. Trends in incidence of adult-onset psoriasis over three decades: A population-based study. J Am Acad Dermatol 60: 394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incyte. 2013. A study of escalating doses of INCB039110 administered orally in patients with plaque psoriasis. clinicaltrials.gov/ct2/show/NCT01634087?term=01634087&rank=.
- Jabbari A, Suarez-Farinas M, Dewell S, Krueger JG 2012. Transcriptional profiling of psoriasis using RNA-seq reveals previously unidentified differentially expressed genes. J Invest Dermatol 132: 246–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen. 2013. A study to evaluate CNTO 1959 in the treatment of patients with moderate to severe plaque-type psoriasis (X-PLORE). clinicaltrials.gov/ct2/show/NCT01483599.
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS 2012. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483: 227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Yang S, Zhang F, Kong Y, Xiao F, Hou Y, Fan X, Zhang X 2009. Combined effects of HLA-Cw6 and cigarette smoking in psoriasis vulgaris: A hospital-based case-control study in China. J Eur Acad Dermatol Venereol 23: 132–137 [DOI] [PubMed] [Google Scholar]
- Johansen C, Vinter H, Soegaard-Madsen L, Olsen LR, Steiniche T, Iversen L, Kragballe K 2010. Preferential inhibition of the mRNA expression of p38 mitogen-activated protein kinase regulated cytokines in psoriatic skin by anti-TNF-α therapy. Br J Dermatol 163: 1194–1204 [DOI] [PubMed] [Google Scholar]
- Johnston A, Fritz Y, Dawes SM, Diaconu D, Al-Attar PM, Guzman AM, Chen CS, Fu W, Gudjonsson JE, McCormick TS, et al. 2013. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol 190: 2252–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokai H, Szakonyi J, Kontar O, Barna G, Inotai D, Karpati S, Hollo P 2013. Cutaneous lymphocyte-associated antigen as a novel predictive marker of TNF-α inhibitor biological therapy in psoriasis. Exp Dermatol 22: 221–223 [DOI] [PubMed] [Google Scholar]
- Jordan CT, Cao L, Roberson ED, Duan S, Helms CA, Nair RP, Duffin KC, Stuart PE, Goldgar D, Hayashi G, et al. 2012a. Rare and common variants in CARD14, encoding an epidermal regulator of NF-κB, in psoriasis. Am J Hum Genet 90: 796–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CT, Cao L, Roberson ED, Pierson KC, Yang CF, Joyce CE, Ryan C, Duan S, Helms CA, Liu Y, et al. 2012b. PSORS2 is due to mutations in CARD14. Am J Hum Genet 90: 784–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien D, Prinz JC, Langley RG, Caro I, Dummer W, Joshi A, Dedrick R, Natta P 2004. T-cell modulation for the treatment of chronic plaque psoriasis with efalizumab (Raptiva): Mechanisms of action. Dermatology 208: 297–306 [DOI] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A 2010. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 130: 1373–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabudak O, Ulusoy RE, Erikci AA, Solmazgul E, Dogan B, Harmanyeri Y 2008. Inflammation and hypercoagulable state in adult psoriatic men. Acta Derm Venereol 88: 337–340 [DOI] [PubMed] [Google Scholar]
- Kaur S, Zilmer K, Kairane C, Kals M, Zilmer M 2008. Clear differences in adiponectin level and glutathione redox status revealed in obese and normal-weight patients with psoriasis. Br J Dermatol 159: 1364–1367 [DOI] [PubMed] [Google Scholar]
- Kim GK, Del Rosso JQ 2010. Drug-provoked psoriasis: Is it drug induced or drug aggravated? Understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol 3: 32–38 [PMC free article] [PubMed] [Google Scholar]
- Krueger GG, Papp KA, Stough DB, Loven KH, Gulliver WP, Ellis CN 2002. A randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J Am Acad Dermatol 47: 821–833 [DOI] [PubMed] [Google Scholar]
