Abstract
Mutations in either tubby or tubby-like protein 1 (Tulp1) cause retinal degeneration with undefined mechanisms. We recently identified both proteins with unconventional secretion as novel MerTK-specific phagocytosis ligands for retinal pigment epithelium (RPE) cells. Using our newly-developed open reading frame (ORF) phage display as a technology for protein-protein interactions, we identified Tulp1 as a Tubby-binding protein. The interaction of tubby and Tulp1 was verified by yeast two-hybrid and protein pull-down assays. Tubby and Tulp1 form heterodimer or heterooligomer and their interaction was functionally revealed by their synergistic stimulation of RPE phagocytosis. Tubby and Tulp1 mediated phagocytosis through MerTK-dependent signaling with non-muscle myosin II redistribution leading to colocalization of phagocytosed vesicles with rearranged NMMIIA.
Keywords: Synergistic interaction, Tubby, Tubby-like protein 1, Tulp1, Retinal pigment epithelium, Phagocytosis, MerTK, Retinal degeneration, Phagocytosis ligands, ORF phage display
64.1 Introduction
Tubby and tubby-like protein 1 (Tulp1) belong to tubby protein family with four members (tubby, Tulp1, 2 and 3; Tulps), which share the highly conserved C-terminal region of the ‘tubby domain’ with ~ 260 amino acids [1]. A spontaneous mutation in tubby gene causes adult onset obesity, progressive retinal and cochlear degeneration in Tubby mice with undefined mechanisms, whereas mutations in Tulp1 associate only with retinal degeneration [2–3]. Several cellular functions have been characterized for Tulp1, including interactions with F-actin and GTPase dyna-min-1 [4], photoreceptor synaptic development and photoreceptor protein transport pathways [5]. Both tubby and Tulp1 were reported as signaling molecules for G protein-coupled receptors (GPCRs) and putative nuclear factors that regulate gene transcription [6]. Despite their nuclear translocation and putative C-terminal binding to double-stranded DNA, no target gene transcriptionally regulated by tubby or Tulp1 have been identified. We recently reported unconventional secretion of tubby and Tulp1 [7] and characterized both proteins as MerTK-specific ligands that facilitate retinal pigment epithelium (RPE) phagocytosis [8]. Here we describe that tubby interacts with Tulp1. Their interaction synergistically facilitates RPE phagocytosis. Given the broad roles of tubby and Tulp1 in the nuclei, cytoplasm and extracellular environment, tubby and Tulp1 interaction may synergistically modulate an array of cellular functions with broad implication.
64.2 Materials and Methods
64.2.1 Screening of Tubby-Binding Proteins
Recombinant adenovirus expressing FLAG-tubby-F or GFP-FLAG was generated as previously described [9] and used to infect HEK293 cells to express the recombinant protein. Protein was purified using anti-FLAG mAb affinity columns (Sigma), eluted with FLAG peptide (100 mg/ml), and dialyzed against PBS. Purified tubby-F and GFP were immobilized onto the ELISA plates (5 mg/ml, 100 ml/well) and used for phage binding using open reading frame (ORF) phage display [10]. After three rounds of phage selection, individual clones were randomly picked from plates of enriched phages and analyzed for their binding to purified Tubby. Positive clones were identified by DNA sequencing.
64.2.2 Y2H Assay
Tubby-N-terminal cDNA was cloned into pGBT9 plasmid (Clontech) at EcoRI and BamHI sites. The cDNA inserts of identified phages were amplified by PCR and cloned into pGAD424 plasmid (Clontech) at BamHI and SalI sites. Y2H assay was performed according to the manufacturer’s protocol. Briefly, S. cerevisiae CG-1945 strain was co-transformed with cDNA insert/pGAD424 and tubby-N/pGBT9 or pGBT9 control. The transformed yeast was selected on agar plates with double (-Leu/-Trp) or triple (-Leu/-Trp/-His) dropout of the essential amino acids. Dropout- resistant yeast clones were re-grown on YPD plates, lifted onto a filter paper and analyzed for b-galactosidase expression using X-gal as a substrate.
64.2.3 Protein Pull-Down Assay
Full-length coding sequence of mouse Tulp1 or Tubby was amplified by PCR and cloned into pcDNA3 with an N-terminal FLAG tag. HEK293 cells were transfected with the plasmid expressing FLAG-tagged Tulp1 with Lipofectamine reagent (Invitrogen). The cells were harvested at 48 h post-transfection and washed. Cell lysate was prepared in PBS containing 0.5 % Triton X-100, spun and incubated with purified GST-tubby-F or GST control, followed by glutathione resin. The resin was washed and analyzed by Western blot using anti-FLAG mAb.
