Abstract
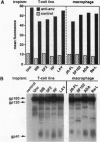
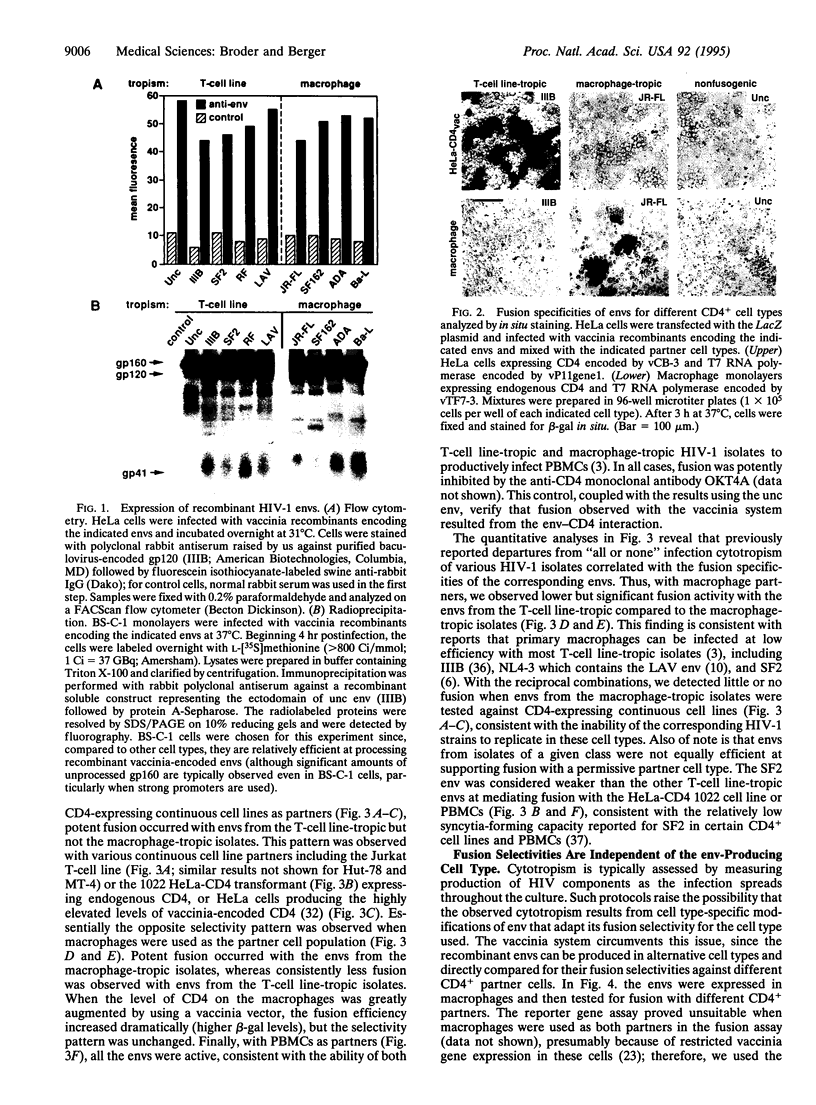
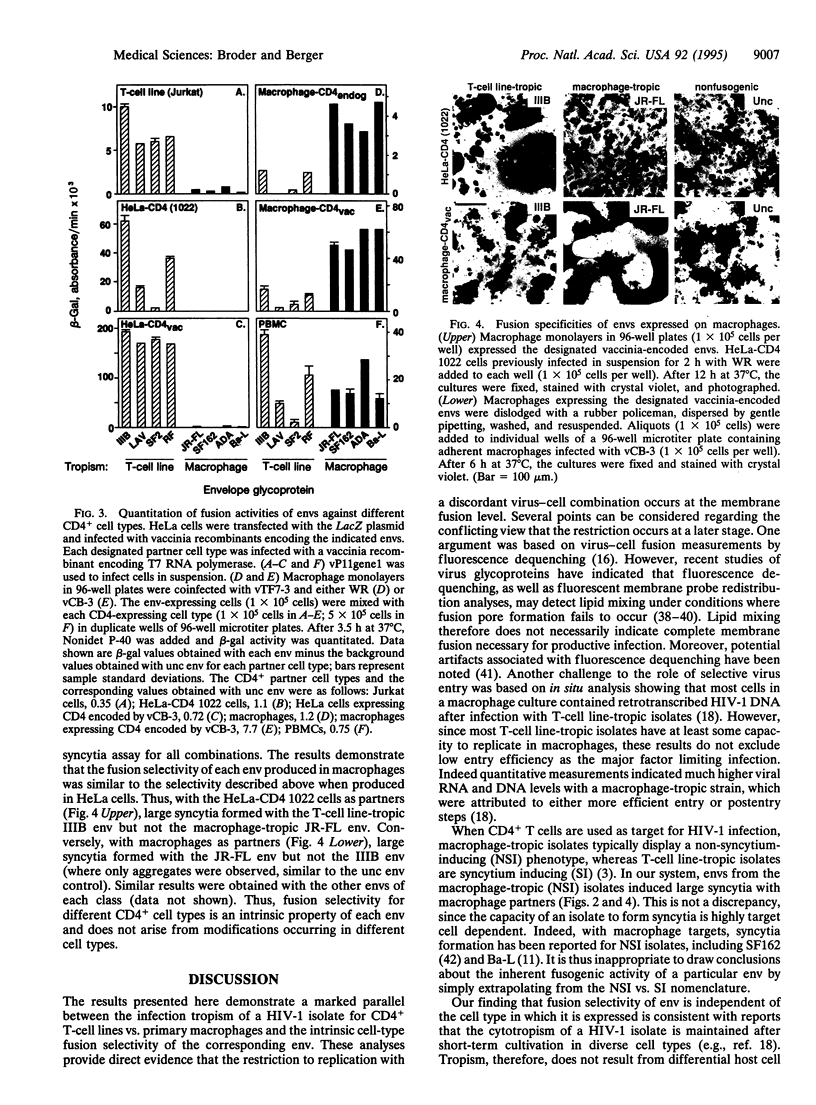
We investigated the relationship between the fusion selectivity of the envelope glycoprotein (env) and the tropism of different human immunodeficiency virus type 1 (HIV-1) isolates for CD4+ human T-cell lines vs. primary macrophages. Recombinant vaccinia viruses were prepared encoding the envs from several well-characterized HIV-1 isolates with distinct cytotropisms. Cells expressing the recombinant envs were mixed with various CD4+ partner cell types; cell fusion was monitored by a quantitative reporter gene assay and by syncytia formation. With CD4+ continuous cell lines as partners (T-cell lines, HeLa cells expressing recombinant CD4), efficient fusion occurred with the envs from T-cell line-tropic isolates (IIIB, LAV, SF2, and RF) but not with the envs from macrophage-tropic isolates (JR-FL, SF162, ADA, and Ba-L). The opposite selectivity pattern was observed with primary macrophages as cell partners; stronger fusion occurred with the envs from the macrophage-tropic than from the T-cell line-tropic isolates. All the envs showed fusion activity with peripheral blood mononuclear cells as partners, consistent with the ability of this cell population to support replication of all the corresponding HIV-1 isolates. These fusion selectivities were maintained irrespective of the cell type used to express env, thereby excluding a role for differential host cell modification. We conclude that the intrinsic fusion selectivity of env plays a major role in the tropism of a HIV-1 isolate for infection of CD4+ T-cell lines vs. primary macrophages, presumably by determining the selectivity of virus entry and cell fusion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander W. A., Moss B., Fuerst T. R. Regulated expression of foreign genes in vaccinia virus under the control of bacteriophage T7 RNA polymerase and the Escherichia coli lac repressor. J Virol. 1992 May;66(5):2934–2942. doi: 10.1128/jvi.66.5.2934-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder C. C., Dimitrov D. S., Blumenthal R., Berger E. A. The block to HIV-1 envelope glycoprotein-mediated membrane fusion in animal cells expressing human CD4 can be overcome by a human cell component(s). Virology. 1993 Mar;193(1):483–491. doi: 10.1006/viro.1993.1151. [DOI] [PubMed] [Google Scholar]