- Krueger JG, Fretzin S, Suarez-Farinas M, Haslett PA, Phipps KM, Cameron GS, McColm J, Katcherian A, Cueto I, White T, et al. 2012. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol 130: 145–154 e149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, Szeliga W, Wang Y, Liu Y, Welling TH, et al. 2008. Induction of IL-17+ T cell trafficking and development by IFN-γ: Mechanism and pathological relevance in psoriasis. J Immunol 181: 4733–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM 2010. The risk of depression, anxiety, and suicidality in patients with psoriasis: A population-based cohort study. Arch Dermatol 146: 891–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, Smith CH, Hayday AC, Nickoloff BJ, Nestle FO 2011. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J Immunol 187: 2783–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Su B, Nestle FO, Zal T, et al. 2007. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449: 564–569 [DOI] [PubMed] [Google Scholar]
- Lebwohl M, Christophers E, Langley R, Ortonne JP, Roberts J, Griffiths CE 2003a. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol 139: 719–727 [DOI] [PubMed] [Google Scholar]
- Lebwohl M, Tyring SK, Hamilton TK, Toth D, Glazer S, Tawfik NH, Walicke P, Dummer W, Wang X, Garovoy MR, et al. 2003b. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med 349: 2004–2013 [DOI] [PubMed] [Google Scholar]
- Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, Dhodapkar M, Krueger JG 2004. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med 199: 125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, Gottlieb AB 2003. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 349: 2014–2022 [DOI] [PubMed] [Google Scholar]
- Leonardi CL, Papp KA, Gordon KB, Menter A, Feldman SR, Caro I, Walicke PA, Compton PG, Gottlieb AB 2005. Extended efalizumab therapy improves chronic plaque psoriasis: Results from a randomized phase III trial. J Am Acad Dermatol 52: 425–433 [DOI] [PubMed] [Google Scholar]
- Leonardi C, Menter A, Hamilton T, Caro I, Xing B, Gottlieb AB 2008a. Efalizumab: Results of a 3-year continuous dosing study for the long-term control of psoriasis. Br J Dermatol 158: 1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, Li S, Dooley LT, Gordon KB 2008b. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371: 1665–1674 [DOI] [PubMed] [Google Scholar]
- Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, Braun D, Banerjee S 2012. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 366: 1190–1199 [DOI] [PubMed] [Google Scholar]
- Li WQ, Han JL, Chan AT, Qureshi AA 2013. Psoriasis, psoriatic arthritis and increased risk of incident Crohn's disease in US women. Ann Rheum Dis 72: 1200–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, Villanueva EC, Shah P, Kaplan MJ, Bruce AT 2011. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol 187: 490–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Helms C, Liao W, Zaba LC, Duan S, Gardner J, Wise C, Miner A, Malloy MJ, Pullinger CR, et al. 2008. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet 4: e1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wu EQ, Bensimon AG, Fan CP, Bao Y, Ganguli A, Yang M, Cifaldi M, Mulani P 2012. Cost per responder associated with biologic therapies for Crohn's disease, psoriasis, and rheumatoid arthritis. Adv Ther 29: 620–634 [DOI] [PubMed] [Google Scholar]
- Lomholt G, ed. 1963. Psoriasis: Prevalence, spontaneous course, and genetics. GEC GAD, Copenhagen [Google Scholar]
- Lonnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF 2013. Heritability of psoriasis in a large twin sample. Br J Dermatol 169: 412–416 [DOI] [PubMed] [Google Scholar]
- Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG 2008. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol 128: 1207–1211 [DOI] [PubMed] [Google Scholar]