64.2.4 RPE Phagocytosis Assay
Membrane vesicles were prepared from Neuro-2a cells transfected with Tubby, Tulp1, and/or control plasmid(s) that also co-expressed plasma membrane-targeted green fluorescent protein (mGFP) [11]. Plasma membrane vesicles were incubated with human ARPE19 RPE cells for 3 h at 37 °C for phagocytosis. Phagocytosed fluorescent signals were analyzed by confocal microscopy [11].
64.2.5 Immunocytochemistry
ARPE19 RPE cells were incubated with membrane targeted GFP (mGFP)-labeled membrane vesicles in the presence or absence of Mer-Fc for 3 h, washed, fixed with 4 % buffered formalin, permeabilized with 0.5 % Triton X-100 in PBS, and incubated with rabbit anti-myosin II-A or anti-Lamp-1 Ab (Sigma). Bound Abs were detected by Texas Red-labeled secondary Abs and analysed by confocal microscopy for colocalization of mGFP and Texas Red signals.
64.3 Results
64.3.1 Tubby and Tulp1 Facilitate Phagocytosis in Synergy
ORF phage display screening with purified tubby N-terminal domain (Tubby-N) as bait identified Tulp1 as a tubby-binding protein. The interaction of tubby and Tulp1 was independently verified by yeast two-hybrid assay and protein pull-down assay (Fig. 64.1). Tubby or Tulp1 alone stimulated RPE phagocytosis of POS. The combination of tubby and Tulp1 synergistically stimulated RPE ingestion of membrane vesicles (Fig. 64.1).
Fig. 64.1.
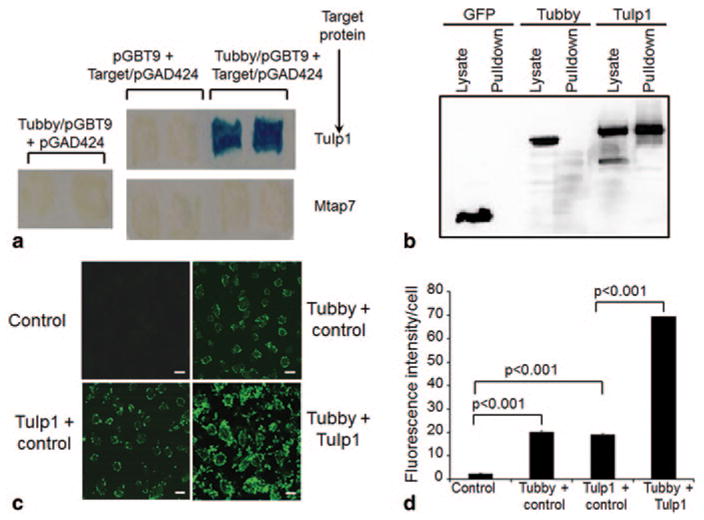
Tubby and Tulp1 interact and synergistically stimulate RPE phagocytosis. a Yeast two-hybrid assay. Tubby N-terminal domain in pGBT9 plasmid and Tulp1 in pGAD424 plasmid were co-expressed in S. cerevisiae CG-1945 stain, selected with -Leu/-Trp/-His triple dropout plates. Individual plasmids as controls were selected on -Leu/-Trp double dropout plates. Mtap7 was included as a negative control. Dropout-resistant yeast colonies were re-grown on a plate, lifted onto filter and analyzed for β-galactosidase expression with X-gal. Blue color indicated that the target protein interacts with tubby. b Protein pull-down assay. Glutathione S-transferase (GST)-tubby fusion protein was expressed, purified, incubated with cell lysates of FLAG-tagged tubby, Tulp1, or GFP (green fluorescent protein), pull-downed with glutathione resin, washed and analyzed by Western blot using anti-FLAG monoclonal antibodies. c A mixture of mGFP-labeled plasma membrane vesicles expressing tubby, Tulp1 or control was analyzed for RPE phagocytosis with human ARPE19 RPE cells for 3 h at 37 °C. Phagocytosed fluorescent signals were analyzed by confocal microscopy. Bar = 10 μm. d Relative fluorescence intensity per cell in c was quantified with > 100 cells counted per group (± SEM, n > 100, t-test). (Caberoy et al. 2010 [10] with permission from J Mol Recognit for a; Caberoy et al. 2010 [11] with permission from Exp Cell Res for b–d)
64.3.2 Tubby and Tulp1 Mediate Phagocytosis Through MerTK
To identify the phagocytic receptor for tubby and Tulp1, we screened their interaction with several known receptors. Tubby and Tulp1 bound to Mer tyrosine kinase (MerTK) [8]. We then analyzed the role of MerTK in tubby- and Tulp1-mediated RPE phagocytosis. MerTK receptor dimerization is known to facilitate receptor activation and phosphorylation. Tubby or Tulp1 alone induced MerTK activation [8] (Fig. 64.2). Excessive soluble Mer-Fc (MerTK extracellular domain fused to human IgG1 Fc domain) blocked tubby- or Tulp1-mediated phagocytosis. Both ligands induced MerTK activation with receptor phosphorylation and signaling cascade, including non-muscle myosin II redistribution and co-localization with phagocytosed vesicles.
