Abstract.
The near-infrared (NIR) fluorescence signal in the 700 to 900 nm from molecular probes used in fluorescence image-guided surgery (FIGS) is usually weak compared to the NIR component from white light-emitting diode surgical light, which is typically switched off during FIGS to enhance the molecular fluorescence contrast of the image. We propose a simple solution to this critical issue in FIGS by removing NIR light from surgical light with a low cost commercial 3M cool mirror film 330.
Keywords: fluorescence imaging, image-guided surgery, imaging system
1. Introduction
Near-infrared (NIR) fluorescence imaging is widely employed as a surgical guidance tool in surgery because of the low autofluorescence of tissue and the deeper penetration of light in tissue in this region.1,2 A major issue in fluorescence image-guided surgery (FIGS) is the negative impact of the surgical lighting on the fluorescence image contrast. Surgical lighting in an operation room uses high illuminance ranging from 40,000 to 160,000 lux to improve the tissue visualization during surgery.3 Light-emitting diode (LED) surgical lights are replacing the traditional tungsten halogen and gas discharge lamps because they can illuminate the subjects’ true colors more accurately than bulb lights and the arrangements of small light sources fixed at different angles minimize shadows for a more consistent light. In addition, LEDs offer a longer lifetime, less power consumption, less heat generation, and low ultraviolet (UV) radiation. While an LED has a much lower NIR component compared to traditional halogen lamps, its NIR light component is still very strong compared to the NIR fluorescence from the fluorescent molecular probes used in FIGS. To obtain high contrast NIR fluorescence image, many FIGS systems require the surgical lights to be turned off or dimmed significantly during fluorescence imaging.1,4 The lighting change in the surgical field interrupts clinical workflow and reduces the convenience of the FIGS to practicing applications. To address this issue, several methods have been developed, including pulsed excitation and homodyne detection.5,6 While those methods can work under normal surgical light, the systems are relatively complicated and expensive.
2. Method to Address the Impact of the Surgical Light
We present a simple and low-cost solution to minimize the impact of white LED surgical light on NIR fluorescence imaging. Similar to the common solution to reduce the impact of the excitation light using a long-pass filter in the imaging path, we minimize the NIR component of the light from the white LED surgical light using a short-pass filter in the illumination path. Due to the large dimensions of the surgical light, it is not feasible to use conventional glass short-pass filters based on the interference thin film because they are expensive and it is difficult to modify the existing LED lamps in the surgical room. Instead, we use a low-cost commercial 3M cool mirror film 330 to minimize the NIR spectrum with a wavelength longer than 650 nm,7 the price of which is less than 1% of the glass short-pass filter. 3M cool mirror film 330 is an all-polymeric mirror film which has high transmission in the visible spectrum with an average reflectance at a normal incidence of 89% or greater for wavelengths longer than 650 nm. Its thickness is only 0.107 mm, and it can be fabricated as large as 48 in., sufficient to cover the entire white LED surgical lamp. The advantage of using this film is that it can be easily cut to shape and laminated to the normal white LED surgical lamps without any additional modification.