- Lowes MA, Russell CB, Martin DA, Towne JE, Krueger JG 2013. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol 34: 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chen H, Nikamo P, Qi Low H, Helms C, Seielstad M, Liu J, Bowcock AM, Stahle M, Liao W 2013. Association of cardiovascular and metabolic disease genes with psoriasis. J Invest Dermatol 133: 836–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, et al. 2008. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest 118: 597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak RK, Hundhausen C, Nestle FO 2009. Progress in understanding the immunopathogenesis of psoriasis. Actas Dermosifiliogr 100: 2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, et al. 2009. Finding the missing heritability of complex diseases. Nature 461: 747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, Gabriel SE 2005. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum 52: 402–411 [DOI] [PubMed] [Google Scholar]
- Marongiu F, Sorano GG, Bibbo C, Pistis MP, Conti M, Mulas P, Balestrieri A, Biggio P 1994. Abnormalities of blood coagulation and fibrinolysis in psoriasis. Dermatology 189: 32–37 [DOI] [PubMed] [Google Scholar]
- Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, Zribi J, Bal E, Cluzeau C, Chrabieh M, et al. 2011. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med 365: 620–628 [DOI] [PubMed] [Google Scholar]
- Menssen A, Trommler P, Vollmer S, Schendel D, Albert E, Gurtler L, Riethmuller G, Prinz JC 1995. Evidence for an antigen-specific cellular immune response in skin lesions of patients with psoriasis vulgaris. J Immunol 155: 4078–4083 [PubMed] [Google Scholar]
- Menter A, Gordon K, Carey W, Hamilton T, Glazer S, Caro I, Li N, Gulliver W 2005. Efficacy and safety observed during 24 weeks of efalizumab therapy in patients with moderate to severe plaque psoriasis. Arch Dermatol 141: 31–38 [DOI] [PubMed] [Google Scholar]
- Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, Li S, Dooley LT, Arnold C, Gottlieb AB 2007. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol 56: 31.e1-e15. [DOI] [PubMed] [Google Scholar]
- Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, Strober BE, Kaul M, Gu Y, Okun M, et al. 2008. Adalimumab therapy for moderate to severe psoriasis: A randomized, controlled phase III trial. J Am Acad Dermatol 58: 106–115 [DOI] [PubMed] [Google Scholar]
- Merck. 2013a. A study to evaluate the efficacy and safety of subcutaneous MK-3222, followed by an optional long-term safety extension study, in participants with moderate-to-severe chronic plaque psoriasis (MK-3222-010 AM3). clinicaltrials.gov/ct2/show/NCT01722331.
- Merck. 2013b. A study to evaluate the efficacy and safety/tolerability of subcutaneous SCH 900222/MK-3222 in participants with moderate-to-severe chronic plaque psoriasis (P07771/MK-3222-011). clinicaltrials.gov/ct2/show/NCT01729754
- Nair RP, Henseler T, Jenisch S, Stuart P, Bichakjian CK, Lenk W, Westphal E, Guo SW, Christophers E, Voorhees JJ, et al. 1997. Evidence for two psoriasis susceptibility loci (HLA and 17q) and two novel candidate regions (16q and 20p) by genome-wide scan. Hum Mol Genet 6: 1349–1356 [DOI] [PubMed] [Google Scholar]
- Nair RP, Stuart P, Henseler T, Jenisch S, Chia NV, Westphal E, Schork NJ, Kim J, Lim HW, Christophers E, et al. 2000. Localization of psoriasis-susceptibility locus PSORS1 to a 60-kb interval telomeric to HLA-C. Am J Hum Genet 66: 1833–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair RP, Stuart PE, Nistor I, Hiremagalore R, Chia NV, Jenisch S, Weichenthal M, Abecasis GR, Lim HW, Christophers E, et al. 2006. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet 78: 827–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, et al. 2009. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nat Genet 41: 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najarian DJ, Gottlieb AB 2003. Connections between psoriasis and Crohn's disease. J Am Acad Dermatol 48: 805–821 [DOI] [PubMed] [Google Scholar]
- Naldi L, Chatenoud L, Linder D, Belloni Fortina A, Peserico A, Virgili AR, Bruni PL, Ingordo V, Lo Scocco G, Solaroli C, et al. 2005. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: Results from an Italian case-control study. J Invest Dermatol 125: 61–67 [DOI] [PubMed] [Google Scholar]
- Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M 2005. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J Exp Med 202: 135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ 2009. Skin immune sentinels in health and disease. Nat Rev Immunol 9: 679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff BJ 1991. The cytokine network in psoriasis. Arch Dermatol 127: 871–884 [PubMed] [Google Scholar]
- Novartis. 2013a. A dose finding study of AEB071 assessing psoriasis area and severity index in patients with plaque psoriasis. http://clinicaltrials.gov/ct2/show/NCT01194219. Accessed July 26, 2013
- Novartis. 2013b. Extension study of secukinumab prefilled syringes in subjects with moderate to severe chronic plaque-type psoriasis completing preceding psoriasis phase iii studies with secukinumab. http://clinicaltrials.gov/ct2/show/NCT01544595?term=NCT01544595&rank=1. Accessed July 26, 2013
- O’Leary CJ, Creamer D, Higgins E, Weinman J 2004. Perceived stress, stress attributions and psychological distress in psoriasis. J Psychosom Res 57: 465–471 [DOI] [PubMed] [Google Scholar]
- Onoufriadis A, Simpson MA, Pink AE, Di Meglio P, Smith CH, Pullabhatla V, Knight J, Spain SL, Nestle FO, Burden AD, et al. 2011. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet 89: 432–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega C, Fernandez AS, Carrillo JM, Romero P, Molina IJ, Moreno JC, Santamaria M 2009. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol 86: 435–443 [DOI] [PubMed] [Google Scholar]
- Ozden MG, Tekin NS, Gurer MA, Akdemir D, Dogramaci C, Utas S, Akman A, Evans SE, Bahadir S, Ozturkcan S, et al. 2011. Environmental risk factors in pediatric psoriasis: A multicenter case-control study. Pediatr Dermatol 28: 306–312 [DOI] [PubMed] [Google Scholar]
- Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, Becher B 2012. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest 122: 2252–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CE, Nakanishi AM, Zitnik R, van de Kerkhof PC, Melvin L 2005. A global phase III randomized controlled trial of etanercept in psoriasis: Safety, efficacy, and effect of dose reduction. Br J Dermatol 152: 1304–1312 [DOI] [PubMed] [Google Scholar]
- Papp KA, Bressinck R, Fretzin S, Goffe B, Kempers S, Gordon KB, Caro I, Walicke PA, Wang X, Menter A 2006. Safety of efalizumab in adults with chronic moderate to severe plaque psoriasis: A phase IIIb, randomized, controlled trial. Int J Dermatol 45: 605–614 [DOI] [PubMed] [Google Scholar]
- Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, Guzzo C, Hsu MC, Wang Y, Li S, et al. 2008. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 371: 1675–1684 [DOI] [PubMed] [Google Scholar]
- Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, Hu C, Day RM 2012a. Efficacy of apremilast in the treatment of moderate to severe psoriasis: A randomised controlled trial. Lancet 380: 738–746 [DOI] [PubMed] [Google Scholar]
- Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, Aras G, Li J, Russell CB, Thompson EH, et al. 2012b. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med 366: 1181–1189 [DOI] [PubMed] [Google Scholar]
- Papp KA, Menter A, Strober B, Langley RG, Buonanno M, Wolk R, Gupta P, Krishnaswami S, Tan H, Harness JA 2012c. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: A Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol 167: 668–677 [DOI] [PubMed] [Google Scholar]
- Papp KA, Poulin Y, Bissonnette R, Bourcier M, Toth D, Rosoph L, Poulin-Costello M, Setterfield M, Syrotuik J 2012d. Assessment of the long-term safety and effectiveness of etanercept for the treatment of psoriasis in an adult population. J Am Acad Dermatol 66: e33–e45 [DOI] [PubMed] [Google Scholar]
- Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, Chan D, Hsu MC, Ho V, Ghislain PD, et al. 2013a. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: Final results from 5 years of follow-up. Br J Dermatol 168: 844–854 [DOI] [PubMed] [Google Scholar]
- Papp KA, Langley RG, Sigurgeirsson B, Abe M, Baker DR, Konno P, Haemmerle S, Thurston HJ, Papavassilis C, Richards HB 2013b. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: A randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol 168: 412–421 [DOI] [PubMed] [Google Scholar]
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM 2013. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Dermatol 133: 377–385 [DOI] [PubMed] [Google Scholar]
- Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R, et al. 2012. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Atherosclerosis 223: 1–68 [DOI] [PubMed] [Google Scholar]
- Perera GK, Di Meglio P, Nestle FO 2012. Psoriasis. Annu Rev Pathol 7: 385–422 [DOI] [PubMed] [Google Scholar]
- Pfizer. 2013. Study evaluating the safety and tolerability of ILV-094 in subjects with psoriasis. http://clinicaltrials.gov/ct2/show/NCT00563524. Accessed July 26, 2013
- Picardi A, Lega I, Tarolla E 2013. Suicide risk in skin disorders. Clin Dermatol 31: 47–56 [DOI] [PubMed] [Google Scholar]
- Piruzian E, Bruskin S, Ishkin A, Abdeev R, Moshkovskii S, Melnik S, Nikolsky Y, Nikolskaya T 2010. Integrated network analysis of transcriptomic and proteomic data in psoriasis. BMC Syst Biol 4: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis C, Cauli A, Pipitone N, Smith C, Barker J, Marchesoni A, Yanni G, Panayi GS 1996. Cutaneous lymphocyte antigen-positive T lymphocytes preferentially migrate to the skin but not to the joint in psoriatic arthritis. Arthritis Rheum 39: 137–145 [DOI] [PubMed] [Google Scholar]
- Ports WC, Khan S, Lan S, Lamba M, Bolduc C, Bissonnette R, Papp K 2013. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol 169: 137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin Y, Langley RG, Teixeira HD, Martel MJ, Cheung S 2009. Biologics in the treatment of psoriasis: Clinical and economic overview. J Cutan Med Surg 13: S49–S57 [DOI] [PubMed] [Google Scholar]
- Prinz JC 2001. Psoriasis vulgaris—A sterile antibacterial skin reaction mediated by cross-reactive T cells? An immunological view of the pathophysiology of psoriasis. Clin Exp Dermatol 26: 326–332 [DOI] [PubMed] [Google Scholar]
- Prinz JC, Vollmer S, Boehncke WH, Menssen A, Laisney I, Trommler P 1999. Selection of conserved TCR VDJ rearrangements in chronic psoriatic plaques indicates a common antigen in psoriasis vulgaris. Eur J Immunol 29: 3360–3368 [DOI] [PubMed] [Google Scholar]
- Punwani N, Scherle P, Flores R, Shi J, Liang J, Yeleswaram S, Levy R, Williams W, Gottlieb A 2012. Preliminary clinical activity of a topical JAK1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol 67: 658–664 [DOI] [PubMed] [Google Scholar]
- Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, et al. 2011. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol 12: 1159–1166 [DOI] [PubMed] [Google Scholar]
- Rashmi R, Rao KS, Basavaraj KH 2009. A comprehensive review of biomarkers in psoriasis. Clin Exp Dermatol 34: 658–663 [DOI] [PubMed] [Google Scholar]
- Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, Li S, Dooley LT, Griffiths CE 2005. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: A phase III, multicentre, double-blind trial. Lancet 366: 1367–1374 [DOI] [PubMed] [Google Scholar]
- Rich P, Sigurgeirsson B, Thaci D, Ortonne JP, Paul C, Schopf RE, Morita A, Roseau K, Harfst E, Guettner A, et al. 2013. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: A randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol 168: 402–411 [DOI] [PubMed] [Google Scholar]
- Roberson ED, Liu Y, Ryan C, Joyce CE, Duan S, Cao L, Martin A, Liao W, Menter A, Bowcock AM 2012. A subset of methylated CpG sites differentiate psoriatic from normal skin. J Invest Dermatol 132: 583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F 2004. The inflammatory response in mild and in severe psoriasis. Br J Dermatol 150: 917–928 [DOI] [PubMed] [Google Scholar]