Fig. 64.2.
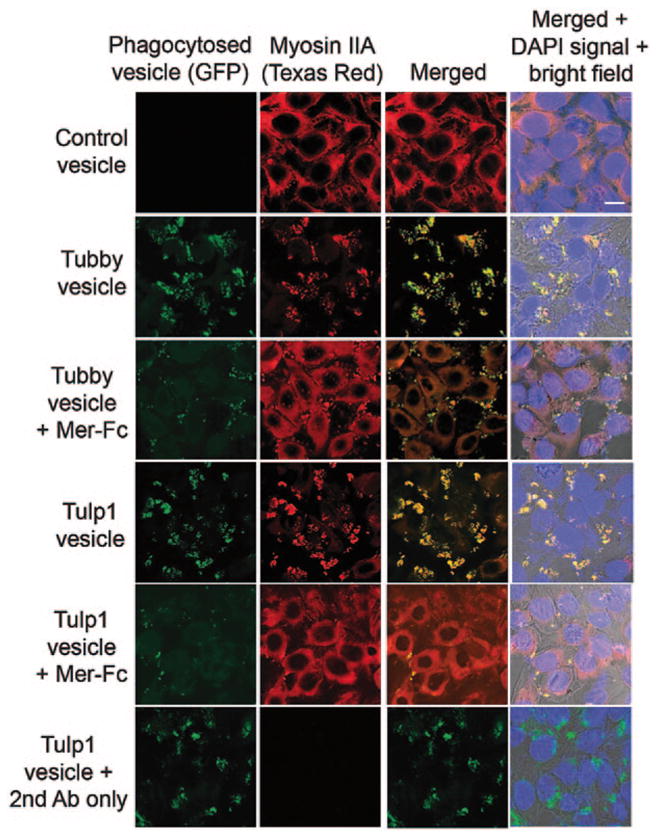
Tubby- and Tulp1-mediated RPE phagocytosis was MerTK-dependent. mGFP-labeled plasma membrane vesicles were prepared from Neuro-2a cells expressing tubby or Tulp1, and incubated with ARPE19 cells in the presence or absence of excess Mer-Fc (MerTK extracellular domain covalently fused to human IgG1 Fc domain, R&D Systems) for phagocytosis assay as in Fig. 64.1. After washing, ARPE19 cells were fixed, permeabilized, detected with anti-non-muscle myosin II (NMMII) A heavy chain antibodies, detected with Texas red-labeled secondary antibodies and analyzed by confocal microscopy. Fluorescence signals for mGFP and NMMII-A were co-localized. Bar = 10 μm. (Caberoy et al. 2010 [8] with permission from EMBO J)
64.4 Discussion
Mutations in either tubby or Tulp1 associate with autosomal recessive retinal degeneration with unknown mechanisms. Tubby and Tulp1 facilitate phagocytosis in RPE cells and both proteins bind to and activate MerTK. RPE phagocytosis has been previously demonstrated to be critical in preventing retinal degeneration in Royal College of Surgeons (RCS) rats with a mutation in the well-characterized phagocytic receptor MerTK [12]. If tubby and Tulp1 facilitates RPE phagocytosis through MerTK, it is tempting to speculate that they are essential ligands to prevent retinal degeneration. An intriguing question is why these two functionally redundant proteins fail to compensate each other in their autosomal recessive mutations. One of the possible explanations is that tubby and Tulp1 synergistically stimulate RPE phagocytosis. The failure of mutant tubby or Tulp1 to stimulate phagocytosis in synergy may be responsible for the lack of their functional compensation, leading to autosomal recessive retinal degeneration with mutation in either gene. However, the caveat is that these data and our previous studies [8, 11] are sufficient only to support tubby and Tulp1 as RPE ligands, but not enough to support a claim that they are essential ligands to prevent retinal degeneration. Moreover, possible accumulation of unphagocytosed vesicles in POS which is characteristic of MerTK mutation in RCS rats [13] is not observed in Tubby mice and Tulp1−/− mice during retinal degeneration [5, 14, 15]. Similarly, ablation of other known MerTK ligand Gas6 results in minimal defect in phagocytosis and retinal homeostasis [16], possibly due to functional compensation by other MerTK ligand like protein S [17]. Hence, the lack of accumulation of unphagocytosed POS debris in Tubby mice or Tulp1−/− mice could be due to functional compensation by other MerTK ligands. A body of evidence, including previously described intracellular functions of tubby and Tulp1, 16 new tubby-binding proteins identified by our ORF phage display [10] and 4 distinct clinical manifestations of retinal degeneration associated with 23 different hTulp1 mutations [3], suggest that tubby and Tulp1 are proteins with diverse functions. Stimulation of RPE phagocytosis is only one of their important functions. Their critical role as eat-me signals in retinal homeostasis is yet to be delineated. It is possible that the interaction of tubby and Tulp1 may also play important roles in their cytoplasmic and nuclear functions.