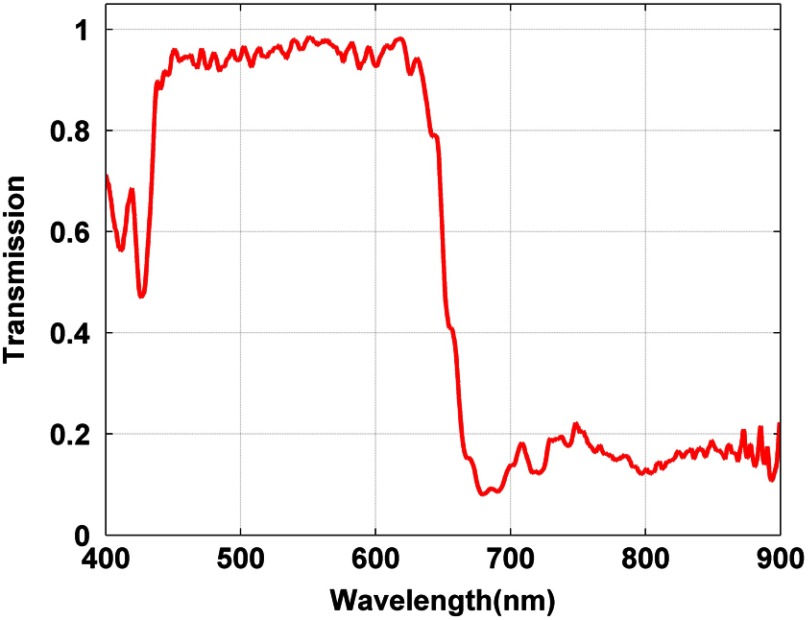
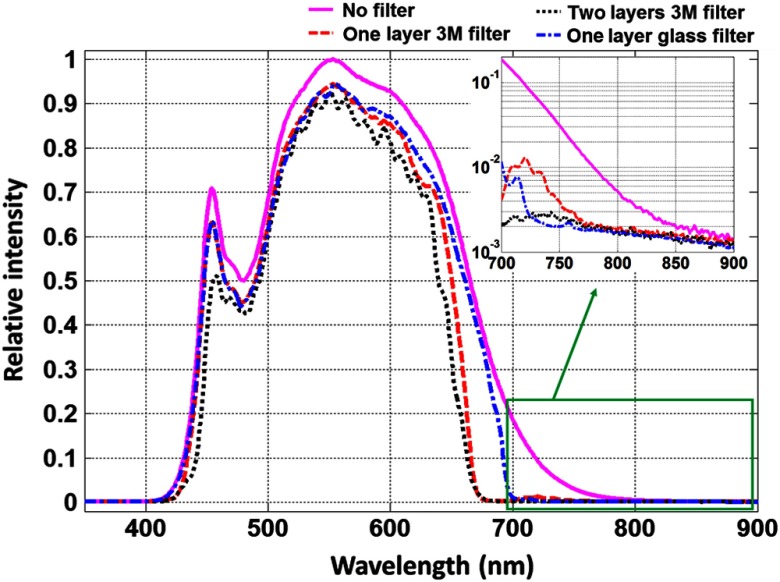
The transmission spectrum of the 3M film 330 was measured via a HR2000+ spectrophotometer (Ocean Optics, Dunedin, Florida) and a broadband spectrum lamp as a light source. The result is plotted in Fig. 1. The transmission over the visible spectrum is higher than 90%, and the reflectance is higher than 80% in NIR. The LED surgical lamp tested in this letter is CHROMOPHARE F628 by BERCHTOLD BERCHTOLD (Tuttlingen, Germany). Figure 2 shows the measured spectrum of the white LED surgical light and spectrums after passing 1 layer of the 3M cool mirror film 330, 2 layers of the 3M cool mirror film 330, and a single glass short-pass filter. As shown in the inserted figure in logarithmic scale, the NIR component of the white LED light is significantly reduced with both the 3M film and glass short-pass filter. The single glass short-pass filter has a slightly better performance in reducing NIR transmission, but it is more difficult and more expensive to use.
Fig. 1.
Transmission spectrum of 3M cool mirror film 330.
Fig. 2.
Spectra of white LED surgical lamp with and without 3M cool mirror film 330.