- Broder C. C., Kennedy P. E., Michaels F., Berger E. A. Expression of foreign genes in cultured human primary macrophages using recombinant vaccinia virus vectors. Gene. 1994 May 16;142(2):167–174. doi: 10.1016/0378-1119(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Cann A. J., Zack J. A., Go A. S., Arrigo S. J., Koyanagi Y., Green P. L., Koyanagi Y., Pang S., Chen I. S. Human immunodeficiency virus type 1 T-cell tropism is determined by events prior to provirus formation. J Virol. 1990 Oct;64(10):4735–4742. doi: 10.1128/jvi.64.10.4735-4742.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Quiroga M., Tung J. W., Dina D., Levy J. A. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990 Sep;64(9):4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Shioda T., Levy J. A. Host range, replicative, and cytopathic properties of human immunodeficiency virus type 1 are determined by very few amino acid changes in tat and gp120. J Virol. 1991 Dec;65(12):6931–6941. doi: 10.1128/jvi.65.12.6931-6941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Nishio J., Perryman S., Cann A., O'Brien W., Chen I. S., Wehrly K. Identification of human immunodeficiency virus envelope gene sequences influencing viral entry into CD4-positive HeLa cells, T-leukemia cells, and macrophages. J Virol. 1991 Nov;65(11):5782–5789. doi: 10.1128/jvi.65.11.5782-5789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Metcalf J., Griffin D. E. Use of a new CD4-positive HeLa cell clone for direct quantitation of infectious human immunodeficiency virus from blood cells of AIDS patients. J Infect Dis. 1991 Jan;163(1):64–70. doi: 10.1093/infdis/163.1.64. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992 Nov;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collman R. Human immunodeficiency virus type 1 tropism for human macrophages. Pathobiology. 1992;60(4):213–218. doi: 10.1159/000163725. [DOI] [PubMed] [Google Scholar]
- Connor R. I., Ho D. D. Transmission and pathogenesis of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1994 Apr;10(4):321–323. doi: 10.1089/aid.1994.10.321. [DOI] [PubMed] [Google Scholar]
- Dragic T., Charneau P., Clavel F., Alizon M. Complementation of murine cells for human immunodeficiency virus envelope/CD4-mediated fusion in human/murine heterokaryons. J Virol. 1992 Aug;66(8):4794–4802. doi: 10.1128/jvi.66.8.4794-4802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T., Picard L., Alizon M. Proteinase-resistant factors in human erythrocyte membranes mediate CD4-dependent fusion with cells expressing human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1995 Feb;69(2):1013–1018. doi: 10.1128/jvi.69.2.1013-1018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Hügin A. W., Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990 May;64(5):2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Koenig S., Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991 Jan;65(1):31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Groenink M., Fouchier R. A., de Goede R. E., de Wolf F., Gruters R. A., Cuypers H. T., Huisman H. G., Tersmette M. Phenotypic heterogeneity in a panel of infectious molecular human immunodeficiency virus type 1 clones derived from a single individual. J Virol. 