- Russell TJ, Schultes LM, Kuban DJ 1972. Histocompatibility (HL-A) antigens associated with psoriasis. N Engl J Med 287: 738–740 [DOI] [PubMed] [Google Scholar]
- Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH 2013. Incidence of cardiovascular disease in individuals with psoriasis: A systematic review and meta-analysis. J Invest Dermatol 133: 2340–2346 [DOI] [PubMed] [Google Scholar]
- Sathish JG, Sethu S, Bielsky MC, de Haan L, French NS, Govindappa K, Green J, Griffiths CE, Holgate S, Jones D, et al. 2013. Challenges and approaches for the development of safer immunomodulatory biologics. Nat Rev Drug Discov 12: 306–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, Unnebrink K, Kaul M, Camez A 2008. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 158: 558–566 [DOI] [PubMed] [Google Scholar]
- Schlaak JF, Buslau M, Jochum W, Hermann E, Girndt M, Gallati H, Meyer zum Buschenfelde KH, Fleischer B 1994. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol 102: 145–149 [DOI] [PubMed] [Google Scholar]
- Setta-Kaffetzi N, Navarini AA, Patel VM, Pullabhatla V, Pink AE, Choon SE, Allen MA, Burden AD, Griffiths CE, Seyger MM, et al. 2013. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol 133: 1366–1369 [DOI] [PubMed] [Google Scholar]
- Shen C, Van Assche G, Rutgeerts P, Ceuppens JL 2006. Caspase activation and apoptosis induction by adalimumab: Demonstration in vitro and in vivo in a chimeric mouse model. Inflamm Bowel Dis 12: 22–28 [DOI] [PubMed] [Google Scholar]
- Shibata S, Saeki H, Tada Y, Karakawa M, Komine M, Tamaki K 2009. Serum high molecular weight adiponectin levels are decreased in psoriasis patients. J Dermatol Sci 55: 62–63 [DOI] [PubMed] [Google Scholar]
- Sivamani RK, Goodarzi H, Garcia MS, Raychaudhuri SP, Wehrli LN, Ono Y, Maverakis E 2013. Biologic therapies in the treatment of psoriasis: A comprehensive evidence-based basic science and clinical review and a practical guide to tuberculosis monitoring. Clin Rev Allergy Immunol 44: 121–140 [DOI] [PubMed] [Google Scholar]
- Sjogren F, Davidsson K, Sjostrom M, Anderson CD 2012. Cutaneous microdialysis: Cytokine evidence for altered innate reactivity in the skin of psoriasis patients? AAPS J 14: 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S 2011. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA 305: 2525–2531 [DOI] [PubMed] [Google Scholar]
- Spits H, Cupedo T 2012. Innate lymphoid cells: Emerging insights in development, lineage relationships, and function. Annu Rev Immunol 30: 647–675 [DOI] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, et al. 2013. Innate lymphoid cells—A proposal for uniform nomenclature. Nat Rev Immunol 13: 145–149 [DOI] [PubMed] [Google Scholar]
- Strange A, Capon F, Spencer CC, Knight J, Weale ME, Allen MH, Barton A, Band G, Bellenguez C, Bergboer JG, et al. 2010. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet 42: 985–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober BE, Crowley JJ, Yamauchi PS, Olds M, Williams DA 2011. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol 165: 661–668 [DOI] [PubMed] [Google Scholar]
- Stuart PE, Nair RP, Ellinghaus E, Ding J, Tejasvi T, Gudjonsson JE, Li Y, Weidinger S, Eberlein B, Gieger C, et al. 2010. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat Genet 42: 1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M, Fuentes-Duculan J, Lowes MA, Krueger JG 2011. Resolved psoriasis lesions retain expression of a subset of disease-related genes. J Invest Dermatol 131: 391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG 2012. Expanding the psoriasis disease profile: Interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol 132: 2552–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LD, Cheng H, Wang ZX, Zhang AP, Wang PG, Xu JH, Zhu QX, Zhou HS, Ellinghaus E, Zhang FR, et al. 2010. Association analyses identify six new psoriasis susceptibility loci in the Chinese population. Nat Genet 42: 1005–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR, Johnston A, Carbajal S, Han G, Wohn C, Lu J, Xing X, Nair RP, Voorhees JJ, Elder JT, et al. 2011. Genome-wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PLoS ONE 6: e18266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CS, Koralnik IJ 2010. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: Clinical features and pathogenesis. Lancet Neurol 9: 425–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejasvi T, Stuart PE, Chandran V, Voorhees JJ, Gladman DD, Rahman P, Elder JT, Nair RP 2012. TNFAIP3 gene polymorphisms are associated with response to TNF blockade in psoriasis. J Invest Dermatol 132: 593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Krueger JG, Li K, Jabbari A, Brodmerkel C, Lowes MA, Suarez-Farinas M 2012. Meta-analysis derived (MAD) transcriptome of psoriasis defines the “core” pathogenesis of disease. PLoS ONE 7: e44274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiilikainen A, Lassus A, Karvonen J, Vartiainen P, Julin M 1980. Psoriasis and HLA-Cw6. Br J Dermatol 102: 179–184 [DOI] [PubMed] [Google Scholar]
- Tollefson MM, Crowson CS, McEvoy MT, Maradit Kremers H 2010. Incidence of psoriasis in children: A population-based study. J Am Acad Dermatol 62: 979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonel G, Conrad C, Laggner U, Di Meglio P, Grys K, McClanahan TK, Blumenschein WM, Qin JZ, Xin H, Oldham E, et al. 2010. Cutting edge: A critical functional role for IL-23 in psoriasis. J Immunol 185: 5688–5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembath RC, Clough RL, Rosbotham JL, Jones AB, Camp RD, Frodsham A, Browne J, Barber R, Terwilliger J, Lathrop GM, et al. 1997. Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome-wide search in psoriasis. Hum Mol Genet 6: 813–820 [DOI] [PubMed] [Google Scholar]
- Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, Ding J, Li Y, Tejasvi T, Gudjonsson JE, et al. 2012. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet 44: 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, et al. 2006. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: Double-blind placebo-controlled randomised phase III trial. Lancet 367: 29–35 [DOI] [PubMed] [Google Scholar]
- Updike J 1976. From the journal of a leper. The New Yorker July19: 28–33 [Google Scholar]
- Updike J 1985. Personal history: At war with my skin. The New Yorker September 2: 39–57 [Google Scholar]
- Updike J 1989. Self-consciousness: Memoirs. Penguin, London [Google Scholar]
- Valdimarsson H, Thorleifsdottir RH, Sigurdardottir SL, Gudjonsson JE, Johnston A 2009. Psoriasis—As an autoimmune disease caused by molecular mimicry. Trends Immunol 30: 494–501 [DOI] [PubMed] [Google Scholar]
- Valeyev NV, Hundhausen C, Umezawa Y, Kotov NV, Williams G, Clop A, Ainali C, Ouzounis C, Tsoka S, Nestle FO 2010. A systems model for immune cell interactions unravels the mechanism of inflammation in human skin. PLoS Comput Biol 6: e1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, et al. 2009. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 182: 5836–5845 [DOI] [PubMed] [Google Scholar]
- van de Kerkhof PCM, Nestle FO 2012. Psoriasis. In Dermatology (ed. Bolognia JL, Jorizzo JL, Schaffer JV). Elsevier, Amsterdam [Google Scholar]
- van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S, Forejtova S, Zwillich SH, Gruben D, Koncz T, et al. 2012. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 367: 508–519 [DOI] [PubMed] [Google Scholar]
- Veal CD, Capon F, Allen MH, Heath EK, Evans JC, Jones A, Patel S, Burden D, Tillman D, Barker JN, et al. 2002. Family-based analysis using a dense single-nucleotide polymorphism-based map defines genetic variation at PSORS1, the major psoriasis-susceptibility locus. Am J Hum Genet 71: 554–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanova F, Di Meglio P, Nestle FO 2013a. Biomarkers in psoriasis and psoriatic arthritis. Ann Rheum Dis 72: ii104–ii110 [DOI] [PubMed] [Google Scholar]