To evaluate the performance of the 3M cool mirror film 330 for surgical lighting, we measured the illuminance, color rendering index (CRI) Ra, CRI R9, and correlated color temperature (CCT) of surgical light before and after passing a 3M film via a HR2000+ spectrophotometer. The CRI Ra is a quantitative measure of the ability of a light source to reveal the colors of various objects compared to an ideal light source. The higher the CRI Ra the better the color rendering. The CRI R9 measures the ability of the light source in rendering the red color, which is an important parameter in evaluating the performance of an LED light source because some of the white LED light sources lack a red spectrum. CCT is a specification of the color appearance of the light. It is recommended that the surgical lamps should have a CRI Ra and CRI R9 higher than 85, and a CCT not less than 3000 K and not greater than 6700 K.8
The results show that the illuminance drops by about 13% after the application of one layer of 3M film 330 and 19% after two layers (Table 1). Since most of the surgical lights have a strong optical output, this optical power loss could be acceptable. The change of CRI Ra after using 3M film is negligible since 3M film only blocks the NIR component. As shown in Table 1, the reading of CRI R9 after passing 1 layer of 3M film 330 changes slightly. The CCT after passing the 3M film increases slightly since the 3M film blocks the red component in the visible spectrum. These results show that the changes of three critical factors (CRI Ra, CRI R9, and CCT) in surgical lighting are small, particularly when a single film 330 is used.
Table 1.
Illuminance and color performance of surgical light without and after 3M film.
| No filter | One layer | Two layers | |
|---|---|---|---|
| Illuminance (lux) | 54,047 | 47,051 | 43,900 |
| CRI Ra | 96.7 | 96.4 | 95.7 |
| CRI R9 | 89.9 | 89.7 | 83.7 |
| CCT | 5264 K | 5392 K | 5402 K |
3. Experimental Evaluation
To evaluate the feasibility of this solution in NIR fluorescence imaging, a custom developed dual-mode camera was used to capture NIR fluorescence images of ICG samples.9 ICG solution samples (Sigma-Aldrich, St. Louis, Missouri) of different concentrations ranging from 130 nM to in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) and a pure DMSO sample were illuminated under white LED surgical light with an illuminance of 40960 lux. A long-pass filter (Semrock BLP01-808R-25, Rochester, New York) was placed in front of the monochromatic camera to block the visible and excitation lights below 800 nm. Two high power NIR laser diodes (LDs) were used to excite the fluorescence signal from ICG samples. The NIR LD has an optical output power of 2 W at a peak wavelength of 785 nm. Bandpass filters (Semrock FF01-769/41-25) were placed in front of the NIR LDs as excitation filters. The exposure time of the camera was 33.3 ms.