1991 Apr;65(4):1968–1975. doi: 10.1128/jvi.65.4.1968-1975.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V. M., Martin J. E., Dapolito G., Elkins W. R., London W. T., Goldstein S., Johnson P. R. Spontaneous substitutions in the vicinity of the V3 analog affect cell tropism and pathogenicity of simian immunodeficiency virus. J Virol. 1994 Apr;68(4):2649–2661. doi: 10.1128/jvi.68.4.2649-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z. B., Potash M. J., Simm M., Shahabuddin M., Chao W., Gendelman H. E., Eden E., Volsky D. J. Infection of macrophages with lymphotropic human immunodeficiency virus type 1 can be arrested after viral DNA synthesis. J Virol. 1993 Nov;67(11):6893–6896. doi: 10.1128/jvi.67.11.6893-6896.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Kemble G. W., Danieli T., White J. M. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994 Jan 28;76(2):383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F., Mori K., Desrosiers R. C. The "V3" domain is a determinant of simian immunodeficiency virus cell tropism. J Virol. 1994 Jun;68(6):3682–3692. doi: 10.1128/jvi.68.6.3682-3692.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdins J. K., Woods-Cook K., Walker M., Alteri E. The lipophilic muramyl peptide MTP-PE is a potent inhibitor of HIV replication in macrophages. AIDS Res Hum Retroviruses. 1990 Oct;6(10):1157–1161. doi: 10.1089/aid.1990.6.1157. [DOI] [PubMed] [Google Scholar]
- Meylan P. R., Spina C. A., Richman D. D., Kornbluth R. S. In vitro differentiation of monocytoid THP-1 cells affects their permissiveness for HIV strains: a model system for studying the cellular basis of HIV differential tropism. Virology. 1993 Mar;193(1):256–267. doi: 10.1006/viro.1993.1121. [DOI] [PubMed] [Google Scholar]
- Miedema F., Meyaard L., Koot M., Klein M. R., Roos M. T., Groenink M., Fouchier R. A., Van't Wout A. B., Tersmette M., Schellekens P. T. Changing virus-host interactions in the course of HIV-1 infection. Immunol Rev. 1994 Aug;140:35–72. doi: 10.1111/j.1600-065x.1994.tb00864.x. [DOI] [PubMed] [Google Scholar]
- Mori K., Ringler D. J., Desrosiers R. C. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J Virol. 1993 May;67(5):2807–2814. doi: 10.1128/jvi.67.5.2807-2814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum O., Broder C. C., Berger E. A. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J Virol. 