- Villanova F, Flutter B, Tosi I, Grys K, Sreeneebus H, Perera GK, Chapman A, Smith CH, Di Meglio P, Nestle FO 2013b. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol 134: 984–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C 2013. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: A real issue, a clinical perspective. Ann Rheum Dis 72: 165–178 [DOI] [PubMed] [Google Scholar]
- Vollmer S, Menssen A, Prinz JC 2001. Dominant lesional T cell receptor rearrangements persist in relapsing psoriasis but are absent from nonlesional skin: Evidence for a stable antigen-specific pathogenic T cell response in psoriasis vulgaris. J Invest Dermatol 117: 1296–1301 [DOI] [PubMed] [Google Scholar]
- Wang F, Lee E, Lowes MA, Haider AS, Fuentes-Duculan J, Abello MV, Chamian F, Cardinale I, Krueger JG 2006. Prominent production of IL-20 by CD68+/CD11c+ myeloid-derived cells in psoriasis: Gene regulation and cellular effects. J Invest Dermatol 126: 1590–1599 [DOI] [PubMed] [Google Scholar]
- Watt FM 1989. Terminal differentiation of epidermal keratinocytes. Curr Opin Cell Biol 1: 1107–1115 [DOI] [PubMed] [Google Scholar]
- Weiss G, Shemer A, Trau H 2002. The Koebner phenomenon: Review of the literature. J Eur Acad Dermatol Venereol 16: 241–248 [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 8: 950–957 [DOI] [PubMed] [Google Scholar]
- Wolfe F, Freundlich B, Straus WL 2003. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol 30: 36–40 [PubMed] [Google Scholar]
- Wolf N, Quaranta M, Prescott NJ, Allen M, Smith R, Burden AD, Worthington J, Griffiths CE, Mathew CG, Barker JN, et al. 2008. Psoriasis is associated with pleiotropic susceptibility loci identified in type II diabetes and Crohn disease. J Med Genet 45: 114–116 [DOI] [PubMed] [Google Scholar]
- Wolf R, Mascia F, Dharamsi A, Howard OM, Cataisson C, Bliskovski V, Winston J, Feigenbaum L, Lichti U, Ruzicka T, et al. 2010. Gene from a psoriasis susceptibility locus primes the skin for inflammation. Sci Transl Med 2: 61ra90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Richman L, Morehouse C, de los Reyes M, Higgs BW, Boutrin A, White B, Coyle A, Krueger J, Kiener PA, et al. 2008. Type I interferon: Potential therapeutic target for psoriasis? PloS ONE 3: e2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA 2011. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol 106: 741–747 [DOI] [PubMed] [Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez-Farinas M, Fuentes-Duculan J, Novitskaya I, Khatcherian A, Bluth MJ, Lowes MA, et al. 2007. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med 204: 3183–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, Gonzalez J, Krueger JG, Lowes MA 2009a. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol 129: 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Krueger JG, Lowes MA 2009b. Resident and “inflammatory” dendritic cells in human skin. J Invest Dermatol 129: 302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaba LC, Suarez-Farinas M, Fuentes-Duculan J, Nograles KE, Guttman-Yassky E, Cardinale I, Lowes MA, Krueger JG 2009c. Effective treatment of psoriasis with etanercept is linked to suppression of IL-17 signaling, not immediate response TNF genes. J Allergy Clin Immunol 124: 1022-10.e1-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XJ, Huang W, Yang S, Sun LD, Zhang FY, Zhu QX, Zhang FR, Zhang C, Du WH, Pu XM, et al. 2009. Psoriasis genome-wide association study identifies susceptibility variants within LCE gene cluster at 1q21. Nat Genet 41: 205–210 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W 2007. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445: 648–651 [DOI] [PubMed] [Google Scholar]