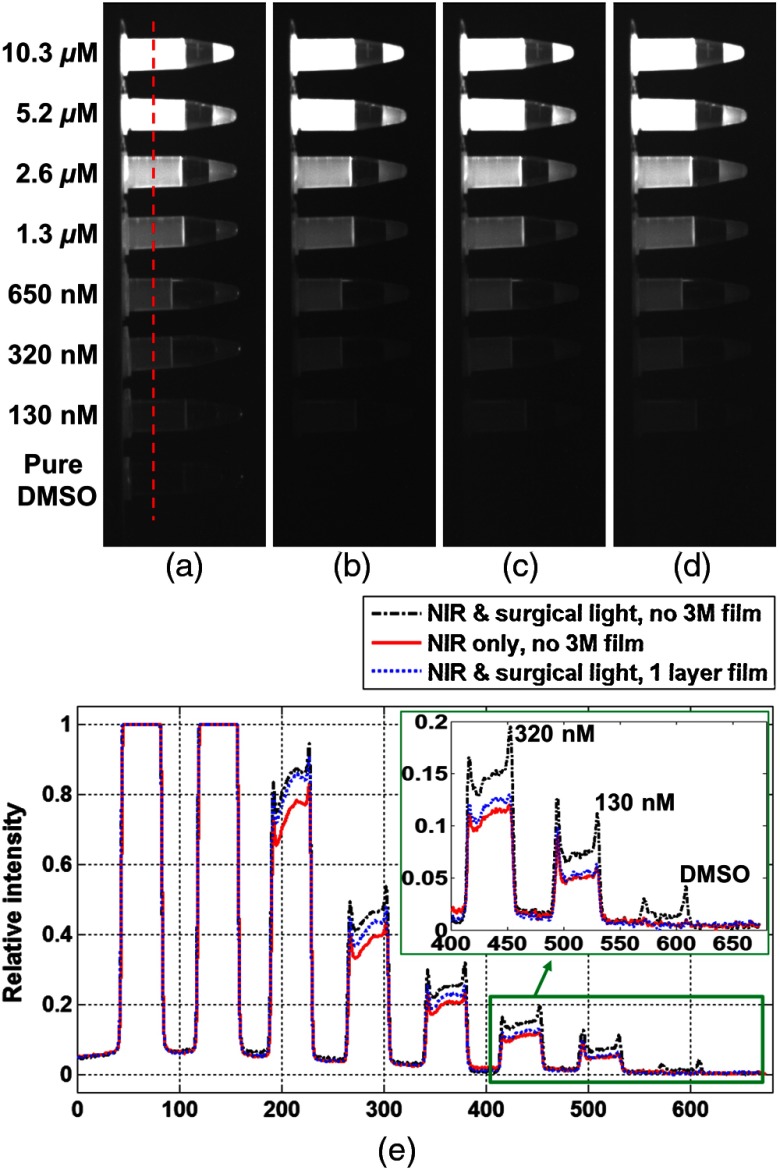
Figure 3 is the experimental result with the concentrations of ICG samples labeled on the left in the figure. Figure 3(a) is the images of the ICG samples and pure DMSO sample when both surgical and NIR fluorescence excitation lights were on but without using 3M film. The container of the pure DMSO sample can be seen because of the NIR spectrum from the surgical light. Figure 3(b) is the images of samples without using 3M film when the surgical light was off and only the NIR excitation light was on. Pure DMSO is not detectable because there is no direct illumination from the surgical light. When both surgical and NIR excitation lights were on, Figs. 3(c) and 3(d) are images with one and two layers of 3M film to block the NIR component from surgical light. Figure 3(e) compares the relative intensities of fluorescence signals in Figs. 3(a) to 3(c) along the vertical red dotted line in Fig. 3(a), while the inserted figure shows that a single layer 3M film 330 is sufficient to minimize the impact of white LED surgical light on ICG NIR fluorescence imaging. Figure 3(e) also shows that, when the ICG concentration is higher, the leakage of NIR light between 700 and 800 nm from the white LED surgical light can excite a reasonable amount of fluorescence.
Fig. 3.
Fluorescence images of ICG samples, (a) under NIR excitation and surgical light without 3M film, (b) NIR excitation only without 3M film, (c) NIR excitation and surgical light with one layer of 3M film, (d) NIR excitation and surgical light with two layers of 3M film and (e) relative intensity curves along the red dotted line in (a) for (a)–(c).
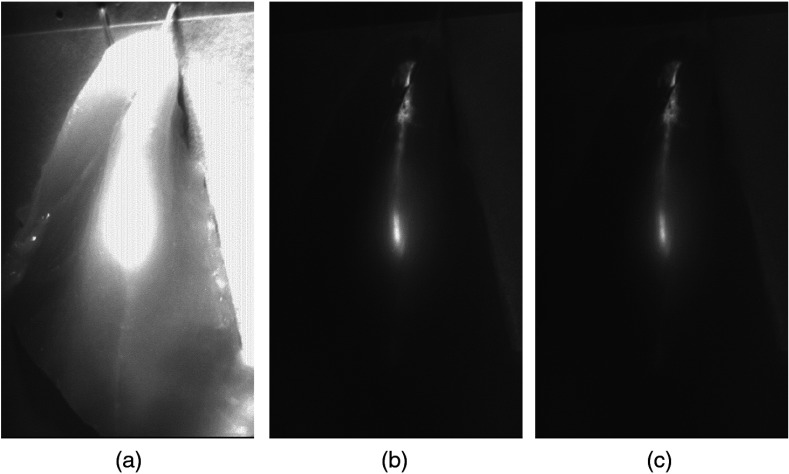
We also demonstrated this solution with fresh chicken breast tissue. ICG solution with a concentration of in DMSO was injected about 5 mm under the surface of the chicken tissue. Figure 4(a) is the NIR fluorescence image of chicken breast tissue under both surgical and NIR excitation lights without using 3M film 330 when the exposure time was 33.3 ms. Because the NIR component of the LED surgical light was not blocked, the surface of the chicken breast tissue was saturated with strong illumination from the surgical light. Figure 4(b) is the fluorescence image under both surgical and excitation lights when one layer of 3M filter film was used. The ICG fluorescence signal is clearly imaged and the fluorescence signal to background contrast ratio is as high as 3.3, showing the effectiveness of the proposed solution in minimizing the impact of white LED surgical light on NIR fluorescence imaging. Figure 4(c) is the fluorescence image under both surgical and excitation lights with two layers of 3M filter film. It is clear that a high contrast NIR fluorescence image can be obtained with one or two layers of 3M film 330 to block the NIR component of white LED surgical light.