Acknowledgments
This study was supported by NIH 1K99EY020865/4R00EY020865 grant to NBC.
References
- 1.Carroll K, Gomez C, Shapiro L. Tubby proteins: the plot thickens. Nat Rev Mol Cell Biol. 2004;5:55–63. doi: 10.1038/nrm1278. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda A, Nishina PM, Naggert JK. The tubby-like proteins, a family with roles in neuronal development and function. J Cell Sci. 2002;115:9–14. doi: 10.1242/jcs.115.1.9. [DOI] [PubMed] [Google Scholar]
- 3.Abbasi AH, Garzozi HJ, Ben-Yosef T. A novel splice-site mutation of TULP1 underlies severe early-onset retinitis pigmentosa in a consanguineous Israeli Muslim Arab family. Mol Vis. 2008;14:675–682. [PMC free article] [PubMed] [Google Scholar]
- 4.Xi Q, Pauer GJ, Ball SL, Rayborn M, Hollyfield JG, Peachey NS, Crabb JW, Hagstrom SA. Interaction between the photoreceptor-specific tubby-like protein 1 and the neuronal-specific GTPase dynamin-1. Invest Ophthalmol Vis Sci. 2007;48:2837–2844. doi: 10.1167/iovs.06-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagstrom SA, Duyao M, North MA, Li T. Retinal degeneration in tulp1 / mice: vesicular accumulation in the interphotoreceptor matrix. Invest Ophthalmol Vis Sci. 1999;40:2795–2802. [PubMed] [Google Scholar]
- 6.Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS, Myszka DG, Shapiro L. G-protein signaling through tubby proteins. Science. 2001;292:2041–2050. doi: 10.1126/science.1061233. [DOI] [PubMed] [Google Scholar]
- 7.Caberoy NB, Li W. Unconventional secretion of tubby and tubby-like protein 1. FEBS Lett. 2009;583:3057–3062. doi: 10.1016/j.febslet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caberoy NB, Zhou Y, Li W. Tubby and tubby-like protein 1 are new ligands for MerTK. EMBO J. 2010;29:3898–3910. doi: 10.1038/emboj.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Huber BT, Grand RJ, Li W. Recombinant adenovirus coexpressing covalent peptide/MHC class II complex and B7-1: in vitro and in vivo activation of myelin basic protein-specific T cells. J Immunol. 2001;167:1297–1305. doi: 10.4049/jimmunol.167.3.1297. [DOI] [PubMed] [Google Scholar]
- 10.Caberoy NB, Zhou Y, Jiang X, Alvarado G, Li W. Efficient identification of tubby-binding proteins by an improved system of T7 phage display. J Mol Recognit. 2010;23:74–83. doi: 10.1002/jmr.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caberoy NB, Maiguel D, Kim Y, Li W. Identification of tubby and tubby-like protein 1 as eat-me signal by phage display. Exp Cell Res. 2010;316:245–257. doi: 10.1016/j.yexcr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 13.Bok D, Hall MO. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol. 1971;49:664–682. doi: 10.1083/jcb.49.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda S, Shiva N, Ikeda A, Smith RS, Nusinowitz S, Yan G, Lin TR, Chu S, Heckenlively JR, North MA, Naggert JK, Nishina PM, Duyao MP. Retinal degeneration but not obesity is observed in null mutants of the tubby-like protein 1 gene. Hum Mol Genet. 2000;9:155–163. doi: 10.1093/hmg/9.2.155. [DOI] [PubMed] [Google Scholar]
- 15.Ohlemiller KK, Hughes RM, Lett JM, Ogilvie JM, Speck JD, Wright JS, Faddis BT. Progression of cochlear and retinal degeneration in the tubby (rd5) mouse. Audiol Neurootol. 1997;2:175–185. doi: 10.1159/000259242. [DOI] [PubMed] [Google Scholar]
- 16.Hall MO, Obin MS, Heeb MJ, Burgess BL, Abrams TA. Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp Eye Res. 2005;81:581–591. doi: 10.1016/j.exer.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]