1994 Sep;68(9):5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien W. A., Koyanagi Y., Namazie A., Zhao J. Q., Diagne A., Idler K., Zack J. A., Chen I. S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990 Nov 1;348(6296):69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Potash M. J., Zeira M., Huang Z. B., Pearce T. E., Eden E., Gendelman H. E., Volsky D. J. Virus-cell membrane fusion does not predict efficient infection of alveolar macrophages by human immunodeficiency virus type 1 (HIV-1). Virology. 1992 Jun;188(2):864–868. doi: 10.1016/0042-6822(92)90543-x. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Schmidtmayerova H., Bolmont C., Baghdiguian S., Hirsch I., Chermann J. C. Distinctive pattern of infection and replication of HIV1 strains in blood-derived macrophages. Virology. 1992 Sep;190(1):124–133. doi: 10.1016/0042-6822(92)91198-4. [DOI] [PubMed] [Google Scholar]
- Schoch C., Blumenthal R. Role of the fusion peptide sequence in initial stages of influenza hemagglutinin-induced cell fusion. J Biol Chem. 1993 May 5;268(13):9267–9274. [PubMed] [Google Scholar]
- Schuitemaker H., Groenink M., Meyaard L., Kootstra N. A., Fouchier R. A., Gruters R. A., Huisman H. G., Tersmette M., Miedema F. Early replication steps but not cell type-specific signalling of the viral long terminal repeat determine HIV-1 monocytotropism. AIDS Res Hum Retroviruses. 1993 Jul;9(7):669–675. doi: 10.1089/aid.1993.9.669. [DOI] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Stamatatos L., Düzgüneş N. Simian immunodeficiency virus (SIVmac251) membrane lipid mixing with human CD4+ and CD4- cell lines in vitro does not necessarily result in internalization of the viral core proteins and productive infection. J Gen Virol. 1993 Jun;74(Pt 6):1043–1054. doi: 10.1099/0022-1317-74-6-1043. [DOI] [PubMed] [Google Scholar]
- Starcich B. R., Hahn B. H., Shaw G. M., McNeely P. D., Modrow S., Wolf H., Parks E. S., Parks W. P., Josephs S. F., Gallo R. C. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986 Jun 6;45(5):637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Schoen P., Bron R., Wey J., Bartoldus I., Ortiz A., Nieva J. L., Wilschut J. Evaluation of viral membrane fusion assays. Comparison of the octadecylrhodamine dequenching assay with the pyrene excimer assay. Biochemistry. 1993 Oct 26;32(42):11330–11337. doi: 10.1021/bi00093a009. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Gendelman H. E. Cellular and viral determinants that regulate HIV-1 infection in macrophages. J Leukoc Biol. 1994 Sep;56(3):278–288. doi: 10.1002/jlb.56.3.278. [DOI] [PubMed] [Google Scholar]
- Weiner D. B., Huebner K., Williams W. V., Greene M. I. Human genes other than CD4 facilitate HIV-1 infection of murine cells. Pathobiology. 1991;59(6):361–371. doi: 10.1159/000163679. [DOI] [PubMed] [Google Scholar]
- Westervelt P., Gendelman H. E., Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]