Fig. 4.
Chicken breast tissue under surgical lighting and excitation illumination (a) without 3M film 330, (b) with one layer of 3M film and (c) with two layers of 3M film.
4. Conclusion
In summary, a simple solution is demonstrated to reduce the impact of a white LED surgical lamp on NIR fluorescence image-guided surgery. The commercial 3M cool mirror film 330 can be laminated to the surgical lamp to block the NIR component of the illumination spectrum. Although this film reduces the illuminance, the illuminance is still sufficient for the surgical procedures. Measured CRI Ra, CRI R9, and CCT with and without 3M film 330 show that the impact of the film on color performance is minimal. The experiments with ICG samples and chicken breast tissue show that a single layer of 3M film 330 can effectively minimize the impact of the surgical light. More layers can be used for lamps with a stronger NIR spectrum. This solution is low cost and can be easily implemented in different types of surgical lamps without any additional modification. Although we have demonstrated the effectiveness of this solution with ICG samples and chicken breast tissue, a more extensive study with human subjects in real surgical procedures is needed.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA171651. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Dr. Farid Gharagozloo, Keith Mass, and Duran Powers at University of Arizona Medical Center for their help on the experiment in the surgical room. We also thank 3M for providing 3M cool mirror film 330.
Biography
Biographies of the authors not available.
References
- 1.Troyan S. L., et al. , “The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping,” Ann. Surg. Oncol. 16(10), 2943–52 (2009). 10.1245/s10434-009-0594-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y., et al. , “Near-infrared fluorescence goggle system with complementary metal–oxide–semiconductor imaging sensor and see-through display,” J. Biomed. Opt. 18(10), 101303 (2013). 10.1117/1.JBO.18.10.101303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Department of Energy, “Technical guidance document: LED surgical task lighting,” Building Technologies Program (2012).
- 4.Themelis G., et al. , “Real-time intraoperative fluorescence imaging system using light-absorption correction,” J. Biomed. Opt. 14(6), 064012 (2009). 10.1117/1.3259362 [DOI] [PubMed] [Google Scholar]
- 5.Sexton K., et al. , “Pulsed-light imaging for fluorescence guided surgery under normal room lighting,” Opt. Lett. 38, 3249–3252 (2013). 10.1364/OL.38.003249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu B., Rasmussen J. C., Sevick-Muraca E. M., “Non-invasive fluorescence imaging under ambient light conditions using a modulated ICCD and laser diode,” Biomed. Opt. Express 5(2), 562–572 (2014). 10.1364/BOE.5.000562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.http://solutions.3m.com/wps/portal/3M/en_US/Renewable/Energy/Product/Films/Cool_Mirror/://solutions.3m.com/wps/portal/3M/en_US/Renewable/Energy/Product/Films/Cool_Mirror/ (July 2014).
- 8.“LED surgical task lighting scoping study: A hospital energy alliance project,” US Department of Energy (2011).
- 9.Zhu N., et al. , “Dual-mode optical imaging system for fluorescence image-guided surgery,” Opt. Lett. 39, 3830–3832 (2014). 10.1364/OL.39.003830 [DOI] [PMC free article] [PubMed] [Google Scholar]